Abstract
Molecular details of μ opioid receptor activations were obtained using molecular dynamics simulations of the receptor in the presence of 3 agonists, 3 antagonists, a partial agonist and on the constitutively active T279K mutant. Agonists have a higher probability of direct interactions of their basic nitrogen (N) with Asp147 as compared to antagonists, indicating that direct ligand-Asp147 interactions modulate activation. Medium size substituents on the basic N of antagonists lead to steric interactions that perturb N-Asp147 interactions, while additional favorable interactions occur with larger basic N substituents, such as in N-phenethylnormorphine, restoring N-Asp147 interactions, leading to agonism. With the orvinols, the increased size of the C19 substituent in buprenorphine over diprenorphine leads increased interactions with residues adjacent to Asp147, partially overcoming the presence of the cyclopropyl N substituent, such that buprenorphine is a partial agonist. Results also indicate different conformational properties of the intracellular regions of the transmembrane helices in agonists versus antagonists.
Keywords: Structure-activity relationship, molecular dynamics, binding orientations, agonists and antagonists
Introduction
Opioids are potent analgesics used for the treatment of moderate or severe pain caused by disease, injury, or surgery1. Many semi-synthetic or synthetic opioids were derived from the classic opioids first obtained from opium poppies and there are a number of peptidic opioids based on the endogenous ligands such as enkephalin. Receptors of opioids are seven transmembrane (TM) G-protein coupled receptors (GPCR) in the Rhodopsin (class A) family and γ subfamily2. Three main receptor subtypes are μ, δ, and κ, which are distributed in the central nervous system and the gastrointestinal tract. Over the years a large number of opioids have been developed to improve the therapeutic efficiency, such as convenient dosing and reduced side effects3. However, improvements, especially with respect to side effects such as tolerance and constipation, are still needed, requiring further understanding of the molecular determinants of opioid activity4 and functional selectivity5 to design new analogs with diminished undesired effects.
Efforts towards understanding the relationship of the structure of both peptidic and non-peptidic opioids to their activity to date have involved ligand-based methods due to the lack of experimental 3D structures of the opioid receptors3. These SAR studies led to identification of the central role of the basic nitrogen and the development of the message/address concept6 allowing for qualitative understanding of features on the opioids responsible for binding affinity versus specificity for the receptor type. However, those models were typically limited to individual classes of ligands and not generalizable across the spectrum of peptidic and nonpeptidic opioids. More recent efforts have overcome this limitation and allowed for quantitative models of efficacy of μ and δ opioids to be developed. These efforts, based on the conformationally sampled pharmacophore (CSP) method7, 8, relied on inclusion of all accessible conformations of each opioid during model development and the overlap of probability distributions of distances and angles between functional groups, thereby avoiding the need to align the molecules during model development and allowing for structurally diverse ligands to be included in the model. In addition, the CSP approach allowed for the posteriori identification of bound orientations of the studied opioids and predictions of their interaction orientations with the receptors8.
Towards better understanding of opioid SAR is the recent elucidation of the 3D structures of the μ, δ, and κ opioid receptors (OR) and the nociceptin/orphanine FQ peptide receptor bound to antagonists9-12 via X-ray crystallography. The 3D structural information allows for validation of many prior hypotheses on opioid SAR based on pharmacological, biochemical, biophysical and computational analyses, such as that determined from CSP studies performed in our laboratory. For example, the close spatial relationship of the basic nitrogen and Asp 147 is confirmed as is the interaction of His 297 with the phenolic moiety present in a large number of opioids. Importantly, the receptor structures may now be utilized to investigate how the opioids induce conformational changes in the ORs and how those changes lead to agonism or antagonism as well as building a structural foundation to understand crosstalk between OR subtypes. While the availability of experimental structures of the opioids represents the foundation for SAR development at the receptor level, computational studies offer the potential to gain additional insights into ligand-receptor interactions as well as receptor conformational properties and dynamical processes associated with receptor activity13, 14. Such studies are particularly relevant given the current lack of an X-ray crystal structure of the receptors with a bound agonist.
In this study interactions between selected opioid ligands and the μ OR using molecular dynamics (MD) simulations are presented. For the MD simulations the dimeric biological assembly suggested by the X-ray crystallography study was used. The dimer involves an extensive protein-protein interface and Portoghese et al. proposed that the transmembrane (TM) 5, 6 dimerization interface is the most probable considering their bivalent ligands which have μ-specific and δ-specific moieties 15. Therefore, MD simulations were undertaken on the μOR homodimer in lipid bilayers and explicit water in the presence of β-funaltrexamine, naloxone, diprenorphine, morphine, etorphine, buprenorphine, N-phenethylnormorphine and DAMGO and on the constitutively active mutant, T279K16. For the ligand-receptor complexes the initial conformations of the ligands was based on predictions made in our laboratory using the CSP approach, such that the present works act as a validation of those predictions.
Methods
The X-ray crystal structures of the μ OR was obtained from the PDB (ID: 4DKL)10. The receptor structure includes a covalently attached antagonist, β-funaltrexamine. One disulfide bond is present between Cys140 and Cys217 in the first and second extracellular loops, respectively. In preparation for the MD simulations, the region of the structure corresponding to T4 lysozyme, which was inserted between TM5 and 6 to facilitate crystallization, was deleted and substituted with the third intracellular loop (ICL3). The missing six residues of ICL3 (Met264-Leu-Ser-Gly-Ser-Lys269) were built using the loop module in Modeller9v817. Out of 1000 models, the best ranking model based on the Modeller energy function18 was selected. No secondary structure for this region was detected from the secondary structure prediction programs PSIPRED19 and JPRED20. The crystal water molecules, Cl− ions, and one cholesterol were maintained from the crystal structure. For the initial 10 ns equilibration simulation (see below) the covalently bound β-funaltrexamine ligand was included in the system. The other ligands were modeled into the final structure from the 10 ns simulation following removal of β-funaltrexamine, as described in the Results and Discussion.
Preparation for the initial 10 ns equilibration simulation involved the OR dimer being inserted into a rectangular POPC membrane containing ~300 lipids with 10% cholesterol using the CHARMM-GUI21 with the remainder of the system filled with 0.15 M NaCl aqueous solution based on the TIP3P water model22. The system, which contained 118,000 atoms, was minimized for 3000 steps using the steepest descent and adopted basis Newton-Raphson algorithms to remove bad contacts. During minimization, positions of protein backbone and all ligands present in the crystal structure were harmonically restrained using a force constant of 10 kcal/mol/Å2 on the non-hydrogen atoms. The side chains were restrained with a force constant of 5 kcal/mol/Å2. Influx of water molecules into the hydrophobic core of the receptor was prevented using a harmonic restraining potential of 2.5 kcal/mol/Å along x,y planes at ±11 Å of the z axis from the center of receptor. The same force was used to keep heads and tails of lipids in place and configurations of the lipids, such as cis double bonds, were maintained with harmonic dihedral restraints with a force constant of 250 kcal/mol/rad2. A short equilibration simulation of 375 ps was performed on the prepared μOR using CHARMM23 with the same restraints used during minimization while gradually reducing the restraint forces, as described in Table S1 in the supporting information. Langevin dynamics were performed for the first 50ps using a friction coefficient of 3 ps−1. The remaining 325 ps of equilibration used constant pressure-temperature (NPT) dynamics using the Langevin Piston Integrator24. The final coordinates were then used to initiate the 10ns equilibration simulation using NAMD25 without any restraints. This and other simulations were performed in the NPT ensemble at 303.15 K and 1atm using Langevin dynamics26, 27 with an integration time step of 2 fs and saving of coordinates every 50 ps. Covalent bonds involving hydrogens were fixed at the equilibrium length by the SHAKE algorithm28. Periodic boundary conditions were used and the Lennard-Jones (LJ) interactions were smoothed from 10 to 12 Å by a switching function29 and non-bonded pair list was generated out to 14 Å and updated heuristically. Electrostatic interactions were calculated by the particle mesh Ewald summation method30 with a real space cutoff of 12 Å. The initial equilibration simulation used the CHARMM22/CMAP force field (FF)13 for the protein and CHARMM36 for the lipids31. The final coordinates from the 10 ns simulation were used to build a hexagonal system of ~ 90,000 atoms with additional simulations performed on that system in the presence of different ligands or the T279K mutant with the recently published CHARMM36 protein FF 32. To prepare the hexagonal systems the protein, ligand and water molecules with one or more atoms within 20 Å of Asp147 were preserved and all remaining atoms deleted. With the exception of the β-funaltrexamine system, β-funaltrexamine was deleted, the terminal atoms of the Lys233 side chain were restored, and the empty binding pocket was filled with additional water molecules before the studied ligands were placed in the binding site. The reversible opioid agonists morphine, etorphine, N-phenethylnormorphine and DAMGO and the antagonists naloxone and diprenorphine, and partial agonist buprenorphine were positioned by taking the CSP predicted conformations and overlaying the ligand atoms onto the β-funaltrexamine based on RMS alignment as described in the Results and Discussion. These systems, as well as the T279K mutant where the binding site is without a ligand were then placed into a hexagonal lattice that contained a POPC membrane with 10% cholesterol with the bilayer faces solvated with 0.15 M NaCl. In this procedure, all water molecules and ions with a non-hydrogen atom within 2.8 Å of the protein, ligand and the maintained water molecules were deleted. Simulations for etorphine, DAMGO, naloxone, β-funaltrexamine, and T279K were continued for 140ns with the last 60ns of the trajectories used for analysis. Simulations for morphine, buprenorphine, diprenorphine, and N-phenethylnormorphine involved two MD simulations for each system with the two simulations initiated with different random number seeds for velocity assignment and run for 70ns, with the last 30ns of each trajectory combined, yielding a total of 60ns sampling for analysis.
Results and Discussion
MD simulations of the μ OR dimer in the presence of selected agonists, antagonists and a partial agonist are presented. Simulations included explicit representation of the lipid bilayer and the surrounding aqueous solvent environment. Four simulations of activated states were performed based on the presence of the constitutively active T279K mutant and the reversible agonists morphine, etorphine and DAMGO. Inactive states of the receptor were simulated based on the presence of the covalently bound antagonist β-funaltrexamine and the reversible antagonists naloxone and diprenorphine. In addition, the partial agonist buprenorphine and full agonist N-phenethylnormorphine were studied to evaluate the impact of substituents on the basic nitrogen (N) and C19 atom. The ligands studied in the present work are shown in Figure S1 of the supporting information and details of how they were initially modeled in the receptor binding site are presented in the following section. Initial analysis of the simulations focused on their stability and convergence based on root mean square (RMS) differences. RMS fluctuations were then analyzed to determine if the simulations reproduced the overall dynamic characteristics of the OR. Subsequent analysis focused on interactions between the ligands and the binding site to determine how those interactions may contribute to receptor activation. The final section presents data on larger scale conformational changes occurring in the receptor that may be representative of the active and inactive states.
Ligand binding regions and ligand placement based on μ OR CSP model
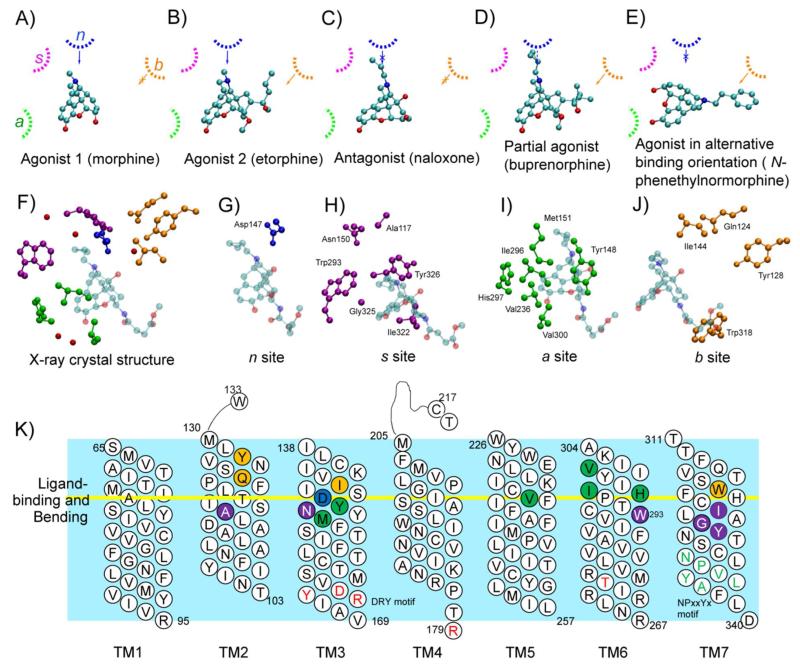
Previously published CSP model development for the μ OR ligands involved the determination of the extent of overlap of the probability distributions of pharmacophoric keys between training set ligands and correlating that with ligand activity. The pharmacophoric keys included the A, B, N, and S functional groups as shown in Figure S1. This effort yielded a consensus model relating interactions of the pharmacophoric keys with corresponding regions on the receptor-binding site to agonism or antagonism (Figure 1). One aspect of the model addressed the puzzling opioid SAR in which short N-substituents (−CH3) elicit agonism, medium length substitutents (−CH2CH=CH2 or cyclopropylmethyl) show antagonism, and longer substitutents (−CH2CH2-Phenyl (phenethyl)) restore agonism. Medium length N substituents in antagonists potentially impact the electrostatic interaction between Asp147 and the basic N as diagrammatically shown in Figure 1A and 1C. Agonism of the longer N substituents was explained by re-orientation of the ligand such that the N-phenethyl group mimicked the C19 substituent of etorphine (Figures 1B and 1E) while keeping the position of the basic nitrogen and phenyl ring close to the orientation of other ligands. Another observation that was addressed was that C19 substituents in orvinols modulate the extent of agonism and mitigate the impact of medium length N-substituent in antagonists thereby bringing partial agonism to some orvinols such as buprenorphine (Figure 1D). While these observations were obtained from a ligand-based model the availability of the OR structures allows the CSP model to be interpreted in the context of specific ligand-receptor interactions.
Figure 1.
Hypothetical binding regions of μOR ligands (adapted from Shim et al.) A through E schematically show how the basic N and other substituents of the various ligands interact with the receptor to bring agonism or antagonism and F) is the 3D spatial arrangement of β-funaltrexamine along with representative residues composing the hypothesized a (green, Ile296, His297 and Val300), b (orange, Gln124, Tyr128 and Ile144), n (blue, Asp147) and s (purple, Trp293 and Tyr326) regions. Note that binding regions in the receptor are identified by lower-case letters a, b, n, and s corresponding to the pharmacophoric keys with which they are interacting (A, B, N, and S) in the ligands. Detailed views of F, in G through J, show all residues composing the a, b, n, and s regions in the X-ray crystal structure, respectively. Side chain non-hydrogen atoms of residues within 5 Å of the pharmacophoric keys of ligand β-funaltrexamine are shown. K is the mapping of key residues on the topology of the receptor.
Creation of the corresponding 3D structure-based ligand OR models involves taking the A, B, N and S pharmacophore keys from the CSP model and hypothesizing that they interact with binding regions, a, b, n, and s on the 3D crystal structure (Figure 1F). A full list of residues whose side chains interact with the A, B, N, and S pharmacophoric keys and their overall locations on the topology of TM helices are described in Figure 1G through 1K. Selection of the residues was based on their location in the crystal structure relative to the pharmacophoric keys of β-funaltrexamine and on the identification of additional residues from analysis of the simulations. We note that certain residues may, in practice, contribute to more than one region and such possibilities are discussed below. For example, Met151 is assigned to the a region, but interacts with atoms from pharmacophoric keys; A, N, and S. The proposed b binding region is largely empty in the crystal structure due to the lack of a functional group on β-funaltrexamine that corresponds to the C19 substitutent of the orvinols (e.g. B key in etorphine, Figure S1). This region is located near the top of the receptor and residues within 8.5 Å of β-funaltrexamine around the b region include Gln124, Tyr128, and Ile144; Trp318 was observed to interact with the B key of other ligands and was included as part of the b region. In general, as shown in Figure 1K, residues interacting with the ligands are clustered around TM regions where the helices are bent indicating the potential influence of ligand binding on the receptor conformation.
While the CSP method does not require a priori conformations of the ligands during model development, once the model is completed conformations of the ligands may be extracted based on common aspects of the pharmacophoric keys that are being sampled by all agonists or antagonists. These conformations were obtained from our previous study and were used here during initial ligand replacement. β-funaltrexamine was one of the molecules in the test set during μ OR CSP model development and was predicted to be an antagonist, consistent with its known biological activity. Superposition of the ligands onto β-funaltrexamine in their CSP conformations therefore allows for placement of the ligands in the binding site. Etorphine, DAMGO and N-phenethylnormorphine overlaid with β-funaltrexamine from this procedure are shown in Figure S2. Superpositioning of the non-peptidic reversible ligands, morphine, etorphine, buprenorphine, diprenorphine and naloxone to β-funaltrexamine was straightforward due to the common 4,5-epoxymorphinan scaffold, including the phenol ring and basic nitrogen. Placement of DAMGO was based on the orientation of etorphine following overlaying of etorphine on β-funaltrexamine. The Tyr residue of DAMGO matches the phenol ring of etorphine, the N-terminal amino group with the basic nitrogen, and the Phe residue with the C19 substituent. For N-phenethylnormorphine, two orientations of the ligand were simulated. The first superposition is based on the 4,5-epoxymorphinan scaffold as done with the other non-peptidic opioids (Figure S2B), with the second orientation based on the CSP model (Figure S2D). In orientation 2 the traditional S key is treated as the B key (Figure S1). These initial orientations were used to initiate the subsequent MD simulations.
Structural characterization of the receptor MD simulations
RMS differences (RMSD) of all backbone non-hydrogen atoms were calculated as a function of simulation time with respect to the X-ray crystal structure. Differences were evaluated for the entire dimer, the TM helices, and for the individual monomers (Figures S3 and S4). The overall RMSD of the full OR backbone non-hydrogen atoms and TM region non-hydrogen atoms of the backbone showed an initial fast relaxation on the 5-10 ns time scale in all cases, with additional gradual relaxation that varied in the individual systems. All systems were assumed to be adequately relaxed following the initial 80 ns with the final 60 ns used for analysis. For those systems that were subjected to two simulations, the last 30ns from each simulation, following 40 ns of equilibration, were taken for analysis yielding 60ns of sampling. The extended relaxation periods allowed for more global shifts in the proteins to occur that may be associated with conformational changes from the assumed initial inactive form to an active conformation in the case of the agonists.
The magnitude of the RMSD of the TM regions of the dimers were approximately 2 Å or less, indicating the overall simulation systems to be stable. Analysis of the RMSD of the individual monomers (Figure S4) reveals the TM regions to be well reproduced in the simulations with values all less than 2 Å. This also indicates that the structural changes in the dimers largely involve relative shifts of the individual monomers with respect to each other. Structural changes of the loops alone in the monomers were significantly larger than those of the TM regions with the RMSD increasing towards the end of the simulations. These analyses indicate that a satisfactory level of structural convergence of the TM regions has occurred on the time scale of the present simulations while additional sampling of the loop regions is clearly warranted. Accordingly, analysis in the present study focused on TM regions of the OR, which includes the opioid binding pocket. However, it should be noted that additional relaxation and conformational changes of the TM regions of receptors may occur in more extended simulations; future studies will address this possibility.
Analysis of the RMS fluctuations (RMSF) as a function of residue from the simulations is presented in Figure S5 and S6 for all the studied systems. The figure includes RMSF based on the published isotropic B factors obtained from the crystallographic analysis. The B factors were averaged over non-hydrogen atoms of each residue and converted to RMSF using the equation (RMSF)2=3B/8π2,33, 34. As expected the TM regions had relatively low flexibility while the intracellular and extracellular loops showed larger fluctuations in the both agonist and antagonist-bound receptors. No trends were obtained differentiating the fluctuations of the agonists and antagonists. Concerning the magnitude of the fluctuations, in the loop regions the degree of fluctuations in the MD simulations is higher than the crystal structures consistent with the simulations being carried out in solution and at room temperature while the temperature of the crystal was 78K. The RMSF of the TM regions are generally lower in the simulations. This is likely due to additional phenomena, such as lattice disorder and additional conformations of the TM helices not sampled in the simulations may contribute. Overall, the simulations are yielding flexibilities that are consistent with the experimental data.
Conformations and orientations of the ligands
Analysis of the ligands focused on both the orientation of the ligands in the binding pocket by calculating the RMS differences of the ligands following alignment of the non-hydrogen backbone atoms of the OR dimer (Figure S7) and on the conformation of the ligands based on RMS differences following alignment of the ligands to themselves (Figure S8). With respect to their orientation in the binding site, for all the ligands, at least one of the two ligands in the dimer maintained an orientation within approximately 2 Å of the initial model (Figure S7). To understand how the conformations of the ligands were maintained, probability distributions of the RMSDs of the ligands following alignment to themselves was analyzed (Figure S8) for both the present simulations and simulations in the absence of the receptor from our previous CSP study, with the later included to gauge the magnitude of the self-aligned RMSD values. With naloxone in monomer A and B, β-funaltrexamine in monomer B, and DAMGO in monomer B the RMSD values in the OR simulations are significantly lower than the values obtained in simulations without receptor, indicating that ligand conformations stay close to CSP predicted conformation. Etorphine also shows small RMSD values of 0.8 Å or less, though these are on the same magnitude as those occurring in the simulation without receptor. Similar results are obtained with β-funaltrexamine in monomer A and buprenorphine. Diprenorphine and N-phenethylnormorphine2 show RMSD values that approach the largest RMSD values obtained in the absence of the receptor, indicating relatively large conformational changes. The change of the orientation of DAMGO in monomer A was associated with a significant change in its conformation, with the images of the conformations shown in Figure S9F. With naloxone, the conformations of both ligands are well maintained though the orientation of the ligand in monomer A changed significantly associated with a shift and rotation of the ligand (Figure S9E). Orientation of diprenorphine changed slightly in one of the monomers but maintained its conformations and key interactions with the receptor (Figure S9D). Overall, these results indicate that the modeling approach using conformations based on the CSP approach yielded conformations and orientations that were stable in a number of cases, though deviations from the initial orientations are present in selected cases suggesting an inherent flexibility of the ligands in the binding pocket or potential limitations in the initial modeling of the ligands. Due to the altered binding orientations of DAMGO and naloxone the monomer A simulations were not used for subsequent analysis, including of the receptor conformational properties. However the binding orientations of diprenorphine were deemed acceptable because it maintained key interactions with receptors and, thus, used for analysis. For the other ligands, both monomers were included in the analyses presented below.
Validation of the CSP-based ligand-receptor hypothesized 3D model
While analyses in the preceding section indicates that the initial orientations and conformations of the ligands from our μ OR CSP study8 were maintained in the majority of cases, further validation of the orientations based on the hypothesized interactions with residues in the different binding regions (Figure 1) was undertaken. Analysis involved the probabilities of interactions of non-hydrogen atoms of the ligands defining the A, B, N and S keys with the residues defining the respective receptor binding regions (i.e. a, b, n and s). Probabilities, normalized to 1, were based on one or more side chain non-hydrogen atoms of the selected residues being within 5 Å of the non-hydrogen atoms in the corresponding A, B, N and S keys of the ligands. In the following figures the occupancies of the agonists morphine, etorphine, and DAMGO were combined (red line) as where those of the antagonists (blue line). Results for the partial agonist buprenorphine are also shown (pink line). The occupancies of individual ligands with the binding region residues are presented in Figure S10 and results for the two conformations of N-phenethylnormorphine will be discussed separately.
The basic nitrogen was considered to be absolutely essential for opioid activity until the discovery of the non-nitrogenous opioid, Herkinorin35; however, the role of the group is still a key feature in the majority of opioids. The most important interaction of the basic N key involves an ionic interaction with Asp147, an interaction long predicted based on mutation studies36, 37 and subsequently confirmed in the OR crystal structures. In the MD simulations of agonists this interaction is present in all cases with occupancies of 1 and partial agonist buprenorphine showed an occupancy of 0.95 (Figure 2A). Notably, the antagonists had lower occupancies; 0.9 in naloxone and 0.75 in diprenorphine, with the exception of β-funaltrexamine, with a value of 1.0. However, closer inspection of the minimum interaction distance probability distributions between the N and Asp147 shows the agonist distances to be systematically shorter (Figure 3), while the antagonists are shifted towards longer distances.
Figure 2.
Probability of residues being within 5 Å of pharmacophoric keys based on the non-hydrogen atoms of the listed residues defining the a, b, n or s sites and the respective A, B, N or S key atoms. Results were combined for the agonists (morphine, etorphine, and DAMGO, red line) and antagonists (naloxone, β-funaltrexamine, and diprenorphine, blue dashed line). Results for the partial agonist buprenorphine are shown as a pink line.
Figure 3.
Minimum distance probability distributions between Asp147 and non-hydrogen atoms in the N pharmacophoric key. The crystallographic values for β-funaltrexamine in the crystal structure are shown as blue crosses on the x axis. Distributions for the individual ligands are shown in Figure S11.
These longer distances are suggested to be due to the presence of the medium-sized S group on naloxone, diprenorphine and β-funaltrexamine, which is associated with those ligands being antagonists. The distribution for buprenorphine is also shifted to longer distance, which would be expected to lead to antagonism. However, its occupancy is greater than diprenorphine (Figure S10) indicating that the size of the C19 substituent is leading to the partial agonist activity. These results suggest that direct interactions of the N key with Asp147 plays a direct role in receptor activation, which is consistent with mutagenesis experiments showing the affinities of agonists to be impacted to a larger extent than antagonists in Asp147 mutants36, 38.
To further investigate molecular details of Asp147 associated with receptor activation, analysis was undertaken of the conformations of the Asp147 side chain based on sampling of the χ1 dihedral (Figure 4A). This analysis shows that Asp147 samples the trans state almost exclusively in the presence of the agonists. However, with the antagonists sampling of the gauche− rotamer is significant, contributing to the longer N-Asp147 distances (Figure 3), as evidenced by the strong correlation between the χ1 dihedral and distances between Asp147 and basic N for all the ligand-OR complexes (Figure S12). These trends are clearly evident in the agonists etorphine, DAMGO and morphine versus the antagonist naloxone (Figure S13). Average populations of trans rotamer in the two monomers are 97%, 97%, 93% and 2% in etorphine, DAMGO, morphine, and naloxone, respectively. As noted above monomers A from naloxone and DAMGO were not included in the analysis due to significant changes in the orientation and conformations of those ligands. Asp147 in β-funaltrexamine (Fig S13) does sample a significant amount of trans (94%), which may be associated with its covalent connectivity, which is suggested to also contribute to its more favorable interactions with Asp147. Thus, the stronger interactions of agonists with Asp147 is associated with the side chain maintaining a trans conformation, with sampling of the gauche− conformation occurring with antagonists associated with longer Asp147-N interaction distances.
Figure 4.
A) Concatenated χ1 rotamer time series and probability distributions of Asp 147 in the presence of the studied opioid ligands. B) Probability of residues being within 5 Å of Asp147 based on the non-hydrogen side chain atoms. Solid and dotted black lines represent the probability when those residues are in the gauche− and trans rotamers, respectively. Probabilities based on the following definitions: trans (−180 ≤ χ1 < −120 or 120 ≤ χ1 < 180), gauche+ (0 ≤ χ1 < 120) and gauche− (−120 ≤ χ1 < 0).
Sampling of the gauche− rotamer by Asp147 changes its interaction with surrounding residues in the receptor. Shown in Figure 4B are the probabilities of selected binding region residues having one or more side chain non-hydrogen atoms within 5 Å of the non-hydrogen atoms of the Asp147 side chain. Probabilities were calculated separately for trans and gauche− rotamers of Asp147. In the gauche− rotamer, interactions of Asp147 with Gln124 and Tyr148 increase and interactions with Asn150 and Tyr326 decrease. These residues are in the b and s sites suggesting that communication between these sites contribute to receptor activation. In particular, this communication may influence the population of trans rotamer in buprenorphine (50%) and diprenorphine (61%) as their C19 substituents are interacting with Gln124.
As discussed above the ligand-based SAR for the S key is complex, with short N substituents corresponding to agonists, medium length substituents being antagonists and long N substituents again being agonists. The CSP model attributed the loss of activity in medium length substituents to be associated with perturbations of interactions with the basic N, a result consistent with the results discussed in the preceding paragraphs, with the regain in activity being due to the S moiety occupying the b region in agonists such as N-phenethylnormorphine (see next section). All ligands with an S key functional group (i.e. a N substitutent) participate in interactions with Trp293 (Figure 2B). The antagonist showed interaction populations greater than 0.6 for Ile322 and Tyr326 while agonists have values of 0.0 and 0.2 for each residue, respectively. The only exception among the agonists was DAMGO, which maintained close contact with Tyr326 with an occupancy close to 0.9 (Figure S10). Additional analysis involved correlation analysis of the minimum basic N-Asp147 to minimum s region residue-S key distances, as shown in Figure 5. The agonists, which include methyl N substituents in morphine and etorphine, do participate in short interactions with the s site; however, these do not lead to longer N-Asp147 interactions. In contrast, the sampling of short s residue-S key distances in the antagonists is increased, associated with increased sampling of longer N-Asp147 distances (Figure 5), most notably with residues Asn150, Trp293 and Tyr326 (Figure S14). A pattern similar to that occurring in the antagonists occurs with the partial agonist buprenorphine. Thus, the present results indicate that antagonism associated with medium sized N substitutents is due to steric interactions with the s site that leads to decreased basic N-Asp147 interactions.
Figure 5.
2D minimum basic N-Asp147 to minimum s site residue-S key distance probability distributions. Distances are based on the side chain non-hydrogen atoms. N-phenethylnormorphine results are for orientation 1. Contour levels used are 0.0005, 0.001, 0.002, 0.006, and 0.024.
Experimental studies indicate that mutation of Asn15039 impacts the binding affinity of agonist more than antagonists. The increased sampling of short Asn150 to the S key in agonists over antagonists (Figure 2) is consistent with those results. However, those short interactions in agonists do not lead to longer N to Asp147 interactions (Figure S14). In contrast, with the antagonists, short interactions with Asn150 often lead to increased N to Asp147 distances. Additional details of s site interactions are presented in the following section on N-phenethylnormorphine.
Residues defining the a region include Tyr148, Met151, Val236, Ile296, His297, and Val300. The occupancy of all the ligands with these residues is greater than 0.6 (Figure 2). With Val236 values of less than one are observed with antagonists while with Ile296 occupancies less than one occur with all ligands with the exception of β-funaltrexamine. The maintenance of A key to a region interactions for all the ligands indicates that this region is important for ligand binding but not directly involved in discrimination of agonism and antagonism. The residues composing the a region are distributed on TM helices 3, 5, and 6 (Figure 1K) such that maintenance of interactions with those residues indicates that the overall integrity of the binding pocket was maintained in the simulations.
The B key is of particular importance in the orvinols, where it has been suggested to modulate receptor activation40 as also predicted by the CSP model. However, there are no site-directed mutagenesis studies on residues in the b region, such that this region remains to be studied. Interactions between B key and b region non-hydrogen atoms are occupied to a larger extent in the agonists (Figure 2D), with the partial agonist buprenorphine similar to the agonists. Interactions with Gln124 are maintained with DAMGO and etorphine with occupancies of 1, but lost in the case of morphine, while buprenorphine had an occupancy is 0.7 (Figure S10). With Tyr128 interactions again only occur with DAMGO, etorphine and buprenorphine, although the occupancies are low, in the vicinity of 0.2 to 0.3. With Ile144 interactions with DAMGO are significant, with an occupancy of 1 and interactions with etorphine and buprenorphine also occur although the occupancy is low, at less than 0.1 and 0.2, respectively. These results are consistent with the role of b region interactions in modulating the activity of the orvinols via their C-19 functionality and indicate that the peptidic opioid DAMGO also exploits this interaction to achieve its biological activity. Notably, residues Gln124 and Ile144 are in the vicinity of Asp147 with the minimum side chain-side chain distances being 4.4 and 5.1 Å, respectively, in the crystal structure. Given the high probability of the B key interacting with those residues in the partial agonist buprenorphine (Figure 2D), while being significantly smaller in the antagonist diprenorphine (Figure S10D) indicates a mechanism by which interactions with the b region impact receptor activation in the orvinols.
Binding orientation of N-phenethylnormorphine
The N-phenethyl moiety is present in many μ-OR agonists such as N-phenethylnormorphine and fentanyl but it was not clear how the longer N-substituent restores agonism. Assuming steric hindrance in the s site by larger N substituents, our CSP models suggested alternative binding orientations where the ligand is rotated in the pocket such that the N-phenethyl group is located near the b site in an orientation which will still allow basic N to Asp147 interactions (Figure 1E). Alternatively, visual inspection of the crystal structure shows the presence of a potential cleft in the s site that may accommodate longer N-substituents. Therefore we tested two possible binding orientations of N-phenethylnormorphine where N-phenethylnormorphine1 indicates the binding orientation in which the N-phenethyl group is oriented as in the other non-peptidic opioids (Figure S2B) and N-phenethylnormorphine2 is the alternative binding orientation based on the CSP model (Figure S2D). N-phenethylnormorphine in both orientations underwent some shifts, with the shifts generally being larger in orientation 2 (Figures S7 and S8).
The probability of the various binding region residues interacting with both N-phenethylnormorphine1 and 2 are shown Figure S10. First, interactions with the basic N were maintained to a larger extent with N-phenethylnormorphine1, with the occupancy of N-phenethylnormorphine2 with Asp147 being less than 0.7. N-phenethylnormorphine1 maintained the classical binding interactions with the a region, while those interactions are lost to a greater extent with N-phenethylnormorphine2. N-phenethylnormorphine2 participates in interactions with b region residues Gln124, Tyr128 and Ile144, while these interactions are not present with N-phenethylnormorphine1, which has an interaction pattern similar to β-funaltrexamine. Finally, at the s region, N-phenethylnormorphine1 maintained more interactions than N-phenethylnormorphine2, consistent with the large phenethyl moiety being located in that region. In addition, short S key to s region interactions typically did not lead to increased N-Asp147 distances, as occurs in the antagonists, with N-phenethylnormorphine1 (Figure 5). Images of the final bound orientations of N-phenethylnormorphine1 from simulation 1, monomer A are shown in Figure 6.
Figure 6.
Images of the interaction of N-phenethylnormorphine in orientation 1 with the μ opioid receptor from the final time frame from simulation 1, monomer A. The ligand is shown in CPK, atom-based coloring, the side chains of the s region residues are in purple and Asp147 is blue. The surface of the receptor is transparent silver. The two panels are different orientations of the same structure.
As is evident, the phenethyl moiety in N-phenethylnormorphine1 is accommodated in the s region, thereby leading to favorable interactions that lead to the maintenance of many of the other interactions with the receptor. Thus, in contrast to our previously predicted orientation based on the CSP model, the present results indicate that the phenethyl moiety has favorable interactions with the s site, yielding the following model for the relationship of N-substituent size to activity. Short substituents allow for the basic N-Asp147 interactions required for activity, while medium size substituents participate in steric interactions with the s region leading to a loss of basic N-Asp147 interactions and antagonism. However, upon going to even longer N substituents, such as the phenethyl moiety, additional favorable interactions with the s site leads to stabilization of the basic N-Asp147 interactions and agonism. Analysis of the van der Waals interactions energies between the S key atoms and the s region residues (Figure S15) shows that while the antagonists have small favorable interactions with the s region, the vdW interactions are significantly more favorable with orientation 1 of N-phenethylnormorphine.
Long-range phenomena related to OR activation
To facilitate the analysis of long-range changes in the OR that may be related to activation an overview of known GPCR conformational characteristics relevant to activation is of utility14, 41. Conformations defining active and inactive states of GPCRs typically involve the movement of TM helices or the presence of specific electrostatic interactions (Figure 1K). For example, in the active state of rhodopsin, TM6 is rotated and shifted away from TM3 and closer to TM5 on the cytoplasmic side and the distance between TM2 and TM7 is also shorter 41. These changes are associated with disruption of the conserved Asp164-Arg165-Tyr166 (DRY motif) ionic network near the intracellular face of TM3. It was proposed that agonist binding shifts the position of a Trp residue thereby triggering the cascade of long-range conformational changes that impact the DRY motif. As another example, in the active state of the β2-adrenergic receptor42 outward movements of TM5 and TM6 were observed in the intracellular side away from other TMs and there were large conformational changes in the NPxxYx conserved motif in TM7.
Prior to analyzing the present simulations for conformational changes similar to those described in the preceding paragraph, unique features in the OR crystal structures need to be noted. First unlike many other GPCRs, inactive ORs have a very accessible binding pocket. Secondly, the ionic interactions associated with the DRY motif on TM3 are not present in the inactive conformations of the ORs. In addition, interactions between Arg165-Thr279 (TM6) and Asp164-Arg179 (TM4) have been suggested to stabilize the inactive conformation; no significant differences in these interactions between the agonists and antagonists were observed in the present study (Figure S16). Thirdly, much attention has been paid to the nature of the dimeric interface in ORs. Different OR subtypes can form heterodimers, and possibly higher-order oligomers, potentially leading to different pharmacological effects. For example, the adverse side effects of μOR agonists can be reduced by the δOR antagonists, which may be related to μ-δ heterodimers. A number of studies support the idea that ORs are constitutively dimerized and that dimerization is independent of activation states or ligands 43, 44.
The crystal structure of the μOR revealed an extended oligomer where TM5 and TM6 comprise the major interface of one possible dimer and TM1 and H8 may interact as a minor dimerization interface. However, the δOR structure, despite its high sequence similarity with the μOR, did not show clear dimer interfaces, which was suggested to be associated with crystal packing. In addition, the κOR primary interface involved TM1, TM2, and H8 and it was pointed out that multiple dimerization interfaces are possible, with these interfaces possibly playing a role in oligomerization and cross talk between OR subtypes.
Analysis of the long range structural changes in the simulations initially involved the distances and angles between helices45, 46. Only continuous segments of the helices were analyzed, such that the definitions of the helices were terminated at bends near the central region of the TM helices (Figure 1K), with the residues that define the regions used in the analysis listed in the legend of Figure 7. Analysis of constitutively active T279K mutant and the partial agonist simulations were included to identify any similarities in the intracellular movements of their TMs. Initial analyses of probability distributions of individual distances and angles between pairs of helices did not reveal trends differentiating active from inactive states, though it was evident that both agonists- and antagonists-bound receptors sample a range of conformational space. Subsequent 2D analysis, as presented as contour plots in Figure 7, where the data from all the agonist/active and antagonist/inactive states were pooled, did show some differentiating characteristics (Individual 2D plots of the agonists and antagonists are shown in Figure S17). In general, the antagonists sampled a wider range of conformations as compared to the agonists. This is most evident in the TM5-TM6 vs. TM6-TM7 and the TM5-TM6 vs. TM6-TM3 angle plots, although in all cases there is significant overlap of the two classes of distributions. Interestingly, T279K mutant sampled different regions as compared to the agonists in the TM5-TM6 vs. TM3-TM6 angle, TM5-TM6 vs. TM7-TM5 distance and the TM5-TM6 vs. TM3-TM6 distance 2D plots, suggesting that the mechanism of activation in T279K may be different that of the agonist ligands. Consistent with this are the Arg165-Thr279 (TM6) and Asp164-Arg179 (TM4) distance distributions in Figure S16. The 2D distributions of the partial agonist buprenorphine were between that of the agonists and antagonists. We note that the 2D sampling overlapped with the helix orientation from the crystal structure with the exception of the TM6-TM7 angle, where a deviation of less the 5° occurred.
Figure 7.
2D distributions of (A) relative helix angles and (B) distances between the ends of the intracellular portions of selected TM helices. A) 2D probability contour plots of TM7-TM6 angle versus TM5-TM6 angle (upper row) and TM3-TM6 angle versus TM5-TM6 angle (lower row) and B) distances between the ends of helices TM7-TM6 versus TM5-TM6 (upper row) and TM3-TM6 versus TM5-TM6 (lower row). The green dot is the respective value in the X-ray crystal structure and contour levels used in each panel are shown in the agonist panels. Segments of the helices used for the analyses are TM3 (Ser154-Val169), TM5 (Met243-Leu259), TM6 (Leu275-Ile290), and TM 7 (Val334-Asp340). Helical axes were defined using the “COOR HELIX” command in CHARMM which is based on the methods of Åqvist and of Chothia et al.45, 46
Thus, the present simulations indicate that the TM helices in the agonists sample a more focused region of conformational space than the antagonists and that the sampling of the constitutively active T279K mutant differs from that of the other agonists, suggesting a different mechanism of activation. We note that shifts in TM3 are of interest as Asp147 is on this helix while TM5 and TM6 comprise the largest potential dimer interface as well as being in direct contact with the bound ligands, such that shifts in their positions due to ligand binding may be communicated to adjacent monomers in a dimer, which may contribute to altered pharmacological profiles in heterodimers. However, the length of the present simulations limit further interpretation of the relationship of TM helix conformation and dynamics to activation and the relationship of ligand-binding site interactions to those phenomena. Additional studies will be required to address these important issues.
Conclusions
In this study, ligand-based SARs previously identified using the CSP method were evaluated in the context of receptor structures. The μOR, starting from the X-ray crystal structure, was simulated with various ligands including agonists, antagonists, and a partial agonist. With the relatively short simulation length limiting the ability to obtain a detailed model of long-range conformational changes, focus was mainly on events occurring in the binding pocket, for example, interactions between pharmacophoric keys of ligands and receptor residues.
As is widely known, we confirmed that interactions of phenol rings of opioids comprising the A key with the receptor are largely conserved in the studied agonists and antagonists. Interestingly, the basic N of agonists showed shorter interaction distances with Asp147 in TM3 than antagonists. The length of N- (i.e. S key) and C19 (i.e. B key) substituents were indicated to modulate basic N-Asp147 interactions. The short N substituents (i.e. S key) of agonists allow for direct basic N-Asp147 interactions while medium sized N-substituents in antagonists interact with s region residues leading to steric effects that are suggested to perturb basic N-Asp147 interactions. However, upon going to longer N substituents, as in the agonist N-phenethylnormorphine, additional stabilizing interactions with the s region occur, leading to sampling of short basic N to Asp147 distances as in the agonists morphine, DAMGO and etorphine. We note that this is not consistent with previous predictions based on the CSP modeling. Basic N to Asp147 interactions are predicted to be influenced by the C19 substituent in orvinols through b region residues Gln124 and Ile144. Interactions of the larger C19 moiety of the partial agonist buprenorphine leads to greater interactions with those residues thereby leading to sampling of shorter basic N to Asp147 distances, a phenomena that does not occur with the small C19 substituent in the antagonist diprenorphine. This observation is consistent with predictions from our ligand-based CSP modeling efforts. In future studies, it will be of interest to investigate in greater detail how interactions of agonists and antagonists with different regions of the binding pocket impact basic N to Asp147 interactions. These insights into the molecular mechanism of efficacy open up a unique approach to the rational design of improved agonists and antagonists including those with substantially differing structures to the traditional morphinan-based skeleton.
The present simulations also indicate that differences in the conformational sampling of the intracellular regions of the TM helices occur between agonists and antagonists, with antagonists sampling a wider range of conformations. In addition, it is indicated that the mechanism of activation in the T279K mutant may differ from that by agonists. Further conclusions about larger scale conformational changes in the receptor are limited by the duration of the present simulations. It is anticipated that future efforts will yield a detailed picture of the cascade of interactions from the binding pocket through the TM helices that propagate information on agonist versus antagonist binding to intracellular loops that participate in interactions with G proteins.
Supplementary Material
Acknowledgement
Financial support from the NIH (DA13583 and DA19634) and computational support from the XSEDE supercomputing consortium and the Computer-Aided Drug Design Center, School of Pharmacy, University of Maryland, Baltimore are acknowledged.
Abbreviations used
- RMSD
root mean square difference
- RMSF
root mean square fluctuation
- OR
opioid receptor
- CSP
conformationally sampled pharmacophore
Footnotes
Author contributions: Participated in research design: A.C. and A.D.M. Conducted experiments and Data analysis: J.S. and A.D.M. Wrote and approved the manuscript: J.S., A.D.M. and A.C.
Supporting Information Available: Tables and Figures. This information is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Martin TJ, Eisenach JC. Pharmacology of Opioid and Nonopioid Analgesics in Chronic Pain States. J. Pharmacol. Exp. Ther. 2001;299:811–7. [PubMed] [Google Scholar]
- 2.Stevens RC, Cherezov V, Katritch V, Abagyan R, Kuhn P, Rosen H, Wuthrich K. The Gpcr Network: A Large-Scale Collaboration to Determine Human Gpcr Structure and Function. Nat. Rev. Drug. Discov. 2012;12:25–34. doi: 10.1038/nrd3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casy AF, Parfitt RT. Opioid Analgesics: Chemistry and Receptors. Plenum Press; New York: 1986. [Google Scholar]
- 4.Coop A, MacKerell AD., Jr. The Future of Opioid Analgesics. Am. J. Pharm. Educ. 2002;66:153–156. [Google Scholar]
- 5.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. Functional Selectivity and Classical Concepts of Quantitative Pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 6.Portoghese PS, Sultana M, Nagase H, Takemori AE. Application of the Message-Address Concept in the Design of Highly Potent and Selective Non-Peptide Delta Opioid Receptor Antagonists. J. Med. Chem. 1988;31:281–2. doi: 10.1021/jm00397a001. [DOI] [PubMed] [Google Scholar]
- 7.Bernard D, Coop A, MacKerell AD., Jr. Quantitative Conformationally Sampled Pharmacophore for Δ Opioid Ligands: Reevaluation of Hydrophobic Moieties Essential for Biological Activity. J. Med. Chem. 2007;50:1799–1809. doi: 10.1021/jm0612463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim J, Coop A, MacKerell AD., Jr. Consensus 3d Model of Mu-Opioid Receptor Ligand Efficacy Based on a Quantitative Conformationally Sampled Pharmacophore. J. Phys. Chem. B. 2011;115:7487–7496. doi: 10.1021/jp202542g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the Delta-Opioid Receptor Bound to Naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal Structure of the Mu-Opioid Receptor Bound to a Morphinan Antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al. Structure of the Nociceptin/Orphanin Fq Receptor in Complex with a Peptide Mimetic. Nature. 2012;485:395–9. doi: 10.1038/nature11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, et al. Structure of the Human Kappa-Opioid Receptor in Complex with Jdtic. Nature. 2012;485:327–32. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filizola M, Devi LA. Grand Opening of Structure-Guided Design for Novel Opioids. Trends Pharmacol. Sci. 2012 doi: 10.1016/j.tips.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nygaard R, Zou Y, Dror RO, Mildorf TJ, Arlow DH, Manglik A, Pan AC, Liu CW, Fung JJ, Bokoch MP, et al. The Dynamic Process of Beta(2)-Adrenergic Receptor Activation. Cell. 2013;152:532–42. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portoghese PS. From Models to Molecules: Opioid Receptor Dimers, Bivalent Ligands, and Selective Opioid Receptor Probes. J. Med. Chem. 2001;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- 16.Huang P, Li J, Chen C, Visiers I, Weinstein H, Liu-Chen LY. Functional Role of a Conserved Motif in Tm6 of the Rat Mu Opioid Receptor: Constitutively Active and Inactive Receptors Result from Substitutions of Thr6.34(279) with Lys and Asp. Biochemistry. 2001;40:13501–9. doi: 10.1021/bi010917q. [DOI] [PubMed] [Google Scholar]
- 17.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen M-Y, Pieper U, Sali A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinformatics. 2006 doi: 10.1002/0471250953.bi0506s15. Chapter 5, Unit 5.6-Unit 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiser A, Do RK, Sali A. Modeling of Loops in Protein Structures. Protein Sci. 2000;9:1753–73. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan DW, Ward SM, Lobley AE, Nugent TC, Bryson K, Jones DT. Protein Annotation and Modelling Servers at University College London. Nucleic Acids Res. 2010;38:W563–8. doi: 10.1093/nar/gkq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole C, Barber JD, Barton GJ. The Jpred 3 Secondary Structure Prediction Server. Nucleic Acids Res. 2008;36:W197–201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo S, Kim T, Iyer VG, Im W. Charmm-Gui: A Web-Based Graphical User Interface for Charmm. J. Comp. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983;79:926–926. [Google Scholar]
- 23.Brooks BR, Brooks CL, MacKerell AD, Jr., Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, et al. Charmm: The Biomolecular Simulation Program. J. Comput. Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feller SE, Zhang Y, Pastor RW, Brooks BR. Constant Pressure Molecular Dynamics Simulation: The Langevin Piston Method. J. Chem. Phys. 1995;103:4613–4613. [Google Scholar]
- 25.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Scalable Molecular Dynamics with Namd. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen MP, Tildesley DJ. Computer Simulation of Liquids. Vol. 1. Oxford University Press; USA: 1989. [Google Scholar]
- 27.Chandrasekhar S. Stochastic Problems in Physics and Astronomy. Rev. Mod. Phys. 1943;15:1–89. [Google Scholar]
- 28.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of N-Alkanes. J. Comput. Phys. 1977;23:327–341. [Google Scholar]
- 29.Steinbach PJ, Brooks BR. New Spherical-Cutoff Methods for Long-Range Forces in Macromolecular Simulation. J. Comput. Chem. 1994;15:667–683. [Google Scholar]
- 30.Darden T, York D, Pedersen L. Particle Mesh Ewald: An N-Log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 31.Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD., Jr. Update of the Charmm All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Best RB, Zhu X, Shim J, Lopes P, Mittal J, Feig M, MacKerell AD., Jr. Optimization of the Additive Charmm All-Atom Protein Force Field Targeting Improved Sampling of the Backbone Phi, Psi and Side-Chain Chi1 and Chi2 Dihedral Angles. J. Chem. Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzmanic A, Zagrovic B. Determination of Ensemble-Average Pairwise Root Mean-Square Deviation from Experimental B-Factors. Biophys. J. 2010;98:861–871. doi: 10.1016/j.bpj.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips GN., Jr. Comparison of the Dynamics of Myoglobin in Different Crystal Forms. Biophys. J. 1990;57:381–3. doi: 10.1016/S0006-3495(90)82540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. Neoclerodane Diterpenes as a Novel Scaffold for Mu Opioid Receptor Ligands. J. Med. Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 36.Li JG, Chen C, Yin J, Rice K, Zhang Y, Matecka D, de Riel JK, DesJarlais RL, Liu-Chen LY. Asp147 in the Third Transmembrane Helix of the Rat Mu Opioid Receptor Forms Ion-Pairing with Morphine and Naltrexone. Life Sci. 1999;65:175–85. doi: 10.1016/s0024-3205(99)00234-9. [DOI] [PubMed] [Google Scholar]
- 37.Surratt CK, Johnson PS, Moriwaki A, Seidleck BK, Blaschak CJ, Wang JB, Uhl GR. -Mu Opiate Receptor. Charged Transmembrane Domain Amino Acids Are Critical for Agonist Recognition and Intrinsic Activity. J. Biol. Chem. 1994;269:20548–53. [PubMed] [Google Scholar]
- 38.Befort K, Tabbara L, Bausch S, Chavkin C, Evans C, Kieffer B. The Conserved Aspartate Residue in the Third Putative Transmembrane Domain of the Delta-Opioid Receptor Is Not the Anionic Counterpart for Cationic Opiate Binding but Is a Constituent of the Receptor Binding Site. Mol. Pharmacol. 1996;49:216–23. [PubMed] [Google Scholar]
- 39.Mansour A, Taylor LP, Fine JL, Thompson RC, Hoversten MT, Mosberg HI, Watson SJ, Akil H. Key Residues Defining the Mu-Opioid Receptor Binding Pocket: A Site-Directed Mutagenesis Study. J. Neurochem. 1997;68:344–53. doi: 10.1046/j.1471-4159.1997.68010344.x. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JW, Husbands SM. The Orvinols and Related Opioids--High Affinity Ligands with Diverse Efficacy Profiles. Curr. Pharm. Des. 2004;10:717–32. doi: 10.2174/1381612043453027. [DOI] [PubMed] [Google Scholar]
- 41.Standfuss J, Edwards PC, D’Antona A, Fransen M, Xie G, Oprian DD, Schertler GF. The Structural Basis of Agonist-Induced Activation in Constitutively Active Rhodopsin. Nature. 2011;471:656–60. doi: 10.1038/nature09795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. Crystal Structure of the Beta2 Adrenergic Receptor-Gs Protein Complex. Nature. 2011;477:549–55. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozenfeld R, Gomes I, Devi LA. Opioid Receptor Dimerization. In: Pasternak GW, editor. The Opiate Receptors. Springer Science; 2011. pp. 407–437. [Google Scholar]
- 44.Bai M. Dimerization of G-Protein-Coupled Receptors: Roles in Signal Transduction. Cell Signal. 2004;16:175–86. doi: 10.1016/s0898-6568(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 45.Åqvist J. A Simple Way to Calculate the Axis of an A-Helix. Comput. Chem. 1986;10:97–99. [Google Scholar]
- 46.Chothia C, Levitt M, Richardson D. Helix to Helix Packing in Proteins. J. Mol. Biol. 1981;145:215–50. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.