Abstract
Background
Unemployment rates are high among chronic methamphetamine (MA) users and carry a significant economic burden, yet little is known about the neurocognitive and psychiatric predictors of employment in this vulnerable population.
Methods
The present study examined this issue in 63 participants with recent MA dependence and 47 comparison subjects without histories of MA use disorders. All participants completed a comprehensive neurocognitive, psychiatric and neuromedical evaluation. Individuals with HIV infection, severe neuropsychological or psychiatric conditions that might affect cognition (e.g., seizure disorder, schizophrenia), or a positive Breathalyzer or urine toxicology screen on the day of testing were excluded.
Results
Consistent with previous research, a logistic regression revealed MA dependence as a significant, independent predictor of full-time unemployment status. Within the MA-dependent sample, greater impairment in global neurocognitive functioning and history of injection drug use emerged as significant independent predictors of unemployment status. The association between worse global cognitive functioning and unemployment was primarily driven by deficits in executive functions, learning, verbal fluency, and working memory.
Conclusion
These findings indicate that neurocognitive deficits play a significant role in the higher unemployment rates of MA-dependent individuals, and highlight the need for vocational rehabilitation and supported employment programs that assess and bolster cognitive skills in this population.
Keywords: Functional status, Substance abuse, Cognitive impairment, Neuropsychological assessment, Employment
1. Introduction
Methamphetamine (MA) is a potent psychostimulant with significant medical, psychiatric, and behavioral consequences that can interfere with psychosocial functioning, daily living skills and result in disability (Scott et al., 2007). Vocational functioning is a particularly important psychosocial outcome with potential psychological and public health implications for MA users. Unemployment is a common adverse functional problem in substance abusing populations; for example, 74% of individuals admitted into an amphetamine treatment program were unemployed (Substance Abuse and Mental Health Services Administration – SAMHSA, 2004). Unemployment is a significant predictor of drug behavioral outcomes, including reduced likelihood of retention in drug and alcohol treatment programs (Platt, 1995) and increased relapse rates after completion of treatment (Wolkstein and Spiller, 1998). In fact, relative to other substances of abuse (i.e., marijuana and cocaine), MA use is a particularly strong predictor of unemployment (Iritani et al., 2007), possibly due to its somewhat higher addictive potential (Gonzalez Castro et al., 2000) and more severe neurotoxic effects (e.g., Scott et al., 2007). Despite the clear clinical importance of employment status among MA users, research has yielded limited information regarding predictors of vocational functioning in this at-risk population. In one of the few studies to date, Webster et al. (2007) found that employment instability in a large cohort of polysubstance abusers was associated with more frequent MA use over the previous 6 months. Importantly, MA use is frequently accompanied by myriad risk factors that have implications for poor vocational outcomes, including impulsivity (Lee et al., 2009), elevated co-occurring psychiatric disorders (e.g., Meredith et al., 2005), unstable housing (Das-Douglas et al., 2008), and limited educational achievement (SAMHSA, 2004).
One particularly relevant predictor of unemployment not yet explored in MA-using samples is neurocognitive impairment. Cross sectional studies conducted across a wide range of clinical populations (e.g., HIV infection, schizophrenia, traumatic brain injury), have identified impaired neurocognitive functioning as a significant independent predictor of employment status and vocational skills (e.g., Heaton et al., 2004a; McGurk and Meltzer, 2000; Fabiano and Crewe, 1995, respectively), above and beyond more traditional non-cognitive predictors of vocational functioning (e.g., depression). A meta-analysis conducted by Kalechstein et al. (2003) confirmed small-to-medium effect sizes for the association between neurocognitive impairment and employment among studies assessing cohorts with a wide range of neurological disorders (e.g., severe traumatic brain injury, HIV infection). The neurocognitive domains most strongly associated with employment status were executive functions, learning and memory, and attention, as well as general intellectual ability. Additionally, a longitudinal study demonstrated that neurocognitive deficits adversely affect one’s ability to successfully return to work after unemployment (e.g., van Gorp et al., 2007).
Neurocognitive impairment may be a particularly relevant risk factor for unemployment in persons with MA use disorders. Chronic MA use is associated with mild-to-moderate deficits across a wide range of neurocognitive functions, most notably in the domains of learning and memory, executive functions, information processing speed, attention/working memory, and motor skills (Scott et al., 2007). While this profile of neurocognitive deficits is consistent with the neurotoxic effects of MA on the structure and function of frontostriatal systems (e.g., Chang et al., 2002, 2007), the nature and extent of MA-associated impairment does not reliably correlate with historical MA use parameters (e.g., age of first MA use; Scott et al., 2007). Importantly, there is some evidence to suggest neural recovery from MA damage following prolonged (i.e., greater than 6 months) periods of abstinence (e.g., Nordahl et al., 2005; Chang et al., 2002) as well as partial cognitive improvement in areas including attention, information processing speed, and psychomotor skills (e.g., Salo et al., 2009; Iudicello et al., 2010; Volkow et al., 2001). However, other MA-associated cognitive deficits may persist or even worsen despite discontinued use of MA (e.g., Johanson et al., 2006; Simon et al., 2004) and continue to adversely impact important everyday functioning abilities. In fact, the magnitude of MA-associated neurocognitive deficits is thought to be larger than that which is observed in association with other substances of abuse, including cannabis (Grant et al., 2003) and cocaine (Jovanovski et al., 2005), which may increase MA users’ relative risk of experiencing declines in everyday functioning.
Despite the known pattern of neurocognitive deficits in MA users and research indicating an association between cognition and everyday functioning, no studies have specifically examined the relationship between cognitive functioning and employment in MA-users. Only three have explored the relationship between MA-associated cognitive impairment and other, non-vocational aspects of daily functioning (Henry et al., 2010; Sadek et al., 2007). Sadek et al. (2007) found that MA dependent adults had higher rates of self-reported dependence in instrumental activities of daily living (IADLs; e.g., managing finances, grocery shopping) relative to a group of healthy comparison participants. Within their mixed clinical sample, neurocognitive deficits were not associated with IADL decline, but were predictive of self-reported cognitive problems in everyday life. Henry et al. (2010) reported that MA-dependent individuals performed significantly worse on nearly all of the functional domains (e.g., finances) measured by the UCSD Performance-based Skills Assessment (UPSA; Patterson et al., 2001). Within the MA-using group, poorer UPSA scores were associated with executive dysfunction (i.e., increased perseverative errors and fewer categories completed on the Wisconsin Card Sorting Test) and greater frequency of MA use. More recently, Cattie et al. (in press) reported that self-reported neurobehavioral symptoms (e.g., impulsivity, executive dysfunction) were uniquely predictive of IADL declines among chronic MA users. Considering this prior research, the present study sought to examine the hypothesis that MA use is associated with higher rates of unemployment, and that neurocognitive deficits would independently predict employment status.
2. Methods
2.1. Participants
This study was approved by the institution’s human research protections program. Participants included 63 MA-dependent and 47 non-MA using comparison adults (total N = 110). Each participant in the MA-dependent group met Diagnostic and Statistical Manual of Mental Disorders (4th edition; DSM-IV; American Psychiatric Association, 1994) criteria for lifetime MA dependence, as well as DSM-IV criteria for MA abuse or dependence within 18 months of their evaluation. All MA-dependent participants were not currently using MA or in the early stages of withdrawal, as indicated by a urine toxicology screen on the day of testing, the limit of detection of which is 72 h. All participants included in this study were HIV-seronegative as confirmed by enzyme linked immunosorbent assays (ELISA) and a Western Blot confirmatory test, and all individuals in the non-MA using comparison group were seronegative for HCV as determined by detection of HCV immunoglobulin G (IgG) antibody in plasma by ELISA. Approximately 35% (n = 22) of the MA-dependent participants were infected with HCV.
Participants were excluded if they tested positive for illicit drugs (other than marijuana) or alcohol on a urine toxicology screen or Breathalyzer, respectively, administered on the day of testing, or if they met DSM-IV criteria for current non-MA substance use disorders (i.e., within 30 days of evaluation). Individuals with histories of alcohol dependence within one year of evaluation or any other substance dependence within five years were also excluded. Additional exclusion criteria included histories of severe psychiatric (i.e., schizophrenia) or neuromedical (e.g., seizure disorders, closed head injuries with loss of consciousness greater than 15 min) conditions known to affect cognition.
Demographic, psychiatric, and substance use characteristics for the participants are presented in Table 1. The study groups were comparable for age and gender (both ps > 0.10), though the non-MA using comparison sample had a significantly greater proportion of non-Caucasian individuals, were more highly educated, and had higher estimates of pre-morbid verbal intelligence as determined by oral word reading scores of the Wide Range Achievement Test – Revision 3 (WRAT; Wilkinson, 1993) (ps < 0.05). The MA-dependent cohort also had significantly lower occupational achievement (p < 0.05) as determined by the Hollingshead employment ranking. The MA-dependent group had greater proportions of individuals with histories of antisocial personality disorder (ASPD) and attention-deficit/hyperactivity disorder (ADHD; both ps < 0.05), though no significant differences were found for current or lifetime rates of major depressive disorder (MDD; ps > 0.10). On average, the MA-dependent participants began using MA at age 22, used MA for approximately 10 years, and had abstained for approximately 5 months (see Table 1). As might be expected, the MA-dependent group had higher lifetime rates of alcohol, cocaine, marijuana, and other substance dependence relative to their non-MA using counterparts (ps ≤ 0.05).
Table 1.
Demographic and psychiatric characteristics of the study participants.
| Characteristics | HA (n = 47) | MA+ (n = 63) | p-value |
|---|---|---|---|
| Demographics | |||
| Sex (% male) | 87.2 | 84.1 | 0.65 |
| Ethnicity (% Caucasian) | 46.8 | 66.7 | 0.04 |
| Age (years) | 40.6 (11.1) | 42.4 (6.9) | 0.37 |
| Education (years) | 13.3 (2.2) | 11.9 (2.1) | <0.01 |
| WRAT-III SS | 102.1 (12.0) | 94.6 (12.1) | <0.01 |
| Hollingshead employment ranking (highest ever)a | 3.6 (1.2) | 4.3 (1.1) | <0.01 |
| Neurocognitive clinical rating | 3.9 (1.3) | 4.6 (1.3) | 0.02 |
| HCV (%) | 0.0 | 34.9 | <0.01 |
| Psychiatric | |||
| MDD (% currently diagnosed) | 6.4 (n = 3) | 12.7 (n = 8) | 0.27 |
| MDD (% lifetime) | 29.8 (n = 14) | 41.3 (n = 26) | 0.22 |
| ASPD (% lifetime) | 4.3 (n = 2) | 23.8 (n = 15) | <0.01 |
| ADHD (% lifetime) | 2.2 (n = 1) | 12.7 (n = 8) | 0.05 |
| MA use characteristics | |||
| Age of first use (years) | – | 22.3 (8.3) | |
| Total duration of use (years) | – | 10.5 (7.2) | |
| Total quantity of use (g) | – | 4565 (6244) | |
| Last use (days) | – | 150 (170) | |
| Injection MA use ever (%) | – | 42.9 (n = 27) | |
| Non-MA dependence | |||
| Cannabis (% lifetime) | 2.1 (n = 1) | 12.7 (n = 8) | 0.05 |
| Alcohol (% lifetime) | 8.5 (n = 4) | 36.5 (n = 23) | <0.01 |
| Cocaine (% lifetime) | 4.2 (n = 2) | 23.8 (n = 15) | <0.01 |
| Other substances (% lifetime) | 0.0 | 12.7 (n = 8) | <0.01 |
Note: HA, healthy adults; MA, methamphetamine; WRAT-III SS, Wide Range Achievement Test – Third Edition, Standard Score; HCV, hepatitis C virus; MDD, major depressive disorder; ASPD, antisocial personality disorder; ADHD, attention-deficit/hyperactivity disorder.
Hollingshead employment rankings: higher scores represent lower job responsibilities.
2.2. Materials and procedure
All eligible participants provided written informed consent and completed comprehensive, standardized psychiatric and neuropsychological research evaluations.
2.2.1. Employment status
Several types of information were collected for characterization of employment status. An item from the Patient Assessment of Own Functioning Inventory (PAOFI; Chelune et al., 1986), a self-report measure in which an individual rates daily functioning difficulties, was used to classify employment status. The Hollingshead (1975) system was used to characterize the highest level of employment ever achieved by the participants. A score (ranging from 1 to 7) was assigned to the participant’s self-reported occupation, which provided a relative rank for that position in terms of sophistication and skill. (Note: Higher raw scores reflect lower level positions.)
2.2.2. Psychiatric assessment
Assessment of relevant psychiatric diagnoses was conducted using the Composite International Diagnostic Interview Version 2.1 (CIDI 2.1; World Health Organization, 1998), which is a computer-assisted structured interview that is administered by trained evaluators. This assessment tool yields lifetime and current (i.e., endorsing clinical symptoms within 1 month of evaluation) diagnoses that are consistent with DSM-IV criteria. The resulting diagnoses of mood and substance use disorders were used for determining eligibility for the study (i.e., exclusion criteria) and for characterization of the sample. (Diagnoses of MDD were assigned only if the reported depressive symptoms were not solely substance induced, per the DSM-IV criteria). For the MA-dependent group, a semistructured timeline follow-back interview (see Rippeth et al., 2004) was used to obtain information regarding MA use characteristics (i.e., frequency, duration, and quantity of MA use).
2.2.3. Neuropsychological functioning
All participants completed an approximately 3-h comprehensive neuropsychological battery comprised standardized clinical tests across several cognitive domains, including the following: learning and memory: Hopkins Verbal Learning Test-Revised (HVLT-R; Benedict et al., 1998), Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997); speed of information processing: Trail Making Test (TMT A; Reitan and Wolfson, 1985); Wechsler Adult Intelligence Scale (WAIS) III processing speed index (Psychological Corporation, 1997); verbal fluency: Controlled Oral Word Association Test (COWAT-FAS; Benton et al., 1994), animal fluency (Benton et al., 1994), actions (Woods et al., 2005a); executive functions: Wisconsin Card Sorting Test-64 Card Version (WCST-64; Kongs et al., 2000), TMT B (Reitan and Wolfson, 1985); Delis-Kaplan Executive Function Scale Color-Word Interference (D-KEFS; Delis et al., 2001); working memory: WMS-III Spatial Span (Psychological Corporation, 1997); Paced Auditory Serial Addition Test (PASAT; Diehr et al.,1998); fine motor coordination: Grooved Pegboard Test (GP: KlØve, 1963). Raw scores from the measures listed above were converted to demographically corrected T-scores using the most comprehensive available normative data (e.g., Diehr et al., 1998; Heaton et al., 2004b; Norman et al., 2011). Clinical ratings were assigned by a neuropsychologist on the basis of these demographically adjusted T-scores in accordance with a manualized procedure (Woods et al., 2004). Specifically, this procedure involves using a detailed set of manualized guidelines for assigning clinical ratings that were directly referenced by the neuropsychologist, which is system that has demonstrated excellent inter-rater reliability (Woods et al., 2004) and construct validity in MA (Carey et al., 2006; Chana et al., 2006; Jernigan et al., 2005). In brief, global and ratings are assigned according to a 9-point scale ranging from 1 (above average) to 9 (severe impairment), with a cutoff of 5 (mild impairment) or greater indicating presence of neurocognitive impairment.
2.3. Data analysis
First, a logistic regression was conducted to examine MA dependence as a predictor of employment status (i.e., employed versus unemployed), while accounting for potentially confounding variables that differed between the study groups (viz., education, ethnicity, Hollingshead, and estimated pre-morbid VIQ). Due to the limited prevalence of HCV infection, ADHD, ASPD, and non-MA substance dependence diagnoses in the non-MA using comparisons, analyses examining the associations between these factors and employment were restricted to the MA-dependent group only. Chi-square and independent samples t-tests (or their non-parametric counterparts for non-normally distributed data) were used to examine potential demographic, neuromedical, psychiatric, and substance-related differences between the employed and unemployed participants in the MA group. Next, we conducted a logistic regression analysis examining global and domain-specific cognitive impairment as predictors of employment status in the MA-dependent group, while also taking into account variables that differed significantly between the employed and unemployed MA-dependent samples (viz., injection MA use). A critical α of 0.05 was used for all statistical tests.
3. Results
A logistic regression model was tested to evaluate the unique contribution of MA use to employment status controlling for ethnicity, education, estimated VIQ, and Hollingshead. The overall model was significant (χ2[5, N = 109] = 11.34, p = 0.045; R2 = 0.08; see Table 3) and MA use was a significant predictor of unemployment (χ2 = 4.75, p = 0.030). All other predictors in the model were non-significant (ps > .10). The odds ratio revealed that non-MA comparison participants were 2.63 times more likely than MA-using participants to be employed full-time (95% confidence interval = 1.10, 6.41).
Table 3.
Logistic regression showing methamphetamine use as a unique predictor of employment status.
| Variable | Model | Parameter (χ2) | p-Value |
|---|---|---|---|
| R2 | 0.08 | ||
| χ2 | 11.34 | 0.045 | |
| Methamphetamine [MA–] | 4.75 | 0.030 | |
| Age | 0.13 | 0.72 | |
| Education | 1.08 | 0.30 | |
| Premorbid functioning | 0.06 | 0.81 | |
| Hollingshead employment ranking (highest ever)a | 0.003 | 0.95 |
Note: premorbid functioning, Wide Range Achievement Test – III reading score.
Hollingshead employment rankings: higher scores represent lower job responsibilities.
Within the MA-dependent sample (n = 63), a series of univariate analyses showed that unemployed individuals differed from those who were employed in several important ways. Unemployed participants reported more frequent lifetime injection drug use than the employed MA participants (χ2 = 7.01, p = 0.008, Φc = 0.334; see Table 2). Additionally, unemployed MA participants had a slightly higher prevalence of lifetime MDD (χ2 = 3.68, p = 0.055, Φc = 0.242) than the employed MA participants. Given the non-significance of this variable and the low prevalence of lifetime MDD in the employed group, this factor was not included in subsequent multivariable analyses due to model instability. Nevertheless, inclusion or exclusion of this variable did not affect the significance of the global cognitive rating as a predictor of employment described below. No other significant differences were found between employed versus unemployed MA groups (all ps > 0.10).
Table 2.
Demographic and psychiatric characteristics of MA+ participants.
| Characteristics | Employed (n = 15) | Unemployed (n = 48) | p-value |
|---|---|---|---|
| Demographics | |||
| Sex (% male) | 93.3 | 81.3 | 0.26 |
| Ethnicity (% Caucasian) | 60.0 | 68.8 | 0.53 |
| Age (years) | 40.3 (7.2) | 43.0 (6.8) | 0.20 |
| Education (years) | 12.3 (1.9) | 11.8 (2.2) | 0.48 |
| WRAT-III SS | 95.3 (10.6) | 94.4 (12.6) | 0.80 |
| Hollingshead employment ranking (highest ever)a | 4.3 (.8) | 4.3 (1.2) | 0.78 |
| Neurocognitive clinical rating | 3.9 (1.2) | 4.8 (1.3) | <0.05 |
| HCV (%) | 20.0 | 40.0 | 0.16 |
| Psychiatric | |||
| MDD (% currently diagnosed) | 6.7 (n = 1) | 14.6 (n = 7) | 0.42 |
| MDD (% lifetime) | 20.0 (n = 3) | 47.9 (n = 23) | 0.06 |
| ASPD (% lifetime) | 26.7 (n = 4) | 22.9 (n = 11) | 0.77 |
| ADHD (% lifetime) | 6.7 (n = 1) | 14.6 (n = 7) | 0.42 |
| MA use characteristics | |||
| Age of first use (years) | 21.9 (7.5) | 22.5 (8.7) | 0.83 |
| Total duration of use (years) | 7.7 (5.6) | 11.4 (7.5) | 0.08 |
| Total quantity of use (g) | 3704 (5322) | 4834 (6534) | 0.55 |
| Last use (days) | 188 (177) | 139 (168) | 0.33 |
| Injection MA use ever (%) | 13.3 (n = 2) | 52.1 (n = 25) | <0.01 |
| Non-MA dependence | |||
| Cannabis (% lifetime) | 20.0 (n = 3) | 10.4 (n = 5) | 0.33 |
| Alcohol (% lifetime) | 40.0 (n = 6) | 35.4 (n = 17) | 0.75 |
| Cocaine (% lifetime) | 33.3 (n = 5) | 20.8 (n = 10) | 0.32 |
| Other substances (% lifetime) | 6.7 (n = 1) | 14.6 (n = 7) | 0.42 |
Note: MA, methamphetamine; WRAT-III SS, Wide Range Achievement Test – Third Edition, Standard Score; HCV, hepatitis C virus; MDD, major depressive disorder; ASPD, antisocial personality disorder; ADHD, attention-deficit/hyperactivity disorder.
Hollingshead employment rankings: higher scores represent lower job responsibilities.
Within the MA-dependent sample, a binary logistic regression (χ2[2, N = 63] = 13.81, p = 0.001; R2 = 0.20) revealed greater global neurocognitive impairment (χ2[1, N = 63] = 6.01, p = 0.014; OR = 1.99, 95% CI = 1.14, 3.86) and history of injection MA use (χ2[1, N = 63] = 9.27, p = 0.002; OR = 9.90, 95% CI = 2.11, 78.84) as independent predictors of unemployment status. A follow-up series of logistic regression analyses indicated that unemployment status was significantly associated with more impaired domain ratings of verbal fluency (p = 0.009; OR = 1.93, 95% CI = 1.16, 3.70), executive functions (p = 0.030; OR = 1.55, 95% CI = 1.04, 2.52), working memory (p = 0.016; OR = 1.60, 95% CI = 1.09, 2.59), and learning (p = 0.036; OR = 1.51, 95% CI = 1.02, 2.36), even after controlling for injection use. Difficulties in fine motor coordination approached significance (p = 0.051; OR = 1.49, 95% CI = 1.0, 2.44). Ratings of information processing speed and memory were not significantly related to employment status (all ps > 0.10). Unit odds ratios for risk of unemployment are depicted in Fig. 1.
Fig. 1.
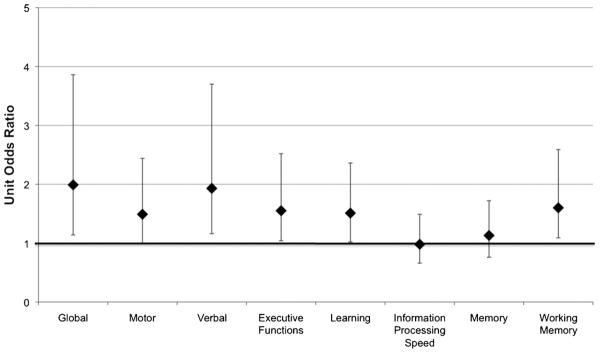
Unit odds ratios (and 95% confidence intervals) for neurocognitive impairment in association with unemployment among chronic MA users.
4. Discussion
Results from this study provide preliminary evidence that MA-dependent individuals with neurocognitive impairment are at significantly greater concurrent risk of unemployment. This is consistent with a broader body of research showing a significant association between neurocognitive functioning and employment status in other clinical conditions with similar risk factors and functional consequences (e.g., HIV infection; Kalechstein et al., 2003). Findings also converge with two fairly recent studies linking neurocognitive deficits to worse outcomes in other aspects of everyday functioning in MA users, including everyday cognitive complaints (Sadek et al., 2007) and a battery of performance-based functional skills (Henry et al., 2010). Of particular note, the present study found that MA-associated cognitive impairment was a unique predictor of unemployment even when considering other potentially confounding factors that may be associated with unemployment (e.g., injection drug use). Thus, consideration of neurocognitive impairment may provide some incremental ecological validity in the context of vocational evaluations of persons with MA use disorders.
Given the multifactorial nature of our global neurocognitive index, we also examined domain level variables that provide greater degree of specificity and may be more informative with regard to hypothesis generation and informing intervention development. Significant associations with employment status in MA users were observed for executive functions (i.e., divided attention, perseverative responses, and prepotent inhibition), verbal fluency, working memory, learning, and fine motor coordination (at a trend level). These domain level associations with employment status are consistent with meta-analyses in mixed clinical populations (Kalechstein et al., 2003), and with the profile of MA-associated neurocognitive impairment (Scott et al., 2007). One possible interpretation of these results is that unemployed MA-dependent persons demonstrated a deficit in the strategic or executive aspects of cognitive functioning (i.e., cognitive dyscontrol). In support of this notion, research has demonstrated that both verbal (Woods et al., 2005b) and visual (Morgan et al., 2012) MA-associated learning deficits are primarily driven by dysregulation of strategic encoding and retrieval, which are closely associated with executive dysfunction. Moreover, working memory and verbal fluency are commonly subsumed under the umbrella of executive functions (Lezak et al., 2004). Interestingly, neither delayed recall nor information processing speed was associated with unemployment in this sample. While both of these constructs can involve some degree of executive control that may depend on prefrontal systems, they are also arguably less specific in that regard than the other domains measured in this study; that is, delayed memory is more often closely linked with automatic processes dependent on medial temporal structures (e.g., Moscovitch, 1992) and processing speed is poorly localizing (Gläscher et al., 2009). While we cannot rule out the influence of a basic attentional deficit, future studies may wish to further explore associations between unemployment and other aspects of executive dysfunction, including multitasking (Scott et al., 2011), decision-making (Duarte et al., 2012), planning, or prospective memory (Iudicello et al., 2011). Furthermore, research aimed at elucidating the executive aspects of verbal fluency (e.g., switching), learning (e.g., semantic clustering), working memory (e.g., central executive), and motor (e.g., sequencing) in the context of everyday functioning declines among chronic MA users shed light on the cognitive mechanisms driving these apparent neurocognitive barriers to employment.
It is important to consider the practical implications of this profile of neurocognitive deficits in the workplace. Across various types of employment, job performance is a complex daily function involving a range of cognitive abilities, and the observed domain impairments could interfere with vocational duties in a variety of ways. For example, aspects of executive functions such as planning, organization, and problem-solving would be essential to almost any type of job (e.g., completing administrative tasks in order of priority), particularly with increased cognitive load (e.g., managing increased demands at a retail store during the holiday season). Impaired ability to learn new material may create difficulties in learning new concepts, policies, procedures, and vocational skills in a timely and efficient manner. Working memory deficits may interfere with work performance in instances where one must maintain and manipulate information (e.g., mental math calculations while assessing inventory). Beyond the scope of job duties, even the act of organizing oneself to attend a job is inherently necessary in the maintenance of employment (e.g., coordinating childcare, transportation planning). Comprehensive neuropsychological evaluations may be a useful component of clinical vocational evaluations by identifying the profile of cognitive strengths and weaknesses that impact employment status in MA-dependent individuals and could assist in tailoring compensatory strategies aimed at maintaining, or even possibly regaining, employment.
Consistent with previous studies (e.g., Kalechstein et al., 2000), we also identified several psychiatric and substance use predictors of unemployment within the MA-dependent group. Specifically, individuals with a history of injection drug use or lifetime MDD were at greater risk for unemployment. Sustained MA use is associated with high rates of affective distress, increased irritability and agitation, impulsive behaviors, poor coping skills, limited social support, and disorganized lifestyles (Newton et al., 2004; Cretzmeyer et al., 2003; Halkitis and Shrem, 2006; Semple et al., 2004), all of which have important implications for successful vocational functioning. Engaging in risky behaviors such as injection drug use is also related to myriad poor health outcomes, including higher rates of hospitalizations (Latkin et al., 2001), infectious disease (e.g., Ghanem et al., 2011), and psychiatric comorbidity (Zweben et al., 2004), which may impact the ability to be present and engaged in vocational responsibilities. Additionally, though it did not reach significance, MA-dependent individuals with a history of lifetime MDD also tended to be unemployed. This is consistent with data from a mixed cohort of individuals with HIV infection and MA dependence in which greater depressive symptoms significantly predicted unemployment (Sadek et al., 2007). Depression is also independently associated with job turnover, and absenteeism in vocational functioning (Lerner et al., 2004). Of course, given the cross-sectional nature of these data we are unable to determine whether individuals with preexisting depression lead to higher rates of unemployment and/or if depressive symptoms might also arise due to the psychosocial stressors that can accompany unemployment. Nevertheless, our finding therefore suggests that the role of depression on employment may also generalize to MA users, and be particularly important given that MA users exhibit higher rates of affective disorders compared to the general population. Relatedly, it remains to be seen whether these results will generalize to other substance-using populations (e.g., heroin) with different neurocognitive deficit profiles.
5. Limitations and future directions
While this study has important clinical implications, several limitations are worth noting and may be addressed in future research. First, the cross-sectional nature of the present study prohibits any causal inferences from being made regarding the effect of neurocognition on employment status. Prospectively examining the role of neurocognition in a sample of individuals actively job searching (i.e., “return to work”) might better clarify this relationship (van Gorp et al., 2007). Different neurocognitive skills may be required for active job searching than those that may be related to job loss, and so the results of such a study may highlight an alternative neurocognitive profile of importance. Second, the classification of employment status is inherently complex, particularly during times of economic downturn, given the myriad unassessed and unrelated factors that may contribute to unemployment (e.g., hiring freezes). The analyses were limited to individuals who reported full-time employment versus unemployment (including disability). We specifically did not include individuals who were employed part-time or volunteering due to the amount of variability of important factors (e.g., time spent working). Third, while our findings indicated that MA use was a significant predictor of unemployment even when considering secondary factors that may be associated with employment status (e.g., depression), there are a number of additional potential factors that are relevant to MA using individuals and could adversely influence employment status that we were unable to measure (e.g., legal history). Additionally, our MA sample may not be fully representative of the MA-dependent population given our inclusion and exclusion criteria (e.g., HIV seronegative, exclusion of other recent substance use disorders), which may limit generalizability. Relatedly, the MA status groups were imbalanced on important clinical factors (e.g., HCV) that were not amenable to statistical modeling. Despite these limitations, this is the first study to examine the role of neuropsychological functioning on unemployment in a relatively large cohort of well-characterized MA users.
In summary, findings from this study suggest that neuropsychological impairment is an important predictor of unemployment for MA-dependent individuals. Importantly, some aspects of MA-associated neurocognitive impairment (e.g., psychomotor speed) may recover to some degree following prolonged periods of abstinence (Iudicello et al., 2010; Salo et al., 2009; Volkow et al., 2001) and/or targeted cognitive rehabilitation interventions (e.g., Bickel et al., 2011). Thus, to the degree that targeted neurocognitive interventions are effective specifically for MA-users, incorporating individualized cognitive rehabilitation interventions into traditional MA-use treatment programs may be highly beneficial in terms of both cognitive and functional outcomes. In addition, specific vocational rehabilitation techniques may be useful in this population. For example, Individual Placement and Support and supported employment are two work rehabilitation interventions that aim to directly place clients into integrated work settings that specifically are tailored to the client’s needs. These placements provide explicit job training based on the client’s relative difficulties, which may help to address existing cognitive difficulties in the workplace. Both interventions have been found to be particularly effective in individuals with serious mental illness (Twamley et al., 2003), which provides some parallels to the vocational challenges MA users face. Research on the added value of incorporating neuropsychological factors into the design and implementation of such vocational rehabilitation efforts for MA users may therefore be worthwhile.
Acknowledgments
Role of funding source
Funding for this study was provided by NIH grants T32-DA31098, P01-DA12065, P50-DA026306, and P30-MH62512; the NIH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of this report, or in the decision to submit the paper for publication.
The Translational Methamphetamine AIDS Research Center (TMARC) group is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute. The TMARC is comprised Director: Igor Grant, M.D.; Co-Directors: Ronald J. Ellis, M.D., Ph.D., Cristian Achim, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Steven Paul Woods, Psy.D.; Aaron Carr (Assistant Center Manager); Clinical Assessment and Laboratory Core: Scott Letendre, M.D. (P.I.), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric Core: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas Marcotte, Ph.D.; Neuroimaging Core: Gregory Brown, Ph.D. (P.I.), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models Core: Cristian Achim, M.D., Ph.D., Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D.; Participant Unit: J. Hampton Atkinson, M.D., Rodney von Jaeger, M.P.H. (Unit Manager); Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D., Clint Cushman (Unit Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; Project 1: Arpi Minassian, Ph.D. (P.I.), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (P.I.), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (P.I.), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (P.I.), Athina Markou, Ph.D.; Project 5: Marcus Kaul, Ph.D. (P.I.). This research was supported by National Institutes of Health grants T32-DA31098, P01-DA12065, P50-DA026306, and P30-MH62512. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
Contributors
Authors Woods, Moore, and Grant designed the study and wrote the protocol. Authors Weber, Blackstone, Iudicello, and Morgan managed the literature searches and summaries of previous related work. Authors Woods and Weber undertook the statistical analysis. Authors Weber, Blackstone, Iudicello, and Morgan wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
The authors report no conflicts of interest.
Conflict of interest
The authors declare no conflicts of interest, aside from the NIH grant support listed above.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test – Revised. Psychological Assessment Resources, Inc; Odessa, FL: 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test-revised: normative data and analysis of inter-form and test–retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. AJA Associates; Iowa City, IA: 1994. [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Addictive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Posada C, Iudicello JE, Grant I The TMARC Group. Elevated neurobehavioral symptoms are associated with poorer everyday functioning in chronic methamphetamine users. J Neuropsychiatr Clin Neurosci. doi: 10.1176/appi.neuropsych.11080192. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E, HNRC Group. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 (Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability. In: Tarter RE, Goldstein G, editors. Advances in Clinical Neuropsychology. Vol. 3. Plenum Press; New York: 1986. pp. 95–126. [Google Scholar]
- Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24:267–277. doi: 10.1016/s0740-5472(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Das-Douglas M, Colfax G, Moss AR, Bangsberg DR, Hahn JA. Tripling of methamphetamine/amphetamine use among homeless and marginally housed persons, 1996–2003. J Urban Health. 2008;85:239–249. doi: 10.1007/s11524-007-9249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function Scale. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Duarte NA, Woods SP, Rooney A, Grant I, Atkinson JH The TMARC Group. Working memory deficits affect risky decision-making in metham-phetamine users with attention-deficit/hyperactivity disorder. J Psychiatric Res. 2012;46:492–499. doi: 10.1016/j.jpsychires.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano RJ, Crewe N. Variables associated with employment following severe traumatic brain injury. Rehabil Psychol. 1995;40:223–231. [Google Scholar]
- Ghanem A, Little SJ, Drumright L, Liu L, Morris S, Garfein RS. High-risk behaviors associated with injection drug use among recently HIV-infected men who have sex with men in San Diego CA. AIDS Behav. 2011;15:1561–1569. doi: 10.1007/s10461-011-9970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Castro F, Barrington EH, Walton MA, Rawson RA. Cocaine and methamphetamine: differential addiction rates. Psychol Addict Behav. 2000;14:390–396. [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Shrem MT. Psychological differences between binge and chronic methamphetamine using gay and bisexual men. Addict Behav. 2006;31:549–552. doi: 10.1016/j.addbeh.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Rivera-Mindt M, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004a;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead–Reitan. Psychological Assessment Resources; Odessa, FL: 2004b. [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Yale University; New Haven, CT: 1975. Four-factor index of social status. http://www.yale-university.com/sociology/faculty/docs/hollingsheadsocStat4factor.pdf. [Google Scholar]
- Iritani BJ, Hallfors DD, Bauer DJ. Crystal methamphetamine use among young adults in the USA. Addiction. 2007;102:1102–1113. doi: 10.1111/j.1360-0443.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Weber E, Dawson M, Grant I, Weinborn M, Woods SP HNRC Group. Misremembering future intentions in methamphetamine dependent individuals. Clin Neuropsychol. 2011;25:269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, Atkinson JH, Grant I HNRC Group. Longer term improvement in neurocognitive functioning and affective distress amongst methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology. 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Longshore D, Anglin MD, van Gorp WG, Gawin FH. Psychiatric comorbidity of methamphetamine dependence in a forensic sample. J Neuropsychiatry Clin Neurosci. 2000;12:480–484. doi: 10.1176/jnp.12.4.480. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, van Gorp W. Neurocognitive functioning is associated with employment status: a quantitative review. J Clin Exp Neuropsych. 2003;25:1186–1191. doi: 10.1076/jcen.25.8.1186.16723. [DOI] [PubMed] [Google Scholar]
- Kløve H. Grooved Pegboard. Lafayette Instruments; Lafayette, IN: 1963. [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Computerized Version. Psychological Assessment Resources; Odessa, FL: 2000. [Google Scholar]
- Latkin CA, Knowlton AR, Sherman S. Routes of drug administration, differential affiliation, and lifestyle stability among cocaine and opiate users: implications to HIV prevention. J Subst Abuse. 2001;13:89–102. doi: 10.1016/s0899-3289(01)00070-0. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Momford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner D, Adler DA, Chang H, Lapitsky L, Hood MY, Perissinotto C, Reed J, McLaughlin TJ, Berndt ER, Rogers WH. Unemployment, job retention, and productivity loss among employees with depression. Psychiatric Services. 2004;55:1371–1378. doi: 10.1176/appi.ps.55.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. Oxford University Press; New York: 2004. [Google Scholar]
- McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. 2000;45:175–184. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harvard Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Poquette AJ, Vigil O, Heaton RK, Grant I The Translational Methamphetamine Research Center (TMARC) Group. Visual memory in methamphetamine dependent individuals: deficient strategic control of encoding and retrieval. Aust N Z J Psychiatry. 2012;46:141–152. doi: 10.1177/0004867411433212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. J Cog Neuro. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Metham-phetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Jr, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK HNRC Group. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Platt JJ. Vocational rehabilitation of drug abusers. Psychol Bull. 1995;117:416–433. doi: 10.1037/0033-2909.117.3.416. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III Technical Manual. San Antonio, TX: 1997. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I HNRC Group. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Vigil O, Grant I, Heaton RK. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Exp Neuropsychol. 2007;29:266–276. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine dependent individuals. J Subst Abuse Treat. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, Grant I, Marcotte TD, HNRC Group. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology. 2011;25:511–519. doi: 10.1037/a0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. A comparison of injection and non-injection methamphetamine-using HIV positive men who have sex with men. Drug Alcohol Depend. 2004;76:203–212. doi: 10.1016/j.drugalcdep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat. 2004;27:59–66. doi: 10.1016/j.jsat.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Characteristics of Primary Amphetamine Treatment Admissions: 2001. The DASIS Report. 2004 Retrieved from http://store.samhsa.gov/product/Characteristics-of-Primary-Amphetamine-Treatment-Admissions-2001/SR016.
- Twamley EW, Jeste DV, Lehman AF. Vocational rehabilitation in schizophrenia and other psychotic disorders: a literature review and meta-analysis of randomized controlled trials. J Nerv Ment Dis. 2003;191:515–523. doi: 10.1097/01.nmd.0000082213.42509.69. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Rabkin JG, Ferrando SJ, Mintz J, Ryan E, Borkowski T, McElhiney M. Neuropsychiatric predictors of return to work in HIV/AIDS. J Int Neuropsychol Soc. 2007;13:80–89. doi: 10.1017/S1355617707070117. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Webster JM, Staton-Tindall M, Duvall JL, Garrity TF, Leukefeld CG. Measuring employment among substance-using offenders. Subst Use Misuse. 2007;42:1187–1205. doi: 10.1080/10826080701409800. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. 3. Wide Range, Inc; Wilmington, DE: 1993. [Google Scholar]
- Wolkstein E, Spiller H. Providing vocational services to clients in substance abuse rehabilitation. Directions in Rehabilitation Counseling. 1998;9:65–78. [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I, HNRC Group. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005b;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI The HNRC Group. Action (verb) fluency: test–retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 2005a;11:408–415. [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, Version 2.1) World Health Organization; Geneva: 1998. [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M Methamphetamine Treatment Project. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]


