Summary
Cre/LoxP-mediated DNA recombination allows for gene function and cell lineage analyses during embryonic development and tissue regeneration. Here, we describe the derivation of a K19CreERT mouse line in which the tamoxifen-activable CreERT was knocked into the endogenous cytokeratin 19 locus. In the absence of tamoxifen, leaky Cre activity could be detected only in less than 1% of stomach and intestinal epithelial cells, but not in pancreatic or hepatic epithelial tissues. Tamoxifen administration in postnatal animals induced widespread DNA recombination in epithelial cells of pancreatic ducts, hepatic ducts, stomach, and intestine in a dose-dependent manner. Significantly, we found that Cre activity could be induced in the putative gut stem/progenitor cells that sustained long-term gut epithelial expression of a Cre reporter. This mouse line should therefore provide a valuable reagent for manipulating gene activity and for cell lineage marking in multiorgans during normal tissue homeostasis and regeneration.
Keywords: lineage tracing, pancreas, small intestine, colon, liver, kidney, stomach, Cre
The Cre/LoxP-based technology allows for functional analyses of essential genes in specific organs by gene inactivation or controlled ectopic gene expression (Branda and Dymecki, 2004; Lewandoski, 2001; Sauer and Henderson, 1988). When combined with detectable marker protein expression, Cre-LoxP allows for cell lineage analyses in living animals (Branda and Dymecki, 2004; Gu et al., 2003). Upon modifying Cre to produce a tamoxifen (TM)-dependent molecule, CreERT, it is now possible to control Cre activity both spatially and temporally (Metzger and Chambon, 2001). This feature allows for dissecting the genetic requirements for cell/tissue homeostasis and for following cell lineages during tissue regeneration.
We have derived a K19CreERT knockin allele to recombine DNA in epithelial cells of several adult organs. K19 encodes an intermediate filament protein (Moll et al., 1982) that is expressed in multiple cell types from the epiblast stage and is maintained in multiple epithelial cell types of later embryonic and postnatal stages (Bosch et al., 1988; Lane et al., 1983; Moll et al., 1982; Quinlan et al., 1985). For example, K19 is highly expressed in the pancreatic ducts of the adult pancreas (Deramaudt et al., 2006), but is absent or weak in acini and islets (Brembeck et al., 2001). Similarly, K19 is highly expressed in the liver duct cells but not in the mature hepatocytes (Nishikawa et al., 1996). Because studies have suggested that the pancreatic duct and liver duct cells may behave as progenitor or stem cells for tissue regeneration (Dorrell and Grompe, 2005; Hu et al., 2007; Xu et al., 2006), K19CreERT-based cell lineage studies could reveal the potential fate of these ductal cells during regeneration and tissue homeostasis. Additionally, K19CreERT could allow for temporally controlled gene inactivation and/or activation in epithelial cells to analyze gene function during cell renewal and tissue maintenance, a tribute that cannot be addressed with the existing K19Cre mouse line that is active in progenitors for all tissues of the embryo proper (Means et al., 2005).
We derived a K19CreERT allele by replacing K19 ATG with a CreERT-cDNA followed by a SV40 polyadenylation signal (see Fig. 1). This design minimally altered K19 transcription regulatory elements while producing a CreERT message with a short 3′-UTR. Inclusion of a polyadenylation signal 3′ to the CreERT sequence prevented the transcription of the five noncoding exons of the endogenous K19 gene. Otherwise, the presence of these noncoding exons in CreERT mRNA could trigger nonsense- mediated mRNA degradation (Conti and Izaurralde, 2005; Doma and Parker, 2007). Thus, adding an extra polyadenylation signal immediately down-stream of CreERT cDNA is designed to enhance CreERT production. We expected that K19CreERT would produce CreERT, which could be activated by application of TM, in most, if not all K19-expressing cells.
FIG. 1.
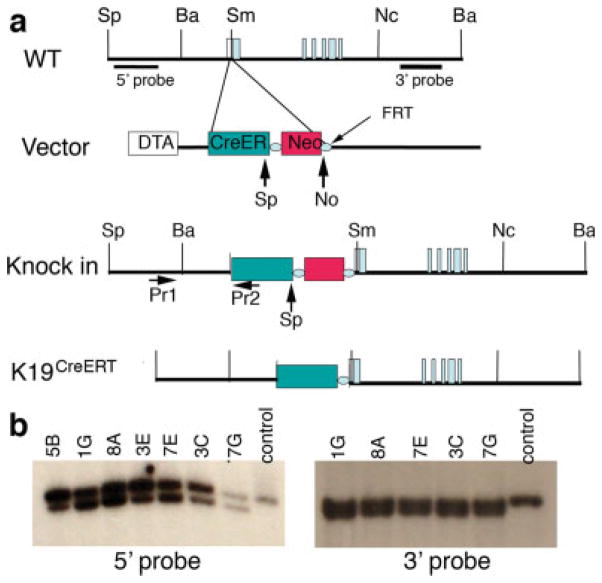
K19 locus targeting strategy. (a) Structures of K19 alleles and targeting vector. The targeting vector has a 1.3-kb 5′-arm, which locates immediately upstream of the K19 ATG. The 3′-arm is a 7.2-kb fragment that has a 156-base pair overlap with the 5′-arm. The targeting vector uses Diphteria Toxin A (DTA) subunit for negative selection and a FRT-flanked neomycin resistant gene for positive selection. Pr1 and Pr2 indicate the oligos utilized for genotyping. (b) Southern blots to show several targeted ES cell clones that give expected hybridization patterns (see Materials and Methods section for the expected size of DNA fragments). Sp, SpeI, Ba. BamHI, Sm, SmaI, Nc, NcoI, No, NotI.
R26REYFP reporter mouse was utilized to monitor Cre activity by virtue of EYFP expression that is dependent upon Cre-mediated recombination (Srinivas et al., 2001). Because EYFP is a small protein, it readily diffuses between the cytoplasm and the nucleus but often appears more abundant in the nucleus. TM was administered to K19CreERT/+; R26REYFP/+ animals of different ages, at P0 (Day 1 after birth), P3, or P6 with 0.2–0.5 mg TM or at 8 weeks of age with (4 mg × 3 doses) TM. Four days after the last TM injection, EYFP expression in several endodermally derived organs was monitored.
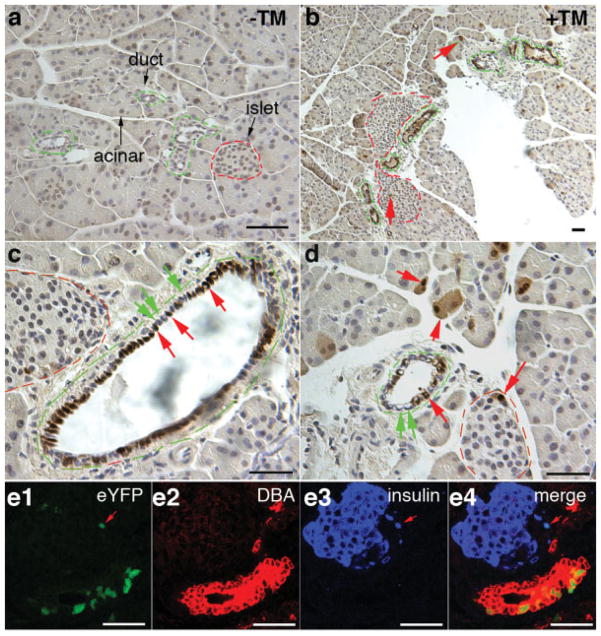
We first examined EYFP expression in K19CreERT/+; R26REYFP/+ pancreas, which contains the exocrine acini, pancreatic ducts, and endocrine islet cells, with TM administration at several stages (Slack, 1995). Immunohistochemical assay (IHC, Fig. 2a–d) or direct EYFP fluorescence observation (FL, Fig. 2e) detected no pancreatic cells expressing EYFP without TM administration (Fig. 2a and data not shown). Yet TM administration to adult (Fig. 2b–d) or neonatal (Fig. 2e) K19CreERT/+; R26REYFP/+ animals induced widespread EYFP expression in the pancreatic ducts 1 week after TM administration. In animals that received 0.5 or 12 mg of TM at P0 or 8 weeks of age, respectively, from 10–45% of interand intralobular duct cells (n = 4 for P0 and n = 6 for adults) produced EYFP. No EYFP was detected in intercalated ducts, which do express K19 but may have a lower level of expression than the cuboidal epithelial ducts. To our surprise, rare, yet detectable, acinar cells (<1%) and some islets cells (<1%) also express EYFP after TM administration (Fig. 2b,d). Co-IF staining with insulin antibodies or a ductal marker recognized by Dolichos biflorus agglutinin (DBA) confirmed that a small portion of the EYFP+ (positive) cells are endocrine cells (Fig. 2e). Although K19 protein production in acinar or islet cells has not been shown, this low amount of recombination may be due to low, immunologically undetectable level of expression or to the CreERT insertion cassette altering expression of the allele. Despite the leakiness in acinar and islet cells, our K19CreERT mouse is the first inducible Cre line that allows for DNA recombination preferably in pancreatic ductal cells and it should complement other existing pancreas-specific Cre lines for pancreatic lineage and development-related studies. K19CreERT does not induce recombination in all ductal cells as discovered by sampling multiple pancreatic sections in each of six adult pancreata. Yet this low activity is sufficient for many lineage and gene deletion studies. The lower K19CreERT activity could create marked mutant cell clones in largely normal tissue/organs, allowing cell-specific analyses without confounding effects from lethality or complete loss of tissue.
FIG. 2.
K19CreERT allows inducible recombination in pancreatic duct cells. Panels (a–d) utilized antibodies to GFP/YFP that were then visualized colorimetrically (brown). Panels in (e) directly visualized yellow fluorescence emitted by the EYFP protein. (a) Control K19-CreERT; R26REYFP pancreas without TM administration shows lack of any EYFP expression in the pancreas. Acinar, duct (green broken lines) and islets (red broken lines) were marked. (b–d) Pancreata of animals that received 12 mg TM at 8 weeks of age, 1 week before sacrifice. Note the abundance of labeled cells (red arrows) in ducts and a small number of labeled cells in islets and in acinar cells. Also note the presence of EYFP− duct cells shown at high magnification (green arrows) in (c and d). (e) Pancreata of animals that received TM 1 day after birth and were sacrificed 1 week later. Even at this age, most labeled cells are ductal but there are rare labeled cells within islets (red arrows in e) and acini. Labeled cells were not randomly assorted in ducts; while the ducts shown have many labeled cells, other ducts have very few. In (e), immunofluorescence staining showed the presence of EYFP fluorescence (green) in DBA-reactive duct cells (red) and insulin+ β cells (blue) on a same microscopic field. Three separate channels (e1–e3) and a merge (e4) were shown. Bars = 50 μm.
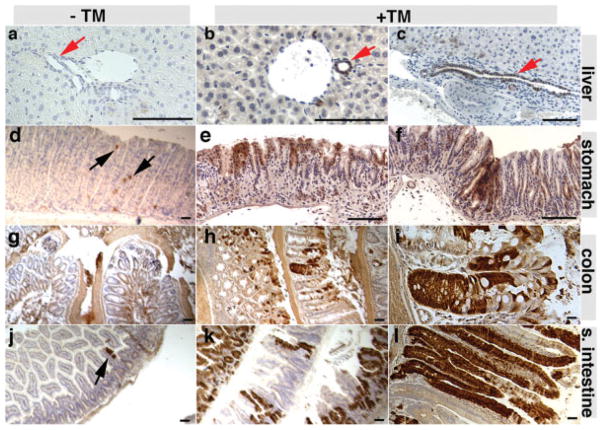
We determined whether K19CreERT could induce recombination in the liver, stomach, the intestine, and the kidney (Fig. 3 and data not shown) by administering TM at 8 weeks of age and characterizing EYFP production after 1 week. TM induced widespread recombination in the epithelial portions of these organs (see Fig. 3). Only rare recombination events (~0.15% epithelial cells in six animals inspected) could be detected in the absence of TM in the stomach (Fig. 3d) and proximal intestinal epithelial cells (Fig. 3j) of the K19CreERT/+; R26REYFP/+ animals. Quantification showed that 30.8% ± 9.8%, 48.0% ± 19.5%, 54.1% ± 14.1%, 42.8% ± 14.4%, and 20.6% ± 6.8% (n = 6) of the epithelial cells in the bile duct, duodenum, small intestine, large intestine, and stomach cells produced EYFP after TM administration, respectively. Within the kidney, recombination was also observed principally in the papillary ducts, with a lower amount of recombination in collecting ducts (data not shown).
FIG. 3.
K19CreERT induces recombination in liver duct and gastrointestinal epithelial cells in the adults. Twelve milligram TM was administered at 8 weeks of age. EYFP expressions were analyzed after 1 week. EYFP production, detected by antibody staining (brown), was shown in the absence (a, d, g, and j) or presence (b, c, e, f, h, i, k, and l) of TM. (a–c) The liver. Note the both bile ductule cells (b) and intrahepatic bile duct cells (c) were labeled. (d–e) Stomach. (g–i) Large intestine. (j–l) Small intestine. Note that in the absence of TM, only stomach and small intestinal cells showed rare cells that express EYFP (black arrows). The red arrows in the liver sections point to the duct. Bars = 100 μm.
We further examined whether K19CreERT could label putative gut stem cells. Intestinal stem cells are thought to divide slowly giving rise to transient amplifying cells that rapidly divide to produce mature cells. However, label retention experiments indicate that most transient amplifying cells in the small intestine turn over approximately every 7 days (Potten, 1998). Therefore, by examining EYFP labeling 6 weeks after TM-induced cell marking, most labeled intestinal cells likely have arisen from intestinal stem cells. The identity and duration of transient amplifying cells in the stomach epithelium are not as well characterized. Yet, direct lineage tracing has suggested that each gastric gland is derived from one to three stem cells (Qiao et al., 2007). Therefore K19CreERT- based clonal analyses could likely reveal the presence of clone-forming cells in stomach as well.
K19CreERT/+; R26REYFP/+ mice were administered with TM at P3 (0.1 mg TM) or at 8 weeks of age (4 mg TM). One or 6 weeks after TM injection, the EYFPlabeled cells in the whole gut were characterized. In non- TM treated animals (n = 3 at each stage), only a small number of single cell or small cell clusters (less than 20 cells) were observed to express EYFP (Fig. 4a,e). One week after TM injection, about 5% of all gut cells express EYFP in all animals, with some appeared as large EYFP+ cell clusters (data not shown). Six weeks after TM administration, large EYFP+ clones in all animals were frequently observed in TM-administered animals at P3 and 8 weeks of age (Fig. 4b–d,f–h, and data not shown). Several features of the EYFP+ clones suggest that they were derived from single gut stem cells. First, at the TM dosage utilized in this experiment, K19CreER only marked about 5% of all gut cells shortly (1 week) after TM administration. These marked cells appeared to distribute randomly in the intestinal epithelium, suggesting that it is unlikely to introduce DNA recombination simultaneously in multiple progenitor cells within a single crypt. Therefore, if all cells within a villus produced EYFP 6 weeks after TM administration, it is likely that this villus was derived from a marked stem cell. Second, EYFP+ clones containing multiple adjacent villus structures were frequently observed 6 weeks after TM administration, suggesting that each of these clones could arise from a single stem cell by expansion and fission during growth (Yen and Wright, 2006). Consistent with this notion, more than half of the large EYFP+ clones contained multiple villi or glands in animals treated with TM at P3. Most of the EYFP+ clones in animals treated with TM at adult ages only contain single villus or glands, indicating that the putative gut stem cells in the adults are less likely to expand than in neonatal stages. Third, all cells in some EYFP+ villi produced EYFP (Fig. 4d), suggesting that each villus was derived from a single, self-renewing EYFP+ cell, instead of a portion of several transient amplifying progenitor cells within a single villus. Similar EYFP-labeling features were observed in the stomach (Fig. 4g) after TM exposure, indicating the marking of putative gastric stem cells. These data suggest, but do not prove, that K19CreERT could be used to label gut stem cells.
FIG. 4.
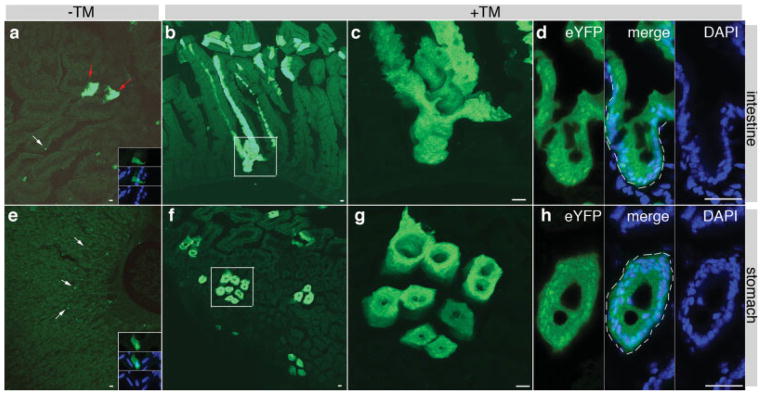
K19CreERT labels putative gut stem cells. Direct fluorescence showed gut EYFP expression 6 weeks after 0.5 mg TM injection at P3. (a–d) Intestine. (e–h) Stomach. (a and e) No TM administration. Low magnification pictures were provided for a global view on the leakiness of K19CreERT deletor. White arrows point to single cells. Red arrows point to two cell clusters. Insets in (a) and (e) are high amplification of single YFP+ cells costained with DAPI. EYFP, DAPI, and a merge channel were shown. (b–d and f–h) EYFP expression after TM administration. (c) and (g) are amplified areas (boxed) in (b) and (f), respectively. (d) and (h) showed an intestinal crypt and a gastric gland, respectively. Cells were stained with DAPI to show nucleus of each cell. Note that in the crypt or gland (white broken lines), all cells are EYFP+. Bars = 40 μm.
The non-essential nature of K19 allowed us to derive K19CerERT/CreERT; R26REYFP/+ animals. We found that 12 mg of TM administration to these mice at 8 weeks increased cell labeling to about 50–70% in the intestine (data not shown), about a 1.5-fold increase over that of K19CerERT/+;R26REYFP/+animals. Similar labeling enhancement in other organs was also observed. Notably, the percentage of labeled cells without TM administration increased as well to 0.4–0.7% (n = 4) in the duodenum. These data suggest that two doses of K19CreERT could induce a higher recombination rate, more suitable for loss of gene function analysis.
In summary, we have demonstrated that the K19CreERT mouse line can be utilized to introduce DNA manipulation in a variety of adult epithelial cells. Thus, it allows for temporally controlled gene recombination and cell lineage tracing during tissue regeneration in multiple cell types of endodermally derived organs.
MATERIALS AND METHODS
Mouse care and experimental procedures followed standard protocols in accordance with Vanderbilt animal usage guidelines. For initial knockout mice production, the C57BL/6 strain was used (Charles River Laboratories, Wilmington, MA). Subsequent strain maintenance and crosses utilized CD1 mice (Charles River Laboratories). Flpe, R26REYFP mice and genotyping methods were previously reported (Dymecki, 1996; Srinivas et al., 2001). The K19 targeting vector construction followed standard techniques. The final targeting vector contains the following DNA elements sequentially: 1.3 kb 5′ K19 arm-CreERT-SV 40 PolyA-FRT-pGKneo-FRT-7.2 kb 3′ K19 arm. The K19 recombination arms are derived from a BAC clone [RP23-217I3, purchased from the Children’s Hospital of Oakland, Oakland, CA (Warming et al., 2005)] using direct PCR or DNA recombineering, respectively. The oligos for PCR-amplifying the 5′ arm: forward: 5′-GTCGACCTTTGAGCTAGGAAGTGGT-3′; reverse: 5′-GAATTCGATGAGGAGGGAGACCAGAGCG-3′. The oligos for retrieving 3′ arm: 5′-GGCCGCATAACTTCGTATAGCATACATTATACGAAGTTATTTAGGGCATAAAAAGCCACAGGTGAGGGCCTTGTCACTCCTCCTGCGGCCAGCAGTTCTCAG-3′ and 5′-CCATGGCAGAAAGACCCTCTCTACTCTCAACCCCATCTCTGTCCCCCCGATCACCCCATAAACAACTCTCAGGCCTTTCCACCCTGCACACACAAAGAAAGGCTTCGTCTCACACTTC-3′. ES cell electroporation (129 SvEv-derived TL1 cell) and clone selection followed standard protocol. For ES cell screening, both PCR and Genomic Southern blot-based methods were used. First, a DNA oligo outside of the 5′ arm (Pr1: 5′-AGGACTGACTTTGAACACTCTTC-3′) and an oligo complementary to the 5′-end of CreERT (Pr2: 5′-ATTTTGGTGTACGGTCAGTAAATT-3′) were utilized to screen for the clones that had correct homologous recombination in the 5′-end of the K19 locus. Targeted clones were expected to produce a 1.5-kb band, which is absent from nontargeted clones. For 3′ recombination verification, a-1.4 kb DNA fragment outside the 3′-arm was PCR-amplified (forward: 5′-TCTTTCCATCTTCTTTTTGGGA-3′ and reverse: 5′-GCTCTGCCTCAACACTATGAG-3′) and used for southern blot. If digested with Bam HI and Not I, the wild type or targeted allele produces a 14- or 12-kb band, respectively. Subsequent genotyping used PCR-based techniques. The oligos used are as follows: Pr3 (5′-GCAGAATCGCCAGGAATTGACC-3′) and Pr2 (5′-GTTCTTGCGAACCTCATCACTC-3′). The unique band from the K19CreERT gene is 300 bp. Mouse lines with the targeted K19 allele were derived by standard blastocyst injection, using C57BL/6 mouse lines as acceptor. After germ line transmission was achieved, the FRT-flanked pGKneo selection cassette was deleted using a Flpe transgenic mouse line to obtain the K19CreERT allele (Dymecki, 1996). TM administration followed previous method (Gu et al., 2002) with minor modification. Briefly, TM was dissolved in corn oil at 40 mg/ml. Desired dose was then injected subcutaneously into different-aged animals.
Immunohistochemistry followed standard protocols. Briefly, mouse tissues were fixed in 4% paraformaldehyde overnight at 4°C or 4 h at room temperature. Tissues were prepared as 6-μm paraffin sections or 20-μm frozen sections. Direct fluorescence observation was utilized to detect EYFP in frozen sections. Indirect staining was visualized within paraffin-embedded tissues using Rabbit anti-GFP antibody (ABCam, Cambridge, MA). Antibody binding was visualized with the Vectastain Elite ABC kit (Vector Labs, Burlingame, CA) followed by incubation in diamino benzidine tetrahydrochloride substrate (Invitrogen, Carlsbad, CA). Colabeling was done with guinea pig anti-insulin and Cy5-conjugated donkey anti-guinea-pig IgG (Jackson Immunoresearch, West Grove, PA) and with DBA-biotin (Sigma-Aldrich, St. Louis, MO) and Cy3-conjugated streptavidin (Jackson Immunoresearch, West Grove, PA). All antibodies utilized a 1:500–1:2,000 dilution.
For quantification of labeling efficiency, frozen tissue sections were utilized. Specifically, fixed tissues were prepared as 20-μm frozen sections. One section per 200 μm was collected. For counting YFP+ cells in the pancreatic ducts, DBA IF staining (as red fluorescence) was utilized to locate the duct. Pictures were taken at both green and red channels on same fields to compare EYFP+ and BDA-binding cells. The labeling efficiency was calculated as the area of EYFP+ cells/area of all epithelial tissues. For cell counting in other tissues, similar approaches were used, except that no immunofluorescence is required for visualizing the epithelial structures.
Acknowledgments
The authors thank the staff of the Vanderbilt Transgenic/ES Cell Shared Resource for expert performance of the ES cell selection and blastocyst microinjection experiments. We also thank Sean Schaffer for help with confocal microscopy.
Contract grant sponsor: NIH, Contract grant number: DK065949 and JDRF 1-2006-759
LITERATURE CITED
- Bosch FX, Leube RE, Achtstatter T, Moll R, Franke WW. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol. 1988;106:1635–1648. doi: 10.1083/jcb.106.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Moffett J, Wang TC, Rustgi AK. The keratin 19 promoter is potent for cell-specific targeting of genes in transgenic mice. Gastroenterology. 2001;120:1720–1728. doi: 10.1053/gast.2001.24846. [DOI] [PubMed] [Google Scholar]
- Conti E, Izaurralde E. Nonsense-mediated mRNA decay: Molecular insights and mechanistic variations across species. Curr Opin Cell Biol. 2005;17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Deramaudt TB, Sachdeva MM, Wescott MP, Chen Y, Stoffers DA, Rustgi AK. The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter. J Biol Chem. 2006;281:38385–38395. doi: 10.1074/jbc.M605891200. [DOI] [PubMed] [Google Scholar]
- Doma MK, Parker R. RNA quality control in eukaryotes. Cell. 2007;131:660–668. doi: 10.1016/j.cell.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Dorrell C, Grompe M. Liver repair by intra- and extrahepatic progenitors. Stem Cell Rev. 2005;1:61–64. doi: 10.1385/SCR:1:1:061. [DOI] [PubMed] [Google Scholar]
- Dymecki SM. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc Natl Acad Sci USA. 1996;93:6191–6196. doi: 10.1073/pnas.93.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, Sylvester KG. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Lane EB, Hogan BL, Kurkinen M, Garrels JI. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature. 1983;303:701–704. doi: 10.1038/303701a0. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Means AL, Chytil A, Moses HL, Coffey RJ, Jr, Wright CV, Taketo MM, Grady WM. Keratin 19 gene drives cre recombinase expression throughout the early postimplantation mouse embryo. Genesis. 2005;42:23–27. doi: 10.1002/gene.20119. [DOI] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Tokusashi Y, Kadohama T, Nishimori H, Ogawa K. Hepatocytic cells form bile duct-like structures within a threedimensional collagen gel matrix. Exp Cell Res. 1996;223:357–371. doi: 10.1006/excr.1996.0091. [DOI] [PubMed] [Google Scholar]
- Potten CS. Stem cells in gastrointestinal epithelium: Numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RA, Schiller DL, Hatzfeld M, Achtstatter T, Moll R, Jorcano JL, Magin TM, Franke WW. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S. GLP-1/exendin-4 facilitates beta-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract. 2006;73:107–110. doi: 10.1016/j.diabres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]