Abstract
Background
Ischemic postconditioning (IPostC) has been shown to attenuate brain injury in rat stroke models, but a mouse model has not been reported. This study establishes an IPostC model in mice and investigates how IPostC affects infiltration of leukocytes in the ischemic brain and lymphopenia associated with stroke-induced immunodepression.
Material and Methods
A total of 125 mice were used. IPostC was performed by a repeated series of brief occlusions of the middle cerebral artery (MCA) after reperfusion, in a focal ischemia model in mice. Infarct sizes, neurological scores, inflammatory brain cells and immune cell populations in lymph nodes, spleen and bone marrow were analyzed with FACS.
Results
IPostC performed immediately, 2 min and 3 hr after reperfusion significantly reduced infarct sizes and attenuated neurological scores as measured up to 3 days post-stroke. In the group with strongest protection, infarct sizes were reduced from 49.6 ± 2.8% (n=16) to 27.9 ± 2.9% (n=10, P<.001). The spared infarct areas were seen in the ischemic penumbra or ischemic margins, i.e., the border zones between the cortical territories of the anterior cerebral artery (ACA) and those of the MCA, as well as in the ventromedial and dorsolateral striatum. FACS analyses showed that IPostC significantly blocked increases in the numbers of microglia (CD45intCD11b+), macrophages (CD45hiCD68+), CD4 T cells (CD45+CD4+) and CD8 T cells (CD45+CD8+) as well as B lymphocytes (CD45+CD19+) in the ischemic brain (n=5/group). Reduced-immune cell numbers in the peripheral blood and spleen were increased by IPostC while immune cell populations in the bone marrow were not altered by IPostC.
Conclusions
IPostC reduced brain infarction and mitigated neurological deficits in mice, likely by blocking infiltration of both innate and adaptive immune cells in the ischemic brain. In addition, IPostC robustly attenuated peripheral lymphopenia and thus improved systemic immunodepression.
Keywords: cerebral ischemia, postconditioning, infarction size, mouse model
1 Introduction
Ischemic postconditioning (IPostC), an emerging concept for stroke treatment, refers to a series of brief occlusions of cerebral blood vessels after reperfusion(Zhao et al., 2003b; Zhao et al., 2006; Zhao, 2009). We and others have reported that IPostC reduces infarction and improves neurological deficits after stroke in rats(Lee et al., 2008; Pignataro et al., 2008; Ren et al., 2008; Xing et al., 2008; Zhao, 2009). The protective mechanisms are associated with the ability of IPostC to attenuate free radical generation, inhibit infiltration of neutrophils in ischemic brain and attenuate pro-inflammatory cytokine and adhesion molecule expression of IL-1β and TNF-α, and ICAM-1(Zhao, 2009). IPostC also promotes neuronal survival molecular signaling—such as Akt and εPKC—and inhibits pro-apoptotic protein expression— such as Bax and caspase(Zhao, 2009). Most of these studies, however, are descriptive and lack insight into the underlying protective mechanisms of IPostC. To begin to address the underlying mechanisms better genetic approaches, such as those available in various gene mutant or transgenic mice, will be required. Our study establishes the first mouse model of IPostC.
As mentioned, brain injury after stroke is exacerbated by the inflammatory response, which is additionally modulated by both innate and adaptive immunity(Iadecola and Anrather, 2011). Macrophages responsible for innate immunity have been shown to contribute to acute brain injury after stroke. Macrophages can be derived from either residential microglia (Lehrmann et al., 1998; Gregersen et al., 2000; Mabuchi et al., 2000; Liu et al., 2001) and/or monocytes migrated from peripheral blood (Schilling et al., 2003; Tanaka et al., 2003; Schilling et al., 2005; Jin et al.). Recent studies suggest that adaptive immune cells T cells and B cells also affect stroke-induced brain injury (Yilmaz et al., 2006; Hurn et al., 2007; Liesz et al., 2009; Kleinschnitz et al., 2010a; Chen et al., 2012) and that brain inflammation and infarction are followed by immunodepression in peripheral immune organs. Stroke-induced immunodepression (SIID) results in pneumonia, a major cause of delayed mortality in stroke patients(Prass et al., 2006; Hug et al., 2009; Urra et al., 2009). As a result, several leading research centers have shifted from targeting brain injury to targeting SIID(Meisel et al., 2004; Prass et al., 2006; Hug et al., 2009; Urra et al., 2009). Taken together, both local brain inflammation and systemic SIID play a critical role in stroke pathology, yet the effect of IPostC on brain inflammation has been poorly studied particularly the effect of IPostC on SIID.
We have established an IPostC model in mice to provide for future studies on the underlying mechanisms of IPostC against stroke in mice with various available genetic tools. As the ischemic penumbra is the primary target of most neuroprotectants, we analyzed whether or not IPostC spares ischemic penumbras. We also used FACS techniques to further investigate the effects of IPostC on infiltration of inflammatory cells in the ischemic brain—such as macrophages, T cells and B cells—and examined the effects of IPostC on SIID caused by reductions of immune cells in the immune organs.
2 Materials and Methods
2.1 Focal cerebral ischemia and ischemic postconditioning
All experimental protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC). Male C57BL/6 mice aged 8–10 weeks weighing 22–25g purchased from Jackson laboratory (Bar Harbor, Maine) were used. Animals were maintained on a standard diet and water ad libitum, and kept in a temperature-controlled environment with alternating 12-hour light and dark cycles. Efforts were made to minimize the number of animal used and the suffering animals experienced. Anesthesia was induced by 5% isoflurane and maintained at 1% to 2% isoflurane during surgery. Body core temperature was monitored with a rectal probe and maintained at 37 ± 0.5°C with a surface heating pad during the entire procedure. Focal ischemia was generated as described previously with slight modifications(Xiong et al., 2012). Briefly, under the operating microscope, the left common carotid artery (CCA) and external carotid artery (ECA) were exposed through a ventral midline neck incision, and were ligated proximally. A 6-0 silicon-coated nylon suture with a 0.23 mm tip diameter (Doccol, Redlands, CA, USA) was inserted through the arteriotomy in the CCA just below the carotid bifurcation and advanced approximately 8 ± 0.5 mm until a mild resistance was felt. The inserted 6-0 nylon suture was tied by a 5-0 black silk suture at the proximal CCA bifurcation to prevent withdrawal. In the ischemic control group, reperfusion was achieved by permanently withdrawing the suture after 45 min of ischemia. IPostC was induced by brief, repetitive MCA occlusions via withdrawal and reinsertion of the suture into the ICA. To induce IPostC, the suture was withdrawn only approximately 2 to 3 mm to allow temporal reperfusion and re-insertion
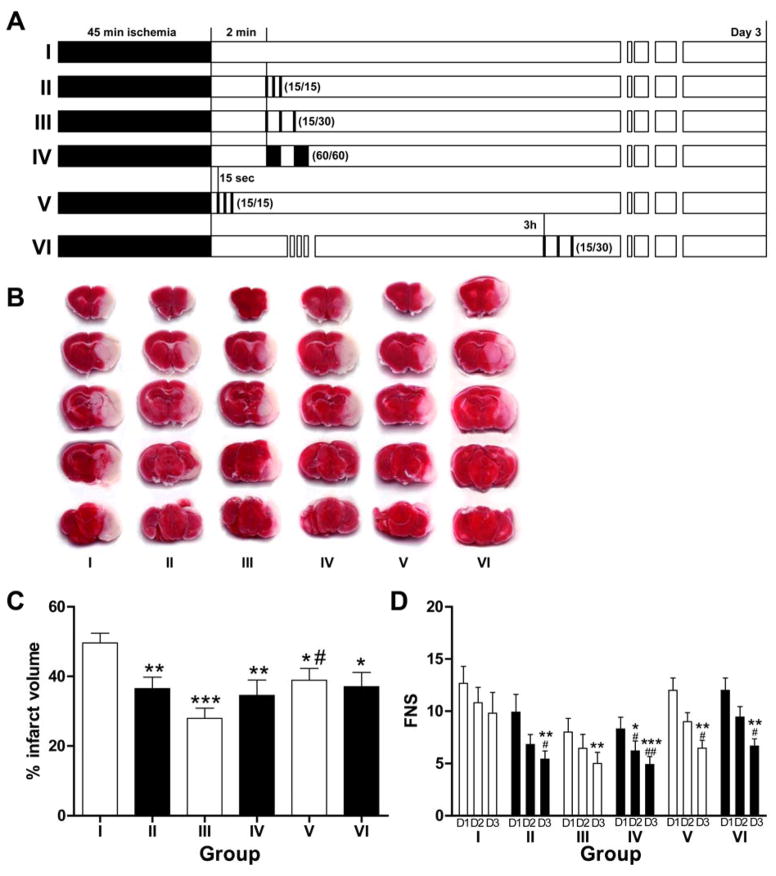
Animals were randomly divided into 6 groups (Fig. 1): I. Control group (n=16): animals were subjected to 45 min ischemia followed by 72 hrs reperfusion; II. IpostC was initiated at 2 min after reperfusion with 3 cycles of 15 sec occlusion/15 sec release (IP15/15, n=10); III. IpostC with 3 cycles of 15 sec occlusion/30 sec release started at 2 min after reperfusion (IP15/30, n=10); IV. IpostC with 2 cycles of 60 sec occlusion/60 sec release starting from 2 min of reperfusion (IP60/60, n=10); V. IpostC with 3 cycles of 15 sec occlusion/15 sec reperfusion initiated immediately after stroke (n=10); VI. IPostC with 3 cycles of 15 sec occlusion/30 sec release (IP15/30, n=9) starting from 3 hrs post-reperfusion.
Fig. 1.
IPostC reduced infarct volumes and neurologic scores. A. Experimental protocols. Mice were divided into 6 groups. Group I is ischemic control without IPostC (n=16). In groups II, III and IV, IPostC was initiated from 2 min after reperfusion, with 3 cycles of 15 s occlusion/15 s reperfusion, 3 cycles of 15 s occlusion/30 s reperfusion, and 2 cycles of 60 s occlusion/60 s reperfusion, respectively. In group V, IPostC was conducted by 3 cycles of 15 s occlusion/ 15 s reperfusion initiated 15 sec after reperfusion. In group VI, IPostC was performed from 3 h after reperfusion, with 3 cycles of 15 s occlusion/ 30 s reperfusion. B. Representative infarction of TTC staining in the ischemic mouse brain in the 6 groups as described in A. C. The histogram shows average infarct volume in each group. Data are presented as the mean ± SEM. *P<0.05, **P<0.01, and *** P<0.001 compared to control group; #P<0.05 compared to group III. D. The histogram shows average focal neurological scores (FNS). Higher scores indicate greater impairment. From Day 1 to Day 3 after reperfusion, FNS was improved significantly in all IPostC groups. *P<0.05, **P<0.01, and *** P<0.001 compared to control (group I) for a corresponding day; #P<0.05 and ##P<0.01 compared to Day 1 in a same group. D1, D2, D3, represent Day 1, Day 2 and Day 3, respectively.
2.2 Neurological score evaluation
Animals were returned to their cages after surgery and were given free access to food and water. The neurological score of mice was assessed blindly by an investigator who did not know the animal conditions, at day 1, 2 and 3 after stroke. A 28 point scale of focal neurological scores (FNS) was used (Hill et al., 1999), which was based on the following 7 tests: (1) body symmetry, 0 to 4 points; (2) gait, 0 to 4 points; (3) climbing, 0 to 4 points; (4) circling behavior, 0 to 4 points; (5) frontal limb symmetry, 0 to 4 points; (6) compulsory circling, 0 to 4 points; (7) whisker response, 0 to 4 points. The 7 individual test scores were summed up at the end of the evaluation (the total score for each animal is 0 to 28).
2.3 Measurement of infarct sizes
After neurological evaluation, the mice were deeply anesthetized by isoflurane and decapitated 72 hrs after stroke. The brains were rapidly removed and cut into 5 coronal sections with 2-mm thickness using a brain matrix, stained in 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO) at 37 °C for 15 min, and fixed in 4% paraformaldehyde for 24 hrs. The brain slices were scanned. Infarct areas of all sections were measured using NIH imageJ software. Infarct volumes were calculated by multiplying the infarct area by the thickness of the brain sections, normalized to the volumes of non-ischemic hemisphere to exclude the effect of cerebral edema induced by ischemia on the infarct size, and expressed as percentage of the hemisphere according to the following formula: (Zhao et al., 2003a; Zhao et al., 2005).
2.4 FACS analyses for immune cells
Mice were deeply anesthetized by isoflurane 72 hr after reperfusion, and tail vein blood was collected. After euthanization, spleens, right inguinal lymph nodes, and right tibia were collected. Ischemic ipsilateral brains were dissected after transcardial perfusion with 100 ml of PBS. Spleens were separated by passing through a 70 μm cell strainer. Bone marrow was flushed from the tibia with a 5 ml syringe. Red blood cells in spleens, lymph nodes, blood and bone marrow were lysed using ACK Lysing buffer (GIBCO, Invitrogen, Carlsbad, CA, USA), and then cells were resuspended in PBS containing 1% fetal bovine serum (FACS buffer) for FACS analysis. Brain microglia and mononuclear leukocytes were collected as described previously (Lim et al., 2011; Xiong et al., 2012). In brief, ipsilateral cortices were homogenized and filtered through a 70 μm cell strainer. After centrifugation, cells were resuspended in 7 ml FACS buffer, completely mixed with 3 ml of 90% Percoll (GE Healthcare, Pittsburgh, PA), and 1 ml of 70% Percoll was loaded under the cell suspension. The cell suspension was then centrifuged at 2470 rpm for 30 min at 4°C. Leukocytes at the interphase were collected for immunostaining and FACS analysis. These cells were stained with differentially fluorochrome-labeled mAbs against CD45, CD4, CD8, CD19, and CD68 (BioLegend Inc, San Diego, CA, USA) on ice for 30 min to identify CD4+ T cells, CD8+ T cells, B cells, macrophages and microglia. Data on stained samples were acquired on a BD LSR II flow cytometer using Diva software (v6.1.2, Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (v7.6.2, Tree Star, Ashland, OR).
2.5 Statistical analysis
All data are expressed as mean ± SEM, and analyzed with GraphPad Prism (GraphPad Software, San Diego, CA). Statistical analysis for infarct volumes was performed with analysis of variance (ANOVA) followed by Newman-Keuls post hoc or Dunnet’s tests. Functional neurological scores (FNS) were analyzed with the Kruskal-Wallis test followed by the Mann-Whitney U-test with Bonferroni correction. FACS results were analyzed by one-way ANOVA followed with Bonferroni post hoc test. P<0.05 was considered significant.
3 Results
3.1 Ischemic postconditioning reduced infarct sizes and attenuated neurological scores after stroke
Of 125 adult male C57BL/6 mice that underwent surgery, 38 died during the observation period. Of the 87 survivors, 65 were randomly assigned to 6 groups to measure infarcts. Two mice were excluded due to no infarction and the remaining 20 mice were used for FACS analysis in 4 groups (n=5 mice/group).
Stroke resulted in 49.6 ± 2.8% infarction of the ischemic hemisphere as measured 3 days after stroke, which was significantly reduced to 36.5 ± 3.3%, 27.9 ± 2.9%, 34.6 ± 4.4%, 38.9 ± 3.4, and 37.1 ± 4.1% in groups II to VI receiving IPostC with different paradigms, respectively (Fig. 1). Among these groups, IPostC with 3 cycles of 15 sec/30sec occlusion/reperfusion started at 2 min after reperfusion in group III generated the strongest protection.
Focal neurological scores were evaluated at post-stroke day 1, 2 and 3. Consistent with the protective effects on infarct sizes, IPostC significantly lowered neurological scores compared with ischemic controls at day 3. In addition, scores were significantly reduced from day 1 to day 3 in animals receiving IPostC treatment but not in animals with control ischemia alone.
3.2 Ischemic areas spared by IPostC are evident in the ischemic penumbra
Two areas of border zone showed protective effects of IPostC: the inter-ACA-MCA cortical area betweenand the ventromedial–dorsolateral interstriatal area (Fig. 2A and 2B). These territories are the “watershed area” between 2 adjacent arteries. We hypothesized that these spared ischemic areas belong to the ischemic penumbra of the focal ischemia. To prove this hypothesis, we identified ischemic core and penumbra by subjecting animals to a series of ischemic periods ranging from 10 to 60 min. The infarction stained by TTC 3 days post-stroke suggests that the infarction progressively expanded after ischemia from the dorsolateral striatum in ischemic brains at 10 and 15 min, to the ventromedial striatum at 20 min, to the cortex at 30 min, and eventually reached nearly the entire area of the frontoparietal cortex at 45 min after ischemia (Fig. 2C). We define the ischemic core as the initial site of injury and the penumbra as the infarcted area that develops over time. The 2 regions spared by IPostC, as analyzed above, are thus located in the penumbra.
Fig. 2.
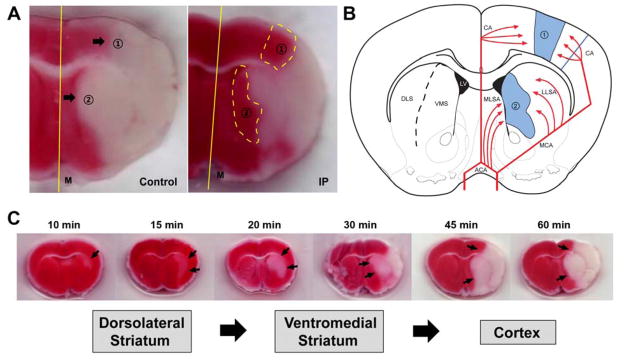
The ischemic regions spared by IPostC. A. The spared regions of infarcted area by IPostC are observed in border zones between the cortical territories of the ACA and those of the MCA (area 1), as well as in the ventromedial and dorsolateral striatum (area 2). B. The schematic diagram shows brain regions with collateral blood supply. The red lines signify blood supplies by cerebral arteries, and the blue regions in the schematic drawing are the “fighting area” between collaterals of ACA and MCA, as well as medial lenticulostriate artery (MLSA) of ACA and lateral lenticulostriate artery (LLSA) of MCA. CA; cortical artery, DLS; dorsolateral striatum, M; midline, VMS; ventromedial striatum. C. Development of infarction in animals with from brief ischemia (10 min) to various longer periods of ischemia up to 60 min. By 10 min of ischemia, only slight subcortical injury was seen in the lateral striatum. By 30 min of ischemia, infarct was rapidly increased and evident in the cortex. Severe cortical injury was observed after 45 min of ischemia.
3.3 IPostC inhibited brain inflammation and attenuated peripheral lymphopenia
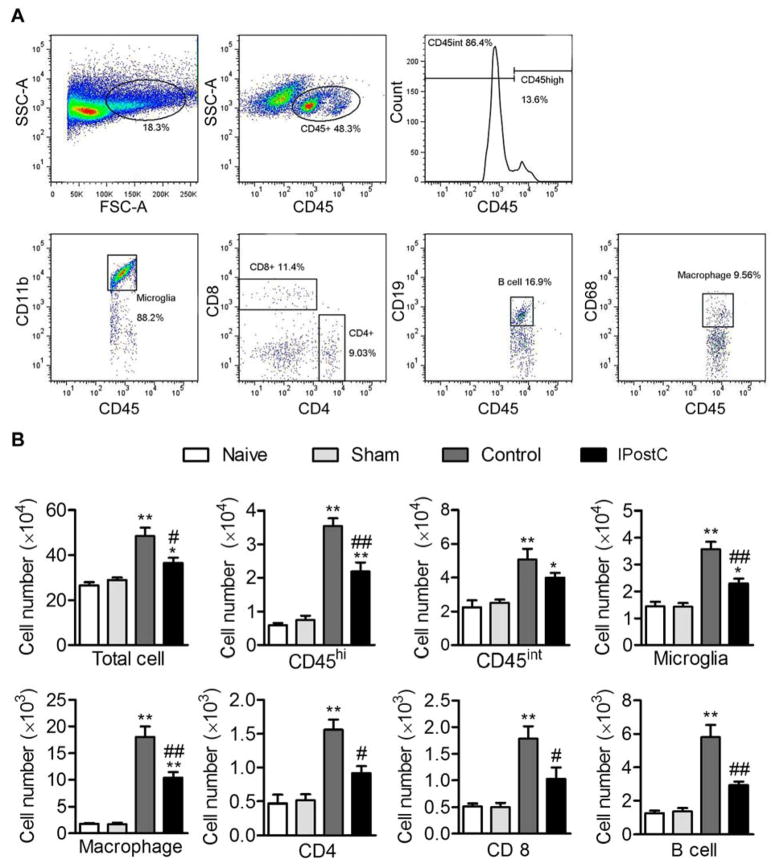
We measured microglial cells and immune cell populations of macrophages, CD8 T cells, CD4 T cells, and B cells in the ischemic brain 3 days after stroke (Fig. 3). Microglial cells are identified as CD45intCD11b; macrophages as CD45hiCD68+; CD4 T cells and CD8 T cells by CD45+CD4+ and CD45+CD8+ cells, respectively. Results show that the numbers of all cell types were significantly increased in the ischemic brains, but IPostC attenuated their increases.
Fig. 3.
The effects of IPostC on infammatory cell infiltration in the ischemic brain 3 days after stroke. A. Gating strategies to identify immune and microglial cells in the ischemic brain. Both microglial and leukocytes are identified by expression of CD45 antigen. Microglia is identified as CD45intCD11b+ cells, while monocyte-derived macrophages are defined as CD45hiCD68+ cells. CD4 T cells, CD8 T cells and B cells are identified as CD45+CD4+, CD45+CD8+ and CD45+CD19+ cells, respectively. B. Mean and SD of individual subsets for each group, *P<0.05 and **P<0.01 between naive and sham groups; #P<0.05 and ##P<0.01 compared to control group. n=5 mice/group.
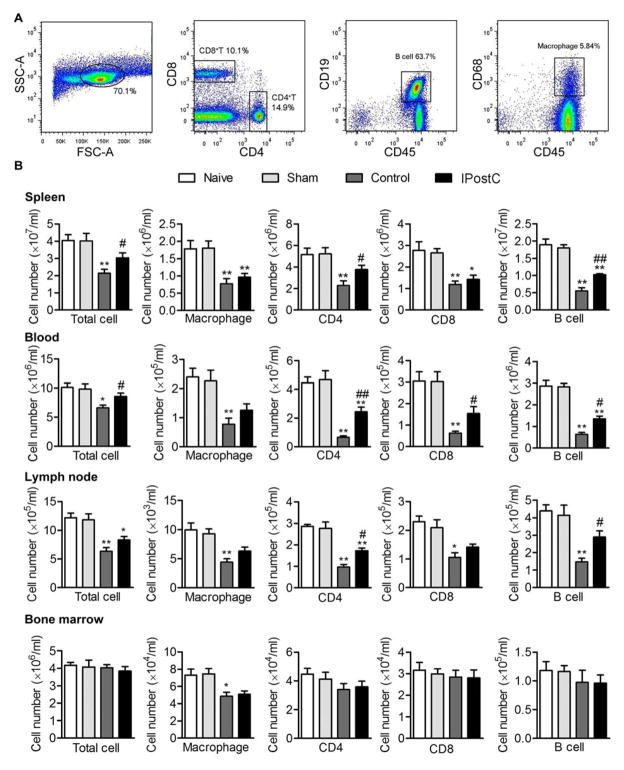
We further examined the numbers of CD4 T cells, CD8 T cells, B cells and macrophages in the peripheral blood, lymph node, spleen and bone marrow (Fig. 4). We found that these cell numbers were robustly reduced in both blood, lymph nodes and spleen, a phenomenon of lymphopenia typically observed in SIID. In the spleen, IPostC significantly helped maintain CD4 T cells and B cells, but not CD8 T cells or macrophages. In blood, IPostC significantly increased ooth T cell subsets and B cells.
Fig. 4.
Changes in numbers of immune cells in peripheral blood, spleen and bone marrow 3 days after stroke. A. Gating strategies to identify immune cell types. CD4 T cells, CD8 T cells, B cells and macrophages are identified similarly as in Fig. 3. B. Mean and SD of individual subsets for each group. The bar graphs show statistical results of immune cell populations in the spleen, peripheral blood, and bone marrow. *P<0.05 and **P<0.01 between naive and sham group; #P<0.05 and ##P<0.01 compared to control group (stroke). n=5 mice/group.
4 Discussion
We establish an IPostC model in mice. IPostC along various paradigms of brief occlusion/reperfusion of the MCA initiated immediately, 2 min or 3 hr after stroke reduced infarction and improved neurological scores. We show that IPostC blocked infiltration of leukocytes, including macrophages, T cells and B cells in the ischemic brain. We also found that IPostC attenuated lymphopenia in the peripheral blood and spleen.
IPostC was initially defined in the field of myocardial ischemia research as a series of brief mechanical occlusions induced after reperfusion (Na et al., 1996; Zhao et al., 2003b), in contrast to ischemic preconditioning. Our laboratory demonstrated that IPostC also reduces cerebral infarction after stroke in a distal MCA occlusion model in rats (Zhao et al., 2006). Several laboratories have confirmed the protective effects of IPostC in MCA suture occlusion and transient global ischemic models in rats(Zhao et al., 2006; Steiger and Hanggi, 2007; Pignataro et al., 2008). Our previous studies defined rapid IPostC as that induced immediately or a few minutes after reperfusion (Zhao et al., 2006; Steiger and Hanggi, 2007; Pignataro et al., 2008; Zhao, 2009) and delayed IPostC as that initiated a few hours or days post-stroke(Ren et al., 2008; Leconte et al., 2009). We and others have found that rapid and delayed IPostC attenuated brain injury, as measured by infarct size and neurological function, and that protective effects depend on the cycle number and duration of reperfusions/occlusions as well as stroke severity(Zhao, 2009). This study supports our hypothesis that IPostC generates protective effects in mice comparable to those seen in rats. As several genetic mutant mice are available, IPostC mouse models may be superior for future studies that investigate underlying cellular and molecular protective mechanisms against stroke injury.
We further analyzed and examined if the spared infarct areas are located in the ischemic penumbra. Our results show that the spared infarct areas were seen in border zones between the cortical territories of the ACA and the MCA, as well as in the ventromedial and dorsolateral striatum. These regions were confirmed to develop sequentially over longer periods, after ischemic core onset in the dorsolateral striatum, and to progress into the ventromedial striatum and cerebral cortex of MCA territory. The ischemic regions spared by IPostC can thus be regarded as ischemic penumbra.
Intracranial vascular collaterals are known to provide residual blood flow to penumbral tissue in acute ischemic stroke and to contribute to infarct size variability in humans. Recruitment of this subsidiary vascular network may therefore provide a source of residual blood flow to potentially salvageable cerebral regions (Miteff et al., 2009). The field of collateral therapeutics remains largely unexplored in pre-clinical studies, however, mostly because of the technical difficulty in quantifying the hemodynamic response of collateral vessels to treatment. Although translational stroke research in this area is necessary, the few basic pertinent studies so far have reported morphological evidence for dynamic recruitment of collaterals in the ACA–MCA and lenticulostriate arteries (LSA) border zone territories after MCA embolic occlusion (Phan et al., 2005; Kleinschnitz et al., 2010b). In both humans and rodents, a significant source of collateralization after MCA occlusion occurs via the circle of Willis through the ACA and lenticulostriate anastomoses (medial and lateral) between deeply penetrating branches of ACA and MCA. The corpus striatum is especially vulnerable to unrecoverable injury after occlusion of the proximal MCA(Bozzao et al., 1989). Whereas obstruction of the proximal MCA causes an immediate drop in blood flow in the territories supplied by the LSA, the superficial cortex may be salvaged by leptomeningeal collaterals (Adams et al., 2007). Our findings suggest that intracranial collateral blood supplies may be attributable to the protective effect of IPostC in mice.
During inflammation innate immune cells, including neutrophils and macrophages, are recruited into the ischemic brain and generate overproduction of free radicals, which contributes to further brain injury (Myers et al., 1991; Matsumoto et al., 2008; Gelderblom et al., 2009). Brain macrophages can be derived from both microglia and circulating monocytes. Recent studies suggest that macrophages derived from circulating monocytes can be identified by high level expression of CD45 antigen (CD45hi) while intraneous microglia can be defined as low level of CD45 expression(Gliem et al., 2012). Our results using these markers suggest that both microglia and monocyte-derived macrophages were robustly increased in the ischemic brain, and both were significantly blocked by IPostC. In addition, recently adaptive immune cells, such as T cells and B cells, have also been reported to infiltrate into the ischemic brain(Yilmaz et al., 2006; Hurn et al., 2007; Liesz et al., 2009; Kleinschnitz et al., 2010a; Chen et al., 2012). In general, T cells worsens brain injury while the role of B cells in ischemic injury remains controversial. Our results suggest that IPostC reduced infiltration of both T cell subsets as well as B cells, which may be involved in the protective effects of IPostC.
We have shown for the first time that IPostC can attenuate systemic lymphopenia in the peripheral immune organs, including lymph nodes and spleen, suggesting that IPostC may inhibit SIID. In recent years research centers have shifted their focus from targeting primary brain injury to peripheral immunity, as SIID is a major factor in delayed infection and mortality(Meisel et al., 2004; Prass et al., 2006; Hug et al., 2009; Urra et al., 2009). One characteristic of SIID is lymphopenia in the peripheral blood and spleen. We found that stroke resulted in lymphopenia, which was robustly attenuated by IPostC, suggesting IPostC may block SIID. The mechanisms underlying this attenuation of lymphopenia are not known. The severity of SIID may be determined by infarct size and degree of brain inflammation. More studies are required to understand how and to what extent IPostC reduces SIID.
In conclusion, we have successfully established an IPostC model in mice, and found that IPostC spared ischemic penumbra in the cortex and striatum, reduced infiltration of both innate and adaptive immune cells, and attenuated lymphopenia in the peripheral blood and spleen associated with immunodepression.
Highlights.
An ischemic postconditioning (IPostC) model is established in mice.
Various paradigms of IPostC reduce infarction and improve neurological scores.
IPostC blocks the infiltration of leukocytes, including macrophages, T cells and B cells in the ischemic brain.
IPostC attenuates lymphopenia in the peripheral blood and spleen.
Acknowledgments
The authors thank Ms. Cindy H. Samos and Felicia Beppu for manuscript assistance. This project is partly supported by AHA grant in aid and 1R01NS064136-01 (HZ).
Footnotes
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989;20:735–740. doi: 10.1161/01.str.20.6.735. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, Offner H. Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice. Metab Brain Dis. 2012;27:487–493. doi: 10.1007/s11011-012-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, Jander S. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71:743–752. doi: 10.1002/ana.23529. [DOI] [PubMed] [Google Scholar]
- Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Hill JK, Gunion-Rinker L, Kulhanek D, Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP, Eckenstein FP. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res. 1999;820:45–54. doi: 10.1016/s0006-8993(98)01140-8. [DOI] [PubMed] [Google Scholar]
- Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, Liesz A, Veltkamp R. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010a;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010b;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leconte C, Tixier E, Freret T, Toutain J, Saulnier R, Boulouard M, Roussel S, Schumann-Bard P, Bernaudin M. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 2009;40:3349–3355. doi: 10.1161/STROKEAHA.109.557314. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24:437–448. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J Immunol. 2011;186:471–478. doi: 10.4049/jimmunol.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Bartels M, Lu A, Sharp FR. Microglia/macrophages proliferate in striatum and neocortex but not in hippocampus after brief global ischemia that produces ischemic tolerance in gerbil brain. J Cereb Blood Flow Metab. 2001;21:361–373. doi: 10.1097/00004647-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Chuai M, Imai Y, Takahashi H, Tanaka J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2008;28:149–163. doi: 10.1038/sj.jcbfm.9600519. [DOI] [PubMed] [Google Scholar]
- Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, Halle E, Volk HD, Dirnagl U, Meisel A. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- Na HS, Kim YI, Yoon YW, Han HC, Nahm SH, Hong SK. Ventricular premature beat-driven intermittent restoration of coronary blood flow reduces the incidence of reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am Heart J. 1996;132:78–83. doi: 10.1016/s0002-8703(96)90393-2. [DOI] [PubMed] [Google Scholar]
- Phan TG, Donnan GA, Wright PM, Reutens DC. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. 2005;36:986–991. doi: 10.1161/01.STR.0000163087.66828.e9. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37:2607–2612. doi: 10.1161/01.STR.0000240409.68739.2b. [DOI] [PubMed] [Google Scholar]
- Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Hanggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir (Wien) 2007;149:1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Urra X, Obach V, Chamorro A. Stroke induced immunodepression syndrome: from bench to bedside. Curr Mol Med. 2009;9:195–202. doi: 10.2174/156652409787581574. [DOI] [PubMed] [Google Scholar]
- Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–2369. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- Xiong X, Gu L, Zhang H, Xu B, Zhu S, Zhao H. The protective effects of T cell deficiency against brain injury are ischemic model-dependent in rats. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003a;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003b;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]