Abstract
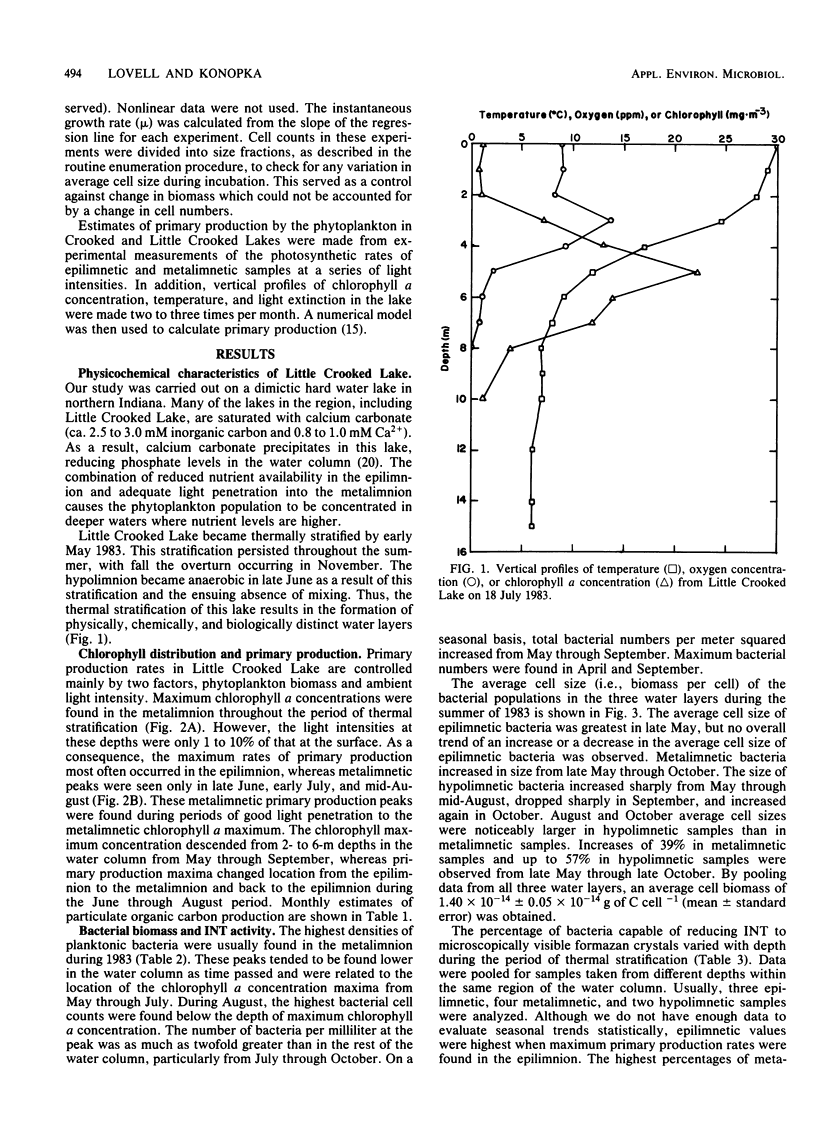
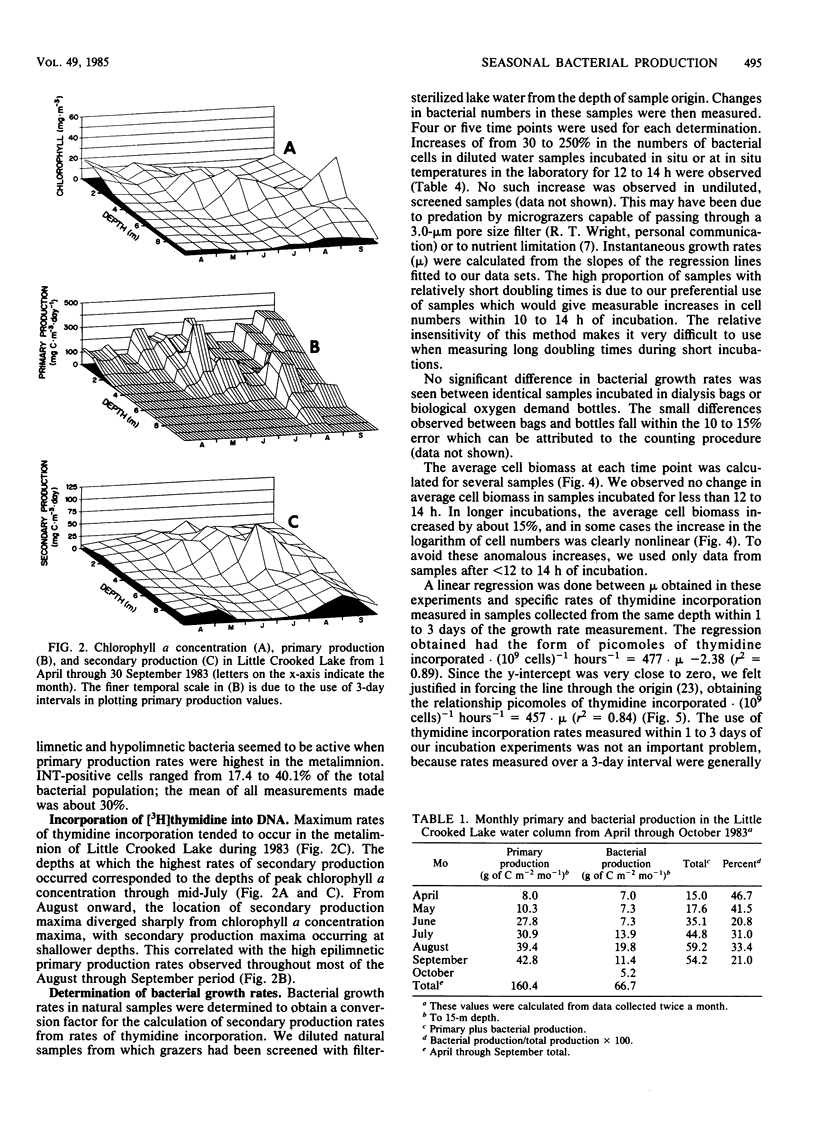
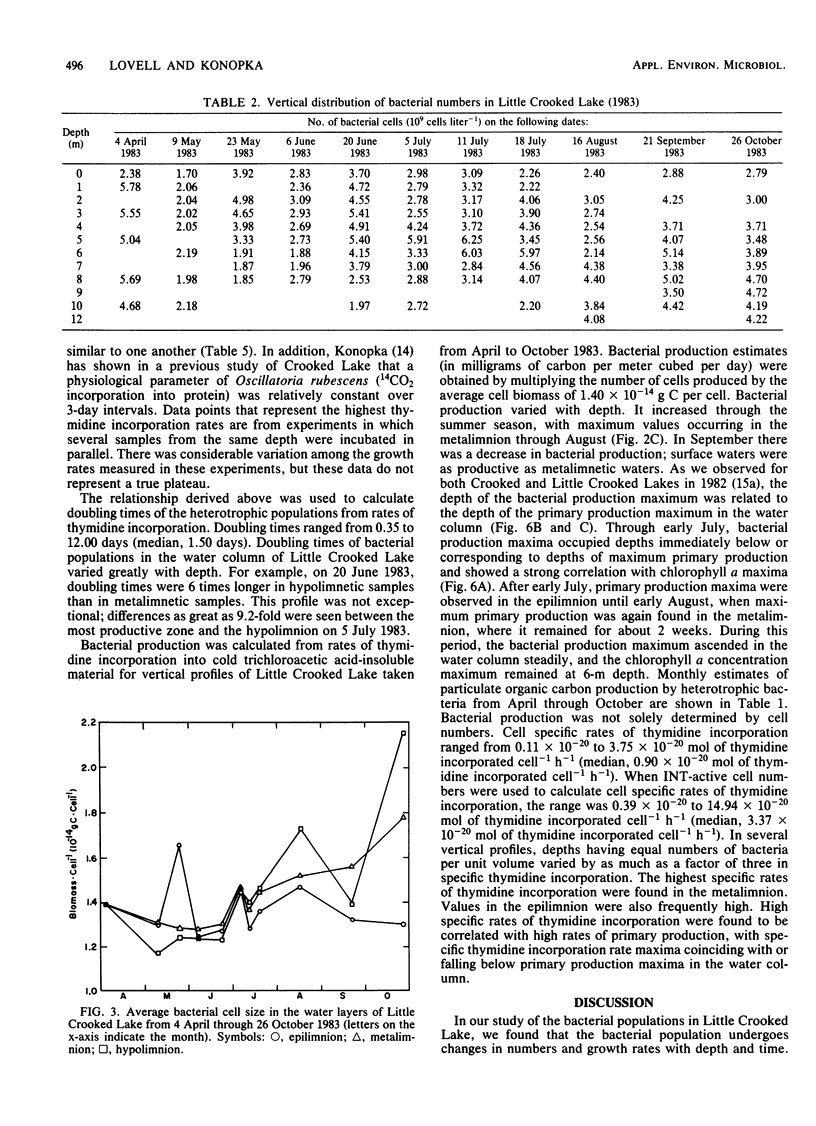
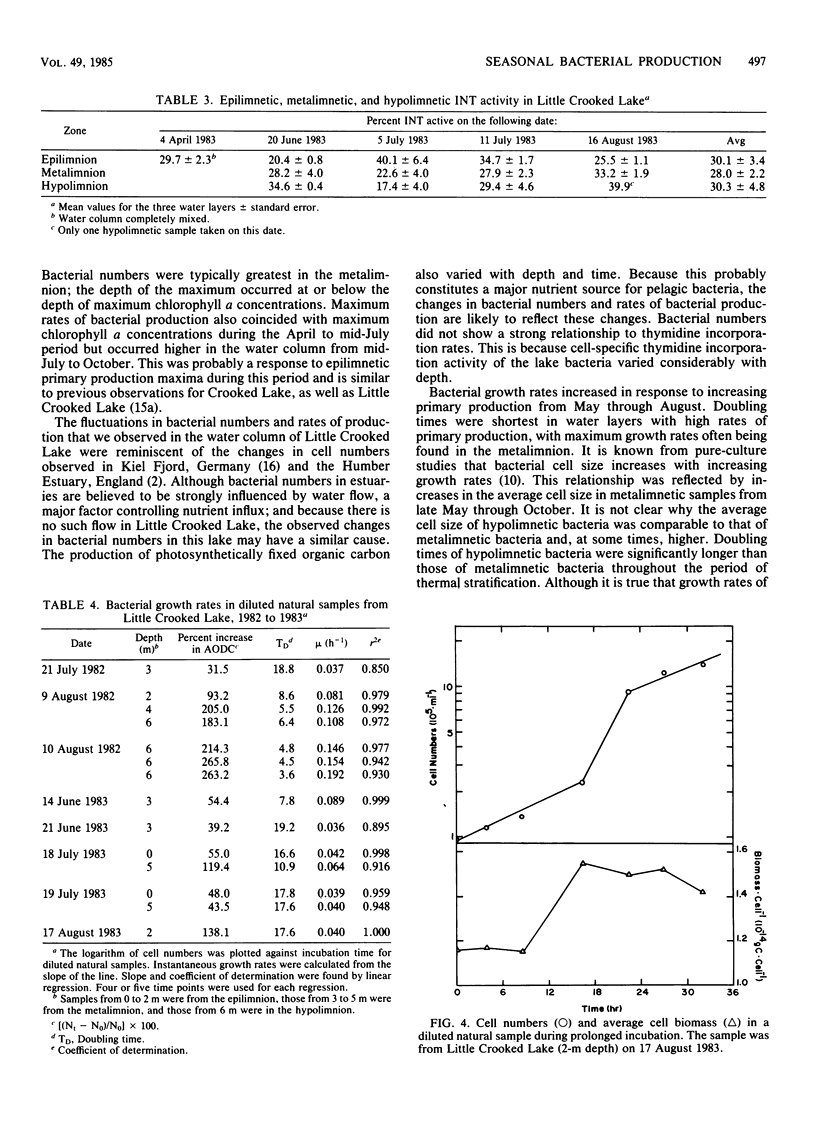
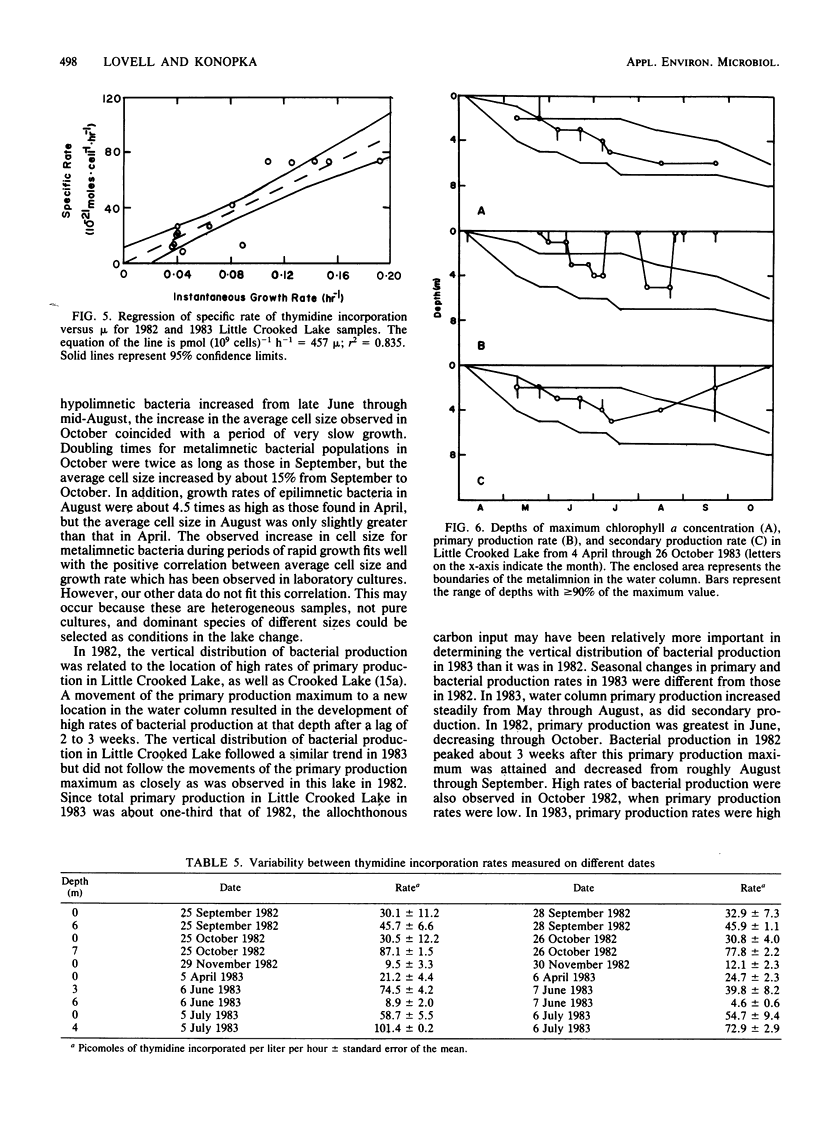
Rates of primary and bacterial production in Little Crooked Lake were calculated from the rates of incorporation of H14CO3− and [methyl-3H]thymidine, respectively. Growth rates of bacteria in diluted natural samples were determined for epilimnetic and metalimnetic bacterial populations during the summers of 1982 and 1983. Exponential growth was observed in these diluted samples, with increases in cell numbers of 30 to 250%. No lag was observed in bacterial growth in 14 of 16 experiments. Correlation of bacterial growth rates to corresponding rates of thymidine incorporation by natural samples produced a conversion factor of 2.2 × 1018 cells produced per mole of thymidine incorporated. The mass of the average bacterial cell in the lake was 1.40 × 10−14 ± 0.05 × 10−14 g of C cell−1. Doubling times of natural bacteria calculated from thymidine incorporation rates and in situ cell numbers ranged from 0.35 to 12.00 days (median, 1.50 days). Bacterial production amounted to 66.7 g of C m−2 from April through September, accounting for 29.4% of total (primary plus bacterial) production during this period. The vertical and seasonal distribution of bacterial production in Little Crooked Lake was strongly influenced by the distribution of primary production. From April through September 1983, the depth of maximum bacterial production rates in the water column was related to the depth of high rates of primary production. On a seasonal basis, primary production increased steadily from May through September, and bacterial production increased from May through August and then decreased in September.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden W. B. Comparison of two direct-count techniques for enumerating aquatic bacteria. Appl Environ Microbiol. 1977 May;33(5):1229–1232. doi: 10.1128/aem.33.5.1229-1232.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Primary and bacterial production in two dimictic indiana lakes. Appl Environ Microbiol. 1985 Mar;49(3):485–491. doi: 10.1128/aem.49.3.485-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved method for determination of respiring individual microorganisms in natural waters. Appl Environ Microbiol. 1982 Jun;43(6):1249–1255. doi: 10.1128/aem.43.6.1249-1255.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982 Oct;44(4):945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



