Abstract
Objective
Schizophrenia is treated with medications that raise serum anticholinergic activity and are known to adversely affect cognition. The authors examined the relationship between serum anticholinergic activity and baseline cognitive performance and response to computerized cognitive training in outpatients with schizophrenia.
Method
Fifty-five patients were randomly assigned to either computerized cognitive training or a computer games control condition. A neurocognitive battery based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was performed at baseline and after the intervention. Serum anticholinergic activity, measured at study entry by radioreceptor assay, was available for 49 patients.
Results
Serum anticholinergic activity showed a significant negative correlation with baseline performance in verbal working memory and verbal learning and memory, accounting for 7% of the variance in these measures, independent of age, IQ, or symptom severity. Patients in the cognitive training condition (N=25) showed a significant gain in global cognition compared to those in the control condition, but this improvement was negatively correlated with anticholinergic burden. Serum anticholinergic activity uniquely accounted for 20% of the variance in global cognition change, independent of age, IQ, or symptom severity.
Conclusions
Serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in MATRICS-based measures of verbal working memory and verbal learning and memory and is significantly associated with a lowered response to an intensive course of computerized cognitive training. These findings underscore the cognitive cost of medications that carry a high anticholinergic burden. The findings also have implications for the design and evaluation of cognitive treatments for schizophrenia.
Schizophrenia is commonly treated with a range of medications that raise serum anticholinergic activity (1–3). Preclinically, these medications are known to affect nicotinic and muscarinic receptors (4). Clinically, medication-related muscarinic anticholinergic effects adversely affect cognitive performance, especially on measures of learning and memory, in both elderly persons and patients with schizophrenia (1, 2, 5–11). This finding is consistent with a wealth of basic science research demonstrating that cholinergic projections from the basal forebrain throughout the cerebral cortex play a key role in attention and memory (12–17).
Given that we now know it is imperative to treat the cognitive dysfunction of schizophrenia, and given that deficits in attention and memory are critically related to functional outcome, it becomes especially important to understand the role of medication-induced anticholinergic effects in both baseline cognitive performance and in response to treatment interventions. For example, emerging work in basic neuroscience indicates that cholinergic projections play an active role in cortical neuroplasticity by optimizing the detection of relevant signals during both bottom-up and top-down information processing (13, 14, 17–20). These data suggest that the use of medications with muscarinic-blocking effects may have serious consequences for any cognitive treatment that attempts to harness mechanisms of cortical plasticity, particularly in view of the apparently synergistic interactions between muscarinic and nicotinic systems in the optimal functioning of attention and working memory processes in humans (16, 21).
In this study, we took a first step toward understanding the relationship between serum anticholinergic activity in schizophrenia patients and their response to cognitive treatment in our ongoing study of “neuroplasticity-based” computerized cognitive training that targets auditory processing (22). We posed the following questions:
What is the association between medication-induced anticholinergic effects and baseline neurocognitive performance in a well-characterized sample of patients with chronic schizophrenia? Would we replicate earlier findings of a negative association (1, 2, 5, 8–11), using a more direct method of assessing anticholinergicity, a larger sample, and a comprehensive cognitive battery based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative?
What portion of the variance in baseline cognition is uniquely accounted for by anticholinergic burden in a clinically stable sample of schizophrenia patients, after age, IQ, and symptom severity have been controlled for?
What is the relationship between anticholinergic burden and the response to intensive “neuroplasticity-based” auditory training (i.e., to cognitive change)? What portion of the response to this cognitive training is uniquely accounted for by serum anticholinergic activity after age, IQ, and symptom severity have been controlled for?
Method
Participants
Fifty-five clinically stable, chronically ill schizophrenia outpatients 20–59 years of age were recruited from community mental health centers to undergo a randomized clinical trial of a computerized neuroplasticity-based auditory training program. After the study procedures were explained, participants gave written informed consent and underwent baseline clinical and cognitive assessments over a 2–3 week period, including blood draws for serum anticholinergic activity. Participants were then stratified by age, gender, education, and symptom severity and randomly assigned to either the auditory training condition or a control condition of commercial computer games. Because four patients refused blood draws and blood results for two patients were lost to laboratory error, serum anticholinergic levels were available for 49 participants. Table 1 summarizes the basic demographic characteristics of the sample.
TABLE 1.
Basic Demographics and Clinical Characteristics of Schizophrenia Outpatients Receiving Computerized Auditory Training or a Computer Games Control Condition
| Characteristic | Total Sample (N=49) | Auditory Training Group (N=25) | Control Group (N=24) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
| Female | 14 | 29 | 8 | 32 | 6 | 25 |
| Mean | SD | Mean | SD | Mean | SD | |
|---|---|---|---|---|---|---|
| Age | 43.86 | 10.29 | 41.44 | 11.06 | 46.38 | 8.97 |
| Education | 13.08 | 2.20 | 12.88 | 2.22 | 13.29 | 2.20 |
| Positive and Negative Syndrome Scale | ||||||
| Positive symptom subscore | 17.90 | 5.44 | 17.84 | 5.71 | 17.96 | 5.25 |
| Negative symptom subscore | 18.14 | 6.54 | 17.48 | 7.51 | 18.83 | 5.43 |
| General psychopathology subscore | 34.78 | 8.31 | 35.02 | 8.90 | 34.52 | 7.85 |
| Total score | 70.81 | 17.19 | 70.34 | 19.08 | 71.30 | 15.38 |
Participants engaged in either auditory training (N=25) or a computer games control condition (N=24) for 1 hour a day, 5 days a week, for an average of 10 weeks (verified by training software). All participants received nominal payment for participation. Participants remained on stable doses of medications during the study, defined as no dose change >10%, as prescribed by their community psychiatrists. Participants' medication regimens varied widely, ranging from monotherapy with a second-generation antipsychotic to several antipsychotics plus other psychotropic medications.
Clinical and Neurocognitive Assessments
The Positive and Negative Syndrome Scale (PANSS) (23) and all MATRICS-recommended measures (24), with the exception of the mazes subtest of the Neuropsychological Assessment Battery, were administered at baseline and after the intervention. The Tower of London subtest of the Brief Assessment of Cognition in Schizophrenia (25) was substituted for the mazes subtest to assess problem-solving. Because of software difficulties, data from the Continuous Performance Test–Identical Pairs Version were not interpretable. At the time this study was initiated, the MATRICS Consensus Cognitive Battery (MCCB) was not yet available, but the list of recommended measures for the MCCB beta version was available on the MATRICS web site. We obtained the MATRICS-recommended measures from test publishers and converted raw scores to z scores using normative data, stratified by age, published by test authors. All measures were distinct and independent from tasks practiced during the auditory training, and assessment personnel were blind to group assignment. Alternate forms of tests were administered and counterbalanced at baseline and after the intervention for tests sensitive to practice effects.
Neuroplasticity-Based Computerized Auditory Training
Training of auditory/verbal processing was provided by software developed by Posit Science, Inc. In these computerized exercises (described in detail in reference 22), participants were driven to make progressively more accurate distinctions about the spectrotemporal fine structure of auditory stimuli and speech under conditions of increasing working memory load, and to incorporate and generalize their improvements in auditory signal salience into working memory rehearsal and verbal learning. The exercises were continuously adaptive: they first established the precise parameters within each stimulus set required for the participant to maintain 80% correct performance; once that threshold was determined, task difficulty increased systematically and parametrically as performance improved. In all exercises, correct performance was heavily rewarded in a game-like fashion through novel and amusing visual and auditory embellishments as well as the accumulation of points.
Serum Anticholinergic Activity
During baseline testing, two blood draws were taken from all patients 1 week apart and averaged to enhance the reliability of the measure. In addition, 13 randomly selected participants had blood draws again 10 weeks later to determine the correlation of levels at study entry with levels drawn after the intervention. This correlation was highly significant (Pearson's r=0.89, p<0.003), indicating that levels remained constant throughout the intervention period in our participants.
Serum anticholinergic activity (25) was measured using the method of Tune and Coyle (7, 26–28). The method is a competitive radioreceptor binding assay using prepared muscarinic membranes from rat forebrain (cerebral cortex and striatum, Sprague-Dawley males) and 3-hour quinuclidinyl benzilate (QNB) as a radioligand with various concentrations of atropine (Sigma-Aldrich, St. Louis, Mo.) used in a standard curve for displacement. Patient serum is added and incubated for 1 hour at 22°C. Ice-cold phosphate buffer is added and the ligand-receptor complex is isolated by aspiration over Whatman GF/B filters (Piscataway, N.J.) using a Brandel Cell Harvester (Gaithersburg, Md.). Filters are added to vials with scintillation cocktail and counted using a Beckman Liquid Scintillation Counter (Fullerton, Calif.). This assay measures the free drug in sera that binds to the muscarinic receptors. Anticholinergic activity is reported in picomoles of atropine equivalents per milliliter (pmol/ml), based on the amount of 3-hour QNB displacement that would have been caused by a standard amount of atropine in a 200-μl sample. The results are then normalized to 1 ml.
Serum anticholinergic activity is thought to reflect the cumulative muscarinic anticholinergic effect of all exogenous substances the person has taken (prescribed and over-the-counter medications and supplements) and their metabolites. The limit of detection for this assay is 0.25 pmol/ml, with a standard linear curve (r=0.99) from 0.50 to 25.00 pmol/ml and good interassay and intraassay reproducibility for these concentrations (coefficient of variations <12%).
Data Analysis
The distributions of all variables were evaluated for normality and screened for outliers. The baseline serum anticholinergic activity distribution was skewed (consistent with other studies [6, 7]) and was normalized using square-root transformation. All neurocognitive variables were normally distributed after winsorizing of outlying values. Measures of cognitive performance were computed for six MATRICS-defined cognitive domains—speed of processing, verbal working memory, nonverbal working memory, verbal learning and memory, visual learning and memory, and problem solving—and a composite score of global cognition was computed as the average of all measures. Repeated-measures analysis of variance (ANOVA) was used to test group differences in cognitive performance from baseline to postintervention assessments. Pearson correlations were computed to determine the association between serum anticholinergicity and baseline cognitive performance in the total sample and between anticholinergicity and response to treatment in the auditory training group, defined as change in the cognitive measures (postintervention minus baseline performance). Multiple regression analyses were used to test the contributions of serum anticholinergic activity, age, IQ, and symptom severity to baseline cognitive measures in the total sample and to response to treatment, defined as z score change in the global cognition composite score, in the auditory training group. Multivariate ANOVA was used to test the difference between participants from the highest and lowest quartiles of serum anticholinergicity in the multivariate effect and main effects of serum anticholinergic activity, response to treatment (defined as z score change in global cognition), and PANSS total score.
Results
Baseline Measures
Table 2 summarizes the baseline neurocognitive data and mean serum anticholinergic activity for the sample and subgroups. This clinically stable group of adult patients with chronic schizophrenia manifested the characteristic pattern of deficits (on average, 0.97 standard deviations below age-stratified norms and 2.00 standard deviations on measures of verbal learning and memory), with no significant differences in baseline profiles between subgroups. Mean anticholinergic activity level was 6.9 pmol/ml, with a median of 3.1 and a range of 0–31; there were no significant differences between subgroups. These levels are slightly lower than those reported by Tracy et al. (5) in 38 state hospital patients but are consistent with those reported by Chew et al. (28).
TABLE 2.
Baseline and Postintervention Cognitive Performance (Age-Adjusted z Scores) and Baseline Serum Anticholinergic Activity Levels in Schizophrenia Outpatients Receiving Computerized Auditory Training or a Computer Games Control Condition
| Auditory Training Group (N=25) |
Control Group (N=24) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample at Baseline (N=49) |
Baseline |
Postintervention |
Baseline |
Postintervention |
Analysisa |
|||||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Test Statistic | p |
| Global cognition | −1.06 | 0.74 | −1.22 | 0.69 | −0.83 | 0.73 | −0.89 | 0.78 | −0.84 | 0.76 | F=10.10 | <0.01 |
| Speed of processing | −0.71 | 0.70 | −0.78 | 0.70 | −0.52 | 0.59 | −0.64 | 0.69 | −0.68 | 0.77 | F=2.55 | 0.12 |
| Verbal working memory | −1.09 | 1.60 | −1.20 | 1.39 | −0.75 | 1.49 | −0.99 | 1.83 | −1.03 | 1.72 | F=3.01 | 0.09 |
| Nonverbal working memory | −0.45 | 0.93 | −0.51 | 0.89 | −0.24 | 0.96 | −0.40 | 1.00 | −0.10 | 1.02 | F=0.08 | 0.79 |
| Verbal learning and memory | −2.22 | 0.98 | −2.54 | 0.75 | −2.08 | 1.08 | −1.89 | 1.10 | −2.25 | 1.24 | F=9.04 | <0.01 |
| Visual learning and memory | −1.05 | 1.39 | −1.27 | 1.28 | −0.71 | 1.31 | −0.84 | 1.48 | −0.46 | 1.38 | F=0.01 | 0.93 |
| Problem solving | −0.28 | 0.87 | −0.46 | 0.90 | −0.08 | 0.75 | −0.10 | 0.82 | 0.08 | 0.87 | F=1.00 | 0.33 |
| Serum anticholinergic activity level (pmol/ml) | 6.92 | 9.16 | 7.70 | 9.94 | 6.11 | 8.40 | t=0.26 | 0.80 | ||||
Between-group comparisons of change in cognition (repeated-measures analysis of variance, time-by-group interaction).
Relation of Anticholinergic Effects to Baseline Neurocognitive Performance
At study entry, anticholinergic activity showed a significant negative correlation with performance in verbal working memory (r=−0.41, p<0.04) and verbal learning and memory (r=−0.29, p<0.04) but not with other MATRICS-based domains (Table 3). Unlike the finding by McGurk et al. (9) of a deleterious effect of benztropine on a computerized spatial working memory task, we found no effect of anticholinergic activity on nonverbal working memory, possibly because of the less sensitive nature of our measure.
TABLE 3.
Correlation of Serum Anticholinergic Activity Levels With Cognitive Variables in Schizophrenia Outpatients Receiving Computerized Auditory Training or a Computer Games Control Condition
| Total Sample (N=49) Baseline Measures |
Auditory Training Group (N=25) z Score Change |
|||
|---|---|---|---|---|
| Measure | r | p | r | p |
| Global cognition | −0.12 | 0.41 | −0.46 | 0.02 |
| Speed of processing | 0.01 | 0.96 | −0.11 | 0.58 |
| Verbal working memory | −0.41 | 0.02 | −0.12 | 0.59 |
| Nonverbal working memory | −0.06 | 0.67 | −0.06 | 0.78 |
| Verbal learning and memory | −0.29 | 0.04 | −0.12 | 0.58 |
| Visual learning and memory | −0.04 | 0.78 | −0.28 | 0.17 |
| Problem solving | 0.22 | 0.21 | −0.06 | 0.81 |
We performed two multiple regression analyses to determine the variance in baseline verbal working memory and verbal learning and memory that was uniquely accounted for by anticholinergic activity, age, IQ, and PANSS score. These four variables accounted for 56% of the variance in verbal working memory and 37% of the variance in verbal learning and memory (adjusted R2) (Table 4). Squared semipartial correlations indicated that anticholinergic activity uniquely accounted for 7% of the variance in both verbal working memory and verbal learning and memory, independent of the effects of age, IQ, and symptom severity, while IQ uniquely accounted for 41% and 33% of the variance in these two cognitive domains, respectively.
TABLE 4.
Contribution of Serum Anticholinergic Activity Level, Age, IQ, and Symptom Severity to Baseline Measures of Cognition (N=49) and to Change in Global Cognition Score After Auditory Cognitive Training in Schizophrenia Outpatients (N=25)
| Regression, Dependent Variable, and Predictors | Standard B | t | df | p | Partial r2 | R | Adjusted R2 | SE | F | df | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression 1 | |||||||||||
| Dependent variable, verbal working memory (baseline) | 0.78 | 0.56 | 1.06 | 10.99 | 4, 44 | <0.001 | |||||
| Predictors | |||||||||||
| Serum anticholinergic activity | −0.28 | −2.22 | 44 | 0.04 | 0.07 | ||||||
| Age | 0.10 | 0.77 | 44 | 0.45 | 0.01 | ||||||
| IQ | 0.72 | 5.42 | 44 | <0.001 | 0.41 | ||||||
| Positive and Negative Syndrome Scale score | 0.08 | 0.58 | 44 | 0.57 | 0.005 | ||||||
| Regression 2 | |||||||||||
| Dependent variable, verbal learning and memory (baseline) | 0.65 | 0.37 | 0.79 | 7.92 | 4, 44 | <0.001 | |||||
| Predictors | |||||||||||
| Serum anticholinergic activity | −0.27 | −2.24 | 44 | 0.03 | 0.07 | ||||||
| Age | 0.11 | 0.93 | 44 | 0.36 | 0.01 | ||||||
| IQ | 0.61 | 4.96 | 44 | <0.001 | 0.33 | ||||||
| Positive and Negative Syndrome Scale score | 0.08 | 0.66 | 44 | 0.51 | 0.006 | ||||||
| Regression 3 | |||||||||||
| Dependent variable, global cognition z score change | 0.62 | 0.25 | 0.32 | 2.90 | 4, 20 | 0.05 | |||||
| Predictors | |||||||||||
| Serum anticholinergic activity | −0.48 | −2.50 | 20 | 0.02 | 0.20 | ||||||
| Age | 0.13 | 0.60 | 20 | 0.55 | 0.01 | ||||||
| IQ | 0.44 | 1.84 | 20 | 0.08 | 0.10 | ||||||
| Positive and Negative Syndrome Scale score | 0.34 | 1.60 | 20 | 0.13 | 0.08 |
Relation of Anticholinergic Effects to the Response to Cognitive Training
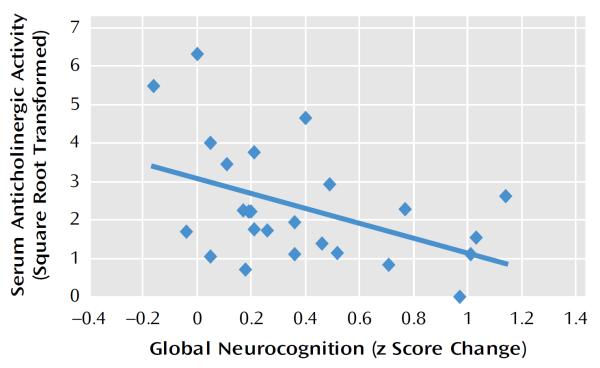
The complete details of the cognitive improvements seen in the full sample of the auditory training group (N=29) after 50 hours of cognitive training have been reported elsewhere (22). In participants for whom serum anticholinergic activity levels were available, statistically significant gains in global cognition, verbal learning and memory, and verbal working memory (trend level) were seen in the 25 auditory training patients relative to the 24 computer games control patients (Table 2). These data indicate that neuroplasticity-based auditory training drives significant improvements in cognition, independent of the nonspecific effects of computer exposure, engagement with the intervention, or staff contact. However, anticholinergic activity was negatively correlated with improvement in global cognition in the auditory training participants (Pearson's r=−0.46, p<0.02) (Table 3, Figure 1). These results indicate that anticholinergic burden had a negative impact on schizophrenia participants' global response to the cognitive training.
FIGURE 1.
Association of Serum Anticholinergic Activity Level and Response to Computerized Auditory Training (Change in Global Cognition Score) in 25 Adult Patients With Chronic Schizophreniaa
aThe bivariate correlation was −0.46 (p<0.02), while regression analysis revealed that serum anticholinergic activity level uniquely accounted for 20% of the variance in change in global cognition, independent of the effects of IQ, age, and symptom severity (R2=0.20).
Clinical Implications
We investigated the relative contributions of serum anticholinergic activity, age, IQ, and symptom severity to cognitive training response. These four variables accounted for approximately 25% of the variance (adjusted R2) in the global cognition change score in the training group (Table 4). Squared semipartial correlations indicated that anticholinergic activity uniquely accounted for 20% of the variance in global cognition change, while IQ accounted for 10% of the variance. These findings suggest that patients' medication regimens play an important role in their response to cognitive training, independent of their age, IQ, or clinical acuity.
In order to investigate the clinical implications of these findings, we compared participants from the lowest quartile of the serum anticholinergic distribution (N=6, mean level=0.8 pmol/ml, mean global cognition z score change=0.55) to those from the highest quartile (N=6, mean level=22 pmol/ml, mean global cognition z score change=0.10). The demographic characteristics, baseline cognitive performance, and PANSS ratings of these two groups are presented in Table 5. There were no differences between groups in age, education, or baseline cognition; however, patients in the lowest quartile had significantly higher symptom scores. The multiple ANOVA revealed a significant difference between groups in the multivariate effect of anticholinergic activity, global cognition change, and PANSS score (F=18.35, df=3, 8, p<0.001). Participants with lower serum anticholinergic activity showed greater cognitive gains and slightly higher symptom ratings compared to participants with high serum anticholinergic activity. The main effects of both anticholinergic level (F=62.24, df=1, 10, p<0.001) and global cognition z score change (F=5.78, df=1, 10, p<0.04) were significant, and the main effect of PANSS score approached statistical significance (F=3.59, df=1, 10, p<0.09). The medication regimens for the patients in these two groups are presented in Table 5. Higher doses of medications with known anticholinergic effects and polypharmacy (28) characterize patients in the upper quartile.
TABLE 5.
Clinical Implications: A Comparison of Subjects From the Lowest and Highest Quartiles of the Serum Anticholinergic Activity Distributiona
| Lowest Quartile (N=6) |
Highest Quartile (N=6) |
|||
|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD |
| Serum anticholinergic activity level (pmol/ml)b | 0.80 | 0.49 | 22.32 | 10.83 |
| Age (years) | 42.50 | 5.72 | 43.67 | 15.37 |
| Education (years) | 11.67 | 1.75 | 13.17 | 3.31 |
| Positive and Negative Syndrome Scale | ||||
| Positive symptomsc | 24.00 | 4.34 | 17.67 | 3.20 |
| Negative symptoms | 18.67 | 4.97 | 19.83 | 8.50 |
| General psychopathologyd | 41.42 | 5.22 | 33.67 | 6.02 |
| Totale | 84.08 | 10.40 | 71.17 | 13.08 |
| Baseline verbal working memory, z score | −1.31 | 1.29 | −2.16 | 0.69 |
| Baseline verbal learning and memory, z score | −2.42 | 0.60 | −2.64 | 0.54 |
| Baseline global cognition, z score | −1.17 | 0.73 | −1.28 | 0.65 |
| Improvement after cognitive training, z score change in global cognitionf | 0.55 | 0.41 | 0.10 | 0.19 |
Daily medication regimens for the patients in the lowest quartile were risperidone 6 mg; risperidone 4 mg and valproic acid 1000 mg; aripiprazole 15 mg and sertraline 200 mg; “herbal medications”; ziprasidone 180 mg; and olanzapine 7.5 mg and citalopram 10 mg. Daily medication regimens for those in the highest quartile were haloperidol 20 mg and benztropine 2 mg; clozapine 800 mg and valproic acid 1000 mg; quetiapine 800 mg, lithium 450 mg, gabapentin 600 mg, and lorazepam 2 mg; quetiapine 800 mg, risperidone 4 mg, buspirone 30 mg, and lamotrigine 100 mg; clozapine 600 mg; and olanzapine 20 mg, mirtazapine 30 mg, and trazodone 150 mg.
Difference between groups statistically significant, p<0.001.
Difference between groups statistically significant, p=0.02.
Difference between groups statistically significant, p=0.04.
Difference between groups approached statistical significance (p=0.09).
Difference between groups statistically significant, p=0.04.
Discussion
We examined serum anticholinergic activity, determined via radioreceptor binding assay, and neurocognitive performance, assessed via the MATRICS battery, in a moderately sized sample of clinically stable adult schizophrenia outpatients receiving community-based medication treatment. Only a small minority of patients (N=3) of this sample of 49 patients had no detectable anticholinergic activity, while over half had levels ≥3 pmol/ml. To place this in a meaningful clinical and cognitive context, Mulsant et al. (7) found that healthy community-dwelling older adults with anticholinergic activity levels greater than 2.8 pmol/ml were 13 times more likely than those with undetectable levels to have a score ≤24 on the Mini-Mental State Examination. Similarly, we found a significant relationship between anticholinergic burden and baseline performance in working memory and in verbal learning and memory in these schizophrenia patients, with serum anticholinergicity accounting for 7% of the variance in these crucial cognitive functions, independent of IQ, age, and symptom severity.
These results were obtained in patients who volunteered to participate in a demanding and time-intensive research study, and who thus were treatment responsive, compliant, and relatively higher-functioning. As indicated by our quartile data in Table 5, one might anticipate that anticholinergic burden would be greater in patients identified as “treatment resistant” and treated with clozapine and/or multiple medications at higher doses (28). Although any generalizations from our study must be undertaken with caution, our results suggest that clinically significant medication-induced anticholinergic activity may be relatively common in the community treatment of schizophrenia. Furthermore, it has meaningful deleterious consequences on cognitive function in patients, suggesting a need for caution when prescribing medications with known anticholinergic effects, such as clozapine, olanzapine, quetiapine, benztropine, and certain first-generation agents. It is likely that anticholinergic burden exacerbates the underlying neurocognitive pathology of schizophrenia, similar to what has been seen with cerebrovascular disease in the elderly (29).
Of perhaps even greater concern is our finding that anticholinergic burden is significantly associated with a lowered response to the effects of intensive “neuroplasticity-based” computerized cognitive training. In our cognitive training group, serum anticholinergic activity accounted for 20% of the variance in the global cognition change score—significantly more than IQ, age, or symptom severity. The relative contribution of anticholinergic activity and of IQ to measures of baseline cognition differed from the relative contribution of these variables to cognitive change, with anticholinergicity playing a greater role in the latter measure. Serum anticholinergic activity was negatively associated with verbal working memory and long-term memory at baseline, but with global cognition change after training. This pattern of results is consistent with emerging research indicating that baseline variables that show significant cross-sectional associations with outcome measures in schizophrenia are not always identical to the variables that are associated with change in outcome (30, 31). Anticholinergic burden may be an important mediator, with effects on specific cognitive domains when measured at a single time point, but with even larger effects on cognitive change resulting from training. Interestingly, patients in the lowest anticholinergicity quartile, who were on more conservative medication regimens, had higher ratings on positive symptoms but also showed a greater response to cognitive training compared to patients in the upper quartile. We speculate that a conservative approach to the pharmacologic treatment of schizophrenia, combined with behavioral techniques for managing low-level psychotic symptoms, might optimize patients' ability to benefit from cognitive interventions.
At this point, we cannot say whether the negative relationship between anticholinergic activity and cognitive outcome is unique to our particular neuroplasticity-based computerized cognitive training approach or whether this finding would also be observed using other remediation methods. As far as we know, no other study has investigated the association between anticholinergic activity in schizophrenia and the response to a behavioral treatment. These results become especially germane in light of the growing awareness that cognitive function influences the ability of people with schizophrenia to benefit from psychosocial interventions (32–34). It appears that if we wish to maximize patients' response to rehabilitation, we must take care to minimize their anticholinergic burden.
There were several limitations to this study. The sample was of modest size, and it was not representative of the schizophrenia population as a whole. Because we did not obtain serial anticholinergic activity measures on all participants in the auditory training group throughout the course of the 10 weeks, we cannot be certain that the measures obtained at study entry remained consistent throughout the duration of the intervention; however, the highly significant correlation we found between levels at study entry and 10 weeks later in 13 randomly selected participants strongly suggests that this was the case.
Finally, because of technical difficulties, we were unable to obtain reliable data on the Continuous Performance Test–Identical Pairs Version, a computerized attentional measure, and therefore do not know whether anticholinergic activity would show a significant negative association with this task, either at baseline or after training. Harvey et al. (35) found that patients being treated with risperidone showed practice-related improvements in visual performance on the Continuous Performance Test, but patients being treated with conventional antipsychotic agents did not.
Although it is well established that older adults are highly susceptible to cognitive impairment and delirium as a result of anticholinergic burden, this study demonstrates that effects are also detectable in younger individuals with schizophrenia, are quantifiable, and are therapeutically relevant. For clinical investigators, these effects deserve further research in other behavioral and perhaps pharmacologic paradigms; if we reduce anticholinergic load, we may foster patients' ability to benefit from cognitive enhancement methods by maximizing their verbal memory functions. For the treating clinician, the “memory” implications are simpler: we would all do well to remember the cognitive cost of medications that carry a high anticholinergic burden.
Acknowledgments
Dr. Pollock has received research support from NIH, has been a member of speakers bureaus and advisory boards of Lundbeck and Forest Laboratories, has been a faculty member of the Lundbeck Institute, and has served as a consultant to Takeda and Wyeth. All other authors report no competing interests. The cognitive training software used in this study was supplied to the first author free of charge by Posit Science, Inc. None of the authors have any financial interest in Posit Science, Inc.
Supported by NIMH grant RO1 MH068725-01A1, the San Francisco VA Medical Center, and NIH/National Center for Research Resources, University of California San Francisco–Clinical and Translational Science Institute grant UL1 RR024131. The article's content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Clinicaltrials.gov ID number: NCT00312962.
References
- 1.Perlick D, Stastny P, Katz I, Mayer M, Mattis S. Memory deficits and anticholinergic levels in chronic schizophrenia. Am J Psychiatry. 1986;143:230–232. doi: 10.1176/ajp.143.2.230. [DOI] [PubMed] [Google Scholar]
- 2.Tune LE, Strauss ME, Lew MF, Breitlinger E, Coyle JT. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry. 1982;139:1460–1462. doi: 10.1176/ajp.139.11.1460. [DOI] [PubMed] [Google Scholar]
- 3.Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Kirshner MA, Bies RR, Kapur S, Gharabawi G. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res. 2006;88:63–72. doi: 10.1016/j.schres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Waller JL. Chronic treatment with first or second generation antipsychotics in rodents: effects on high affinity nicotinic and muscarinic acetylcholine receptors in the brain. Neuroscience. 2006;140:1277–1287. doi: 10.1016/j.neuroscience.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Tracy JI, Monaco C, Giovannetti T, Abraham G, Josiassen RC. Anticholinergicity and cognitive processing in chronic schizophrenia. Biol Psychol. 2001;56:1–22. doi: 10.1016/s0301-0511(00)00083-1. [DOI] [PubMed] [Google Scholar]
- 6.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF., 3rd Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 7.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60:198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 8.Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- 9.McGurk SR, Green MF, Wirshing WC, Wirshing DA, Marder SR, Mintz J, Kern R. Antipsychotic and anticholinergic effects on two types of spatial memory in schizophrenia. Schizophr Res. 2004;68:225–233. doi: 10.1016/S0920-9964(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 10.Spohn HE, Strauss ME. Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. J Abnorm Psychol. 1989;98:367–380. doi: 10.1037//0021-843x.98.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Strauss ME, Reynolds KS, Jayaram G, Tune LE. Effects of anticholinergic medication on memory in schizophrenia. Schizophr Res. 1990;3:127–129. doi: 10.1016/0920-9964(90)90045-9. [DOI] [PubMed] [Google Scholar]
- 12.Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn Mem. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risbrough V, Bontempi B, Menzaghi F. Selective immunolesioning of the basal forebrain cholinergic neurons in rats: effect on attention using the 5-choice serial reaction time task. Psychopharmacology (Berl) 2002;164:71–81. doi: 10.1007/s00213-002-1170-7. [DOI] [PubMed] [Google Scholar]
- 15.Leanza G, Muir J, Nilsson OG, Wiley RG, Dunnett SB, Bjorklund A. Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur J Neurosci. 1996;8:1535–1544. doi: 10.1111/j.1460-9568.1996.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 16.Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- 17.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 19.Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27:14442–14447. doi: 10.1523/JNEUROSCI.4104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 21.Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav. 2005;81:575–584. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 24.Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery Manual. MATRICS Assessment, Inc; Los Angeles, Calif: 2006. [Google Scholar]
- 25.Nebes RD, Pollock BG, Mulsant BH, Kirshner MA, Halligan E, Zmuda M, Reynolds CF., 3rd Low-level serum anticholinergicity as a source of baseline cognitive heterogeneity in geriatric depressed patients. Psychopharmacol Bull. 1997;33:715–720. [PubMed] [Google Scholar]
- 26.Tune L, Coyle JT. Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry. 1980;37:293–297. doi: 10.1001/archpsyc.1980.01780160063007. [DOI] [PubMed] [Google Scholar]
- 27.Pollock BG, Mulsant BH, Nebes R, Kirshner MA, Begley AE, Mazumdar S, Reynolds CF., III Serum anticholinergicity in elderly depressed patients treated with paroxetine or nortriptyline. Am J Psychiatry. 1998;155:1110–1112. doi: 10.1176/ajp.155.8.1110. [DOI] [PubMed] [Google Scholar]
- 28.Chew ML, Mulsant BH, Pollock BG. Serum anticholinergic activity and cognition in patients with moderate-to-severe dementia. Am J Geriatr Psychiatry. 2005;13:535–538. doi: 10.1176/appi.ajgp.13.6.535. [DOI] [PubMed] [Google Scholar]
- 29.Nebes RD, Pollock BG, Meltzer CC, Saxton JA, Houck PR, Halligan EM, DeKosky ST. Serum anticholinergic activity, white matter hyperintensities, and cognitive performance. Neurology. 2005;65:1487–1489. doi: 10.1212/01.wnl.0000183152.16690.26. [DOI] [PubMed] [Google Scholar]
- 30.Fiszdon JM, Choi J, Goulet J, Bell MD. Temporal relationship between change in cognition and change in functioning in schizophrenia. Schizophr Res. 2008;105:105–113. doi: 10.1016/j.schres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Reeder C, Smedley N, Butt K, Bogner D, Wykes T. Cognitive predictors of social functioning improvements following cognitive remediation for schizophrenia. Schizophr Bull. 2006;32(suppl 1):S123–S131. doi: 10.1093/schbul/sbl019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGurk SR, Mueser KT, Harvey PD, LaPuglia R, Marder J. Cognitive and symptom predictors of work outcomes for clients with schizophrenia in supported employment. Psychiatr Serv. 2003;54:1129–1135. doi: 10.1176/appi.ps.54.8.1129. [DOI] [PubMed] [Google Scholar]
- 33.McGurk SR, Mueser KT. Cognitive functioning, symptoms, and work in supported employment: a review and heuristic model. Schizophr Res. 2004;70:147–173. doi: 10.1016/j.schres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Brekke JS, Hoe M, Long J, Green MF. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophr Bull. 2007;33:1247–1256. doi: 10.1093/schbul/sbl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey PD, Moriarty PJ, Serper MR, Schnur E, Lieber D. Practice-related improvement in information processing with novel antipsychotic treatment. Schizophr Res. 2000;46:139–148. doi: 10.1016/s0920-9964(00)00033-5. [DOI] [PubMed] [Google Scholar]