SUMMARY
Root morphogenesis is controlled by the regulation of cell division and expansion. We isolated an allele of the eto1 ethylene overproducer as a suppressor of the auxin-resistant mutant ibr5, prompting an examination of crosstalk between the phytohormones auxin and ethylene in control of root epidermal cell elongation and root hair elongation. We examined the interaction of eto1 with mutants that have reduced auxin response or transport and found that ethylene overproduction partially restored auxin responsiveness to these mutants. In addition, we found that the effects of endogenous ethylene on root cell expansion in eto1 seedlings were partially impeded by dampening auxin signaling, and were fully suppressed by blocking auxin influx. These data provide insight into the interaction between these two key plant hormones, and suggest that endogenous ethylene directs auxin to control root cell expansion.
Keywords: auxin, cell elongation, ethylene, hormone crosstalk, root hair
INTRODUCTION
Plants rely on differential cell expansion to shape root architecture. Root growth is indeterminate, proceeding by the continual succession of cell division, regulated cell expansion, and differentiation in the meristem and adjacent root regions. The outermost epidermal cell layer in radially symmetric Arabidopsis thaliana roots arises from the division of the meristematic epidermal/lateral root cap initials. Root epidermal cells have two fates, differentiating into files of either non-hair cells (atrichoblasts) or hair cells (trichoblasts), which bear root hairs that emerge as tube-shaped outgrowths of the root surface and function in water and nutrient uptake (reviewed in Grierson and Schiefelbein, 2002).
Epidermal cell lengthening and root hair tip growth are sensitive to a variety of environmental and developmental cues, including the plant hormones auxin and ethylene. Auxin inhibits root elongation (reviewed in Parry and Estelle, 2006) and promotes root hair lengthening (reviewed in Grierson and Schiefelbein, 2002). For example, mutants defective in AUXIN RESISTANT 1 (AUX1), which moves the auxin indole-3-acetic acid (IAA) into cells (reviewed in Vieten et al., 2007), are resistant to the inhibitory effects of exogenous and endogenous auxin on root elongation (Pickett et al., 1990), and have short root hairs (Pitts et al., 1998). Auxin response is regulated by a family of AUXIN RESPONSE FACTOR (ARF) transcription factors, which are repressed by interaction with Aux/IAA family members (reviewed in Parry and Estelle, 2006). This repression is relieved by the SCFTIR1/AFB family of ubiquitin-protein ligases, which promote the degradation of Aux/IAA proteins when bound to auxin (reviewed in Parry and Estelle, 2006). Several mutants that are defective in auxin response because of a failure to degrade Aux/IAA proteins, including axr1-12 and axr2-1, display auxin-resistant roots with root hair elongation defects (Masucci and Schiefelbein, 1996; Cernac et al., 1997). The dual-specificity protein phosphatase IBR5 also is required for full auxin responsiveness (Monroe-Augustus et al., 2003; Lee et al., 2009); however, IBR5 promotes auxin response without stimulating Aux/IAA protein degradation (Strader et al., 2008a).
The gaseous hormone ethylene decreases root cell length (Le et al., 2001) and increases root width (reviewed in Smalle and Van Der Straeten, 1997) and root hair length (Tanimoto et al., 1995; Masucci and Schiefelbein, 1996). 1-Aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) enzymes catalyze the rate-limiting step in ethylene biosynthesis. Several ACS isozymes, including ACS5/ETHYLENE OVERPRODUCER 2 (ETO2), are targeted for degradation by the ETO1 ubiquitin-protein ligase (Chae et al., 2003; Christians et al., 2009). Slowing degradation of these ACS enzymes results in ethylene overproduction, which lengthens root hairs, shortens hypocotyls and roots of dark-grown seedlings, and shortens roots of light-grown seedlings (reviewed in Chae and Kieber, 2005). Ethylene is detected by transmembrane histidine kinase receptors that no longer activate the CTR1 Raf-like kinase upon ethylene binding: this relief from CTR1 repression allows ETHYLENE INSENSITIVE 2 (EIN2) to activate the EIN3 family of transcription factors to promote ethylene-responsive transcription (reviewed in Schaller and Kieber, 2002). Consequently, ctr1 mutants have short roots with long root hairs (Kieber et al., 1993; Cho and Cosgrove, 2002), whereas ethylene-resistant mutants, such as ein2, have long roots with short root hairs (Guzman and Ecker, 1990; Pitts et al., 1998).
Multilevel crosstalk between ethylene and auxin affects the synthesis, signaling, and transport of these hormones. Auxin increases ACS transcription, thus stimulating ethylene synthesis (reviewed in Yang and Hoffman, 1984; Tsuchisaka and Theologis, 2004). Similarly, ethylene application promotes the expression of IAA biosynthetic genes (Stepanova et al., 2005, 2008), increases IAA synthesis (Swarup et al., 2007), and increases IAA levels (Růžička et al., 2007) in root tips. Furthermore, root ethylene responses require basipetal (rootward) auxin transport (Růžička et al., 2007), and blocking IAA influx or efflux results in ethylene resistance in the root (Pickett et al., 1990; Luschnig et al., 1998). Moreover, some aspects of auxin response require ethylene response, and some facets of ethylene response require auxin response,as evidenced by auxin resistance in many ethylene signaling mutants and ethylene resistance in many auxin signaling mutants (Stepanova et al., 2007).
We have isolated and characterized modifier mutations that restore auxin responsiveness to ibr5 (Strader et al., 2008b). Here we report that the causative mutation in one of these suppressors is a new allele of the ethylene overproducer eto1. We examined the interaction of eto1 mutants with other mutants that have reduced auxin responsiveness or transport, and found that the effects of endogenous ethylene on root cell expansion in untreated eto1 seedlings were fully suppressed by blocking auxin influx. Our data suggest that endogenous ethylene directs auxin to control root cell expansion, and provide insight into the interaction between the plant hormones auxin and ethylene in shaping the root.
RESULTS
Ethylene overproduction suppresses the weak auxin-resistant mutant ibr5-1
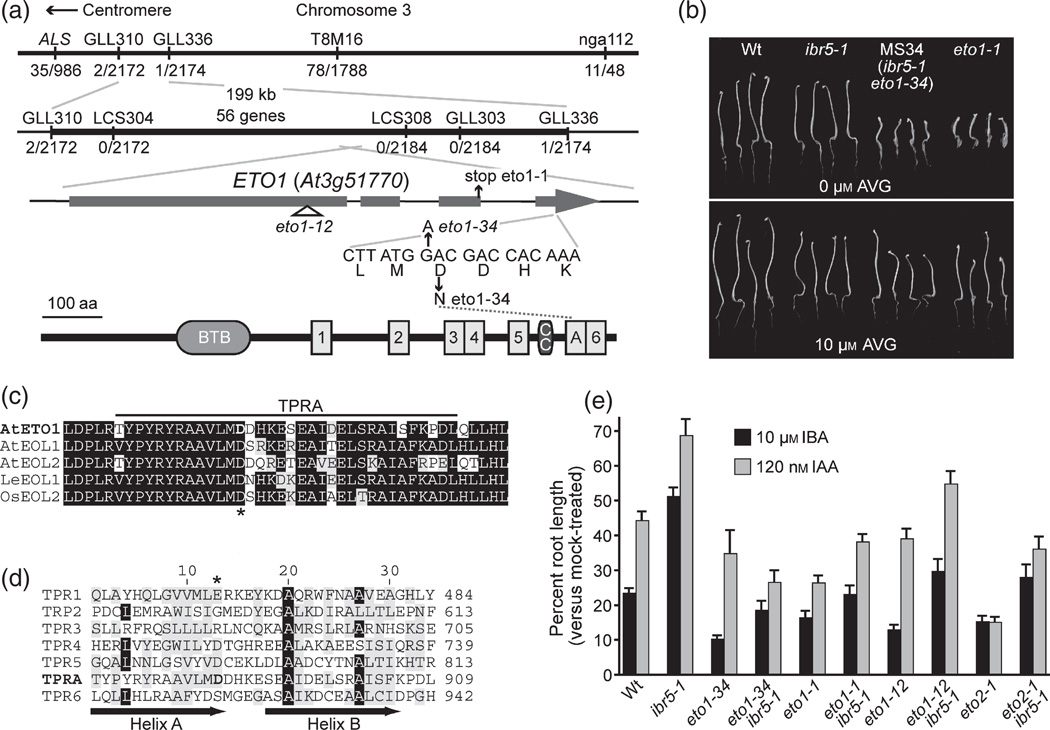
The ibr5 suppressor isolate MS34 displays restored responses to the naturally occurring auxins IAA and indole-3-butyric acid (IBA) (Strader et al., 2008b). We mapped the ibr5-suppressing mutation to an interval containing ETO1 (At3g51770; Figure 1a). Mutation of ETO1 results in ACS protein hyperaccumulation and ethylene overproduction, resulting in short hypocotyls in dark-grown seedlings that can be rescued by the ACS enzyme inhibitor aminoethoxy-vinylglycine (AVG) (Guzman and Ecker, 1990). Like eto1-1, dark-grown MS34 seedlings had short hypocotyls that were rescued by AVG (Figure 1b). Sequencing ETO1 in MS34 revealed a mutation causing an Asp-to-Asn missense mutation in a conserved amino acid (Figure 1a,c), disrupting a previously unannotated tetratricopeptide repeat (TPR) domain immediately N-terminal to the sixth previously annotated (Wang et al., 2004) TPR domain (Figure 1c,d). We found that eto1-1 ibr5 and eto1-12 ibr5 recapitulated MS34 auxin-response phenotypes (Figure 1e), confirming that the identified lesion, which we named eto1-34, was the suppressing mutation in MS34. The eto2-1 mutation, which overproduces ethylene by rendering the ACS5 (ETO2) enzyme resistant to ETO1-mediated degradation (Chae et al., 2003; Christians et al., 2009), also suppressed ibr5 auxin resistance (Figure 1e), suggesting that the observed suppression was a general consequence of ethylene overproduction, and was not specific to eto1.
Figure 1. Ethylene overproduction suppresses the ibr5 auxin-response mutant.
(a) Recombination mapping with the indicated PCR-based markers (Table S1) localized the suppressing mutation in MS34 to a region containing 56 predicted genes between GLL310 and GLL336, with 2/2172 north and 1/2174 south recombinants. Examination of the ETO1 (At3g51770) gene in this region revealed a G→A mutation at position 3426, resulting in an Asp879 → Asn substitution. The ETO1 protein schematic, based on output from the domain-predicting programs smart (Schultz et al, 1998) and ProSite (de Castro et al, 2006), illustrates the ETO1 BTB/POZ domain (BTB), a coiled-coil region (CC) and seven TPR domains (labeled 1–6 and A).
(b) The MS34 (ibr5-1 eto1-34) short hypocotyl is rescued by AVG: photographs of 5-day-old dark-grown seedlings grown in the absence (top panel) or presence (bottom panel) of AVG.
(c) and (d) The eto1-34 mutation alters a conserved Asp in TPR repeat A (asterisks).
(c) Alignment showing the newly recognized TPR domain in Arabidopsis thalianaETO1 (At3g51770), Arabidopsis ETO1-LIKE1 (At4g02680), Arabidopsis ETO1-LIKE2 (At5g58550), LeEOL1 (DQ223268) and Oryza sativaETO1-LIKE2 (AP003826).
(d) Alignment of TPR domains from Arabidopsis ETO1. Sequences were aligned using the MegAlign program (DNAStar, http://www.dnastar.com). Identical and chemically similar residues are boxed in black and gray, respectively.
(e) Normalized primary root lengths (mean + SE versus mock-treated control) of 8-day-old Col-0 (Wt), ibr5-1, eto1-34, eto1-34 ibr5-1 (MS34), eto1-1, eto1-1 ibr5, eto1-12, eto1-12 ibr5, eto2-1 and eto2-1 ibr5-1 seedlings grown under yellow-filtered light at 22°C on medium supplemented with the indicated auxin (n≥ 12). eto1-34 ibr5-1, eto1-1 ibr5-1, eto1-12 ibr5-1 and eto2-1 ibr5-1 normalized root lengths on indole-3-butyricacid (IBA) were significantly shorter than ibr5-1 on 10 µm IBA (P≤ 0.0001) and 120 nM indole-3-acetic acid (IAA) (P≤ 0.01) in two-tailed Student’s t-tests assuming unequal variance.
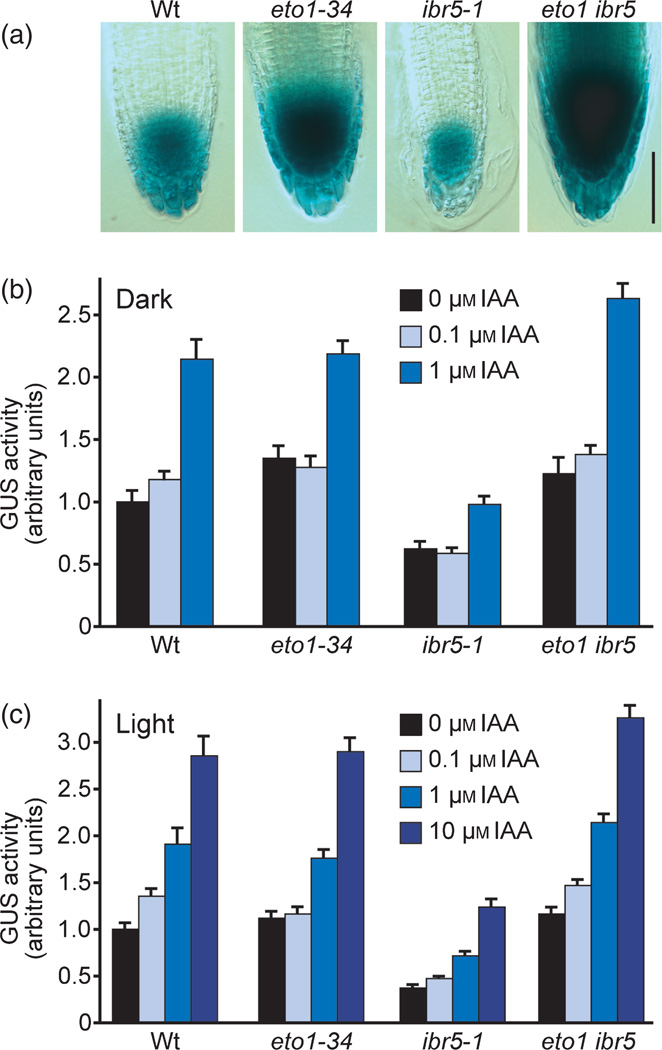
Because examining auxin-responsive root elongation in the eto1 ibr5 double mutant is confounded by the short root of eto1, we examined the activity of the auxin-responsive transcriptional reporter DR5-GUS (Ulmasov et al., 1997) in these lines. ibr5 has reduced DR5-GUS activity both without treatment and in response to auxin (Figure 2; Monroe-Augustus et al., 2003; Strader et al., 2008a). We found that light- and dark-grown eto1-34 seedlings displayed near wild-type DR5-GUS activity in whole seedling extracts (Figure 2b,c), and elevated activity in root tips (Figure 2a). Moreover, eto1-34 fully restored DR5-GUS activity levels to both untreated and auxin-treated ibr5 seedlings (Figure 2), demonstrating that ethylene overproduction restored auxin-responsive transcription to the ibr5 auxin-response mutant.
Figure 2. eto1 restores auxin-responsive transcription to ibr5.
(a) Primary root tips of untreated 8-day-old Col-0 (Wt), eto1-1, ibr5-1 and eto1-1 ibr5-1 seedlings carrying the DR5-GUS construct (Ulmasov et al., 1997) stained to visualize GUS activity. Scale bar: 50 µm.
(b, c) Col-0 (Wt), eto1-1, ibr5-1 and eto1-1 ibr5-1 seedlings carrying the DR5-GUS construct were grown for 5 days in the dark (b) or 8 days in the light (c), and were then transferred to medium supplemented with various concentrations of indole-3-acetic acid (IAA) for 2 h. GUS activity in seedling extracts is presented as normalized fluorescence (mean + SE). GUS activity in ibr5-1 was significantly lower than GUS activity in the wild type in both dark- and light-grown seedlings in both the absence and presence of IAA treatment (P ≤ 0.001 in two-tailed Student’s t-tests assuming unequal variance). GUS activity in eto1-34 ibr5-1 was significantly higher than GUS activity in ibr5-1 in both dark- and light-grown seedlings in both the absence and presence of IAA treatment (P ≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance).
Ethylene overproduction partially restores auxin responsiveness to auxin-resistant mutants
To determine the impact of ethylene overproduction on other mutants with deficient auxin responsiveness, we compared the eto1-1 ibr5-1 phenotypes with eto1-1 combined with the auxin receptor mutant tir1-1, the RUB-activating enzyme mutant axr1-3, the auxin influx mutant aux1-7 or the ethylene response mutant ein2-1 (Table 1). We found that eto1-1 partially restored the responsiveness of tir1, axr1 and aux1 to IBA in root elongation assays (Figure 3a). Because interpreting auxin responses in eto1 is complicated by its short root, we also examined IBA-induced lateral root formation. We found that eto1 produced more emerged lateral roots per unit root length than wild type in response to auxin application, and fully restored auxin-responsive lateral root formation to the auxin-resistant mutants ibr5, tir1 and aux1 (Figure 3b). In contrast, eto1 failed to restore auxin-responsive lateral root formation to the pleiotropic axr1 mutant (Figure 3b).
Table 1.
Mutant alleles used in this study
| Allele | Gene | Gene product | Original isolation | References |
|---|---|---|---|---|
| eto1-1 | At3g51770 | BTB protein | Ethylene overproducing |
Guzman and Ecker (1990); Wang et al. (2004) |
|
eto1-12 (SALK_061581) |
At3g51770 | BTB protein | Reverse genetics | Gingerich et al. (2005) |
| eto1-34 | At3g51770 | BTB protein | Suppressor of ibr5 IBA resistance |
Strader et al. (2008b) |
| eto2-1 | At5g65800 | ACC synthase | Ethylene overproducing |
Kieber et al. (1993); Vogel et al. (1998) |
| ibr5-1 | At2g04550 | MAP kinase phosphatase |
IBA resistance |
Zolman et al. (2000); Monroe-Augustus et al. (2003) |
| tir1-1 | At3g62980 | F-box protein auxin receptor |
Auxin transport inhibitor resistance |
Ruegger et al. (1998) |
| axr1-3 | At1g05180 | Subunit of RUB-activating enzyme |
2,4-D resistance |
Estelle and Somerville (1987); Leyser et al. (1993) |
| aux1-7 | At2g38120 | Auxin influx carrier | 2,4-D resistance |
Maher and Martindale (1980); Bennett et al. (1996) |
| ein2-1 | At5g03280 | Transmembrane protein | Ethylene resistance |
Roman et al. (1995); Alonso et al. (1999) |
Figure 3. eto1 partially restores auxin responsiveness to auxin-resistant mutants.
(a) Mean primary root lengths of Col-0 (Wt), eto1-1, ibr5-1, eto1-1 ibr5-1, tir1-1, eto1-1 tir1-1, axr1-3, eto1-1 axr1-3, aux1-7, eto1-1 aux1-7, ein2-1 and eto1-1 ein2-1 seedlings grown under yellow-filtered light at 22°C on medium supplemented with ethanol (mock) or 10 µm indole-3-butyricacid (IBA; n≥ 15; error bars indicate SE). The percentage of root length of seedlings grown in the presence of 10 µm IBA compared with control seedlings is indicated above the bars. Root lengths of eto1 ibr5, eto1 tir1, eto1 axr1 and eto1 aux1 were significantly shorter than the root lengths of the respective auxin-resistant parent when grown in the presence of 10 µm IBA (P≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance).
(b) Mean numbers of emerged lateral roots per centimeter root length of Col-0 (Wt), eto1-1, ibr5-1, eto1-1 ibr5-1, tir1-1, eto1-1 tir1-1, axr1-3, eto1-1 axr1-3, aux1-7, eto1-1 aux1-7, ein2-1 and eto1-1 ein2-1 seedlings 4 days after transfer of 4-day-old seedlings to medium supplemented with ethanol (mock) or 10 µm IBA (n≥ 10; error bars indicate SE). The number of emerged lateral roots per cm root length in eto1 was significantly higher than the number in the wild type when grown in the presence of 10 µm IBA (P≤ 0.01 in two-tailed Student’s t-tests assuming unequal variance). The number of emerged lateral roots per cm root length in eto1 ibr5,eto1 tir1 and eto1 aux1 was significantly greater than the number for the respective auxin-resistant parent when grown in the presence of 10 µm IBA (P≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance).
(c) Mean primary root lengths of Col-0 (Wt), ibr5-1, tir1-1, axr1-3 and aux1-7 seedlings grown under yellow-filtered light at 22°C on medium supplemented with ethanol (mock) or the indicated auxins (n≥ 15; error bars indicate SE).
Blocking auxin transport suppresses the short-root phenotype of ethylene overproduction
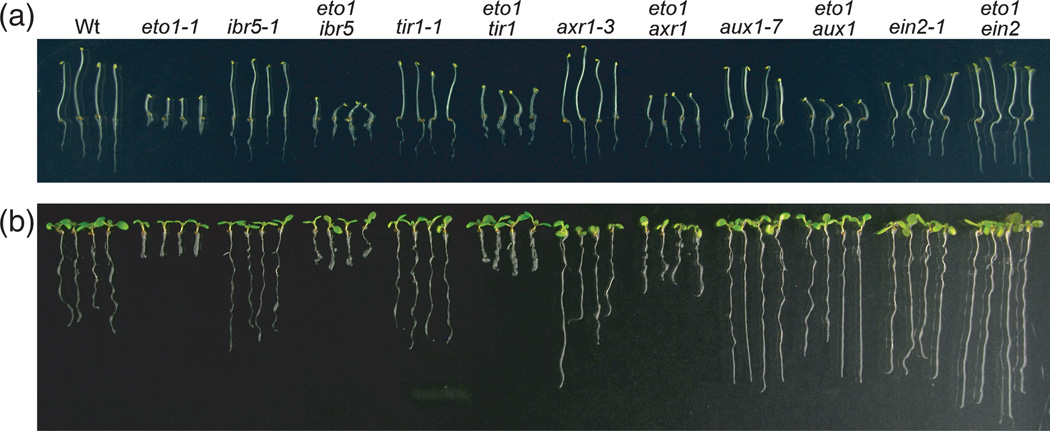
Loss of ETO1 confers ethylene overproduction, resulting in short hypocotyls and roots in dark-grown seedlings, and short roots in light-grown seedlings (reviewed in Chae and Kieber, 2005). As previously reported (Roman et al., 1995), ein2 fully suppressed eto1 root and hypocotyl elongation defects in dark- and light-grown seedlings (Figure 4). In contrast, hypocotyls of dark-grown eto1 ibr5, eto1 tir1, eto1 axr1 and eto1 aux1 seedlings resembled hypocotyls of the eto1-1 parent (Figure 4a), indicating that impeding auxin response or transport does not affect ethylene response in dark-grown hypocotyls, consistent with previous results of ACC application to auxin-response mutants (Stepanova et al., 2007). However, ibr5, tir1, axr1 and aux1 each rescued eto1 root elongation in both the dark (Figure 4a) and the light (Figure 4b) to a degree consistent with the relative auxin resistance of the parent (ibr5-1 = tir1-1 < axr1-3 < aux1-7; Figure 3c). These data suggest that the short-root phenotype of eto1 seedlings requires auxin signaling and transport. Indeed, blocking IAA influx with the aux1 mutation restored eto1 root elongation as efficiently as blocking ethylene signaling with the ein2 mutation (Figure 4a,b).
Figure 4. Blocking auxin response or influx restores eto1 root phenotypes.
Five-day-old dark-grown (a) and 8-day-old light-grown (b) Col-0 (Wt), eto1-1, ibr5-1, eto1-1 ibr5-1, tir1-1, eto1-1 tir1-1, axr1-3, eto1-1 axr1-3, aux1-7, eto1-1 aux1-7, ein2-1 and eto1-1 ein2-1 seedlings representing the range of observed pheno-types.
Endogenous ethylene directs auxin to inhibit longitudinal root cell expansion
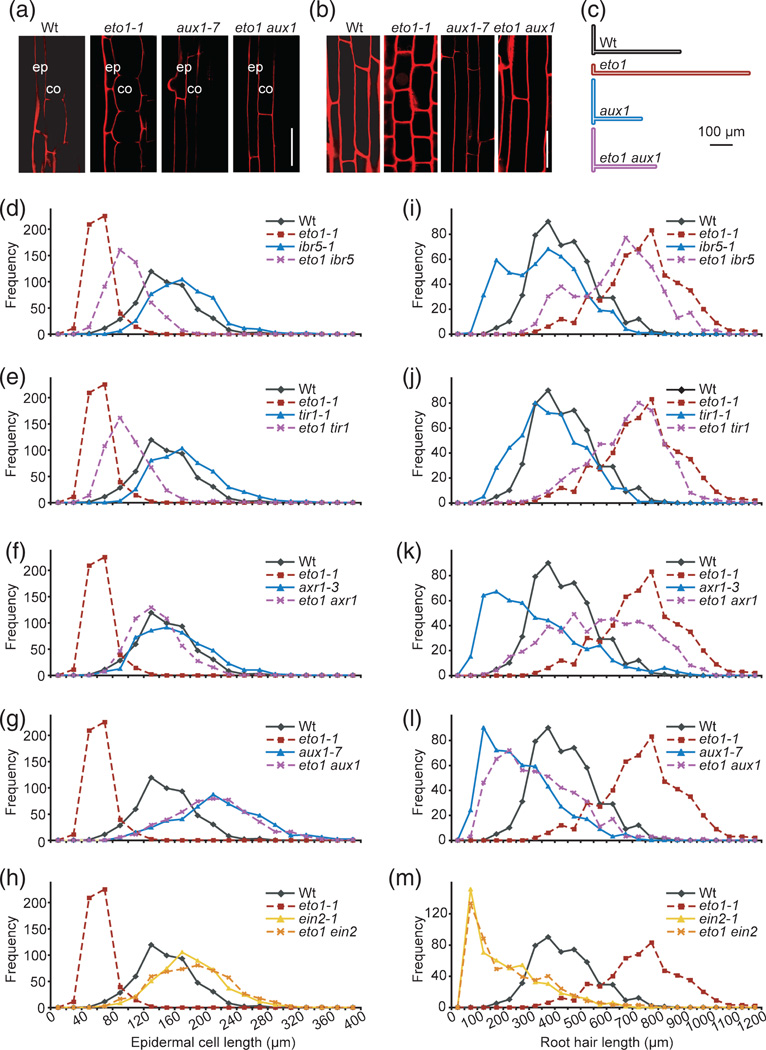
Because both cell division and cell elongation contribute to root length, we used confocal microscopy to examine fully differentiated (no longer expanding) root cells from light-grown wild-type, eto1, aux1 and eto1 aux1 seedlings. As expected, we found that epidermal and cortex cells (the two outermost root cell layers) of eto1 were shorter than those of the wild type (Figure 5a,b), suggesting that ethylene inhibits root elongation, at least in part, by inhibiting elongation of these cells. Moreover, eto1 cortex cells were wider than those of the wild type (Figure 5a). In contrast, the epidermal and cortex cell lengths were longer in aux1 than in the wild type (Figure 5a,b, Rahman et al., 2002), although these cells were often longer than the objective allowed us to image. The length and width of eto1 aux1 epidermal and cortex cells resembled those of aux1 (Figure 5a,b), indicating that AUX1 is necessary for ethylene to affect root cell expansion.
Figure 5. Blocking auxin response or influx restores eto1 root cell elongation phenotypes.
(a, b) Confocal images of differentiated root cells stained with propidium iodide from 8-day-old Col-0 (Wt), eto1-1, aux1-7 and eto1-1 aux1-7 seedlings. (a) Longitudinal section of the root showing epidermal (ep) and cortex (co) cells. Scale bar: 50 µm.
(b) Longitudinal section of epidermal cells. Scale bar: 50 µm.
(c) Schematic depicting mean dimensions of root hair cells from Col-0 (Wt), eto1-1, aux1-7 and eto1-1 aux1-7 seedlings. Mean epidermal cell lengths of eto1 were significantly shorter, and cell lengths of aux1 were significantly longer, than those of the wild type (P≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance). Mean epidermal cell lengths of eto1 aux1 were significantly longer than those of eto1 (P ≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance). Mean root hair lengths of eto1 were significantly longer, and root hair lengths of aux1 were significantly shorter, than those of the wild type (P≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance). Mean root hair lengths of eto1 aux1 were significantly shorter than mean root hair lengths of eto1 (P ≤ 0.0001 in two-tailed Student’s t-tests assuming unequal variance).
(d–h) A decreased auxin response or influx suppresses eto1 inhibition of root cell elongation. Histograms show epidermal cell lengths of 8-day-old Col-0 (Wt) seedlings compared with the indicated single and double mutants. For each genotype, 500 cells from ~12 seedlings were measured. The same wild-type and eto1-1 data are depicted in each panel for ease of comparison.
(i–m) Decreased auxin response or influx suppresses eto1 promotion of root hair elongation. Histograms show root hair lengths of 5-day-old Col-0 (Wt) seedlings compared with the indicated single and double mutants. For each genotype, 500 root hairs from ~12 seedlings were measured. The same wild-type and eto1-1 data are depicted in each panel for ease of comparison.
We quantified the effects of auxin-response mutants on eto1 cell length. eto1 displayed shorter epidermal cells than the wild type (Figure 5c,d), whereas the auxin-resistant mutants ibr5 (Figure 5d), tir1 (Figure 5e), axr1 (Figure 5f) and aux1 (Figure 5c,g), and the ethylene-response mutant ein2 (Figure 5h), all displayed longer epidermal cells than the wild type. As expected, ein2 fully suppressed the short epidermal cell length of eto1: eto1 ein2 epidermal cells were as long as ein2 cells (Figure 5h). The mild auxin-resistant mutants ibr5 (Figure 5d) and tir1 (Figure 5e) partially suppressed eto1 epidermal cell elongation defects, and axr1 restored eto1 cells to wild-type lengths (Figure 5f). Like ein2, aux1 was fully epistatic to eto1: eto1 aux1 epidermal cells were as long as aux1 cells (Figure 5c,g). We concluded that auxin transport and signaling are required for epidermal cell elongation inhibition in response to endogenous ethylene.
Endogenous ethylene directs auxin to promote root hair expansion
In addition to inhibiting root epidermal cell elongation, both auxin and ethylene promote root hair elongation (reviewed in Grierson and Schiefelbein, 2002). As expected, eto1 displayed longer root hairs than the wild type (Figure 5c,i), whereas the auxin-resistant mutants ibr5 (Figure 5i), tir1 (Figure 5j), axr1 (Figure 5k) and aux1 (Figure 5c,l), and the ethylene response mutant ein2 (Figure 5m), all had shorter root hairs than the wild type. Also as expected, ein2 fully suppressed the long root hairs of eto1: eto1 ein2 root hairs were as short as ein2 root hairs (Figure 5m). We found that the mild auxin-resistant mutants ibr5 (Figure 5i) and tir1 (Figure 5j) slightly reduced eto1 root hair length, whereas the more severe axr1-3 mutant more fully suppressed eto1 root hair length (Figure 5k), suggesting that auxin signaling is required for ethylene-promoted root hair lengthening. The auxin influx mutant aux1 completely suppressed the long root hair phenotype of eto1 (Figure 5c,l), indicating that AUX1-mediated IAA influx is required for root hair elongation in response to endogenous ethylene.
Blocking auxin transport does not disrupt ethylene signaling
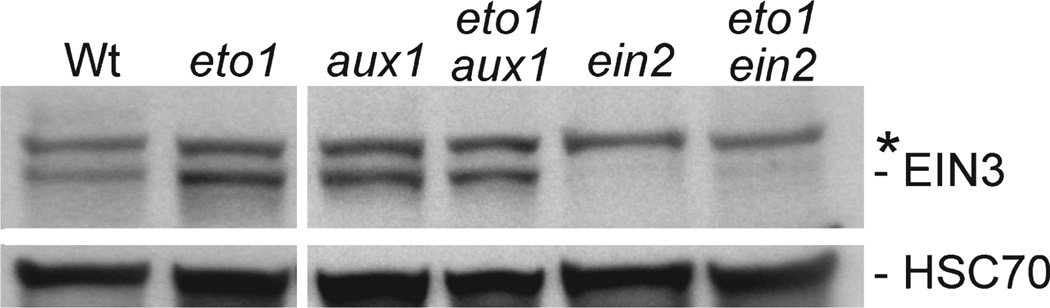
Because auxin upregulates transcription of the ACS ethylene biosynthesis genes (reviewed in Yang and Hoffman, 1984; Tsuchisaka and Theologis, 2004), aux1 might suppress eto1 effects on root cell expansion by preventing ethylene overproduction, by blocking ethylene signal transduction, or by acting downstream of ethylene signaling. We therefore examined a late-acting component of ethylene signaling, the EIN3 transcription factor, which accumulates in response to ethylene application and is elevated in eto1 (Guo and Ecker, 2003) (Figure 6). Because ein2 blocks ethylene response upstream of EIN3, EIN3 levels were low in both ein2 and eto1 ein2 (Figure 6). Unlike eto1 ein2, however, we found that eto1 aux1 accumulated EIN3 to eto1-like levels (Figure 6). Because aux1 prevents phenotypes associated with ethylene overproduction in light-grown eto1 seedlings (Figures 4 and 5) without reducing EIN3 levels (Figure 6), we concluded that AUX1 is needed downstream of ethylene signaling to control root hair expansion in response to endogenous ethylene. Because the only known role of AUX1 is to bring IAA into cells (reviewed in Vieten et al., 2007), this epistatic relationship implies that auxin signaling acts downstream of ethylene signaling to promote root hair elongation (Figure 7).
Figure 6. EIN3 protein accumulates in eto1 aux1.
Anti-EIN3 (top panels) and anti-HSC70 (bottom panels) antibodies were used to probe immunoblots of protein prepared from 5-day-old light-grown Col-0 (Wt), eto1-1, aux1-7, eto1-1 aux1-7, ein2-1 and eto1-1 ein2-1 seedlings. The asterisk marks a protein that cross-reacts with the EIN3 antibody.
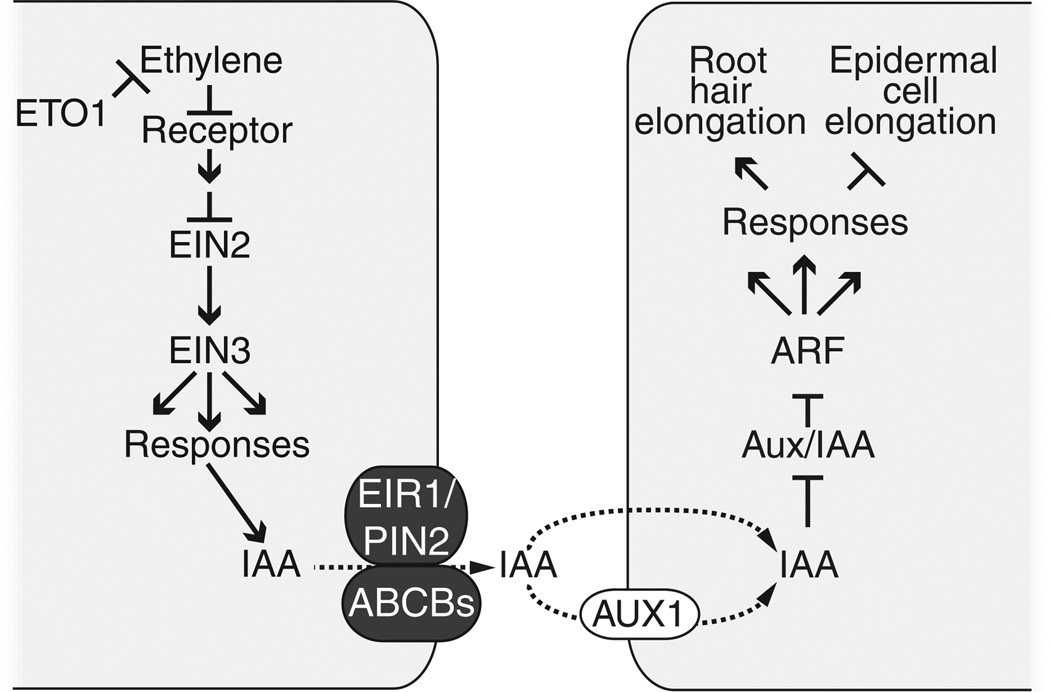
Figure 7. Model for ethylene–auxin interactions controlling root cell expansion.
Ethylene promotes auxin biosynthesis (Swarup et al., 2007) and/or auxin transport (Růžička et al., 2007) to direct auxin in the epidermal cell layer to inhibit root epidermal cell elongation and promote root hair elongation. This process is dependent on atrichoblast-localized AUX1 (Pitts et al., 1998; Jones et al., 2009), suggesting that this increased auxin biosynthesis or transport may occur in cells other than the trichoblast.
DISCUSSION
Both ethylene and auxin alter root morphology by reducing longitudinal cell expansion and promoting root hair elongation. Ethylene decreases primary root length by reducing cell elongation (Le et al., 2001; De Cnodder et al., 2005; Růžička et al., 2007; Swarup et al., 2007). Mutants with decreased ethylene or auxin responsiveness display shorter root hairs (reviewed in Grierson and Schiefelbein, 2002); conversely, mutants with increased ethylene response or production (reviewed in Grierson and Schiefelbein, 2002), or decreased IAA (Santelia et al., 2005; Cho et al., 2007) or IBA (Strader and Bartel, 2009) efflux, display longer root hairs.
The similar influences of ethylene and auxin on root cell morphology suggest that the respective signaling or response pathways are dependent or interdependent. Indeed, many auxin-resistant mutants are also ethylene resistant, and many ethylene-resistant mutants also are auxin resistant, suggesting that some aspects of auxin and ethylene response require responsiveness to the other hormone (Stepanova et al., 2007). In addition, ethylene responses in the root require basipetal (rootward) auxin transport machinery (Pickett et al., 1990; Luschnig et al., 1998; Růžička et al., 2007), and ethylene enhances acropetal (shootward) auxin transport (Negi et al., 2008). However, interpretation of these studies is complicated by the use of applied hormones or inhibitors. Applied hormones may or may not mimic endogenous hormone production, and recent studies reveal that the commonly used ethylene inhibitors AVG and AgNO3 are not suited for studying auxin–ethylene interactions. AVG not only inhibits the pyridoxal phosphate-requiring ACS ethylene biosynthesis enzymes (Yang and Hoffman, 1984), but also inhibits members of the recently described (Stepanova et al., 2008; Tao et al., 2008) pyridoxal phosphate-requiring TAA1 family of auxin biosynthesis enzymes (Soeno et al., 2010). Thus AVG application can directly reduce both ethylene and IAA biosynthesis, and is not informative for dissecting auxin–ethylene interactions. AgNO3 inhibits ethylene perception, but is also unsuitable for dissection of auxin–ethylene interactions because it promotes auxin efflux independently of its effects on ethylene responsiveness (Strader et al., 2009).
We became interested in the interaction between auxin and ethylene response when we identified an eto1 allele that suppressed certain phenotypes of the weak auxin-resistant mutant ibr5, including auxin-responsive root elongation inhibition (Figures 1e and 3a), lateral root promotion (Figure 3b) and gene expression (Figure 2). IBR5 is a dual-specificity protein phosphatase (Lee et al., 2009) that promotes both auxin (Monroe-Augustus et al., 2003) and ethylene (Strader et al., 2008b) responsiveness. Intriguingly, IBR5 promotes auxin responsiveness without stimulating Aux/IAA protein degradation (Strader et al., 2008a), unlike the well-characterized auxin-response components TIR1 and AXR1 (reviewed in Woodward and Bartel, 2005). Because IBR5 is unique among characterized factors that promote auxin-responsive transcription, we determined whether the eto1 restoration of the ibr5 auxin response was also unique.
We found that eto1-1 partially restored the responsiveness of the auxin receptor mutant tir1-1, the RUB activating enzyme mutant axr1-3 and the auxin influx mutant aux1-7 to IBA in root elongation assays (Figure 3a). Moreover, eto1-1 fully restored IBA-responsive lateral root formation in ibr5, tir1, and aux1, so we were surprised to find that eto1-1 did not restore IBA-responsive lateral root formation to axr1-3 (Figure 3b). AXR1 controls RELATED TO UBIQUITIN (RUB) modification of the CULLIN1 subunit of E3 ligase complexes to regulate E3 activity (reviewed in Schwechheimer and Caldero´n Villalobos, 2004). RUB-regulated E3 ligases include not only the TIR1 family of auxin receptors, but potentially hundreds of additional CULLIN-containing E3 ligases in Arabidopsis. As a result, disruption of RUB modification disrupts many plant processes, including responses to auxin (del Pozo et al., 1998, 2002; Bostick et al., 2004) and ethylene (Guo and Ecker, 2003; Potuschak et al., 2003; Stepanova et al., 2007), as well as ethylene production (Bostick et al., 2004; Woodward et al., 2007). Because RUB modification of CULLIN affects diverse processes, it is difficult to pinpoint the cause of the eto1 failure to restore auxin-responsive lateral root formation in axr1. In any case, our results clearly demonstrate that ethylene overproduction can compensate for attenuated auxin responsiveness caused not only by lesions in IBR5, but also by reduced TIR1 or AUX1 function, indicating that the observed suppression is not specific to the IBR5 pathway.
The eto1 mutant was previously reported to produce fewer lateral roots than the wild type in the absence of treatment (Negi et al., 2008). We also found that eto1 exhibited fewer emerged lateral roots without treatment; however, it produced a greater lateral root density (emerged lateral roots per unit root length) in response to exogenous auxin than the wild type (Figure 3b). Treatment with low ACC levels promotes lateral root initiation and emergence in the wild type, but does not bypass the lateral root-deficient phenotype of mutants that lack lateral roots because of a strong block in auxin signaling, such as solitary root-1 and arf7 arf19 (Ivanchenko et al., 2008), consistent with the possibility that ethylene promotion of lateral root initiation and emergence requires some residual auxin signaling. However, our demonstration that eto1 restores auxin-responsive lateral root production to ibr5, tir1 and aux1 is not directly comparable with previous results showing that ACC treatment does not restore lateral root initiation and emergence to slr-1 and arf7 arf19 (Ivanchenko et al., 2008), because Ivanchenko et al. used low levels of ACC that may not yield as much ethylene as is found in eto1. It will be interesting to learn whether eto1 can restore auxin-responsive lateral root production to slr-1 or arf7 arf19. Our demonstration that eto1 restores auxin-responsive lateral root formation to several mutants with attenuated auxin responsiveness (Figure 3b) suggests that increased ethylene levels can prime the root for auxin-responsive lateral root production.
We used eto1 to examine the effects of endogenous ethylene on root cell expansion in untreated auxin-response mutants and found that the effects of elevated ethylene on root cell expansion (inhibition of epidermal cell elongation and promotion of root hair elongation) were fully suppressed by blocking auxin influx (Figure 5). These data are consistent with previous reports that the application of 1-naphthaleneacetic acid (NAA) partially restores root hair elongation in the ein2-1 mutant (Rahman et al., 2002), and that the aux1 mutant is resistant to the effects of the ethylene precursor ACC on the inhibition of root cell elongation (Swarup et al., 2007). Our epistasis data suggest that ethylene directs auxin to both promote root hair lengthening and inhibit root cell longitudinal expansion.
Animals require cell movement to determine the final form of organs. Because there is no morphogenic cell movement in plants, and because the cell wall is usually formed immediately after cell division, plant morphogenesis depends upon cell expansion. The plant hormones ethylene and auxin alter root morphology by decreasing root cell length and by increasing root hair length. We found that the ethylene effects on these processes are entirely dependent on auxin influx and responsiveness. Because ethylene is volatile, using the tightly regulated phytohormone auxin to influence root growth and morphology may provide additional control and specificity to ethylene effects. Our data suggest a model (Figure 7) in which ethylene promotes auxin biosynthesis (Růžička et al., 2007; Swarup et al., 2007) and/or auxin transport (Růžička et al., 2007) to increase auxin levels in the epidermal cell layer, which inhibits longitudinal cell elongation and promotes root hair elongation. Future studies may reveal whether modulation of auxin biosynthesis or auxin transport by ethylene play a larger role in the control of root cell expansion.
EXPERIMENTAL PROCEDURES
eto1-34 identification
The previously described (Strader et al., 2008b) ibr5-1 suppressor MS34 (in the Col-0 background) was outcrossed to Ws-introgressed ibr5-1 for mapping (Strader et al., 2008b). The resulting F2 seeds were plated on 10 lM IBA, and DNA from sensitive individuals was used for mapping using polymorphic markers (Table S1). A candidate gene (ETO1/At3g51770) within the mapping interval was sequenced from 51 bp upstream of the putative translation start site to 159 bp downstream of the stop codon to reveal the eto1-34 missense mutation.
Plant materials, growth conditions and phenotypic assays
Mutants were in the Colombia (Col-0) accession of A. thaliana. PCR analysis of F2 plants was used to identify double mutants (Table S2). Surface-sterilized seeds were plated on plant nutrient medium (PN; Haughn and Somerville, 1986) supplemented with 0.5% (w/v) sucrose (PNS) solidified with 0.6% (w/v) agar. Seedlings were grown at 22°C under continuous illumination unless indicated otherwise.
To examine hypocotyl elongation, seedlings were grown for 1 day in the light, followed by 4 days in darkness.
To examine auxin-responsive root elongation, seedlings were grown under yellow long-pass filters to slow indolic compound breakdown (Stasinopoulos and Hangarter, 1990) for 8 days on PNS with the indicated auxin.
To examine root hairs, 4-mm root sections adjacent to the root–shoot junction from 5-day-old vertically grown seedlings were imaged using a dissecting microscope, and root hair lengths were measured using the public domain NIH Image program (developed at the US National Institutes of Health and available at http://rsb.info.nih.gov/nih-image).
To examine epidermal cell lengths, 8-day-old seedlings were fixed in 3:1 ethanol:acetic acid, mounted in a chloral hydrate solution (80 g chloral hydrate, 20 ml glycerol and 10 ml water) and imaged using a Zeiss Axioplan 2 microscope (http://www.zeiss.com). Epidermal cell lengths (from both trichoblast and atrichoblast cell files) were measured using NIH Image.
GUS assays
For histochemical GUS assays, 5-day-old dark-grown seedlings carrying DR5-GUS (Ulmasov et al., 1997) were incubated in 90% acetone for 20 min at –20°C. Seedlings were rinsed twice in GUS buffer {0.1 m NaPO4, pH 7.0, 0.5 mm K3[Fe(CN6)], 0.5 mm K4[Fe(CN6)], 10 mm EDTA, 0.01% Triton X-100}, and then incubated overnight at 37°C in GUS buffer supplemented with 0.5 mg ml−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, fixed in 3:1 ethanol:acetic acid, mounted and imaged using a Zeiss Axioplan 2 microscope.
For 4-methylumbelliferyl-β-d-glucuronide hydrate (MUG) assays, 16 replicates of three 8-day-old seedlings carrying DR5-GUS (Ulmasov et al., 1997) were treated for 2 h on a PNS plate supplemented with the indicated auxin. Extracts were prepared and GUS activity was monitored as previously described (Strader and Bartel, 2009).
Confocal fluorescence microscopy
Eight-day-old seedlings were stained for 10 min in an aqueous solution of 10 µg ml−1 propidium iodide (Sigma-Aldrich, http://www.sigmaaldrich.com), then mounted in water under a cover-slip. Samples were excited with an argon 488-nm laser line and imaged with a Zeiss LSM 510 Meta laser scanning confocal microscope through a 63x oil-immersion lens. Fluorescence was filtered through a 560-nm high-pass filter, and detected pixels were false-colored red. Images were converted using NIH Image software.
Immunoblot analysis
To monitor EIN3 levels, protein from 12 5-day-old seedlings was analyzed by immunoblotting as previously described (Strader et al., 2009), except that a 1:4000 dilution of anti-EIN3 antibody (Guo and Ecker, 2003) was used.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Joseph Ecker for the EIN3 antibody; Mark Estelle for axr1-3 and aux1-7; the Arabidopsis Biological Resource Center at Ohio State University for ein2-1, eto1-1, eto1-12 (SALK_061581), eto2-1 and tir1-1; James McNew for fluorometer use; and Sarah Christensen, Naxhiely Ramo´n, Sarah Ratzel, and Andrew Woodward for critical comments on the manuscript. This research was supported by the National Science Foundation (MCB-0745122 to BB), the Robert A. Welch Foundation (C-1309 to BB), the National Institutes of Health (1K99-GM089987 to LCS), and the Arnold and Mabel Beckman Foundation (Beckman Scholars Program to GLC).
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Table S1. Markers used in MS34 mapping.
Table S2. PCR-based determination of mutant genotypes.
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
REFERENCES
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress response in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schultz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Bostick M, Lochhead SR, Honda A, Palmer S, Callis J. Related to ubiquitin 1 and 2 are redundant and essential and regulate vegetative growth, auxin signaling, and ethylene production in Arabidopsis. Plant Cell. 2004;16:2418–2432. doi: 10.1105/tpc.104.024943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A, Lincoln C, Lammer D, Estelle M. The SAR1 gene of Arabidopsis acts downstream of the AXR1 gene in auxin response. Development. 1997;124:1583–1591. doi: 10.1242/dev.124.8.1583. [DOI] [PubMed] [Google Scholar]
- Chae HS, Kieber JJ. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005;10:291–296. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell. 2003;15:545–559. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ. Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell. 2002;14:3237–3253. doi: 10.1105/tpc.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Lee SH, Cho H-T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2007;19:3930–3943. doi: 10.1105/tpc.107.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians MJ, Gingerich DJ, Hansen M, Binder BM, Kieber JJ, Vierstra RD. The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 2009;57:332–345. doi: 10.1111/j.1365-313X.2008.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP. Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: a matter of apoplastic reactions. New Phytol. 2005;168:541–550. doi: 10.1111/j.1469-8137.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol. Gen. Genet. 1987;206:200–206. [Google Scholar]
- Gingerich DJ, Gagne JM, Salter DW, Hellmann H, Estelle M, Ma L, Vierstra RD. Cullins 3a and 3b assemble with members of the broad complex/tramtrack/bric-a-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J. Biol. Chem. 2005;280:18810–18821. doi: 10.1074/jbc.M413247200. [DOI] [PubMed] [Google Scholar]
- Grierson C, Schiefelbein J. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. Root Hairs. http://www.aspb.org/publications/arabidopsis/ [Google Scholar]
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn GW, Somerville C. Sulfonylurea-resistant mutants of Arabidopsis thaliana . Mol. Gen. Genet. 1986;204:430–434. [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana . Plant J. 2008;55:335–347. doi: 10.1111/j.1365-313X.2008.03528.x. [DOI] [PubMed] [Google Scholar]
- Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HM, Grierson CS. Auxin transport through non-hair cells sustains root-hair development. Nat. Cell Biol. 2009;11:78–84. doi: 10.1038/ncb1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber J, Rothenberg M, Roman G, Feldmann KA, Ecker J. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol. 2001;125:519–522. doi: 10.1104/pp.125.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Wang S, Sritubtim S, Chen JG, Ellis BE. Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J. 2009;57:975–985. doi: 10.1111/j.1365-313X.2008.03741.x. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M. Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature. 1993;364:161–164. doi: 10.1038/364161a0. [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana . Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EP, Martindale SJB. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem. Genet. 1980;18:1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell. 1996;8:1505–1517. doi: 10.1105/tpc.8.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe-Augustus M, Zolman BK, Bartel B. IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell. 2003;15:2979–2991. doi: 10.1105/tpc.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana . Plant J. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Estelle M. Auxin receptors: a new role for F-box proteins. Curr. Opin. Cell Biol. 2006;18:152–156. doi: 10.1016/j.ceb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–560. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/s0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Timpte C, Tan S, Callis J, Estelle M. The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science. 1998;280:1760–1763. doi: 10.1126/science.280.5370.1760. [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M. AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis Cullin AtCUL1 is required for auxin response. Plant Cell. 2002;14:421–433. doi: 10.1105/tpc.010282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002;130:1908–1917. doi: 10.1104/pp.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Duchtig P, Mancuso S, Martinoia E, Geisler M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005;579:5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Schaller GE, Kieber JJ. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. Ethylene. http://www.aspb.org/publications/arabidopsis/ [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signalling domains. Proc. Natl. Acad. Sci. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Calderón Villalobos LIA. Cullin-containing E3 ubiquitin ligases in plant development. Curr. Opin. Plant Biol. 2004;7:677–686. doi: 10.1016/j.pbi.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D. Ethylene and vegetative development. Physiol Plant. 1997;100:593–605. [Google Scholar]
- Soeno K, Goda H, Ishii T, Ogura T, Tachikawa T, Sasaki E, Yoshida S, Fujioka S, Asami T, Shimada Y. Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant Cell Physiol. 2010;51:524–536. doi: 10.1093/pcp/pcq032. [DOI] [PubMed] [Google Scholar]
- Stasinopoulos TC, Hangarter RP. Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol. 1990;93:1365–1369. doi: 10.1104/pp.93.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Strader LC, Bartel B. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell. 2009;21:1992–2007. doi: 10.1105/tpc.109.065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Bartel B. The IBR5 phosphatase promotes Arabidopsis auxin responses through a novel mechanism distinct from TIR1-mediated repressor degradation. BMC Plant Biol. 2008a;8:41. doi: 10.1186/1471-2229-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Rogers KC, Lin GL, Bartel B. Arabidopsis iba response5 (ibr5) suppressors separate responses to various hormones. Genetics. 2008b;180:2019–2031. doi: 10.1534/genetics.108.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Beisner ER, Bartel B. Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell. 2009;21:3585–3590. doi: 10.1105/tpc.108.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana . Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl Acad. Sci. USA. 1998;95:4766–4771. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KL, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–950. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Ratzel SE, Woodward EE, Shamoo Y, Bartel B. Mutation of E1-CONJUGATING ENZYME-RELATED1 decreases RELATED TO UBIQUITIN conjugation and alters auxin response and development. Plant Physiol. 2007;144:976–987. doi: 10.1104/pp.107.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984;35:155–189. [Google Scholar]
- Zolman BK, Yoder A, Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics. 2000;156:1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.