Abstract
Silk protein-biomaterial wound dressings with epidermal growth factor (EGF) and silver sulfadiazine were studied with a cutaneous excisional mouse wound model. Three different material designs (silk films, lamellar porous silk films, electrospun silk nanofibers) and two different drug functionalization techniques (drug coatings or drug loading into the materials) were studied to compare wound healing responses. Changes in wound size and histological assessments of wound tissues over time confirmed that functionalized silk biomaterial wound dressings increased wound healing rate, including reepithelialization, dermis proliferation, collagen synthesis, epidermal differentiation into hair follicles and sebaceous glands, and reduced scar formation, when compared to air-permeable Tegaderm™ tape (3M) (− control) and a commercially sold wound dressing (Tegaderm™ Hydrocolloid dressing) (+ control). All silk biomaterials studied were effective for wound healing, while the porous features of the silk biomaterials (lamellar porous films and electrospun nanofibers) and the incorporation of EGF/silver sulfadiazine, via drug loading or coating, provided the most rapid wound healing responses. This systematic approach to evaluate functionalized silk biomaterial wound dressings demonstrates a useful strategy to select formulations for further study towards new treatment options for chronic wounds.
Keywords: Silk, Wound dressing, Wound healing, EGF, Silver sulfadiazine
1. Introduction
The phases of normal wound healing include hemostasis, inflammation, proliferation, and remodeling.[1] Successful wound healing requires appropriate treatments to modulate a complex series of interactions between the different cell types, cytokine mediators and the extracellular matrix through all phases of healing. To evaluate the wound healing effect of various wound dressings and medications, a full thickness skin wound model (i.e. cutaneous excisional wound) has been commonly utilized.[2–6] The healing of this cutaneous excision wound includes reepithelialization, granulation tissue proliferation and collagen synthesis.
There are numerous wound dressing biomaterials on the market today, including those composed of hydrocolloids,[7] alginates,[8] polyurethane,[6] collagen,[9] chitosan,[10] pectin[3] and hyaluronic acid.[11] To exhibit improved healing, wound dressings should demonstrate biocompatibility, an ability to retain hydration of the wound for a favorable moist environment, protection against dust and bacteria, structural control for gaseous permeation, and degradability of the material to avoid disrupting the wound bed.
Silk fibroin has been utilized as a promising material for biomedical applications such as tissue engineering and regenerative medicine due to its biocompatibility,[12, 13] material versatility and mechanical robustness,[14, 15] controllable degradability [16–18] and positive impact on wound healing effect.[5, 19] Silk has been engineered into various formats including films,[19, 20] sponges,[22, 23] hydrogels,[24] electrospun mats [21, 22] and tubes.[27, 28] On the basis of acceleration of wound healing, silk biomaterials have been applied as wound healing dressings in diverse structural forms such as films,[4] freeze-dried sponges,[5] and electrospun fibers.[21, 23] Silk wound dressings demonstrated high cell attachment and spreading of keratinocytes and fibroblasts in vitro [24] and in vivo, less inflammation and neutrophil and lymphocyte infiltration of wounds than a commercial wound healing product, Duo Active™ dressing.[4] Safety studies of silk films on rat skin confirmed that silk dressings were safe in acute dermal toxicity, acute dermal irritation and skin sensitization.[25] Moreover, the ability of stabilization and controlled release of combined signaling molecules (e.g. EGF) improved outcomes versus direct administration of growth factors, wherein rapid diffusion and enzymatic digestion/deactivation are problematic.[23]
In the present study, EGF and silver sulfadiazine were incorporated with silk biomaterials to assess impact on wound healing. EGF stimulates proliferation and migration of keratinocytes in the wound healing process,[26–28] has a high affinity receptors expressed in both fibroblasts and keratinocytes,[29, 30] and accelerates wound healing in vivo.[27, 29–31] In our previous wound healing evaluation using an in vitro model of wounded human skin-equivalents, silk wound dressings with EGF accelerated wound closure by the epidermal tongue over the silk dressings in comparison to treatments without EGF and empty wounds.[23] In this previous study, we successfully demonstrated controlled release of EGF from the silk mats (24.7% of release over 7 days). Silver sulfadiazine has been widely used as a topical antimicrobial agent for prophylaxis and treatment of wound infections,[6, 37] including in hydrocolloid and polyurethane dressings.[6, 7, 32, 33] This compound has demonstrated rapid reepithelialization and less scar formation due to increased keratinocyte proliferation in wound settings.[33]
In the present study, our goal was two-fold. First, we wanted to evaluate silk material format in terms of impact on wound healing in comparison to control materials. Second, we examined the impact of functionalization of the silk materials with EGF and silver sulfadiazine, via two different strategies – simple coatings or inclusion direction within the biomaterial matrices. Silk films (non-porous), lamellar porous silk films and silk electrospun mats were studied in comparison to air permeable Tegaderm™ tape (3M) (− control) and a commercial wound dressing product, Tegaderm™ Hydrocolloids (3M) (+ control). These silk biomaterials were fabricated as described in our previous studies.[19, 21, 25, 40] For functionalized versions of these silk materials, we demonstrated increased wound healing rate, reepithelialization, dermis proliferation and collagen formation, and reduced scar formation compared to empty (i.e. air permeable Tegaderm™ tape) and hydrocolloid treatments.
2. Results
2.1. Morphology of silk materials
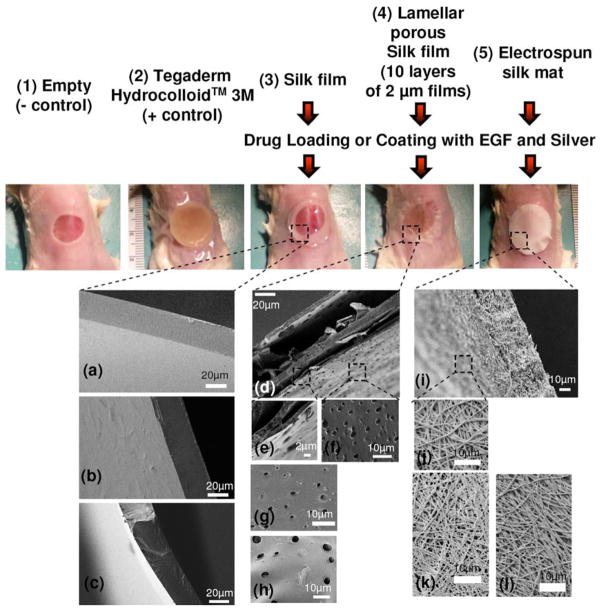
The SEM images display side views of the silk films, drug loaded silk films and drug coated silk films with a thickness of 30–40 μm (Figure 1a–c). The SEM image of the assembled lamellar porous silk film is shown in Figure 1d. Porous features (2–5 μm) were also observed in the drug loaded and coated porous silk films, as shown in the unmodified porous silk film (Figure 1f–h). The electrospinning process was used to generate silk mats, 40 μm in thickness, consisting of 100s of nanometer diameter fibers (Figure 1i–j). The morphology of the fibers was similar, whether silk alone or the silk mats with EGF and silver sulfadiazine. The presence of EGF and silver sulfadiazine did not impact the morphology of electrospun silk mats (Figure 1k–l).
Figure 1.
Silk biomaterials or Tegaderm™ Hydrocolloid Dressing on the mouse wound sites: 1) (−) control covered with air-permeable Tegarderm™ tape (3M), 2) (+) control covered with Tegaderm™ Hydrocolloid dressing (3M), 3) silk film, 4) porous silk films and 5) electrospun silk mat, with/without EGF/silver sulfadiazine loading or coating. SEM pictures of silk biomaterials: (a) silk film, (b) silk film loaded with EGF/silver sulfadiazine, (c) silk film coated with EGF/silver sulfadiazine, (d–f) lamellar porous silk film, (g) lamellar porous silk film loaded with EGF/Silver sulfadiazine, (h) lamellar porous silk film coated with EGF/Silver sulfadiazine, (i–j) electrospun silk mat, (k) electrospun silk mat loaded with EGF/silver sulfadiazine and (l) electrospun silk mat loaded with EGF/silver sulfadiazine.
2.2. Wounds
The silk biomaterials and Tegaderm™ Hydrocolloid dressing were applied on the excisional wounds (Figure 1). To visualize changes in wound size over time, photographs of the wound region were taken on days 3, 6, 9 and 12 (Figure 2). The extent of wound healing was calculated by comparing wound size at each time point with the original wound size on day 0 (Figure 3, Table 1). The wound size was significantly reduced on day 3 with the lamellar porous silk films, while not significantly different than empty (controls) wounds or the silk film. In addition, the size of the empty control wound did not change after 6 days. On days 6, 9 and 12, there was a significant difference between the empty wound and the wounds covered with all silk biomaterials and the Hydrocolloid dressing. When wounds were covered with the silk biomaterials containing EGF and silver sulfadiazine, drug loading and coating enhanced wound healing rate except for the silk films loaded with drug. Wound closure with the drug coated lamellar film (52 ± 2% and 32 ± 1%, respectively) was significantly greater than the wound closure with the no-drug applied lamellar films (52 ± 2 % and 26 ± 3 %, respectively) on day 6 and 9. We also evaluated a positive control with Tegaderm™ Hydrocolloid (3M), which is a commercially sold wound healing dressing. Compared to this positive control, wound healing with all of the silk biomaterial patches were not significantly different, or in some cases were significantly faster with the silk systems, such as for the drug coated lamellar films, drug loaded electrospun mats and drug-coated electrospun mats on day 6.
Figure 2.

Extent of wound healing on days 3, 6 and 12. (a) empty, (−) control covered with air-permeable Tegarderm (3M) tape, (b) (+) control covered with Tegaderm™ Hydrocolloid dressing (3M), (c) silk film, (d) silk film loaded with EGF/silver sulfadiazine, (e) silk film coated with EGF/silver sulfadiazine, (f) lamellar porous silk film, (g) lamellar porous silk film loaded with EGF/silver sulfadiazine, (h) lamellar porous silk film coated with EGF/silver sulfadiazine, (i) electrospun silk mat, (j) electrospun silk mat loaded with EGF/silver sulfadiazine and (k) electrospun silk mat coated with EGF/silver sulfadiazine. Scale bars 1.5 cm.
Figure 3.

Changes in wound size with silk film (a), lamellar silk film (b) and electrospun silk mat (c). These silk biomaterials were loaded or coated with drug (EGF and silver sulfadiazine). Wound size with silk biomaterials was compared with wound size with air-permeable Tegaderm™ (3M) tape (empty) and Tegaderm™ Hydrocolloid dressing (3M).
Table 1.
Changes in wound sizes with silk biomaterials and Hydrocolloid (3M)
| Samples | Functionalization | Wound Size (%)
|
|||
|---|---|---|---|---|---|
| Day 3 | Day6 | Day 9 | Day 12 | ||
|
| |||||
| Film | No Drug | 92 ± 21 | 53 ± 14* | 29 ± 27* | 13 ± 13* |
| Drug Loaded | 107 ± 5 | 66 ± 16* | 43 ± 5* | 20 ± 4* | |
| Drug Coated | 92 ± 25 | 45 ± 7*$ | 24 ± 14* | 9 ± 9* | |
|
| |||||
| Lamellar Film (porous) | No Drug | 89 ± 6* | 52 ± 2* | 32 ± 1* | 17 ± 4* |
| Drug Loaded | 71 ± 11* | 56 ± 4* | 34 ± 17* | 14 ± 11* | |
| Drug Coated | 74 ± 14* | 46 ± 2*#$ | 26 ± 3*# | 9 ± 5* | |
|
| |||||
| E-spun Mat | No Drug | 66 ± 10* | 5 ± 1* | ||
| Drug Loaded | 53 ± 4*$ | 6± 1* | |||
| Drug Coated | 48 ± 12*$ | 5± 1* | |||
|
| |||||
| Emptya) | 115 ± 12 | 99 ± 3 | 69 ± 17 | 29 ± 5 | |
|
| |||||
| + Control (Hydrocolloid, 3M) | 67 ± 5* | 10 ± 8* | |||
Empty wound sites were covered with air-permeable Tegarderm (3M) tape, while the other sites were covered with a silk patch or Hydrocolloid patch (control) and then Tegarderm (3M) tape.
indicates statistical significance between means of sample group and empty group at same time points (p < 0.05, N = 3 Mean ± SD).
indicates statistical significance between means of drug-functionalized group and no-drug group at same time points (p < 0.05, N = 3 Mean ± SD).
indicates statistical significance between means of sample group and +control (Hydrocolloid) group at same time points (p < 0.05, N = 3 Mean ± SD).
2.3. Histology and Immunostaining
Given the improved wound healing with the silk biomaterials over empty defects and the Tegaderm™ Hydrocolloid dressing, we examined the histology of the wound tissues (Figures 4, 5) at days 6 and day 12. Histological data showed that the silk biomaterials reduced the presence of inflammatory cells compared to empty defects (Figures 5c, 6i). Compared with the empty group, silk wound dressings provided structural support for cell attachment, growth and migration on the wound sites, and the epidermis in the silk biomaterial covered wounds was thicker than that on the empty wounds. There was ingrowth of capillaries and the presence of fibroblasts with the drug coated silk biomaterials. After 12 days, all wounds covered with the silk biomaterials showed a regenerated dermis and a concurrent epithelium with several viable layers (Figure 4). Masson’s Trichrome staining of wounds on day 6 and 12 indicated that the collagen regenerated in the silk biomaterial covered wounds was denser than those in the empty wound and the Hydrocolloid dressing covered wounds (Figure 6). EGF-loaded and -coated silk biomaterials (Figure 6l,o,r and Figure 6d,f,h,m,p,s, respectively) produced more obvious collagen production than no EGF-incorporated ones (Figure 6c,e,g,k,n,q).. Interestingly, bud-like structures were visible with EGF-loaded and -coated silk biomaterials, especially in cases of porous silk biomaterials (i.e. lamellar porous films and electrospun fibers), appearing like epithelial in-growth and hair follicle/sebaceous gland differentiation (Figure 6l,m,o,p,r,s).
Figure 4.
Hematoxylin and Eosin staining images of wounds on day 6: (a,b) (−) control covered with air-permeable Tegarderm (3M) tape (i.e. empty) (edge and middle section, respectively), (c,d) (+) controlcovered with Tegaderm™ Hydrocolloid dressing (3M) (edge and middle section, respectively), (e) normal mouse skin (f,g) silk film (edge and middle section, respectively), (h,i) silk film coated with EGF/silver sulfadiazine (edge and middle section, respectively), (j,k) lamellar porous silk film (middle section), (l,m) lamellar porous silk film coated with EGF/Silver sulfadiazine (edge and middle section, respectively), (n,o) electrospun silk mat (edge and middle section, respectively), (p,q) electrospun silk mat coated with EGF/silver sulfadiazine (edge and middle section, respectively). Arrow points to the punched site. D - dermis. E - Epidermis. HC -Hydrocolloid dressing (3M). SF - silk film. LPSP - Lamellar porous silk film. E-mat- electrospun silk mat or electrospun silk mat coated with EGF/silver sulfadiazine. Scale bars 200μm.
Figure 5.
Hematoxylin and Eosin staining photographs of wounds on day 12: (a–c) (−) control covered with air-permeable Tegarderm (3M) tape (i.e. empty), (d,e) (+) control covered with Tegaderm™ Hydrocolloid dressing (3M), (f,g) silk film, (h,i) silk film loaded with EGF/silver sulfadiazine, (j,k) silk film loaded with EGF/silver sulfadiazine, (l,m) lamellar porous silk film, (n,o) lamellar porous silk film loaded with EGF/silver sulfadiazine, (p,q) lamellar porous silk film coated with EGF/silver sulfadiazine, (r,s) electrospun silk mat, (t,u) electrospun silk mat loaded with EGF/silver sulfadiazine (v,w) electrospun silk mat coated with EGF/silver sulfadiazine. Arrow points to the punched site. D - dermis. E - Epidermis. * - inflammatory cells. Scale bars 800 μm in (a, d, f, h, j, l, n, p, r, v) and 200 μm in (b, c, e, g, i, k, m, o, q, s, u, w).
Figure 6.
Masson’s Trichrome staining images of wounds on day 6 and 12: (a) normal mouse skin, (b, j) (−) control covered with air-permeable Tegaderm™ (3M) tape (i.e. empty) on day 6 and 12, respectively, (c,k) (+) control covered with Tegaderm™ Hydrocolloid dressing (3M) on day 6 and 12, respectively, (d,l) silk film on day 6 and 12, respectively, (e,m) silk film coated with EGF/Silver sulfadiazine on day 6 and 12, respectively, (f,n) lamellar porous silk film on day 6 and 12, respectively, (g,o) lamellar porous silk film coated with EGF/silver sulfadiazine on day 6 and 12, respectively, (h,p) electrospun silk mat on day 6 and 12, respectively, (i,q) electrospun silk mat coated with EGF/silver sulfadiazine on day 6 and 12, respectively. Blue color represents collagen staining. Black arrow points to the site of epidermal differentiation into hair follicles and sebaceous glands. D - dermis. E- Epidermis. * - inflammatory cells. HC -Hydrocolloid dressing (3M). E-mat - electrospun silk mat or electrospun silk mat coated with EGF/silver sulfadiazine. Scale bars 200 μm..
Next, we examined suprabasal (cytokeratin 10-CK10) and basal (cytokeratin 14-CK14) expression on the regenerated epithelial layers on day 6 and at week 5 (Figure 7 and 8). On day 6, we observed CK10 expression as a biochemical marker for keratinocyte differentiation on all the regenerated epithelial layers in the granulation sites of the all the wound edges (Figure 7a,c,e,g). CK10 expression was monitored on the regenerated thin epithelial layers in the middle sections of the wounds covered with silk biomaterials (lamellar porous film and drug-coated lamellar porous films) (Figure 7f,h), whereas we did not observe epithelial layers and CK10 expression in the middle sections of the wounds covered with Hydrocolloid or without biomaterials (empty) (Figure 7b,d). CK10 and CK14 staining at Week 5 demonstrated suprabasal and basal expression, respectively, on all the regenerated epithelial layers in the wounds, without significant difference between the groups as shown in the normal skin (Figure 8).
Figure 7.

CK10 immunostaining photographs of wounds on day 6: (a,b) (−) controlcovered with air-permeable Tegaderm™ (3M) tape (i.e. empty), (edge and middle section, respectively), (c,d) (+) controlcovered with Tegaderm™ Hydrocolloid dressing (3M) (edge and middle section, respectively), (e,f) lamellar porous silk film (edge and middle section, respectively), (g,h) lamellar porous silk film coated with EGF/silver sulfadiazine (edge and middle section, respectively). Black arrow points to the site of CK10 expression. Scale bars 50 μm.
Figure 8.
CK10 and CK14 immunostaining photographs of wounds at week 5: (a,b) (−) control covered with air-permeable Tegaderm™ (3M) tape (i.e. empty), (CK10 and CK14, respectively), (c,d) (+) controlcovered with Tegaderm™ Hydrocolloid dressing (3M) (CK10 and CK14, respectively), (e,f) lamellar porous silk film (CK10 and CK14, respectively), (g,h) lamellar porous silk film coated with EGF/silver sulfadiazine (CK10 and CK14, respectively), (i,j) normal mouse skin (CK10 and CK14, respectively). Scale bars 50 μm.
2.4. Scar formation
At 5 weeks after wounding, the wounds covered with or without biomaterials and the Hydrocolloid dressings were visualized (Figure 9). The area of the wounds covered with the silk biomaterials and Hydrocolloid dressing appeared smaller when compared with the empty skin wounds at week 5. Interestingly, the shape of the wounds covered with the silk biomaterials and Hydrocolloid dressing were round, representing wound closure through re-epithelialization and epidermal growth, whereas the shape of the empty wounds was elongated, likely due to contraction of the mouse skin. In addition, the area of the wounds covered with the silk biomaterials appeared smooth when compared with that covered with Hydrocolloid dressing. Histology images (H&E staining, 10a–d, Masson trichrome staining, Figure 10e–m) of the wounds with or without silk biomaterials and the Hydrocolloid dressing are shown at Week 5. The findings showed significantly reduced scar formation at both the visual and histological levels in the mouse wounds compared with empty wounds and wounds covered with Hydrocolloid dressing at week 5.
Figure 9.

Images of the wound sites at week 5. (a) empty, (−) controlcovered with air-permeable Tegaderm™ (3M) tape, (b) (+) controlcovered with Tegaderm™ Hydrocolloid dressing (3M), (c) silk film, (d) film coated with EGF/silver sulfadiazine, (e) lamellar porous silk film, (f) lamellar porous silk film coated with EGF/silver sulfadiazine, (g) electrospun silk mat, (h) electrospun silk mat coated with EGF/silver sulfadiazine. Scale bars 1cm.
Figure 10.
Histology images of wounds at week 5. Hematoxylin and Eosin staining photographs: (a, b) empty, (−) control covered with air-permeable Tegaderm™ (3M) tape, (c,d) electrospun silk mat coated with EGF/silver sulfadiazine. Masson’s Trichrome staining images: (e) empty, (−) control covered with air-permeable Tegaderm™ (3M) tape, (f) (+) control covered with Tegaderm™ Hydrocolloid dressing (3M), (g) silk film, (h) film coated with EGF/silver sulfadiazine, (i) lamellar porous silk film, (k) lamellar porous silk film coated with EGF/silver sulfadiazine, (k) electrospun silk mat, (l) electrospun silk mat coated with EGF/silver sulfadiazine and (m) normal mouse skin. Blue color in Masson’s Trichrome staining represents collagen staining. White arrow points to the punch site. Black arrow points to the site of epidermal differentiation into hair follicles and sebaceous glands. Scale bars 800 μm in (a,c) and 200 μm in (b, d, e–m).
3. Discussion
A successful biomaterial dressing for wound healing should demonstrate proper material selection and optimized design to alleviate infection and inflammation and direct cells and their production to promote wound healing and less scar formation. In the previous work, we focused on the preparation of electrospun mats with controllable porosity [21] and the incorporation of EGF on silk mats with their in vitro evaluation.[23] The latter study showed that EGF was incorporated into electrospun silk mats and slowly released in a time-dependent manner to shorten the time of wound closure by the epidermal tongue, using a model of wounded human skin-equivalents displaying the same structure as human skin. Through this work, it was found that silk biomaterials, probably through the benefits of intrinsic material biocompatibility, growth factor loading and controlled release capability and the ability to form porous structures, enhanced the wound healing process. However, it was important to evaluate functionalized silk biomaterials in an in vivo environment as a next step. Even though silk films have been evaluated using in vivo wound healing models,[4] the effects of functionalization and various structures of silk biomaterials in vivo for wound repair have not been studied.
In the present study, using an in vivo cutaneous excisional mouse wound model, functionalized silk biomaterial treatments increased wound healing rates, reepithelialization, dermis proliferation, collagen synthesis and epidermal differentiation into hair follicles and sebaceous glands, and reduced scar formation, when compared with empty (air permeable Tegaderm tape) and Tegaderm™ Hydrocolloid (3M) treatments. Wound healing dressings should be properly designed to establish and maintain an optimal wound healing environment. For example, the biomaterial design includes electrospun nanofibers which have structural advantages for wound healing due to high surface area and gas permeation. Thus, to evaluate suitable wound healing dressing designs, we prepared three different types of silk biomaterials (film, lamellar porous film and electrospun mat) and examined the influence of these different material formats on wound healing. As reported in our previous study, porous films can be obtained by incorporating PEO into silk films during processing and then leaching out the PEO domains to generate ~2 μm sized pores.[19, 34] The thickness of the porous film was around 2 μm and a total of 10 films were stacked per construct to form an easily-handled, porous and transparent wound dressing. As mentioned above, a porous feature of wound healing biomaterials is desirable for air exchange to promote wound healing for oxygen exchange[35] vital for fibroblast proliferation, collagen synthesis, and polymorphonuclear cell functions.[36–38] Therefore, two different porous biomaterials (porous films, electrospun mats) were compared with non-porous silk films. Although silk films showed oxygen permeation in physiological environments,[39] oxygen permeability through non-porous silk membranes is not comparable to that through the physically generated pores in electrospun mats and porous films. Furthermore, we note that the lamellar porous silk films were transparent, similar to the silk films (Figure 1), which may provide aesthetic advantages in certain applications as well as for visual observations of the wound site. In contrast, the electrospun silk mats were opaque. The studies for wound closure time and histological evaluation of wound sites over time demonstrated that all the silk biomaterials increased wound healing rate, fibroblast proliferation, reepithelialization and collagen synthesis in comparison to the empty wounds (air-permeable Tegaderm™ tape, 3M) and in some cases over the Hydrocolloid dressing; however, the difference in wound closure rate between non-porous silk biomaterials (silk films) and the porous silk biomaterials (lamellar silk films, electrospun mat) was not statistically significant. In contrast, the porous silk biomaterials promoted more collagen production than the non-porous silk biomaterials on days 6 and 12.
We observed that EGF and silver sulfadiazine in all silk biomaterials improved overall wound healing. These results confirmed the previous in vitro studies of EGF incorporated silk electrospun nanofibers.[23] In the composite organotypic co-culture wound healing model, the percentage of wound closure was statistically improved with the silk mats containing EGF (wound closure of 84.8%) compared to the control (wound closure of 2.6%) and the silk mat without EGF (wound closure of 15.7%) after 24 hours.[23] Interestingly, the effect of EGF in the in vivo mouse wound model was not as significant as in the previous in vitro model study. In contrast, the difference between the wound size changes with and without silk biomaterials was significant in the present in vivo model (Table 1). Considering that the in vivo environment naturally produces various growth factors, the biocompatibility of silk biomaterials might promote the surrounding healthy cells in the wound site to generate the growth factors required for wound healing. On the other hand, the effect of EGF incorporation in silk biomaterials on collagen production and the development of bud-like structures appearing like epithelial in-growth and hair follicle/sebaceous gland differentiation was noted. It was reported that EGF increased collagen production in granulation tissue by the stimulation of fibroblast proliferation and not by activation of the procollagen gene.[31] Therefore, more collagen accumulation (Figure 6) with EGF-incorporated silk biomaterials appears to occur due to more fibroblast proliferation (Figs. 4 and 5).
Two different drug incorporation techniques were used in this study: drug loading and coating. Drug loading was performed by directly mixing EGF and silver sulfadiazine in silk solution before casting films or electrospining mats. Using these drug loading techniques, silk biomaterials have shown their ability to stabilize bioactive molecules such as enzymes[40] and growth factors.[41, 42] Moreover, controlled release of EGF from silk biomaterials and normal bioactivity of the released EGF were confirmed in our previous study.[23] However, the process to generate porous structures in the lamellar porous films and electrospun mats requires the removal of PEO by soaking the biomaterials in water, which can result in drug loss. In addition, sterilization such as by autoclaving might reduce the bioactivity of EGF. Therefore drug coating was carried out by simple dipping of the silk biomaterials into EGF and silver sulfadiazine mixture solution for 48 hours at 4°C. The effect of two drug incorporation techniques was not significantly different, whereas drug coating resulted in apparently faster wound close rate and more epidermal differentiation into hair follicles and sebaceous glands than drug loading.
The influence of biodegradation of silk biomaterials on the wound healing process has not been characterized. Silk is biocompatible and less immunogenic and inflammatory than collagens or polyesters such as polylactic acid.[12, 43, 44] Silk biomaterials can be engineered to degrade at a slow or fast rate based on processing. Our previous metabolic and osteogenesis studies confirmed that more rapidly degrading scaffolds exhibited higher glucose consumption, lactate synthesis and mineralization in comparison to the more slowly degrading scaffold system.[45] Interestingly, in the present study, we observed cell migration into the lamellar porous films and electrospun fibers on day 6 (Figures 4j–q and 6e–h). Given that the pore sizes of electrospun mats and porous films are 10s–100s nm and 2–5 μm, respectively, the migration of cells should be related to partial deformation of the biomaterials due to fractional biodegradation of the thin porous film layers and entangled nanofibers, dependent on the rate of degradation.
Another interesting result from this study was the formation of bud-like structures under the epidermis when the wounds were covered with the EGF and silver sulfadiazine-incorporated silk biomaterials. Although the signaling cascades required for epidermal differentiation are mainly unknown, epidermal stem cells have three fates: hair follicles, sebaceous glands and epidermis. Sebaceous glands and hair follicle differentiation are related to overexpression of c-Myc in the basal layer of the epidermis.[46, 47] Growth factors, such as Neuregulin3, regulate cell fate of epidermal morphogenesis and differentiation into hair follicles by activating c-Myc.[48] However, EGF has been known to inhibit hair follicle induction upon subcutaneous injections,[49–51] while daily topical applications of EGF did not affect the follicles and other skin components.[51] It is unknown, however, if down-regulation of epidermal differentiation to the hair follicles with EGF occurs after wounding. It was reported that after wounding, hair follicles form de novo in genetically normal adult mice, where overexpression of Wnt ligand in the epidermis increases the number of regenerated hair follicles.[52] The epidermal environment after wounding is different than normal skin, so the effect of EGF, especially in controlled release, on the regulation of epidermal differentiation fate after wounding will require additional inquiry. Additional animal wound models may also be required, such as a swine model with a wound healing process similar to human skin.[53] The mouse skin model used in the present work has the limitation of rapid wound contraction combined with the formation of granulation tissue.
4. Conclusions
Three different silk biomaterials with two different drug functionalization techniques were engineered to study the effect of silk material architecture and drug functionalization on wound healing. The silk biomaterial wound dressings increased wound healing rate, reepithelialization, dermis proliferation, collagen synthesis and epidermal differentiation into hair follicles and sebaceous glands, and reduced scar formation, when compared to air-permeable Tegarderm™ tape (empty) and commercially sold wound dressing, Tegaderm™ Hydrocolloid dressing. The porous silk biomaterials (lamellar porous films and elecrospun mats) promoted more rapid wound healing responses than the non-porous silk biomaterials (films). EGF and silver sulfadiazine in all silk biomaterials improved overall wound healing, whereas drug coated silk biomaterials resulted in slightly faster wound close rates and more epidermal differentiation into hair follicles and sebaceous glands than the drug loaded silk biomaterials. However, the difference between the wound size changes with and without silk biomaterials was more significant than that between the wound size changes with and without the drugs. This result was the opposite of our previous in vitro study and demonstrated that the biocompatibility of silk biomaterials might promote the surrounding healthy cells in the wound site to generate the growth factors required for wound healing. Altogether, the results indicated that the functionalized silk biomaterials outperformed empty and commercially sold wound dressing in terms of wound-closure and scar formation and suggest the promise of a new wound healing system with superior material biocompatibility, degradability to avoid removal from the healing wound, and options for porous structure and functionalization.
5. Experimental Section
Preparation of silk solution
Silk solution was prepared from Bombyx mori silkworm cocoons according to the procedures described in our previous studies.[16, 22] Cocoons were supplied by Tajima Shoji Co. (Yokohama, Japan). Briefly, the cocoons were degummed in a boiled 0.02M Na2CO3 (Sigma–Aldrich, St. Louis, MO) solution for 20 min. The fibroin extract was then rinsed three times in Milli-Q water, dissolved in a 9.3 M LiBr solution yielding a 20% w/v solution, and subsequently dialyzed (MWCO 3,500) against distilled water for 2 d to obtain a silk fibroin aqueous solution (ca. 8% w/v).
Preparation of EGF/silver sulfadiazine-loaded and -coated silk films
Silk films were cast on Polydimethylsiloxane (PDMS) molds according to the procedures described in our previous studies.[21, 41] Briefly, a 100 μL of 8% silk solution was cast on prepared PDMS substrates, 14 mm in diameter, to generate films with a thickness of 25–30 μm. The as-cast silk films were water-annealed in a water-filled desiccator at 24 mmHg vacuum for 5 h. The films were sterilized with 70% EtOH and UV, and washed three times in sterile PBS (pH 7.4). We selected two approaches to incorporate human epidermal growth factor (human EGF, Sigma-Aldrich) and silver sulfadiazine (Sigma-Aldrich) into silk films as follows: 1) for loading EGF/silver sulfadiazine, EGF (125 μg/g of silk fibroin) and silver sulfadiazine (10 mg/g of silk fibroin) were mixed in silk solution before casting, 2) for coating EGF/silver sulfadiazine, the sterilized silk films were soaked in a mixture of EGF (125 μg/ml) and silver sulfadiazine (10 mg/ml) for 48 hours at 4 C and washed in PBS (pH 7.4). The EGF/silver sulfadiazine mixture was filtered with 0.2 micron filter before coating.
Preparation of EGF/silver sulfadiazine-loaded and -coated porous silk films
Porous silk films was formed according to the procedures described in our previous studies.[20] Briefly, a mixture of 1% silk fibroin and 0.035% polyethylene oxide (PEO, MW = 900,000 Daltons; Sigma-Aldrich) solution was prepared to induce pore formation within the silk film matrix. One hundred μL of solution was cast on PDMS substrates to produce films 2 μm film thick. Post-casting, silk films were water-annealed and then placed into a water bath overnight to leach out the PEO. The films were sterilized with 70% EtOH and UV, and then washed three times in sterile PBS (pH 7.4). In order to incorporate EGF and silver sulfadiazine, two approaches were adapted as above. The porous silk films were then carefully stacked using applied pressure from a 12 mm biopsy punch; applying pressure after each film layer was stacked. A total of 10 films were stacked per construct. The applied pressure was used to seal the stacked film construct together near the film edges.
Preparation of EGF/silver sulfadiazine-loaded and -coated electrospun mats
Electrospinning was performed according to the procedures described in our previous studies.[23] Briefly, electrospinning equipment was built with a high voltage power supply (Gamma High Voltage Research ES-30P, Ormond Beach, Fl.), Thermo Orion Pump Syring Sage Mod M362 (Thermo Scientific, Waltham, MA), potential and ground stages, 1.5 mm polyethylene tubing and a 16-gauge 5.08 cm steel capillary tube. The syringe needle was maintained at a high electric potential (12.0–15.0 kV) for electrospinning and mounted above the grounded collection plate in the parallel plate geometry. The distance between the syringe needle and the grounded collection plate was 20–25 cm and a constant volume flow rate of 0.025 ml min−1 was maintained using a syringe pump. A mixture of 4 mL of 8% silk fibroin and 1 mL of 5% polyethylene oxide (PEO, Mw = 900,000; Sigma-Aldrich) solution was prepared. Four mL of the silk/PEO solution was electrospun on a collection plate covered with aluminum foil, 10 cm in diameter to generate mats ~40 μm thick. EGF and silver sulfadiazine were incorporated with the silk electrospun mat through the loading and coating processes described above. The electrospun mats were treated with methanol for 10 min to obtain water insoluble silk mats. The average thickness of the dressings was around 40 μm.
Implants in mice
All surgical procedures were conducted under animal care protocols approved by the Tufts Institutional Animal Care and Use Committee. All animals used in this study were five to seven weeks old BALB/C female mice (Charles River Labs, Boston, MA). The mice were distributed by three experimental groups each with three time points: 6 days, 12 days and 5 weeks. The mice were randomly assigned to the experimental groups. After anaesthetization (1.5–3 vol % Isoflurane), the dorsal surface was shaved and then punched with a sterile 8-mm punch biopsy punch. Empty wound sites were covered with air-permeable Tegarderm™ (3M, St. Paul, MN) tape and used as a control group, while the other wound sites were covered with 3M™ Tegaderm™ Hydrocolloid Dressing (3M) or the silk biomaterial patches and then the Tegarderm™ (3M) tape. The wound region of the test group was compared to that of the control group. Mice were sacrificed by CO2 exposure after 6 days, 12 days and 5 weeks and samples were collected along with the overlying tissue for histological examination. Wound size was evaluated for empty, silk film-covered, porous silk film-covered wound sites on day 3 and 9 under anesthesia without removing the patches because these wound sites were visible through the transparent patches. The wound area was analyzed with ImagePro6 (Media Cybernetics, Bethesda, MD) software. The extent of wound healing is expressed as the percentage of area remaining exposed. Wound size (%) = [R(3,6,9,12)/R(0)] × 100, where R(0) and R(3,6,9,12) represents the exposed wound area at postoperative days 0 and 3, 6, 9 and 12, respectively.
Scanning electron microscopy (SEM)
Fractured and surface sections of the silk biomaterials were sputter coated with Pt/Pd. The fractured sections were obtained in liquid nitrogen using a razor blade. Morphology was examined with a Field Emission Scanning Electron Microscope (FESEM) Zeiss EVO10 (Carl Zeiss AG, Germany) at 3 kV.
Histological evaluation and Immunostaining
On day 6, the wounds with silk biomaterials were excised together, while on day 12 and week 5, the wounds were excised after removing the silk biomaterials. After fixation with 4% phosphate-buffered formaldehyde for at least 24 h, the specimens were embedded in paraffin and sectioned into a thickness of 10 μm. The samples underwent routine histological processing with hematoxylin and eosin, and Masson’s Trichrome. For immunohistochemical staining, the sections underwent antigen retrieval under heated, low pH conditions. Primary antibodies for mouse Ki67, cytokeratin 10 and cytokeratin 14 were purchased from Abcam (Cambridge, MA). Antibody diluent was purchased from Cell Signaling Technologies (Danvers, MA). The secondary antibodies, ABC (avidin, biotin complex) kit, DAB substrate, hematoxylin counterstain and, antigen retrieval solution were purchased from Vector Labs (Burlingame, CA). Non-specific binding was avoided by incubation with normal blocking serum. After the excess serum was removed, the sections were incubated for 30 minutes with anti-mouse Ki67 (diluted 1:1000 in antibody diluent), anti-mouse cytokeratin 10 (1:100), and anti-mouse cytokeratin 14 (1:200). Sections were washed in PBS and, then incubated with a secondary antibody for 30 minutes. The sections were washed in PBS and then incubated with VECTASTAIN Elite ABC reagent for 30 minutes, washed in PBS, incubated with ImmPACT DAB enzyme substrate for 5 minutes and washed in water. The sections were then counterstained with hematoxylin and mounted.
Statistical methods
Results were statistically analyzed using one-way analysis of variance (ANOVA). A statistically significant difference was reported if p < 0.05. Data are reported as the mean ± standard deviation (SD) with an N = 3 or more.
Acknowledgments
This study was supported by NIH/NIBIB EB002520 via the P41 Tissue Engineering Resource Center.
References
- 1.Diegelmann RF, Evans MC. Front Biosci. 2004;9:283. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 2.Strehin IA, Nahas Z, Rich M, Marti GP, Zhang X, Harmon JW, Elisseeff JH. Wound Repair Regen. 2011;19:A54. [Google Scholar]
- 3.Lim HJ, Kim HT, Oh EJ, Choi JH, Ghim HD, Pyun DG, Lee SB, Chung DJ, Chung HY. Polym-Korea. 2010;34:363. [Google Scholar]
- 4.Sugihara A, Sugiura K, Morita H, Ninagawa T, Tubouchi K, Tobe R, Izumiya M, Horio T, Abraham NG, Ikehara S. P Soc Exp Biol Med. 2000;225:58. doi: 10.1046/j.1525-1373.2000.22507.x. [DOI] [PubMed] [Google Scholar]
- 5.Roh DH, Kang SY, Kim JY, Kwon YB, Kweon HY, Lee KG, Park YH, Baek RM, Heo CY, Choe J, Lee JH. J Mater Sci-Mater M. 2006;17:547. doi: 10.1007/s10856-006-8938-y. [DOI] [PubMed] [Google Scholar]
- 6.Konrad D, Tsunoda M, Weber K, Corney SJ, Ullmann L. J Exp Anim Sci. 2002;42:31. [Google Scholar]
- 7.Wyatt D, Mcgowan DN, Najarian MP. J Trauma. 1990;30:857. doi: 10.1097/00005373-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Ligouri PA, Peters KL. J Wound Ostomy Cont. 2009;36:S46. [Google Scholar]
- 9.Powell HM, Supp DM, Boyce ST. Biomaterials. 2008;29:834. doi: 10.1016/j.biomaterials.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Biomaterials. 2008;29:4323. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Uppal R, Ramaswamy GN, Arnold C, Goodband R, Wang Y. J Biomed Mater Res B. 2011;97B:20. doi: 10.1002/jbm.b.31776. [DOI] [PubMed] [Google Scholar]
- 12.Panilaitis B, Altman GH, Chen JS, Jin HJ, Karageorgiou V, Kaplan DL. Biomaterials. 2003;24:3079. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 13.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. Biomaterials. 2005;26:147. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Shao ZZ, Vollrath F. Nature. 2002;418:741. doi: 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- 15.Motta A, Fambri L, Migliaresi C. Macromol Chem Physic. 2002;203:1658. [Google Scholar]
- 16.Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Kaplan DL. Adv Funct Mater. 2005;15:1241. [Google Scholar]
- 17.Hu X, Shmelev K, Sun L, Gil ES, Park SH, Cebe P, Kaplan DL. Biomacromolecules. 2011;12:1686. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. Biomaterials. 2008;29:3415. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL. Biomaterials. 2009;30:1299. doi: 10.1016/j.biomaterials.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil ES, Mandal BB, Park SH, Marchant JK, Omenetto FG, Kaplan DL. Biomaterials. 2010;31:8953. doi: 10.1016/j.biomaterials.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wharram SE, Zhang XH, Kaplan DL, McCarthy SP. Macromol Biosci. 2010;10:246. doi: 10.1002/mabi.200900274. [DOI] [PubMed] [Google Scholar]
- 22.Wittmer CR, Claudepierre T, Reber M, Wiedemann P, Garlick JA, Kaplan D, Egles C. Adv Funct Mater. 2011;21:4232. doi: 10.1002/adfm.201190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Acta Biomater. 2009;5:2570. doi: 10.1016/j.actbio.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Biomaterials. 2004;25:1289. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Padol AR, Jayakumar K, Shridhar NB, Narayana Swamy HD, Narayana Swamy M, Mohan K. Toxicology international. 2011;18:17. doi: 10.4103/0971-6580.75847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrandon Y, Green H. Cell. 1987;50:1131. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- 27.Buckley A, Davidson JM, Kamerath CD, Wolt TB, Woodward SC. P Natl Acad Sci USA. 1985;82:7340. doi: 10.1073/pnas.82.21.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin JD, Lynch JB. Plast Reconstr Surg. 1979;64:766. doi: 10.1097/00006534-197912000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Okeefe E, Battin T, Payne R. J Invest Dermatol. 1982;78:482. doi: 10.1111/1523-1747.ep12510246. [DOI] [PubMed] [Google Scholar]
- 30.Knauer DJ, Wiley HS, Cunningham DD. J Biol Chem. 1984;259:5623. [PubMed] [Google Scholar]
- 31.Laato M, Kahari VM, Niinikoski J, Vuorio E. Biochem J. 1987;247:385. doi: 10.1042/bj2470385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afilalo M, Dankoff J, Guttman A, Lloyd J. Burns. 1992;18:313. doi: 10.1016/0305-4179(92)90153-l. [DOI] [PubMed] [Google Scholar]
- 33.Madden MR, Nolan E, Finkelstein JL, Yurt RW, Smeland J, Goodwin CW, Hefton J, Staianocoico L. J Trauma. 1989;29:924. doi: 10.1097/00005373-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Gil ES, Park SH, Marchant J, Omenetto F, Kaplan DL. Macromol Biosci. 2010;10:664. doi: 10.1002/mabi.200900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt TK, Dunphy JE. Brit J Surg. 1969;56:705. [PubMed] [Google Scholar]
- 36.Hunt TK, Zederfel B, Goldstic Tk. Am J Surg. 1969;118:521. doi: 10.1016/0002-9610(69)90174-3. [DOI] [PubMed] [Google Scholar]
- 37.Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. New Engl J Med. 1979;301:77. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- 38.Babior BM. New Engl J Med. 1978;298:659. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence BD, Wharram S, Kluge JA, Leisk GG, Omenetto FG, Rosenblatt MI, Kaplan DL. Macromol Biosci. 2010;10:393. doi: 10.1002/mabi.200900294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Q, Wang XQ, Hu X, Cebe P, Omenetto F, Kaplan DL. Macromol Biosci. 2010;10:359. doi: 10.1002/mabi.200900388. [DOI] [PubMed] [Google Scholar]
- 41.Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. J Biomed Mater Res A. 2004;71A:528. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 42.Wongpanit P, Ueda H, Tabata Y, Rujiravanit R. J Biomat Sci-Polym E. 2010;21:1403. doi: 10.1163/092050609X12517858243706. [DOI] [PubMed] [Google Scholar]
- 43.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 44.Meinel L, Betz O, Fajardo R, Hofmann S, Nazarian A, Cory E, Hilbe M, McCool J, Langer R, Vunjak-Novakovic G, Merkle HP, Rechenberg B, Kaplan DL, Kirker-Head C. Bone. 2006;39:922. doi: 10.1016/j.bone.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Park SH, Gil ES, Shi H, Kim HJ, Lee K, Kaplan DL. Biomaterials. 2010;31:6162. doi: 10.1016/j.biomaterials.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koster MI, Huntzinger KA, Roop DR. J Investig Dermatol Symp Proc. 2002;7:41. doi: 10.1046/j.1523-1747.2002.19639.x. [DOI] [PubMed] [Google Scholar]
- 47.Lo Celso C, Berta MA, Braun KM, Frye M, Lyle S, Zouboulis CC, Watt FM. Stem Cells. 2008;26:1241. doi: 10.1634/stemcells.2007-0651. [DOI] [PubMed] [Google Scholar]
- 48.Panchal H, Wansbury O, Parry S, Ashworth A, Howard B. BMC Dev Biol. 2007;7:105. doi: 10.1186/1471-213X-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adelson DL, Hollis DE, Merchant JC, Kelley BA. Reprod Fertil Dev. 1997;9:493. doi: 10.1071/r96118. [DOI] [PubMed] [Google Scholar]
- 50.Richardson GD, Bazzi H, Fantauzzo KA, Waters JM, Crawford H, Hynd P, Christiano AM, Jahoda CA. Development. 2009;136:2153. doi: 10.1242/dev.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapman RE, Hardy MH. Aust J Biol Sci. 1988;41:261. doi: 10.1071/bi9880261. [DOI] [PubMed] [Google Scholar]
- 52.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Nature. 2007;447:316. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. Wound Repair Regen. 2001;9:66. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]