Abstract
Background
Human T cell lymphotropic virus type 1 (HTLV-1) infection has been associated with recurrent and disseminated strongyloidiasis and adult T cell leukemia/lymphoma (ATLL).
Methods
We compared immunological aspects and markers for ATLL in HTLV-1 patients with or without strongyloidiasis, and evaluated the influence of Strongyloides stercoralis treatment on the immune response and clinical outcomes of HTLV-1 infection.
Results
Levels of TNFα and IFNγ were lower in patients coinfected with HTLV-1 and S. stercoralis than in patients with HTLV-1 only (p < 0.05), and there was an increase in TNFα levels after anthelmintic treatment. Levels of sIL-2R were higher in patients with HTLV-1 coinfected with S. stercoralis and anthelmintic treatment decreased sIL-2R levels (p < 0.05). The one patient who developed ATLL was coinfected with S. stercoralis.
Conclusion
These data show that helminthic infection has a modulatory role in HTLV-1 infection and that S. stercoralis may be a cofactor in the development of ATLL.
Keywords: HTLV-1, Strongyloides stercoralis, Leukemia, ATLL, Interleukin-2 receptor, sIL-2R
Introduction
Human T cell lymphotropic virus type 1 (HTLV-1) is the causal agent of HTLV-1 associated myelopathy (HAM/TSP), adult T cell leukemia/lymphoma (ATLL) and manifestations related to the myelopathy, such as overactive bladder and erectile dysfunction.1–3
HTLV-1 infects T cells inducing lymphocyte proliferation and an exaggerated production of Th1 cytokines such as IFNγ and TNFα.2 In contrast, helminthic infections are characterized by Th2 immune response. As individuals coinfected with HTLV-1 and Strongyloides stercoralis have decreased levels of IL4, IL5, IL13 and S. stercoralis IgE levels, disseminated and recurrent strongyloidiasis are common in patients with HTLV-1.4–6 There are also reports that S. stercoralis influences the development of ATLL.7–9 Indeed, helminthes down-modulate the exaggerated inflammatory response observed in HTLV-1 infection, which could prevent the development of neurological disease.10
The aim of this study was to evaluate in a cohort of patients infected with HTLV-1 and S. stercoralis if strongyloidiasis modifies the immunological response and clinical outcomes of patients with HTLV-1, before and after anthelmintic treatment.
Materials and methods
Patients and clinical outcomes
Thirty patients with S. stercoralis and HTLV-1, and 60 patients with HTLV-1 only were followed in a HTLV-1 clinic at the Hospital Universitário Professor Edgard Santos (HUPES), Bahia, Brazil. Patients were followed for 2–11 years. Study and control groups were matched by age (±5 years) and gender. Exclusion criteria included HIV positive patients or patients with HAM/TSP.
Five clinical outcomes were analyzed: recurrence of strongyloidiasis, development of HAM/TSP, ATLL, overactive bladder and erectile dysfunction. Diagnosis for HAM/TSP was based on WHO's established criteria (1989).11 Diagnosis of overactive bladder was based on the International Continence Society's criteria12 and diagnosis of erectile dysfunction was based on the International Index of Erectile Function (IIEF-5).13 Strongyloidiasis was treated with cambendazol 5 mg/kg (n = 22) or ivermectin 200 µg/kg/day (n = 8). Written informed consent was obtained from each participant and the study was approved by the ethical committee of the Hospital Universitário Professor Edgard Santos.
Assays to detect HTLV-1 infection and S. stercoralis infection
Diagnosis of HTLV-1 infection was made by ELISA (Cambridge Biotech Corp., Worcester, MA, USA) and confirmed by western blot analysis (HTLV blot 2.4, Genelab, Singapore). At least three stool examinations were performed prior to treatment and S. stercoralis infection was determined by the Baermann technique.
Cytokine profile
The cytokine levels were determined by ELISA in supernatants of unstimulated lymphocyte cultures as previously described.2,4 INFγ, TNFα, IL5 and IL10 levels, cytokines that are enhanced in HTLV-1 or strongyloidiasis, were measured by ELISA sandwich technique (R&D Systems, Minneapolis, MN, USA). In 17 patients coinfected with HTLV-1 and S. stercoralis, cytokine levels were measured before and after treatment. Serum sIL-2R was determined by ELISA (Human IL-2sR alpha [Quantikine], R&D Systems, Minneapolis, MN, USA). The HTLV-1 proviral load in peripheral blood mononuclear cells was measured by real time PCR as previously described.14
Statistical analyses
The Pearson χ2 test and Student's t test were used to assess differences between gender and ages, respectively. The Mann-Whitney U test was used to compare cytokines levels and proviral load among groups. Wilcoxon signed-rank test was used to compare cytokines levels before and after helminthiasis treatment. Fisher's exact test and McNemar's test were used to compare proportions. A p value <0.05 was considered significant.
Results
There were no differences in age, gender and clinical manifestations related to HTLV-1 in HTLV-1 infected patients with or without strongyloidiasis. With S. stercoralis infection, 26 patients were asymptomatic at entry, 3 presented with diarrhea and/or abdominal pain and 1 had disseminated strongyloidiasis.
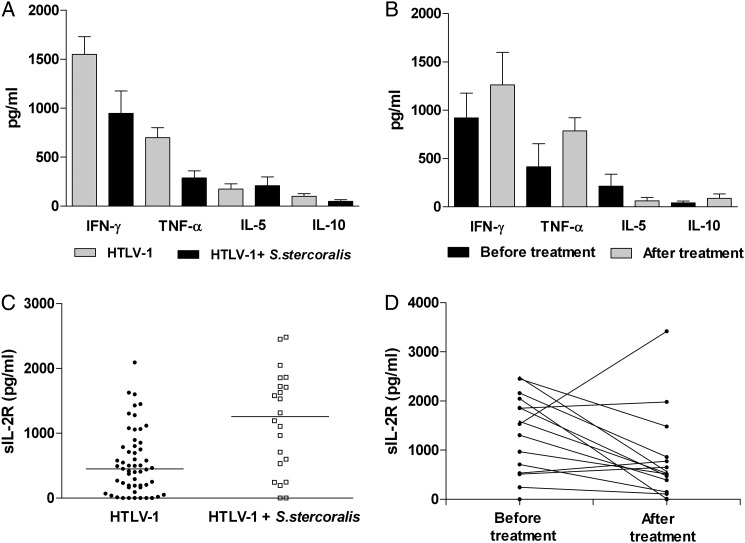
The levels of IFNγ, TNFα, IL5, IL10 and sIL-2R in patients with HTLV-1 with or without strongyloidiasis and after strongyloidiasis treatment are shown in Figure 1. The levels of IFNγ and TNFα were higher in the 60 patients with HTLV-1 without strongyloidiasis than in the 26 patients coinfected with HTLV-1 and strongyloidiasis (p < 0.05). There was an increase in TNFα levels after treatment compared with levels before treatment. This increase was observed in 14 of the 17 (82%) patients treated. INFγ levels increased and IL5 levels decreased after treatment but these differences were not significant (p > 0.05). The serum levels of sIL-2R of 22 patients coinfected with HTLV-1 and strongyloidiasis were higher than in the 55 patients with HTLV-1 without strongyloidiasis (p < 0.05), and sIL-2R levels reduced significantly after anthelmintic treatment (p < 0.05). This decrease was observed in 10 of 15 (67%) patients. The proviral load did not differ (p > 0.05) between the two groups.
Figure 1.
Cytokine profile and sIL-2R levels in patients with human T cell lymphotropic virus type 1 (HTLV-1) with or without strongyloidiasis before and after treatment for strongyloidiasis. (A) The levels (median; range) of IFNγ and TNFα were higher in the 60 HTLV-1 patients without strongyloidiasis (1144 pg/ml; 0–6025 pg/ml and 485 pg/ml; 0–4686 pg/ml, respectively) than in the 26 HTLV-1 patients with strongyloidiasis (744 pg/ml; 0–4538 pg/ml and 113 pg/ml; 0–1157 pg/ml, respectively) (p < 0.05; Mann-Whitney U test). (B) There was an increase in TNFα levels after strongyloidiasis treatment (885 pg/ml; 0–1642 pg/ml) compared with levels before treatment (109 pg/ml; 0–4145 pg/ml) (p < 0.05; Wilcoxon Signed-Rank Test). (C) The levels of sIL-2R in the serum of 22 patients with HTLV-1 and strongyloidiasis (1255 pg/ml; 0–2483 pg/ml) were higher than in the 55 patients with HTLV-1 without strongyloidiasis (452 pg/ml; 0–2094 pg/ml) (p < 0.05; Mann-Whitney U test). (D) Higher levels of sIL-2R were noted before anthelmintic treatment (1532 pg/ml; 0–2438 pg/ml) than after it (527 pg/ml; 0–3419) (p < 0.05; Wilcoxon Signed-Rank test).
Failure of strongyloidiasis treatment was observed in six patients treated with cambendazol, but they responded to ivermectin. In only one case, a 45-year-old woman, recurrence of strongyloidiasis was documented. She was initially treated with cambendazol and then with ivermectin but continued to have gastrointestinal symptoms despite negative stool examination. In 2008 S. stercoralis was again diagnosed and she died due to ATLL.
Neurological outcomes of 90 patients with HTLV-1 with or without past history of strongyloidiasis were determined during follow-up. There were no statistical differences regarding development of erectile dysfunction or overactive bladder between patients with HTLV-1 with or without strongyloidiasis. When the frequency of neurological manifestations was analyzed in all patients enrolled, a significant increase in the number of patients with erectile dysfunction and overactive bladder was observed (p < 0.05) (data not shown).
Discussion
The immune response in HTLV-1 infection is characterized by T cell activation with increased production of inflammatory cytokines and spontaneous T cell proliferation.2 Our observation that levels of IFNγ and TNFα production in patients coinfected with HTLV-1 and S. stercoralis increase after antihelminthic treatment provides an argument in favor of a pivotal role for strongyloidiasis in modifying the immune response of patients with HTLV-1.
The exaggerated production of IFNγ during HTLV-1 infection decreases the defense mechanisms against helminthes and is associated with recurrent and disseminated strongyloidiasis.4–6 This study shows that effective treatment for strongyloidiasis and surveillance for S. stercoralis in patients with HTLV-1 are important in modifying the prognosis for this coinfection. Only one patient remained chronically infected with S. stercoralis and developed ATLL.
Strongyloidiasis has been suggested as a cofactor in the development of ATLL.7,9 Up to 39% of patients coinfected with HTLV-1 and S. stercoralis presented with monoclonal integration of HTLV-1 proviral load DNA in their blood lymphocytes, a characteristic of ATLL.7 Increased levels of sIL-2R and high proviral load are markers for ATLL.15 The sIL-2R levels in patients coinfected with HTLV-1 and S. stercoralis were higher than in patients only infected with HTLV-1 and a significant decrease in these levels occurred after antihelminthic treatment. This, together with the occurrence of ATLL in a patient with strongyloidiasis, provides an argument in favor of a role for S. stercoralis in the development of ATLL.
We did not document that helminthic infection influences the development of neurological diseases in HTLV-1 infection. As all patients with strongyloidiasis were treated, we cannot rule out that helminthic infection, when present, down-modulates the immunological response and may attenuate neurological diseases associated with HTLV-1. However, there was an increase in the prevalence of neurological manifestations in all patients in the study indicating that patients with HTLV-1 may quickly develop neurological symptoms.
This study shows that early and effective treatment for strongyloidiasis in HTLV-1 infection is important to prevent recurrent and disseminated strongyloidiasis. Evidence that after strongyloidiasis treatment there was an increase in TNFα and a decrease in sIL-2R levels reinforces the role of helminthes in the modulation of HTLV-1 immune response and that S. stercoralis may be a cofactor in the development of ATLL.
Acknowledgments
Authors' contributions: FS and EC were responsible for the study design. Clinical follow-up and information about patients were collected by FS, MA, AB and AP. GO, MS and EC participated in the data analysis and in the interpretation of the results. SS was responsible for the immunological studies and proviral load. FS and EC drafted the manuscript. All authors read and approved the final manuscript. EC is guarantor of the paper.
Acknowledgments: We thank Cristiano and Erica for excellent technical assistance.
Funding: This work was supported by the NIH Grants AI-079238, and the Brazilian National Research Council (CNPq); Fundação de Amparo a Pesquisa do Estado da Bahia. EC is a senior investigator with CNPq.
Competing interest: None declared.
Ethical approval: Ethical approval was granted by the ethical committee of the Hospital Universitário Professor Edgard Santos.
References
- 1.Caskey MF, Morgan DJ, Porto AF, et al. Clinical manifestations associated with HTLV type I infection: a cross-sectional study. AIDS Res Hum Retroviruses. 2007;23:365–71. doi: 10.1089/aid.2006.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira P, Castro NM, Muniz AL, et al. Prevalence of erectile dysfunction in HTLV-1 infected patients and its association with overactive bladder. Urology. 2010;75:1100–3. doi: 10.1016/j.urology.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porto AF, Neva FA, Bittencourt H, et al. HTLV-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunol. 2001;23:503–7. doi: 10.1046/j.1365-3024.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 5.Gotuzzo E, Terashima A, Alvarez H, et al. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type-1 infection in Peru. Am J Trop Med Hyg. 1999;60:146–9. doi: 10.4269/ajtmh.1999.60.146. [DOI] [PubMed] [Google Scholar]
- 6.Terashima A, Alvarez H, Tello R, et al. Treatment failure in intestinal strongyloidiasis: an indicator of HTLV-I infection. Int J Infect Dis. 2002;6:28–30. doi: 10.1016/s1201-9712(02)90132-3. [DOI] [PubMed] [Google Scholar]
- 7.Nakada K, Yamaguchi K, Furugen S, et al. Monoclonal integration of HTLV-I proviral DNA in patients with strongyloidiasis. Int J Cancer. 1987;40:145–8. doi: 10.1002/ijc.2910400203. [DOI] [PubMed] [Google Scholar]
- 8.Satoh M, Toma H, Sugahara K, et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)25(+) HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene. 2002;21:2466–75. doi: 10.1038/sj.onc.1205329. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Matutes E, Catovsky D, et al. Strongyloides stercoralis as candidate cofactor for HTLV-I-induced leukaemogenesis. Lancet. 1987;330:94–5. doi: 10.1016/s0140-6736(87)92752-8. [DOI] [PubMed] [Google Scholar]
- 10.Porto AF, Santos SB, Muniz AL, et al. Helminthic infection down-regulates type 1 immune responses in human T cell lymphotropic virus type 1 (HTLV-1) carriers and is more prevalent in HTLV-1 carriers than in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2005;191:612–18. doi: 10.1086/427560. [DOI] [PubMed] [Google Scholar]
- 11.Report of World Health Organization scientific group on HTLV-1 infection and associated diseases. Kagoshima, Japan, 10–15 December 1988. 1989 Manilla, March. [Google Scholar]
- 12.Temml C, Heidler S, Ponholzer A, et al. Prevalence of the overactive bladder syndrome by applying the International Continence Society definition. Eur Urol. 2005;48:622–7. doi: 10.1016/j.eururo.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 14.Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-1 proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-1 carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–93. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 15.Moon Y, Kim Y, Kim M, et al. Plasma soluble interleukin-2 receptor (sIL-2R) levels in patients with acute leukemia. Ann Clin Lab Sci. 2004;34 [PubMed] [Google Scholar]