Abstract
Background
The world health organization (WHO) declared tuberculosis (TB) a global emergency, mainly affecting people in sub-Saharan Africa. However there is little data about the burden of TB among adolescents. We estimated the prevalence and incidence of TB and assessed factors associated with TB among adolescents aged 12–18 years in a rural population in Uganda in order to prepare the site for phase III clinical trials with novel TB vaccines among adolescents.
Methods
In a prospective cohort study, we recruited 5000 adolescents and followed them actively, every 6 months, for 1–2 years. Participants suspected of having TB were those who had any of; TB signs and symptoms, history of TB contact or a positive tuberculin skin test (TST) of ≥10 mm. Laboratory investigations included sputum smear microscopy and culture.
Results
Of the 5000 participants, eight culture confirmed cases of TB were found at baseline: a prevalence of 160/100,000 (95% confidence interval (CI), 69–315). There were 13 incident TB cases detected in an average of 1.1 person years: an incidence of 235/100,000 person years (95% CI, 125–402). None of the confirmed TB cases were HIV infected. Predictors for prevalent TB disease were: a history of TB contact and a cough ≥ 2 weeks at baseline and being out of school, while the only predictor for incident TB was a positive TST during follow-up.
Conclusion
The TB incidence among adolescents in this rural part of Uganda seemed too low for a phase III TB vaccine trial. However, the study site demonstrated capability to handle a large number of participants with minimal loss to follow-up and its suitability for future clinical trials. Improved contact tracing in TB program activities is likely to increase TB case detection among adolescents. Future studies should explore possible pockets of higher TB incidence in urban areas and among out of school youth.
Background
Tuberculosis (TB) is a global emergency causing high morbidity and mortality especially in sub-Saharan Africa [1]. The immune response against Mycobacterium tuberculosis (MTB) is less effective among adolescents [2]. Adolescents have peculiar clinical TB manifestations and may present without symptoms but are more likely to have cavitating disease compared to adults or children [3]. Vaccination against TB using Bacillus Calmette-Guerin (BCG) in neonates has led to a reduction in incidence of severe childhood TB and deaths including miliary TB and TB meningitis [4]. Despite BCG immunization, adult forms of TB continue to emerge implying the current vaccine has limited efficacy against adult TB and therefore a need for new TB vaccines with high protective efficacy [5-7]. The risk of developing TB following infection starts to rise from 12–19 years making it a suitable age group for new TB vaccines [8].
Diagnosed TB among adolescents in most countries is aggregated and reported together with adult TB cases, disaggregated as 0–14 and 15–25 years, with limited information specific to adolescents. Very few studies have investigated TB prevalence and incidence of TB among adolescents [9]. Uganda is ranked 16th among the 22 TB high burden countries globally, with estimated incidence rates of about 209 per 100,000 population per year for all forms of TB in 2010 [10]. The estimated prevalence was 193/100,000 population for all forms of TB in 2010. However, the country notified an incidence of 128/100,000 population, only 66% of the estimated incidence [1].
The objectives of the study were to estimate the prevalence and incidence of TB and assess factors associated with TB among adolescents aged 12–18 years in a rural population in Uganda in order to prepare the site for phase III clinical trials with novel TB vaccines among adolescents.
Methods
The study was conducted in the Makerere University School of Public Health Demographic Surveillance Site (DSS) in Iganga and Mayuge districts, located 120 km east of Kampala between September 2009 and September 2011. The study area is 80% rural and 20% peri-urban with nine health facilities offering free TB smear microscopy and treatment. The study area had a population of approximately 12,000 adolescents aged 12–18 years.
Participants aged 12–18 years who were resident in the study area for the previous three months were approached both in school and at home. Those who gave written assent after the parents/legal guardian had provided written informed consent were enrolled into the study. Participants who were planning to move away from the study area within one year were excluded.
Adolescents were stratified into 12–14 years, 15–16 years and 17–18 year age groups and conveniently sampled from schools and in the community with the sample size in each age stratum proportional to the size of the stratum population in the DSS. They were recruited consecutively until the sample size of 5000 was attained. Recruitment was mainly from rural areas and all had an equal chance of inclusion in the study. Given the World Health Organization (WHO) estimated incidence for 2007 of 330/100,000 population at time of study start (September 2009) [10] and 10-20% loss to follow-up; we expected to find among 5000 adolescents a cumulative TB incidence of 28 (0.56%) cases in two years, with a 95% CI of {26 (0.37%) - 51 (0.73%)}. These estimates were amended by WHO downwards in the later reports.
At enrolment, socio-demographic variables, relevant medical history including symptoms and signs suggestive of TB were collected. The participant’s weight and temperature were taken and a physical examination done. All participants had a tuberculin skin test (TST) 2 Tuberculin Units (Danish type from Staten’s Serum Institute) placed and read after 48–72 hours. Those with a negative result had a repeat TST done at one and two years of follow up. Participants were followed up every 6 months for 1–2 years and targeted history and physical examination were done. The study closed early and therefore those recruited later had a shorter duration of follow-up. Active TB suspicion was defined as participants found with any one of the following; TST induration of ≥10 mm, or at least one TB related symptom: cough ≥ 2 weeks, weight loss for ≥2 weeks, fever for ≥2 weeks, haemoptysis) or a history of TB contact since the last study follow up visit. Two sputum samples, a spot sample on day one and an early morning sample on day 2 were collected from all TB suspects and transported in cold boxes (temperature ≤8°C) to the reference laboratory within 24 hours. All collected data were entered onto a pre-coded case report form (CRF).
Laboratory analysis
Sputum samples were processed in the Makerere University mycobacteriology Biosafety Level III laboratory in Kampala. All sputum samples were subjected to fluorescence microscopy and culture after concentration using 1% sodium hydroxide (NAOH)/N-acetyl L-cysteine (NALC) and sodium citrate method [11]. Cultures were performed by both solid (Lowenstein-Jensen, (Becton and Dickson, Franklin Lakes, NJ USA)) and liquid Mycobacterium Growth Indicator Tube (MGIT) culture techniques and incubated for six to eight weeks. Species identification was done using Capilia TB Neo™ (TAUN, Numazu, Japan) assay for all Ziehl-Neelsen (ZN) positive cultures to exclude mycobacteria other than tuberculosis (MOTT) and all isolates of mycobacterium tuberculosis complex (MTBC) were subjected to Hain Genotype MTBC assay for speciation. Participants with at least two positive smears or at least one MTBC culture were defined as confirmed TB cases.
Ethical approval
Ethical approval was obtained from the Makerere University School of Public Health Higher Degrees Research and Ethics Committee, the Uganda National Council for Science and Technology and the Institutional Review Board of Institute of Tropical Medicine (ITM) Antwerp of Belgium.
Data analysis
Analysis was done using STATA statistical software (Release 11.0, Stata Corporation, College Station, Texas, United States of America). Primary analysis was done by calculating frequencies for key demographic variables and overall prevalence and incidence rates; with secondary analyses done to estimate stratum specific prevalence and incidence rates by age group, sex, education, schooling status, TST outcome, TB contact and cough. Person years were calculated by subtracting the day of entry into the study from the last day of contact with the participant divided by 365 days. For those diagnosed with TB, date of diagnosis was taken as date of last contact. For those who died, date of death was taken as last date of contact. 95% confidence intervals (CI) were calculated using Poisson distribution. Odds ratios (OR) were calculated as a measure of TB risk and hazard ratios were calculated for incident TB cases. In multivariate analysis we included variables that were significant in univariate analysis at the P < 0.01 level.
Results
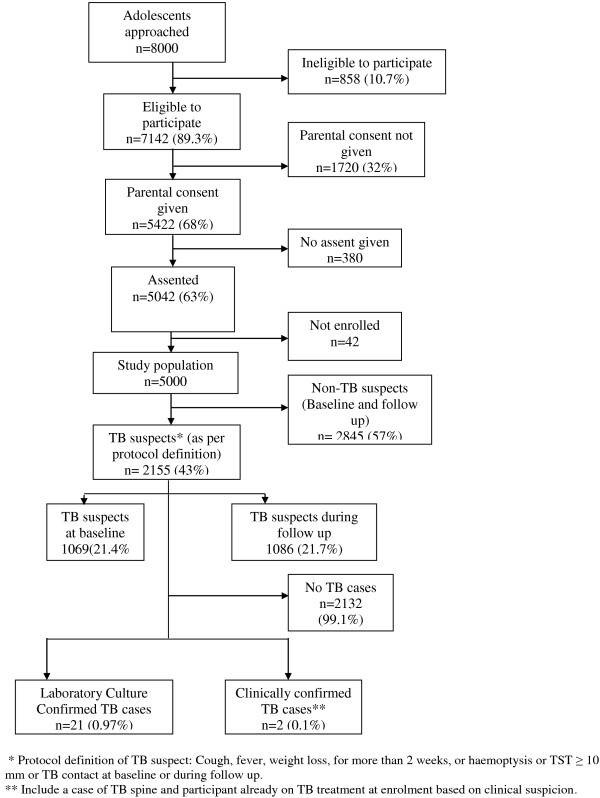
Among the DSS adolescents; 8000 were approached and 5422 (68%) parents agreed to have their children participate in the study and 5042 (63%) adolescents provided assent. Among the screened participants, 858 (10.7%) were ineligible to participate mainly because they were planning to move away from the study area within one year. A total of 5000 adolescents were consecutively enrolled over a 12 month period. Of these, 1069(21.4%) were TB suspects at baseline and 1086 (21.7%) during follow-up. The most common reasons were, a positive TST 1307 (54.7%), symptoms suggestive of TB 735 (30.8%) and a history of TB contact 441 (18.5%), Figure 1.
Figure 1.
Study profile and TB cases among adolescents in a rural cohort in eastern Uganda.
Description of study participants
Study participants were more often male (52%); unmarried (99%), aged 12–14 years (47%), Basoga (82%) the predominant tribe in the area and majority were in school (94%), Table 1. A total of 12,124 follow up visits were planned of which 10,769 (89%) were conducted. The follow up period was at least 12 months and maximum 24 months with an overall loss to follow up of 10.5% and total person years of follow up was 5527 with mean follow up time of 1.1 person years (SD 0.3). Four participants died of: juvenile diabetes, tetanus, sickle cell anemia and a possible cardiac problem and none was due to TB. A total of 143/5000 (3%) participants were never seen again after enrollment.
Table 1.
Baseline characteristics of adolescents enrolled in the Iganga/Mayuge TB Cohort Study, 2009
| Characteristic | Study population | Percentage |
|---|---|---|
|
Total |
5000 |
100 |
|
Age (Years) |
|
|
| 12-14 |
2339 |
47 |
| 15-16 |
1482 |
29 |
| 17-18 |
1179 |
24 |
|
Sex |
|
|
| Male |
2589 |
52 |
|
Marital status |
|
|
| Married |
33 |
0.7 |
|
Level of education |
|
|
| >7 years |
696 |
13.9 |
| ≤ 7 years |
4250 |
85 |
| Never attended school |
14 |
0.3 |
| Unknown |
40 |
0.8 |
|
Enrolment status |
|
|
| In school |
4678 |
94 |
| Out of school |
322 |
6 |
|
Tribe |
|
|
| Basoga |
4089 |
81.8 |
| Others | 911 | 18.2 |
TB prevalence and incidence
A total of nine TB cases out of 5000 adolescents were detected at baseline of which eight cases were confirmed microbiologically, Table 2. Of these eight, seven were smear (at least 1) and culture positive, and one was smear negative but culture positive. One of the TB cases was identified while on TB treatment (smear and culture positive). This resulted in a crude prevalence of culture confirmed TB of 160/100,000 (95% CI, 69–315). Of these eight cases, four had only TB symptoms, three had only a history of TB contact and one had both symptoms and a TB contact. One participant was clinically diagnosed with TB in the health facility. This participant was HIV infected, had TB symptoms and history of TB contact, but sputum samples were negative on smear microscopy and culture, Table 2. There were no prevalent TB cases among adolescents with a negative TST.
Table 2.
Clinical and laboratory characteristics of prevalent TB cases (n = 9)
| Case number | TB symptoms | TB contact | TST ≥10 mm | CXR abnormalities present | Smear positive | Culture positive | Case determination criteria |
|---|---|---|---|---|---|---|---|
| 1. |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Smear and culture |
| 2. |
Yes |
No |
Yes |
No |
Yes |
Yes |
Smear and culture |
| 3. |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Smear and culture |
| 4. |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Smear and culture |
| 5. |
No |
Yes |
Yes |
No |
Yes |
Yes |
Smear and culture |
| 6. |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Smear and culture |
| 7. |
Yes |
No |
Yes |
No |
Yes |
Yes |
Culture only |
| 8. |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Smear and culture |
| 9. *** |
Yes |
Yes |
Yes |
Yes |
No |
No |
Clinical diagnosis |
| Total | 6 | 5 | 9 | 6 | 8 | 8 |
*** HIV positive participant.
Abbreviation: Key: TB Tuberculosis, TST Tuberculin Skin Test, CXR Chest X-Ray.
During follow up, 14 cases of TB were detected of which 13 were culture confirmed (all smear negative) and one was clinically diagnosed with TB. The average duration of follow up was 1.1 years. The incidence of bacteriologically confirmed TB was 235/100,000 person years (95% CI, 125–402). All TB cases were HIV negative. Nine (64%) reported no symptoms and none had CXR abnormalities (CXR was done for only nine cases after start of treatment for clinical care purposes), Table 3.
Table 3.
Clinical and laboratory characteristics of the adolescent incident TB cases (n = 14)
| Case number | Visit detected (months) | TB symptoms | TB contact | TST ≥10 mm | CXR abnormalities | Smear positive | Culture positive | Case determination criteria1 |
|---|---|---|---|---|---|---|---|---|
| 1 |
12 |
Yes |
No |
Yes |
Not done |
No |
Yes |
Culture |
| 2 |
6 |
Yes |
No |
Yes |
Yes** |
No |
No |
Clinical diagnosis▲ |
| 3 |
12 |
No |
No |
Yes |
No |
No |
Yes |
Culture |
| 4 |
12 |
No |
No |
Yes |
No |
No |
Yes |
Culture |
| 5 |
12 |
No |
Yes |
Yes |
No |
No |
Yes |
Culture |
| 6 |
12 |
No |
Yes |
Yes |
No |
No |
Yes |
Culture |
| 7 |
12 |
No |
No |
Yes |
No |
No |
Yes |
Culture |
| 8 |
12 |
No |
No |
Yes |
Not done |
No |
Yes |
Culture |
| 9 |
12 |
No |
No |
Yes |
Not done |
No |
Yes |
Culture |
| 10 |
12 |
Yes |
No |
Yes |
Not done |
No |
Yes |
Culture |
| 11 |
24 |
No |
No |
Yes |
No |
No |
Yes |
Culture |
| 12 |
24 |
No |
No |
Yes |
No |
No |
Yes |
Culture |
| 13 |
12 |
Yes |
No |
Yes |
No |
No |
Yes |
Culture |
| 14 |
12 |
Yes |
No |
Yes |
Not done |
No |
Yes |
Culture |
| Total | 5 | 2 | 14 | None | None | 13 | 14 |
▲POTTS disease.
1 Culture - all smears were negative.
** Necrosis and collapse of thoracic vertebrae 10–12.
Abbreviation: Key: TB Tuberculosis, TST Tuberculin Skin Test, CXR Chest X-Ray.
Predictors for prevalent TB
At baseline there was no significant difference in adolescents with bacteriologically confirmed TB by sex, age groups, and levels of education. Participants who were out of school were seven times more likely to have TB than their in-school counter parts (AOR 6.84; 01.28-36.03). Participants who had contact with a TB patient were sixteen times more likely to have TB compared to those with no TB contact (AOR 15.67; 3.57 -68.77). Those who had had cough for ≥14 days were five times more likely to have TB than those who had had cough for ≤14 days or no cough at all (AOR 5.15; 1.20-22.10), Tables 4 and 5.
Table 4.
Univariate predictors of prevalent TB
| Background characteristic | TB cases n = 8 | Population N = 5000 | Prevalence/100,000 | P-value (Chi-square test) |
|---|---|---|---|---|
|
Gender |
|
|
|
|
| Male |
3 |
2589 |
116 |
0.419 |
| Female |
5 |
2411 |
207 |
|
|
Age (yrs) |
|
|
|
|
| 12-14 |
4 |
2339 |
171 |
|
| 15-16 |
3 |
1482 |
202 |
0.74 |
| 17-18 |
1 |
1179 |
85 |
|
|
Schooling status |
|
|
|
|
| Out of school |
2 |
322 |
621 |
0.032 |
| In school |
6 |
4678 |
128 |
|
|
Level of education |
|
|
|
|
| > 7 years |
1 |
696 |
144 |
|
| ≤ 7 years |
7 |
4264 |
164 |
0.961 |
| Unknown |
0 |
40 |
0 |
|
|
Baseline TST |
|
|
|
|
| Positive (≥10 mm) |
8 |
802 |
998 |
|
| Negative (<10 mm) |
0 |
4179 |
0 |
<0.001 |
| Not done / Not Read |
0 |
19 |
0 |
|
|
TB contact |
|
|
|
|
| Yes |
4 |
237 |
1409 |
0.115 |
| No |
4 |
4763 |
76 |
|
|
Cough duration (ever) |
|
|
|
|
| Cough <14 days |
3 |
2548 |
118 |
|
| No cough |
0 |
1827 |
0 |
<0.001 |
| Cough ≥14 days | 5 | 625 | 800 |
Abbreviation: Key: TST Tuberculin Skin test.
Table 5.
Associations between patient characteristics and prevalence of TB
| Background characteristic | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
|
Gender |
|
|
|
|
| Male |
Reference |
|
Reference |
|
| Female |
1.79 |
0.43 - 7.50 |
1.74 |
0.42 - 7.30 |
|
Age (yrs) |
|
|
|
|
| 12-14 |
Reference |
|
Reference |
|
| 15-16 |
1.18 |
0.27 - 5.30 |
0.95 |
0.12 - 7.47 |
| 17-18 |
0.5 |
0.06 - 4.34 |
0.27 |
0.02 - 3.72 |
|
Schooling status |
|
|
|
|
| Out of school |
4.87 |
0.98 - 24.21 |
6.8 |
1.28 - 36.03 |
| In school |
Reference |
|
Reference |
|
|
Level of education |
|
|
|
|
| > 7 years |
Reference |
|
Reference |
|
| ≤ 7 years |
1.14 |
0.14 - 9.30 |
0.63 |
0.03 - 13.23 |
| Unknown |
-- |
|
-- |
|
|
TB contact (baseline) |
|
|
|
|
| Yes |
20.42 |
5.08 - 82.18 |
15.67 |
3.57 - 68.77 |
| No |
Reference |
|
Reference |
|
|
Cough duration (ever) |
|
|
|
|
| Cough < 14 days |
Reference |
|
Reference |
|
| No cough |
-- |
|
-- |
|
| Cough ≥ 14 days | 6.84 | 1.63 - 28.70 | 5.15 | 1.20 - 22.10 |
Abbreviation: Key: 95% CI 95% Confidence Interval.
Predictors for incident TB
On multivariate analysis, bacteriologically confirmed TB incidence during follow up was significantly higher among participants with a positive TST ≥10 mm than their TST negative counter parts ( adjusted hazard ratio 58.08; 95% CI 7.2-435). The incidence of TB seemed to increase with age though this trend was not significant, p-value = 0.093. Incidence was similar among those with ≥ seven years of schooling compared to those with lower education level (Adjusted hazard ratio 0.9; 0.20, 4.06). Sex specific incidence rates showed males being three times more likely to develop TB than females though this difference was also not statistically significant (Adjusted hazard ratio 0.32: 0.09-1.17). TB incidence was higher among those who had TB contact and those who had coughed for ≥14 days, but this was not significant. Those with incident TB had no prior diagnosis of TB and were all school-going participants, Tables 6 and 7.
Table 6.
Univariate predictors of incident TB
| Determinant | Persons | Pyrs | Cases | Incidence (per 100,000 pyrs) | P-value (chi-square test) |
|---|---|---|---|---|---|
|
Total |
5000 |
5527 |
13 |
235 |
- |
|
Follow up TST outcome * |
|
|
|
|
|
| Negative < 10 mm |
2912 |
3323 |
1 |
30 |
|
| Positive ≥ 10 mm |
582 |
679 |
11 |
1620 |
<0.001 |
| Not done/not read** |
1506 |
1525 |
1 |
66 |
|
|
Baseline TST outcome |
|
|
|
|
|
| Negative < 10 mm |
4179 |
4574 |
12 |
262 |
<0.001 |
| Positive ≥ 10 mm |
802 |
890 |
1 |
112 |
|
|
Prior diagnosis TB |
|
|
|
|
|
| No |
4990 |
5517 |
13 |
236 |
- |
| Yes |
10 |
11 |
0 |
0 |
|
|
Schooling status |
|
|
|
|
|
| In school |
4678 |
5193 |
13 |
250 |
- |
| Out of school |
322 |
335 |
0 |
0 |
|
|
Age group (years) |
|
|
|
|
|
| 12-14 |
2339 |
2778 |
4 |
144 |
|
| 15-16 |
1482 |
1583 |
4 |
253 |
0.093 |
| 17-18 |
1179 |
1167 |
5 |
429 |
|
|
Sex |
|
|
|
|
|
| Male |
2589 |
2898 |
10 |
345 |
|
| Female |
2411 |
2630 |
3 |
114 |
0.028 |
|
Education level |
|
|
|
|
|
| > 7 years |
696 |
733 |
2 |
273 |
|
| None |
40 |
37 |
0 |
0 |
0.752 |
| ≤ 7 years |
4264 |
4757 |
11 |
231 |
|
|
TB Contact during follow-up |
|
|
|
|
|
| No |
4732 |
5196 |
11 |
212 |
0.019 |
| Yes |
268 |
331 |
2 |
604 |
|
|
Cough during follow-up |
|
|
|
|
|
| <14 days |
2250 |
2602 |
5 |
192 |
|
| None |
2102 |
2307 |
6 |
260 |
0.917 |
| ≥14 days | 500 | 581 | 2 | 344 |
Abbreviation: Key: Pyrs Person years, TST Tuberculin Skin Test.
Note:
* One individual with TB during follow up had a positive TST of ≥10 mm at baseline;
**Those with a positive TST at baseline were not given TST again at 1 year and 2 years of follow up.
Table 7.
Associations between patient characteristics and incidence of TB
| Determinant | Unadjusted hazard ratio | 95% CI | Adjusted hazard ratio | 95% CI |
|---|---|---|---|---|
|
Follow up TST outcome * |
|
|
|
|
| Negative < 10 mm |
Reference |
|
Reference |
|
| Positive ≥ 10 mm |
61.28 |
7.95 - 472.38 |
58.08 |
7.23 - 435.21 |
|
Baseline TST outcome |
|
|
|
|
| Negative < 10 mm |
Reference |
|
Reference |
|
| Positive ≥ 10 mm** |
1.30 |
0.37 - 4.63 |
4.70 |
0.56 - 39.41 |
|
Age group (years) |
|
|
|
|
| 12-14 |
Reference |
|
Reference |
|
| 15-16 |
1.58 |
0.39 - 6.33 |
1.58 |
0.39 - 6.33 |
| 17-18 |
3.49 |
1.02 - 11.94 |
2.49 |
0.67 - 9.28 |
|
Sex |
|
|
|
|
| Male |
Reference |
|
Reference |
|
| Female |
0.27 |
0.08 – 0.95 |
0.32 |
0.09 - 1.17 |
|
Education level |
|
|
|
|
| > 7 years |
Reference |
|
Reference |
|
| None |
|
|
|
|
| ≤ 7 years |
0.65 |
0.18 - 2.32 |
0.9 |
0.20 - 4.06 |
|
TB contact during follow-up |
|
|
|
|
| No |
Reference |
|
|
|
| Yes |
2.73 |
0.61 - 12.16 |
|
|
|
Cough during follow-up |
|
|
|
|
| <14 days |
Reference |
|
|
|
| None |
0.71 |
0.14 - 3.54 |
|
|
| ≥14 days | 0.78 | 0.16 - 3.75 |
Key: 95% CI = 95% Confidence Interval.
Note:
* One individual with TB during follow up had a positive TST of ≥10 mm at baseline;
**Those with a positive TST at baseline were not given TST again at 1 year and 2 years of follow up.
Discussion
Among adolescents 12–18 years of age in a rural setting in Uganda, we found a prevalence of TB of 160/100,000 and incidence of 235/100,000 person years. The risk factors for prevalent TB were TB contact, cough of more than 14 days and being out of school whereas for incident TB; the only risk factor was a positive TST ≥ 10 mm.
All incident cases were detected at the scheduled 6 monthly visits and none through the health care system. This is probably due to active case finding leading to early detection of cases before they developed symptoms and presented to the health facilities [3]. All incident cases were culture positive but smear negative. This could have been due to sub-clinical colonization with mycobacteria. Although confidence intervals were wide, the TB prevalence of 160/100,000 in our sample was slightly lower than the estimated TB prevalence for all ages in Uganda (193/100,000). On the other hand the TB incidence of 235/100,000 person years was slightly higher than estimated incidence for all ages in Uganda (209/100,000) [1]. The observed differences in rates may be due to low HIV prevalence in this rural population, active TB case finding and adolescents having a lower TB incidence than the general population [1].
Furthermore, our study sample was mainly from a rural population, prevalence and incidence may have been much higher if an urban/peri-urban population was sampled. One of the few studies on TB prevalence in Uganda done in a slum area in Kampala city among chronic coughers ≥ 15 years reported a TB prevalence of 4,500/100,000 [12]. Future studies should target adolescents in crowded settlements in urban centers. Studies done in adult populations with high HIV prevalence in Africa have reported higher TB prevalence than our study [13-17].
We did not use CXR to screen TB suspects therefore we may have underestimated the TB prevalence and incidence in our population. In our study 4265 (85.3%) of the participants had no TB symptoms. Other prevalence surveys in the general population (usually among those aged over 15 years), that used CXR and/or symptoms to screen for TB have shown that 3852 (23%) of participants in Western Kenya [13] and 2972 (3.4%) in Vietnam [18] had CXR abnormalities suggestive of TB.
Risk factors for TB have been shown previously by others [9,13-15,17]. In our study, risk factors differed slightly between prevalent and incident cases. The high prevalence of TB among adolescents with TB contacts highlights the importance of contact tracing for increased TB case detection mainly in rural areas.
The strength of our study is the relatively large sample size and the fairly low loss to follow up rate (10.5%). This high cohort retention is good for potential future vaccine trials. A weakness of our study however was a relatively short follow up duration of 1.1 years. It is likely that a longer follow up would have yielded more TB cases. Furthermore we did not use chest X-ray as a screening tool. This may have resulted in underestimation of prevalent TB cases as previous studies documented an increase in number of TB cases when chest X-ray results were considered [19-24].
The baseline prevalence may have ‘cleared’ some incident cases, leading to an underestimation of the incidence. Moreover, we documented a high refusal rate from parents and adolescents. As we did not document possible reasons for refusal, we recommend future studies to explore this.
Conclusion
The TB incidence among adolescents in this rural part of Uganda seemed too low for a phase III TB vaccine trial. A positive tuberculin test ≥10 mm, a history of TB contact and a cough ≥ 14 days are the major predictors for TB disease. However, the study demonstrated the capability of the study site to handle a large number of participants with minimal loss to follow-up which is important for future clinical trials. Improved contact tracing in TB program activities is likely to increase TB case detection among adolescents. Future studies should explore possible pockets of higher TB incidence in urban areas and among out of school youth.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JW: analyzed the data and wrote the paper, WS, MJ: managed the laboratory experiments, SV, AW, RC, WS, PM, and HMK: participated in critical review and manuscript writing, PM and HMK: conceived the idea. All authors participated in protocol development and read and approved the final version of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
James Waako, Email: waakojames@yahoo.com.
Suzanne Verver, Email: ververs@kncvtbc.nl.
Anne Wajja, Email: wajja2002@yahoo.com.
Willy Ssengooba, Email: willyssengooba@gmail.com.
Moses L Joloba, Email: moses.joloba@case.edu.
Robert Colebunders, Email: bcoleb@itg.be.
Philippa Musoke, Email: pmusoke@mujhu.org.
Harriet Mayanja-Kizza, Email: hmk@chs.mak.ac.ug.
Acknowledgements
The authors thank the Uganda National TB and Leprosy Program, the various health institutions, the local Government authorities, Iganga/Mayuge Demographic Surveillance Site of the Makerere University School of Public Health for their collaboration. We thank John Baptist Bwanika and Kalungu Michael for helping in statistical data analysis. Our gratitude to the Infectious Diseases Institute (IDI) Makerere, the sponsor of the study, the participants for taking part in the study, all individuals living in the study area and the study staff.
Funding
This study was funded by the European and Developing Countries Clinical Trials Partnership (EDCTP) and one time support for purchasing laboratory reagents from Directorate General for Development Cooperation (DGDC) through the Flemish Interuniversity council (VLIR-UOS).
References
- World Health Organization. Global tuberculosis control. Geneva Switzerland: WHO Report 2011; 2011. whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf. [Google Scholar]
- Nemir RL. Perspectives in adolescent tuberculosis: three decades of experience. Pediatrics. 1986;78(3):399–405. [PubMed] [Google Scholar]
- Kam A, Ford-Jones L, Malloy P, Khan K, Kitai I. Active tuberculosis among adolescents in Toronto, Canada: clinical features and delays in diagnosis. Pediatr Infect Dis J. 2007;26(4):355–356. doi: 10.1097/01.inf.0000258700.86040.b6. [DOI] [PubMed] [Google Scholar]
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- WHO. Department of vaccines and Biologicals. Issues relating to the use of BCG in immunization programmes. WHO/V&B/99.23. Geneva Switzerland; 1999. http://www.who.int/vaccines-documents/DocsPDF99/www9943.pdf. Accessed on Aug 2012. [Google Scholar]
- Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96(1 Pt 1):29–35. [PubMed] [Google Scholar]
- Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Arch Dis Child. 2011;96(9):851–856. doi: 10.1136/adc.2011.214494. [DOI] [PubMed] [Google Scholar]
- Mandalakas AM, Starke JR. Current concepts of childhood tuberculosis. Semin Pediatr Infect Dis. 2005;16(2):93–104. doi: 10.1053/j.spid.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, Ehrlich R, Hanekom WA, Hussey GD. The tuberculin skin test versus QuantiFERON TB Gold(R) in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6(3):17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global tuberculosis control. Geneva Switzerland; 2009. whqlibdoc.who.int/publications/2009/9789241563802_eng_doc.pdf. [Google Scholar]
- Strong BE, Kubica GP. Isolation and identification of Mycobacterium tuberculosis: a guide for the level II laboratory,” Centers for Disease Control. HHS Publication No. (CDC); 1981. pp. 81–8390. http://books.google.co.ug/books?id=VurMQEACAAJ. [Google Scholar]
- Sekandi JN, Neuhauser D, Smyth K, Whalen CC. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis. 2009;13(4):508–513. [PMC free article] [PubMed] [Google Scholar]
- Van't Hoog AH, Borgdorff MW, DeCock KM, Marston BJ, Muchiri BG, Odeny LO, Agaya JA, Meme HK, Githui WA, Laserson KF. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183(9):1245–1253. doi: 10.1164/rccm.201008-1269OC. [DOI] [PubMed] [Google Scholar]
- Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, Churchyard G, Butterworth A, Mason P. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 2007;4(1):e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayles H, Schaap A, Nota A, Sismanidis C, Tembwe R, De Haas P, Muyoyeta M, Beyers N. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One. 2009;4(5):e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelkoop K, Bekker LG, Myer L, Whitelaw A, Grant A, Kaplan G, McIntyre J, Wood R. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African township. Am J Respir Crit Care Med. pp. 1080–1085. [DOI] [PMC free article] [PubMed]
- Corbett EL, Bandason T, Cheung YB, Makamure B, Dauya E, Munyati SS, Churchyard GJ, Williams BG, Butterworth AE, Mungofa S. et al. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis. 2009;13(10):1231–1237. [PMC free article] [PubMed] [Google Scholar]
- Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ. 2010;88(4):273–280. doi: 10.2471/BLT.09.067801. http://www.who.int/bulletin/volumes/88/4/09-067801/en/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Kolappan C, Gopi PG, Chakraborty AK, Sehgal SC. Tuberculosis situation among tribal population of Car Nicobar, India, 15 years after intensive tuberculosis control project and implementation of a national tuberculosis programme. Bull World Health Organ. 2004;82(11):836–843. [PMC free article] [PubMed] [Google Scholar]
- Tupasi TE, Radhakrishna S, Rivera AB, Pascual ML, Quelapio MI, Co VM, Villa ML, Beltran G, Legaspi JD, Mangubat NV. et al. The 1997 Nationwide Tuberculosis Prevalence Survey in the Philippines. Int J Tuberc Lung Dis. 1999;3(6):471–477. [PubMed] [Google Scholar]
- China Tuberculosis Control Collaboration. The effect of tuberculosis control in China. Lancet. 2004;364:417–422. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- Gopi PGSR, Radhakrishna S, Kolappan C, Sadacharam K, Devi TS, Frieden TR, Narayanan PR. A baseline survey of the prevalence of tuberculosis in a community in south India at the commencement of a DOTS programme. Int J Tuberc Lung Dis. 2003;7(12):1154–1162. [PubMed] [Google Scholar]
- Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2(1):27–36. [PubMed] [Google Scholar]
- Datta M, Radhamani MP, Sadacharam K, Selvaraj R, Rao DL, Rao RS, Gopalan BN, Prabhakar R. Survey for tuberculosis in a tribal population in North Arcot District. Int J Tuberc Lung Dis. 2001;5(3):240–249. [PubMed] [Google Scholar]