Abstract
Chronic methamphetamine (MA) use is commonly associated with neural injury and neurocognitive deficits. We examined the nature and correlates of self-reported neurobehavioral symptoms (i.e., apathy, disinihibition, and executive dysfunction) in 73 individuals with histories of MA dependence (MA+) and 85 comparison participants with comparable demographics and risk histories. MA+ individuals endorsed significantly more severe neurobehavioral symptoms on the Frontal Systems Behavioral Scale (FrSBe), especially disinhibition and executive dysfunction. Elevations in neurobehavioral symptoms were independent of common comorbidities, including hepatitis C infection, Attention-Deficit/Hyperactivity Disorder, mood disorders, and other substance use factors. Notably, the severity of neurobehavioral symptoms were uniquely associated with self-reported decrements in instrumental activities of daily living in the MA dependent sample. Findings indicate that chronic MA users may experience elevated neurobehavioral symptoms of disinhibition and executive dysfunction, potentially increasing their risk of functional declines.
Keywords: Methamphetamine, Substance abuse, Executive dysfunction, Behavioral disinhibition, Neuropsychological assessment, Activities of daily living
Methamphetamine (MA) is a potent, highly addictive and neurotoxic psychostimulant with well-documented adverse neural and neuropsychological effects {1}, as well as risk of functional declines {2}. Relative to other neurotoxic substances, MA may be particularly potent due to its high lipid solubility and rapid diffusion across the blood-brain barrier {3}. As a result, MA-associated CNS effects can include a variety of metabolic, structural, and functional brain changes that may impact cognitive functioning. Although some evidence suggests that partial neural and cognitive recovery may occur with stable abstinence {4, 5}, alterations and impairments may nevertheless persist long after MA use is discontinued {6, 7}. Long-term effects of MA may be especially problematic in frontostriatal circuits, where potential consequences include neural injury in the striatum {4} and prefrontal cortex {8}. It has been hypothesized that these neural changes may affect the modulatory functions of the frontostriatal and limbic structures, as well as underlie the neurocognitive deficits observed in chronic MA users {9}.
Approximately 40% of chronic MA users exhibit neurocognitive deficits {10}, with generally moderate impairments observed in the domains of episodic memory, motor skills, language, information processing speed, visuospatial functioning, and executive functions {1}. Within the domain of executive functions, MA has been associated with impulsivity, disinhibition, reduced ability to suppress irrelevant information, risky decision-making, and increased distractibility {7, 11,12}. Neurocognitive impairments, and particularly executive dysfunction, may increase the risk of everyday functioning problems and engagement in high-risk behaviors, which are common among MA users. Self-report measures indicate lower abilities and more disruptions in everyday activities including communication, work, and recreation {13}. These data are corroborated by significant impairments found on performance-based tests, including tests of medication and financial management, arranging travel, and communication skills {2}. MA use has also been associated with lack of health insurance, being on public assistance, and disproportionate representation in burn and trauma units {14}. MA use is negatively correlated with employment stability, and MA users are less likely to be employed full-time {15}. Chronic MA users report elevated rates of psychosocial problems, including domestic conflict {16} and legal complications {17}. Of course, there are long-standing controversies regarding the construct validity of such self-reported versus more objective (e.g., performance-based or observational) assessments of everyday living problems such as these {18}. While self-report approaches are limited by potential bias and current mood, they may also be more sensitive to gross functional difficulties. In addition, self-report measures may more flexibly identify declines specific to the demands of each individual’s life {19}. As such, assessing self-reported neurobehavioral symptoms may nonetheless yield clinically meaningful and incrementally important information with limited investment of time and minimal examiner burden.
To our knowledge, the prevalence and correlates of neurobehavioral disturbances in chronic MA users has not yet been fully established. Here we define neurobehavioral disturbances as self-reported behavioral symptoms that have reliably been associated with brain injury and neuropsychiatric syndromes, particularly those involving dysregulation of frontostriatal systems {20}. Clinicians frequently note behavioral disturbances in MA, and broadly accept that this impulsivity, disinhibition, and predisposition toward stimulus-driven decision-making may reflect the observed neuropsychological deficits and frontal systems injury sustained by MA users. Importantly, studies have found these types of behaviors to be independently related to adverse psychosocial, psychological, substance-related and sexual risk factors. For example, higher levels of impulsivity in MA users have been associated with elevated rates of psychiatric diagnoses, higher rates of unemployment, high risk sexual encounters, and a greater tendency to use MA to cope with mood disturbances {21, 22}. Despite the suggestion that these neurobehavioral symptoms are uniquely associated with important outcomes, the nature and magnitude of neurobehavioral disturbances and their effects on everyday functioning have not been extensively studied in this population.
Though some existing studies {23, 24, 25} have investigated neurobehavioral symptoms in substance-using populations, these designs have included heterogeneous groups of polysubstance abusers rather than examining substance-specific risks (e.g., MA dependence). Given the severity of the aforementioned neural, cognitive, and functional effects in MA relative to other substances, the primary aim of the current study is to examine the presence and severity of neurobehavioral symptoms (e.g., apathy, disinhibition, and executive dysfunction) in individuals with a history of MA dependence using the Frontal Systems Behavior Scale (FrSBe) {20}. The FrSBe has been used to quantify significant neurobehavioral sequelae in other conditions impacting similar brain regions and neural pathways (e.g., Parkinson’s disease {26}). We also sought to explore the potential risks that may be associated with elevated neurobehavioral symptoms in MA, including neurocognitive and functional impairment. Neurobehavioral symptoms themselves may be an important indicator of an individual’s functioning, and as in other populations (e.g., dementia) {27} could predict everyday functioning outcomes over and above any observed cognitive deficits. By clarifying the nature and severity of behavioral disturbances in MA users, clinicians may be able to better identify those at risk for functional impairments and better anticipate and account for difficulties complying with intervention programs.
Method
Participants
A total of 158 eligible participants were included in this study. Participants in the MA+ group met lifetime diagnostic criteria for MA dependence via semi-structured diagnostic interview (Composite International Diagnostic Interview; CIDI version 2.1) {28} and met criteria for MA abuse at least within the last 18 months (n = 73). Participants in the MA− comparison group (n = 85) had never met criteria for MA dependence and reported no prior use of MA, but had been recruited to match the MA+ group on demographic and other substance-related risk factors (e.g., depression) wherever possible. A minimum of 10 days of abstinence from MA was required prior to testing, and a urine toxicology screen confirmed that participants had abstained from use of all illicit substances, except marijuana. Marijuana is detectable up to 30 days after last use, and due to the high comorbidity of marijuana abuse and dependence in MA users, was not considered exclusionary for study participation in either group. Substance-related exclusion criteria included meeting Diagnostic and Statistical Manual-IV (DSM-IV) {29} criteria for current (i.e., within 30 days) abuse or dependence of non-MA substances. Individuals with histories of alcohol dependence within one year of evaluation, or other substance use dependence within five years of evaluation were also excluded. Potential participants were excluded if they reported a past head injury with a loss of consciousness greater than 30 minutes, HIV infection, a history of neurological condition (e.g., seizure disorder, stroke) or psychiatric illness (e.g., mental retardation or schizophrenia spectrum diagnoses) affecting cognitive functioning. All participants in the current study were HIV seronegative, as determined by enzyme-linked immunosorbent assay (ELISA). Given the prevalence of hepatitis C virus (HCV) in substance-abusing populations, HCV infected individuals were included in the analysis. HCV serostatus was determined using standard clinical antibody detection, and HCV RNA was measured in serum using real-time polymerase chain reaction (NGI SuperQuant; National Genetics Institute, Los Angeles, CA, USA; nominal detection limit of 100 IU/mL).
The MA+ and MA− groups were comparable on demographic factors (ps > 0.10; see Table 1). They did not significantly differ on HCV serostatus or lifetime rates of major depressive disorder. As might be expected, the MA group had higher lifetime rates of alcohol, cannabis, and cocaine dependence (all ps < .05), but did not significantly differ on histories of any other substances (p > .10). Though the MA+ group had a significantly higher proportion of individuals diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD; p < .05), differences between the proportions of individuals meeting criteria for Antisocial Personality Disorder (ASPD) approached significance (p > .07).
Table 1.
Demographic and Psychiatric Characteristics of Study Participants
| Variable | MA− (n = 85) | MA+ (n = 73) | p |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 43.6 (12.2) | 41.5 (8.20) | 0.21 |
| Education (years) | 12.7 (1.8) | 12.4 (1.9) | 0.29 |
| Sex (% men) | 76.5 | 86.3 | 0.12 |
| Ethnicity (% Caucasian) | 52.9 | 63.0 | 0.28 |
| Estimated verbal IQ a | 47.8 (8.6) | 47.9 (7.9) | 0.89 |
| Hepatitis C Infection (% seropositive) | 19.7 | 28.6 | 0.23 |
| Psychiatric Characteristics b | |||
| Major Depressive Disorder (%) | |||
| Current | 4.6 | 11.6 | 0.13 |
| Lifetime | 28.8 | 39.1 | 0.20 |
| ADHD | 1.2 | 9.7 | 0.02 |
| ASPD | 9.2 | 20.1 | 0.07 |
| Methamphetamine Use Characteristics c | |||
| Last use (days) | -- | 152.1 (150.5) | -- |
| Cumulative duration of use (days of use) | -- | 3816.3 (3003.9) | -- |
| Cumulative quantity of use d | -- | 4836.0 (7041.0) | -- |
| Onset age (LT dependence) | -- | 25.9 (8.4) | -- |
| Recency age (LT dependence) | -- | 39.5 (8.7) | -- |
| Age of first use (years) | -- | 21.8 (7.6) | -- |
| Other Substance Use Disorders e | |||
| Cannabis dependence | 4.7 | 19.2 | 0.004* |
| Opioid dependence | 4.7 | 4.1 | 0.86 |
| Cocaine dependence | 9.4 | 21.9 | 0.03* |
| Alcohol dependence | 14.1 | 32.9 | 0.005* |
| Other substance dependence f | 0.0 | 1.8 | 0.97 |
Note.
Based on the WRAT-3 Reading Standard Score;
p-values based on Fisher’s Exact Test;
quantity of MA used presented in grams;
lifetime diagnoses;
other substance dependence = hallucinogens and sedatives.
Materials and Procedure
Psychiatric and Substance Abuse Factors
Table 1 presents the psychiatric and substance use characteristics of the two study groups. MA use characteristics (e.g., duration and recency) were obtained via a semi-structured timeline follow-back interview {10}. Trained interviewers administered the CIDI {28}, which is a structured computer-assisted interview that provides lifetime and current substance use and psychiatric (e.g., Major Depressive Disorder) diagnoses according to DSM-IV criteria. ASPD was diagnosed using the SCID {30}, and ADHD diagnoses were determined using the Diagnostic Interview Schedule.
Neurobehavioral Symptoms
As part of their evaluation, all participants completed the self-report version of the Frontal Systems Behavior Scale (FrSBe) {20}, a 46-item self- or other-report behavior rating scale that provides quantitative measurement of behavioral disturbances related to damaged frontal systems. The FrSBe yields a total score in addition to three subscale scores: apathy, disinhibition, and executive dysfunction. Behaviors for apathy (e.g., “sit around doing nothing;” “show little emotion, am unconcerned and unresponsive”), dysregulation (e.g., “laugh or cry too easily;” “talk out of turn; interrupt others in conversation”), and executive dysfunction (e.g., cannot do two things at once [for example, talk and prepare a meal];” “show poor judgment, poor problem solver”) are rated on a 6-point Likert-type scale between 1 (“almost never”) and 5 (“almost always”), where higher scores correspond to more abnormal behavior. Due to the inclusion of retrospective ratings as well as current ratings, the FrSBe also enables the comparison of pre- and post-injury responses. This feature is frequently utilized in patients with acute symptom onset (e.g., following head injury or a surgical intervention), but the current study included only current ratings due to the diffuse nature and insidious onset of MA-related pathology. This practice is consistent with a number of other investigations in which the FrSBe was used to assess behavioral symptoms of neurological conditions that present more gradually (e.g., HCV) {31}. Total and subscale raw scores were converted into demographically adjusted T-scores for analysis. As indicated by the measure’s authors, a T-score cut point of 65 and higher was used as an indicator of clinically elevated behavioral symptoms. FrSBe T-scores for the Total score and three subscales (i.e., Apathy, Disinhibition, Executive Dysfunction) were the primary dependent variables of interest.
Dependence on Activities of Daily Living
Participants also completed a modified version of the Lawton and Brody Activities of Daily Living Scale {32}. This instrument is designed to assess participants’ current functioning and identify improvements or declines relative to their best ever level of functioning in eight areas related to routine daily tasks (e.g., employment, financial management). Participants rated each item on a three (0–2) or four (0–3) point scale, and higher scores indicated poorer functioning. For the current study, a total score was generated to represent the total severity of declines reported in current versus past functioning on all tasks assessed by the measure (range: 0 to 11).
Neurocognitive and Neuromedical Assessments
All participants provided informed consent prior to completing comprehensive neurocognitive and neuromedical assessments. The neuropsychological assessment covered seven cognitive domains: verbal fluency, working memory, speed of information processing, learning, recall, executive functions, and fine motor coordination. Iudicello et al. {5} provides a complete list of neuropsychological tasks included in each domain. Raw test scores were then converted into demographically (age and education) corrected T-scores before deficit scores were computed for each domain. Individual domain deficit scores were averaged to obtain a global deficit score (GDS) {33} for which higher scores reflect poorer neurocognitive performance.
Data Analysis
Due to the non-normality of the variables of interest (e.g., FrSBe T-scores; Shapiro-Wilk test ps < 0.01), all primary between-group analyses were conducted using nonparametric tests. First, a series of Wilcoxon Ranked Sum tests and Cohen’s d statistics {34} were used to compare behavioral symptoms (i.e., FrSBe Total and Apathy, Disinhibition, and Executive Dysfunction T-scores) in MA+ and MA− groups. Chi-square tests and odds ratios were calculated to examine the effects of MA dependence on clinically elevated FrSBe scales (i.e., T-scores > 64) {20}. Next, a planned series of regressions were conducted to examine the unique effects of MA group on FrSBe variables, accounting for the effects of potentially confounding substance use factors on which the groups differed (i.e., lifetime diagnoses of cocaine, cannabis, and alcohol dependence). Due to the limited prevalence of HCV, ADHD, and ASPD in the MA− group, associations between these conditions and the FrSBe ratings were conducted only within the MA+ group using Wilcoxon Ranked Sums tests. Nevertheless, it is important to note that the effect of MA+ group status on FrSBe T scores remained significant in a logistic regression in which these factors were included. Spearman’s rho correlational analyses within the MA+ group were used to explore potential associations between FrSBe ratings and cognitive variables (GDS and domain deficit scores) and MA-use characteristics (i.e., last use, cumulative duration and quantity of use, age of first use, and age at onset and recency of MA-dependence diagnosis). Finally, a series of multiple regression analyses were used to examine each of the FrSBe variables as independent predictors of IADL dependence within the MA+ group while accounting for standard cognitive (i.e., GDS), medical (i.e., HCV status), psychiatric (i.e., current Major Depressive Disorder) and MA-use characteristics (i.e., last use of MA) known to be associated with IADL decline. The critical alpha was set to 0.05 for all analyses.
Results
Table 2 presents the means, standard deviations, and Cohen’s d effect sizes for the FrSBe variables. Relative to the non-MA using comparison participants, the MA-dependent group endorsed a significantly greater level of overall behavioral disturbance (i.e., FrSBe Total T-score; p < .002, d = .51), disinhibition (p < 0.001, d = 0.64) and executive dysfunction (p < 0.001, d = 0.61), but not apathy (p > .10). As shown in Table 3, MA group remained a significant predictor of the Total FrSBe T score as well as the Disinhibition and Executive Dysfunction subscales (ps < .01) even when accounting for potentially confounding factors on which the group differed. Although the proportion of individuals with comorbid ADHD diagnoses was significantly higher the MA+ group than the MA− group, total and subscale T scores and proportions of clinically elevated scores were comparable between MA+ individuals with and without comorbid ADHD (ps > .10). There were no significant effects of ASPD on any FrSBe scale within the MA+ group (all ps > .10). Finally, there were no significant differences between total and subscale T scores or proportion of clinically elevated individuals between MA+ individuals who were and were not infected with HCV (all ps > .10).
Table 2.
FrSBe Subscales and Total T-scores in the MA+ and MA− Groups with Effect Sizes
| FrSBe scale | MA− (n = 85) | MA+ (n = 73) | p value | d |
|---|---|---|---|---|
| Total | 53.8 (22.6) | 65.4 (23.5) | 0.002 | 0.51 |
| Apathy | 54.6 (22.7) | 60.1 (22.6) | 0.130 | 0.24 |
| Disinhibition | 50.2 (17.3) | 61.3 (20.5) | <0.001 | 0.64 |
| Executive dysfunction | 53.6 (18.3) | 64.9 (19.0) | <.001 | 0.61 |
Note. d reflects Hedge’s bias-corrected effect size; FrSBe = Frontal Systems Behavior Scale.
Table 3.
Multiple Regressions Predicting Neurobehavioral Symptoms and IADL Declines
| F | β | Adj. R2 | p | |
|---|---|---|---|---|
| FrSBe Total | 4.39 | - | .08 | .002 |
| MA group | - | −.24 | - | .003 |
| LT alcohol dependence | - | −.18 | - | .029 |
| LT cocaine dependence | - | .02 | - | .850 |
| LT cannabis dependence | - | .15 | - | .063 |
| FrSBe Executive Dysfunction | 5.89 | - | .11 | <.001 |
| MA group | - | −.28 | - | <.001 |
| LT alcohol dependence | - | −.21 | - | .014 |
| LT cocaine dependence | - | .04 | - | .636 |
| LT cannabis dependence | - | .16 | - | .045 |
| FrSBe Disinhibition | 4.76 | - | .09 | .001 |
| MA group | - | −.26 | - | .002 |
| LT alcohol dependence | - | −.16 | - | .060 |
| LT cocaine dependence | - | −.04 | - | .586 |
| LT cannabis dependence | - | .07 | - | .359 |
| FrSBe Apathy | 1.77 | - | .02 | .136 |
| MA group | - | −.14 | - | .085 |
| LT alcohol dependence | - | −.10 | - | .220 |
| LT cocaine dependence | - | .07 | - | .421 |
| LT cannabis dependence | - | .16 | - | .059 |
| IADL Declinea | 4.75 | - | .20 | <.001 |
| Total FrSBe T-score | - | .37 | - | .002 |
| GDS | - | .14 | - | .220 |
| Last MA use (days) | - | −.02 | - | .845 |
| HCV status | - | .08 | - | .458 |
| LT MDD | - | −.20 | - | .087 |
Note. LT = lifetime; FrSBe = Frontal Systems Behavioral Scale; MDD = Major Depressive Disorder; GDS = Global deficit score; HCV = Hepatitis C virus; IADL = Instrumental activities of daily living; MA = methamphetamine;
MA+ group only (n = 73).
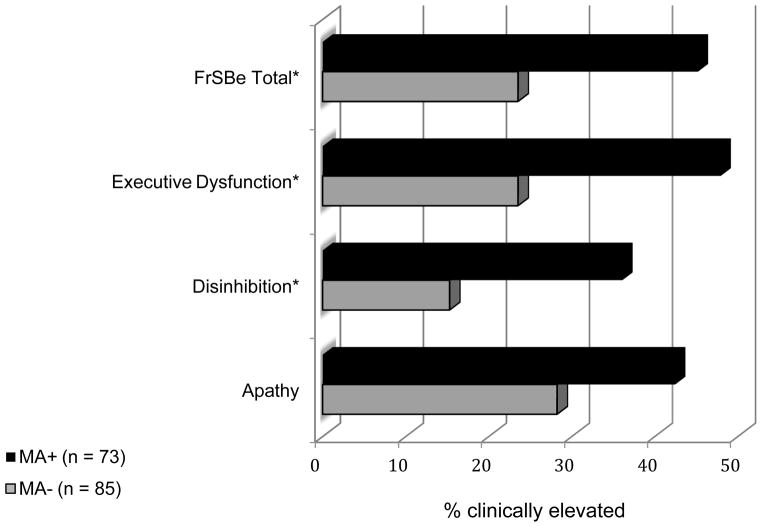
At the group level, the MA+ group had a significantly higher proportion of individuals with clinically elevated T-scores on FrSBe Total (45.2% relative to 23.5%), Disinhibition (36.1% relative to 15.3%), and Executive Dysfunction subscales (48.0% relative to 23.5%) relative to the non-MA users (all ps < .01; see Figure 1). Odds ratios revealed that a history of MA dependence was associated with an approximately threefold risk of clinical elevations on the FrSBe. Odds ratios were 2.68 for Total FrSBe (95% CI = 1.37, 5.37), 3.13 for Disinhibition (95% CI = 1.48, 6.87), and 2.99 (95% CI = 1.53, 5.99) for Executive Dysfunction.
Figure 1.
Proportion of Individuals in the MA+ (n = 73) and MA− (n = 85) Groups with Clinically Elevated T-Scores on the Frontal Systems Behavior Scale (FrSBe)
Within the MA+ group, no significant correlations were found between FrSBe T-scores and GDS or other domain deficit scores (all ps > .10). No significant correlations were observed between FrSBe T scores and MA-use parameters (e.g., age of first use, cumulative quantity, cumulative days in which methamphetamine was used) in the MA+ group.
Finally, MA-associated behavioral symptoms were predictive of functional decline even while accounting for standard cognitive, medical, psychiatric, and MA-use variables that have previously been associated with IADL decline. Specifically, within the MA+ group, identical linear regression models identified the FrSBe Total T score (B = .37, p < .002), Apathy subscale T score (B = .29, p < .005), Disinhibition subscale T score (B = .22, p < .04), and Executive Dysfunction subscale T score (B = .36, p < .0003) as significant, independent predictors of IADL decline severity (see Table 3).
Discussion
Chronic MA use has been associated with neurocognitive and psychosocial complications and adverse neurobehavioral symptoms {7, 12}, potentially reflecting MA-associated neural damage to frontostriatal systems. Moreover, these behavioral symptoms have been linked to MA-specific risk behaviors {21} and adverse psychosocial outcomes {22}. This study extends the literature by demonstrating an increased prevalence and greater severity of self-reported behavioral symptoms in MA-dependent individuals relative to non-MA users. Specifically, we found medium-to-large differences between MA dependent individuals and their non-MA using comparison participants in terms of overall self-reported neurobehavioral symptoms, as well as elevated ratings of self-reported disinhibition and executive dysfunction. In fact, the MA-dependent individuals as a group had a significantly greater proportion of individuals reporting clinically elevated T-scores overall relative to the non-MA users; that is, MA users were approximately three times more likely than the comparison subjects to report a clinically elevated level of disinhibition and executive dyscontrol. This is consistent with evidence of the behavioral disturbances (e.g., impulsivity and discounting of monetary rewards) observed in other stimulant-using populations {35} and the higher levels of neurobehavioral symptoms reported on the FrSBe by polysubstance-using individuals {36}.
To our knowledge, while research has identified neurobehavioral disturbances that are associated with substance dependence {23}, this was the first study to examine potential MA-specific neurobehavioral symptoms assessed using a well-validated, easily administered clinical questionnaire and examined as predictors of everyday functioning declines. Our results speak to the unique risk posed by MA, even in the context of other substances and comorbidities, including psychiatric disorders (e.g., ADHD, ASPD, and MDD) and infectious disease (e.g., HCV). Given the high rates of comorbid substance abuse and dependence in MA using populations, we did not exclude MA-users who also reported current and past use of other substances. To control for these effects, we utilized a comparison sample of individuals who were broadly comparable in terms of substance histories rather than healthy individuals. Moreover, the effects of MA group remained even when dependence on other substances of abuse was included in the statistical models. Thus, the robust effect sizes that we observed between MA+ and MA− groups provide evidence for an independent effect of MA on neurobehavioral symptoms that may be separable from the effects of other illicit substance use.
No significant differences in apathy were observed between the MA+ and MA− individuals. This finding was unexpected given clinically elevated means for apathy symptoms in clinical samples with high rates of stimulant use, including HIV {37} and HCV {38}. However, other investigations of polysubstance dependent individuals have also found trend-level effects for apathy symptoms similar to what we observed {23}. One possible reason for the lack of significant findings on the apathy subscale is the common utilization of amphetamines to treat apathy in a number of disorders (e.g., multiple sclerosis) {38}. Thus, apathy symptoms may not be as pronounced in MA users. Future work is nevertheless necessary to further investigate whether risk for apathy symptoms increases with prolonged abstinence from MA use.
Also contrary to our expectations, we found no significant correlations between neurocognitive deficits and neurobehavioral symptoms in MA+ individuals. However, the observed relationship between neurobehavioral symptoms and everyday functioning in MA suggest that, as in other conditions (e.g., dementia) {27}, neurobehavioral symptoms may provide unique information about an individual’s functioning beyond that which can be gleaned from their neuropsychological profiles. In fact, there is evidence from lesion studies to suggest that the expression of cognitive deficits and clinically observable neurobehavioral symptoms is dissociable at the neural level {39}. Further, correspondence between performance-based and self-report measures is poor in clinical populations {40}. Future studies may continue to explicate this apparent disconnect between neuropsychological performance and neurobehavioral symptoms.
Of particular clinical relevance, MA-associated neurobehavioral symptoms were uniquely associated with declines in instrumental activities of daily living. Specifically, higher levels of overall neurobehavioral disturbance, as well as elevated ratings of disinhibition and executive dyscontrol, were each independently predictive of more severe IADL declines in the MA+ group. This is consistent with research demonstrating both behavioral disturbances {22} and compromised everyday functioning {13} in MA-dependent individuals. These effects were noted independently of well-established predictors of everyday functioning, indicating the potential importance of neurobehavioral symptoms in predicting the IADL declines that may occur in MA+ individuals {2}. Results of this investigation suggest that behavioral disturbances in MA users, while often noted antecdotally in clinical settings, may serve as important indicators of IADL difficulties. These data speak to the potential adjunctive ecological value of self-reported behavioral disturbances when given alongside a standardized neuropsychological and psychiatric assessment of clinical symptoms. In particular, well-validated instruments such as the FrSBe may be useful in this regard. By measuring these neurobehavioral symptoms in MA+ individuals, clinicians and researchers may be able to identify those at risk for poorer functioning and diminished capacity to operate independently in important activities of daily living (e.g., managing finances, cooking, medication management). Future work may extend the current work by exploring whether other objective everyday functioning outcomes (e.g., credit card debt, employment) are associated with neurobehavioral symptoms in MA+ individuals. Further exploring the relationship between neurobehavioral symptoms and everyday functioning in chronic MA users may allow providers of rehabilitation services to better identify and target behavioral problems that may subsequently impair aspects of social and occupational functioning.
While the current study provides strong evidence for sizable neurobehavioral disturbances associated with MA dependence, important etiological questions remain and serve as potential targets for future work. For instance, it is not yet clear whether elevated neurobehavioral symptoms are directly indicative of MA-related frontal systems toxicity, though some functional neuroimaging studies demonstrate behavioral differences associated with prefrontal dysfunction in MA-dependent individuals {41}. It is known that these neurobehavioral symptoms are not specific to frontostriatal circuit involvement, as they have been observed in Multiple Sclerosis, and other disorders with vastly different effects on frontal systems {42}. For this reason, future investigations may incorporate functional and structural neuroimaging methodologies in order to link frontal systems compromise more directly to neurobehavioral symptom elevations in MA.
A few limitations of the current study can also be addressed in future work. For instance, while the high proportion of HCV-infection in our sample is representative of community samples, HCV has been associated with elevated neurobehavioral symptoms in non-stimulant using samples {31}. Although we did not observe an HCV effect in our MA sample, prospective studies controlling for important cofactors (e.g., demographics) are needed to more carefully determine the potential additive or synergistic effects of MA and comorbid infectious disease, perhaps also including HIV infection {10}. Additionally, the current protocol did not collect the caregiver or clinician reports on the FrSBe. Other investigations might prioritize the collection of this data in order to compare self-reported neurobehavioral symptoms with those reported by others. However, as caregivers and clinicians tend to report higher levels of behavioral symptoms than do substance users {20}, we would expect any differences to occur in the conservative direction. In addition, the current study design did not include an analysis of participants’ self-reported neurobehavioral symptoms prior to MA use due to the early age at first use and insidious onset of MA-related changes. Given research to suggest that higher levels of trait impulsivity may serve as both a facilitator and a consequence of substance abuse {43}, future research is necessary in order to investigate whether individuals with premorbid neurobehavioral symptoms are more likely to have elevated symptoms during MA dependence and after long-term abstinence.
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50 DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Burnham Institute for Medical Research. The TMARC is comprised of: Director: Igor Grant, M.D.; Co-Directors: Ronald J. Ellis, M.D., Ph.D., Cristian Achim, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Steven Paul Woods, Psy.D.; Sara Ortiz (Assistant Center Manager); Clinical Assessment and Laboratory Core: Scott Letendre, M.D. (P.I.), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric Core: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas Marcotte, Ph.D.; Neuroimaging Core: Gregory Brown, Ph.D. (P.I.), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences & Animal Models Core: Cristian Achim, M.D., Ph.D., Eliezer Masliah, M.D., Ian Everall, M.D., Ph.D., Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D., Rodney von Jaeger, M.P.H. (PAR Manager); Data Management Unit: Anthony C. Gamst, Ph.D., Clint Cushman (Data Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; Project 1: Arpi Minassian, Ph.D. (P.I.), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (P.I.), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (P.I.), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (P.I.), Athina Markou, Ph.D.; Project 5: Marcus Kaul, Ph.D. (P.I.).
This research was also supported by T32-DA31098 (S.P. Woods). The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- 1.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychological Review. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 2.Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addictive Behaviors. 2010;35(6):593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr AM, Panenka WJ, MacEwan W, Thornton AE, Lang DJ, Honer WG, et al. The need for speed: an update on methamphetamine addiction. Journal of Psychiatry & Neuroscience. 2006;31(5):301–313. [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. Journal of Clinical and Experimental Neuropsychology. 2010;32(7):704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology. 2006;185(3):327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 7.Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research. 2002;111(1):65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 8.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse - A H-1 MRS study. Neurology. 2000;54(6):1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 9.Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(3):317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 10.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 11.Monterosso JR, Aron AR, Cordova X, Xu JS, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188(2):162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 13.Sadek JR, Vigil O, Grant I, Heaton RK. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. Journal of Clinical and Experimental Neuropsychology. 2007;29(3):266–276. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- 14.Baberg HT, Nelesen RA, Dimsdale JE. Amphetamine use: Return of an old scourge in a consultation psychiatry setting. American Journal of Psychiatry. 1996;153(6):789–793. doi: 10.1176/ajp.153.6.789. [DOI] [PubMed] [Google Scholar]
- 15.Webster JM, Staton-Tindall M, Duvall JL, Garrity TF, Leukefeld CG. Measuring employment among substance-using offenders. Substance Use & Misuse. 2007;42(7):1187–1205. doi: 10.1080/10826080701409800. [DOI] [PubMed] [Google Scholar]
- 16.Semple SJ, Strathdee SA, Zians J, Patterson TL. Family Conflict and Depression in HIV-Negative Heterosexuals: The Role of Methamphetamine Use. Psychology of Addictive Behaviors. 2009;23(2):341–347. doi: 10.1037/a0015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartier J, Farabee D, Prendergast ML. Methamphetamine use, self-reported violent crime, and recidivism among offenders in California who abuse substances. Journal of Interpersonal Violence. 2006;21(4):435–445. doi: 10.1177/0886260505285724. [DOI] [PubMed] [Google Scholar]
- 18.Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Clifford DB, Collier AC, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: Self-report versus performance-based assessment of everyday functioning. Journal of the International Neuropsychological Society. doi: 10.1017/S135561771100141X. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore DJ, Palmer BW, Patterson TL, Jeste DV. A review of performance-based measures of functional living skills. Journal of Psychiatric Research. 2007;41(1–2):97–118. doi: 10.1016/j.jpsychires.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Grace J, Malloy PF. Frontal Systems Behavior Scale: Professional manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 21.Semple SJ, Zians JIG, Patterson TL. Impulsivity and methamphetamine use. Journal of Substance Abuse Treatment. 2005;29(2):85–93. doi: 10.1016/j.jsat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Semple SJ, Zians J, Grant I, Patterson TL. Sexual risk behavior of HIV positive methamphetamine-using men who have sex with men: The role of partner serostatus and partner type. Archives of Sexual Behavior. 2006;35(4):461–471. doi: 10.1007/s10508-006-9045-3. [DOI] [PubMed] [Google Scholar]
- 23.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Caracuel A, Verdejo-Garcia A, Vilar-Lopez R, Perez-Garcia M, Salinas I, Cuberos G, et al. Frontal behavioral and emotional symptoms in Spanish individuals with acquired brain injury and substance use disorders. Archives of Clinical Neuropsychology. 2008;23(4):447–454. doi: 10.1016/j.acn.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12(3):405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- 26.Zgaljardic DJ, Borod JC, Foldi NS, Rocco M, Mattis PJ, Gordon MF, et al. Relationship between self-reported apathy and executive dysfunction in nondemented patients with Parkinson disease. Cogn Behav Neurol. 2007;20(3):184–192. doi: 10.1097/WNN.0b013e318145a6f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norton LE, Malloy PF, Salloway S. The impact of behavioral symptoms on activities of daily living in patients with dementia. American Journal of Geriatric Psychiatry. 2001;9(1):41–48. [PubMed] [Google Scholar]
- 28.World Health Organization. Composite International Diagnostic Interview (CIDI) 2.1. Geneva, Switzerland: 1997. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: Author; 1994. [Google Scholar]
- 30.First MB, Spitzer RL, Gibon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 31.Posada C, Woods SP, Vigil O, Ake C, Perry W, Moore DJ. Implications of hepatitis C virus infection for behavioral symptoms and activities of daily living. Journal of Clinical and Experimental Neuropsychology. 2010;32(6):637–644. doi: 10.1080/13803390903418900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 33.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 35.Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11(1):18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- 36.Verdejo-Garcia AJ, Lopez-Torrecillas F, de Arcos FA, Perez-Garcia M. Differential effects of MDMA, cocaine, and cannabis use severity on distinctive components of the executive functions in polysubstance users: A multiple regression analysis. Addictive Behaviors. 2005;30(1):89–101. doi: 10.1016/j.addbeh.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Hoare J, Fouche JP, Spottiswoode B, Joska JA, Schoeman R, Stein DJ, et al. White matter correlates of apathy in HIV-positive subjects: A diffusion tensor imaging study. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(3):313–320. doi: 10.1176/jnp.2010.22.3.313. [DOI] [PubMed] [Google Scholar]
- 38.van Reekum R, Stuss DT, Ostrander L. Apathy: why care? Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(1):7–19. doi: 10.1176/jnp.17.1.7. [DOI] [PubMed] [Google Scholar]
- 39.Sarazin M, Pillon B, Giannakopoulos P, Rancurel G, Samson Y, Dubois B. Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions. Neurology. 1998;51(1):142–148. doi: 10.1212/wnl.51.1.142. [DOI] [PubMed] [Google Scholar]
- 40.Rourke SB, Halman MH, Bassel C. Neurocognitive complaints in HIV infection and their relationship to depressive symptoms and neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology. 1999;21(6):737–756. doi: 10.1076/jcen.21.6.737.863. [DOI] [PubMed] [Google Scholar]
- 41.Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 42.Chiaravalloti ND, DeLuca J. Assessing the behavioral consequences of multiple sclerosis: an application of the Frontal Systems Behavior Scale (FrSBe) Cognitive and Behavioral Neurology. 2003;16(1):54–67. doi: 10.1097/00146965-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 43.de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]