Abstract
The process of cellular transformation involves cascades of molecular changes that are modulated through altered epigenetic, transcription, post-translational and protein regulatory networks. Thus, identification of transformation-associated protein alterations can provide an insight into major regulatory pathways activated during disease progression. In the present protein expression profiling approach, we identified differential sets of proteins in a two-dimensional gel electrophoresis screen of a serous ovarian adenocarcinoma progression model. Function-based categorization of the proteins exclusively associated with pre-transformed cells identified four cellular processes of which RXR-γ is known to modulate cellular differentiation and apoptosis. We thus probed the functional relevance of RXR-γ expression and signaling in these two pathways during tumor progression. RXR-γ expression was observed to modulate cellular differentiation and apoptosis in steady-state pre-transformed cells. Interestingly, retinoid treatment was found to enhance RXR-γ expression in transformed cells and sensitize them towards apoptosis in vitro, and also reduce growth of xenografts derived from transformed cells. Our findings emphasize that loss of RXR-γ levels appears to provide mechanistic benefits to transformed cells towards the acquisition of resistance to apoptosis hallmark of cancer, while effective retinoid treatment may present a viable approach towards sensitization of tumor cells to apoptosis through induction of RXR-γ expression.
Introduction
A contemporary view of tumorigenesis is that transformation results as a multi-step process involving genetic, epigenetic, cellular and tissue-associated changes [1], [2], [3]. These effect alterations in several regulatory and functional networks within the cell that lead to a progressive acquisition of capabilities of self-sufficiency in growth signals, insensitivity to anti-growth signals, unlimited replicative potential, evasion of apoptotic signals, tissue invasion and metastasis, and sustained angiogenesis [4]. More lately, energy metabolism reprogramming and evading immune destruction have received recognition as additional hallmarks of transformation [5].
Epithelial Ovarian Cancer (EOC) is recognized as the fifth most common cancer and the highest cause of cancer-related deaths among woman [6], [7]. A limitation in EOC studies is the lack of identification of pre-neoplastic lesions that lead to rapid and aggressive metastasis, at which stage the disease is most frequently diagnosed. This is further made more complex from recent findings that suggest high-grade EOC to originate in the fallopian tube epithelia, in contrast to the classical opinion of the ovarian surface epithelia being the cell of origin [8], [9]. Though contemporary proteome analyses provide a dynamic and efficient source of identification of tumor suppressors, oncogenes, cancer diagnostics and therapeutics [10], [11], [12], [13], an extended understanding of the multi-step transformation events in EOC vis-a-vis altered molecular expressions among transformed and pre-transformed cells remains to be resolved.
The present study is based on proteomic profiling of an in vitro model of serous ovarian adenocarcinoma (SeOvCa) established earlier in our lab [14]. Briefly, we had established several single-cell clone derived cultures from the malignant ascites of a Grade IV serous ovarian adenocarcinoma patient. Nineteen of these underwent spontaneous immortalization and were established as continuous lines. The A4 clone was one of these clones. In its initial passages, it was seen to be slow-cycling and non-tumorigenic; however, around passage 20–25 it transformed into an aggressively tumorigenic clone with metastatic capabilities. This data suggests that early A4 cells, although lacking tumorigenecity had already acquired some of the features of transformation. Hence we referred to these as being pre-transformed (A4-P), while the transformed cells derived from A4-P cells were termed as A4-T. This provided us a suitable progression model of two functionally discrete cell groups derived from a single clone in the tumor. Proteome profiles of this A4 progression model resolved through 2-Dimension Gel Electrophoresis (2DE) followed by MS (MALDI-TOF/TOF) led to the derivation of specific protein groups based on their exclusive and differential expression patterns.
Characterization of the functional networks defined by such proteins provided a clear insight into altered cellular functionality and major pathways involved in ovarian cell transformation. Of these, RXR-γ modulated cellular differentiation and apoptosis were exclusive to the pre-transformed cells. Modulation of retinol metabolism has been suggested in association with EOC progression [15], [16] in which decreased levels of CRBP1 (cellular retinol-binding protein-1) are considered a crucial step in progression of the transformation process [17]. However, the precise relevance of RXR-γ signaling remains largely uncharacterized. We resolved its functional role in the transformed cells of our progression model through induction of expression by treatment with selective retinoids including 9Cis-Retinoic acid (CRA), Adapalane (ADA) and 4-[(E)-2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl] benzoic acid (TTNPB). Such modulation of cellular differentiation and apoptosis by RXR-γ in SeOvCa further extends our current understanding of cellular transformation.
Results
Comparative protein expression analysis of A4-P and A4-T epithelial ovarian cancer progression model
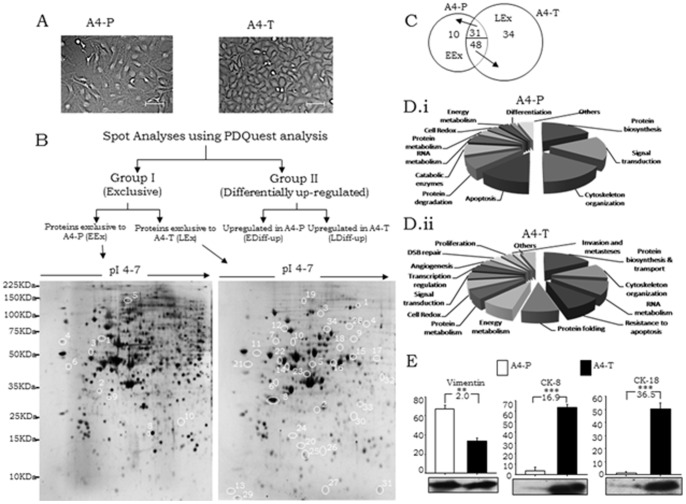
The functionally different A4-P and A4-T epithelial ovarian cancer cells exhibit a distinct phenotype, with the former being spindle-shaped while the latter appear epithelial-like in morphology (Fig. 1A). 2-DE gels were prepared using proteome samples of A4-P and A4-T cells. Two technical sets of 2-DE analytical gels were prepared from each phenotype in each experiment, which was carried out in triplicate (total 6 replicates) and silver-stained. Scanned images were processed using PDQuest and proteins with differential expression were annotated. An average, 400–500 differential protein spots were thus demarcated. Annotation of spots led to the derivation of protein sets based on their expression patterns in each cell type. Towards identification of differential protein expression, selected protein spots were digested and mass spectra was generated in MS/MS analyses. GPS Explorer software (v.3.6) was used to submit the combined MS and MS/MS data from MALDI-TOF/TOF to Mascot against SwissProt database. For all proteins thus analysed, reasonable sequence coverage, low index of mass errors and high confidence interval (CI ≥95%) were obtained.
Figure 1. Expression profiling of the proteome of serous ovarian adenocarcinoma progression model.
A. Morphological differences between A4 pre-transformed (A4-P) and A4 transformed (A4-T) cells (Bar 100μ). B, Analytical pipeline; Lower Panel-Representative gel images showing exclusively expressed proteins in A4-P cells (left – EEx) and A4-T cells (right – LEx). C, Venn diagram showing proteins categorized under all 4 sub-groups. D.i-ii, Pie diagram showing molecular functionality and pathways associated with identified A4-P and A4-T proteins respectively. E, Quantitative protein expression of Vimentin, Cytokeratin-8 and Cytokeratin-18; data shown are representative of 3 separate experiments depicted as mean ± SEM *p<0.05, **p<0.01, ***p<0.00.
Derivation of the protein groups based on expression pattern
MS/MS based protein identification led to derivation of two groups of differentially expressed proteins (Fig. 1B). Group I comprised of proteins that were expressed qualitatively (exclusively) in either A4-P or A4-T (termed as EEx and LEx proteins respectively), while Group II includes proteins expressed at quantitatively different levels (minimum two-fold differential expression between the two cell types). Both groups were further divided into two sub-groups based on their expressions in respective cell types. Annotation of qualitative and quantitative expressions was performed within each replicative set of A4-P and A4-T cells. A total of 10 and 34 Group I proteins and, 31 and 48 Group II proteins were identified as being expressed in A4-P and A4-T cells respectively (Fig. 1C; Table S1). Tables 1 & 2 lists the identified Group I and Group II proteins with specific spot numbers, molecular and functional description along with the details of match peptides, protein score, sequence coverage (%) and relative expression fold-change.
Table 1. Details of proteins identified through 2DE followed by MALDI-TOF (MS/MS) analysis in Group I.
| Sub-group I. Proteins qualitatively expressed in A4-P cells. | ||||||||||||
| Spot No. | Accession No. | Description of identified proteins | Function | SwissProt Accession | Gene name | Gene ID | Mass (Da)/PI | Peptide matched | Score | (%) Sequence coverage | RMS (ppm) | Validation method |
| 1 | P07339 | Cathapsin D precursor | Cell death/proteolysis | CATD_HUMAN | CTSD | 1509 | 44524/6.10 | 11 | 244 | 37 | 43 | 2D |
| 2 | P29992 | Guanine nucleotide binding protein G(y) alpha subunit | Protein amino adic ADP-ribosylation | GNA11_HUMAN | GNA11 | 2767 | 42097/5.11 | 7 | 49 | 20 | 49 | 2D |
| 3 | Q9P2J3 | Kelch like protein -9 | Ubl conjugation pathway | KLHL9_HUMAN | KLHL9 | 55958 | 69383/5.92 | 13 | 39 | 23 | 73 | 2D |
| 4 | Q8NEV4 | Myosin 3A | Autophosphorylation and response to stimulus | MYO3A_HUMAN | MYO3A | 53904 | 185966/9.0 | 21 | 41 | 14 | 64 | 2D |
| 5 | Q8WXW3 | Progestrone induced blocking factor-1 | Progestrone mediator | PIB1_HUMAN | PIBF1 | 10464 | 89719/5.77 | 13 | 43 | 20 | 30 | 2D |
| 6 | P48443 | RXR gamma retinoic acid receptor | Transcriptional regulation, differentiation and proliferation | LMO7_HUMAN | RXRG | 6258 | 50838/7.55 | 11 | 39 | 19 | 69 | 2D: IB |
| 7 | Q15293 | Reticulocalbin 1 precursor | endoplasmic reticulum lumen | RCN1_HUMAN | RCN1 | 5954 | 38866/4.86 | 8 | 68 | 23 | 22 | 2D |
| 8 | P08758 | Annexin A5 | anti-apoptosis/blood coagulation | ANXA5_HUMAN | ANXA5 | 308 | 35783/4.94 | 18 | 243 | 66 | 23 | 2D |
| 9 | Q13162 | Peroxiredoxin 4 | I-kappaB phosphorylation/cell redox homeostasis | PRDX4_HUMAN | PRDX4 | 10549 | 30521/5.86 | 11 | 208 | 49 | 11 | 2D |
| 10 | P09493 | Tropomyosin 1 α chain | Actin binding and cellular dynamics | TMP1_HUMAN | TPM1 | 7168 | 32689/4.69 | 11 | 146 | 21 | 10 | 2D |
Table 2. Details of proteins identified through MALDI-TOF (MS/MS) analysis in Group II.
| Sub-group I. Proteins differentially up-regulated in A4-P cells. | |||||||||||||
| Spot No. | Accession No. | Description of identified proteins | Function | SwissProt Accession | Gene name | Gene ID | Mass (Da)/PI | Peptide matched | Score | Sequence coverage [%] | RMS (ppm) | Fold change (A4-P/T) | Validation method |
| 1 | P84103 | Splicing factor, arginine/serine-rich 3 | RNA splicing and processing | SFRS3_HUMAN | SFRS3 | 6428 | 19318/11.64 | 9 | 153 | 54 | 48 | 2.608228 | 2D: IB |
| 2 | O94925 | Glutaminase | glutamine catabolic process | GLS_HUMAN | GLS | 2744 | 73414/7.85 | 15 | 540 | 36 | 19 | 2.103207 | 2D |
| 3 | P31937 | 3-hydroxyisobutyrate dehydrogenase, mitochondrial | oxidation reduction | HIBADH_HUMAN | HIBADH | 11112 | 35306/8.38 | 8 | 199 | 27 | 33 | 2.88171 | 2D |
| 4 | P08754 | Guanine nucleotide-binding ptn G(k),alpha | negative regulation of adenylate cyclase activity | GNAI3_HUMAN | GNAI3 | 2773 | 40375/5.51 | 14 | 166 | 36 | 30 | 4.086129 | 2D |
| 5 | P62873 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | Ras protein signal transduction/hormone signaling | GNB1L_HUMAN | GNB1L | 2782 | 37353/5.6 | 13 | 311 | 44 | 24 | 2.789543 | 2D |
| 6 | P31930 | Ubiquinol cytochrome C reductase complex core ptn I | aerobic respiration/proteolysis/transport | UQCRC1_HUMAN | UQCRC1 | 7384 | 52585/5.94 | 20 | 592 | 53 | 16 | 3.247917 | 2D |
| 7 | P28331 | NADH ubiquinone oxidoreductase subunit | ATP metabolic process/apoptosis/transport | NDUFS1_HUMAN | NDUFS1 | 4719 | 79465/5.89 | 21 | 249 | 42 | 35 | 13.41311 | 2D: IB |
| 8 | O43707 | Alpha actinin-4 | cellular component movement/protein transport/regulation of apoptosius | ACTN4_HUMAN | ACTN4 | 81 | 104788/5.27 | 31 | 462 | 37 | 38 | 2.19378 | 2D |
| 9 | Q07955 | Splicing factor, arginine/serine-rich 1 | mRNA splice site selection | SFRS1_HUMAN | SFRS1 | 6426 | 27597/10.37 | 6 | 172 | 43 | 29 | 6.715184 | 2D: IB |
| 10 | Q14974 | Importin β subunit | NLS-bearing substrate import into nucleus/protein import & translocation | IMB1_HUMAN | IMB1 | 3837 | 97108/4.68 | 30 | 926 | 50 | 11 | 11.88048 | 2D: IB |
| 11 | Q86UE8 | Serine/threonine-protein kinase tousled-like 2 | intracellular signaling pathway/chromatin modification | TLK2_HUMAN | TLK2 | 11011 | 80606/8.65 | 17 | 45 | 15 | 73 | 6.609533 | 2D |
| 12 | P68363 | Tubulin alpha-ubiquitous chain | microtubule-based movement/protein polymerization | TUBA1B_HUMAN | TUBA1B | 10376 | 50120/4.94 | 11 | 88 | 33 | 28 | 4.449974 | 2D |
| 13 | P20700 | Lamin B1 | protein binding/structural molecule activity | LMNB1_HUMAN | LMNB1 | 4001 | 66237/5.11 | 20 | 220 | 37 | 26 | 2.990194 | 2D |
| 14 | P61978 | Heterogeneous nuclear ribonucleoprotein K (hnRNP K) | RNA splicing/mRNA processing/signal transduction | HNRNPK_HUMAN | HNRNPK | 3190 | 50944/5.39 | 18 | 425 | 40 | 37 | 2.466555 | 2D |
| 15 | P11021 | 78 kDa glucose-regulated protein | ER-associated protein catabolic process/anti-apoptosis | HSPA5_HUMAN | HSPA5 | 3309 | 72288/5.07 | 14 | 753 | 52 | 18 | 26.707 | 2D |
| 16 | O75116 | Rho-associated protein kinase 2 | cytokinesis/protein amino acid phosphorylation | ROCK2_HUMAN | ROCK2 | 9475 | 160812/5.75 | 31 | 73 | 18 | 50 | 7.122232 | 2D |
| 17 | P22314 | Ubiquitin-activating enzyme E1 (A1S9 protein) | cell death/protein modification process | UBA1_HUMAN | UBA1 | 7317 | 117774/5.49 | 24 | 339 | 25 | 20 | 2.52764 | 2D |
| 18 | P05388 | 60S acidic ribosomal protein P0 (L10E) | ribosome biogenesis/translational elongation | RPLP0_HUMAN | RPLP0 | 6175 | 34252/5.71 | 12 | 287 | 43 | 23 | 2.007637 | 2D |
| 19 | P62140 | Serine/threonine protein phosphatase PP1-beta catalytic subunit (PP-1B) | cell cycle/cell division/glycogen metabolic process | PPP1C_HUMAN | PPP1CB | 5500 | 37163/5.84 | 7 | 91 | 19 | 45 | 16.02527 | 2D |
| 20 | Q99497 | DJ-1 protein (Oncogene DJ1) | cell death/regulation of androgen receptor signaling pathway | PARK7_HUMAN | PARK7 | 11315 | 19878/6.33 | 8 | 221 | 53 | 18 | 2.102286 | 2D |
| 21 | P50395 | Rab GDP dissociation inhibitor beta (Rab GDI beta) (GDI-2) | protein transport/regulation of GTPase activity/signal transduction | GDI2_HUMAN | GDI2 | 2665 | 50631/6.11 | 18 | 324 | 44 | 31 | 2.027135 | 2D |
| 22 | P24534 | Elongation factor 1-beta (EF-1-beta) | translational elongation | EEF1B2_HUMAN | EEF1B2 | 1933 | 24617/4.50 | 9 | 329 | 52 | 6 | 2.678404 | 2D |
| 23 | P67936 | Tropomyosin alpha 4 chain (Tropomyosin 4) | cellular component movement/muscle contraction | TPM4_HUMAN | TPM4 | 7171 | 28373/4.67 | 17 | 507 | 51 | 23 | 2.033974 | 2D |
| 24 | Q9Y297 | F-box/WD-repeat protein 1A | Wnt receptor signaling pathway/ubiquitin-dependent protein catabolic process | BTRC_HUMAN | BTRC | 8945 | 68822/8.30 | 17 | 46 | 26 | 50 | 2.223773 | 2D |
| 25 | Q92973 | Transportin 1 (Importin beta-)2 | interspecies interaction between organisms/protein import into nucleus | TNPO1_HUMAN | TNPO1 | 3842 | 101244/4.81 | 15 | 209 | 17 | 12 | 4.892821 | 2D |
| 26 | P06733 | Alpha enolase 2-phospho-D-glycerate hydro-lyase) | glycolysis/negative regulation of cell growth | ENO1_HUMAN | ENO1 | 2023 | 47008/6.99 | 15 | 330 | 42 | 73 | 2.858116 | 2D |
| 27 | P13645 | Cytokeratin 10 | epidermis development | KRT10_HUMAN | KRT10 | 3858 | 59483/5.13 | 18 | 281 | 32 | 15 | 141.9166 | 2D: IB |
| 28 | Q03252 | Lamin B2 | Structural molecule activity | LMNB2_HUMAN | LMNB2 | 84823 | 67647/5.29 | 30 | 603 | 51 | 22 | 7.605702 | 2D |
| 29 | Q9NR28 | Diablo homolog, Mitochonria-deriver caspase cativator | Activation of caspase activity by Cyt C/ Induction of appoptosis | DIABLO_HUMAN | DIABLO | 56616 | 27114/5.68 | 11 | 140 | 39 | 28 | 4.085068 | 2D: IB |
| 30 | P08670 | Vimentin | cellular component movement | VIM_HUMAN | VIM | 7431 | 53488/5.06 | 32 | 939 | 42 | 73 | 3.239119 | 2D: IB |
| 31 | P50224 | Monoamine-sulfating phenol sulfotransferase | catecholamine metabolic process, steroid metabolic process | SULT1A3_HUMAN | SULT1A3 | 4E+05 | 34174/5.68 | 12 | 124 | 49 | 31 | 6.744596 | 2D |
Categorization of functional pathways based on reported ontologies and expression validation
Gene ontology analyses further identified distinct molecular functionalities and pathways associated with the identified protein profiles in the two cell types (Fig. 1D.i). Thus, several cellular regulatory mechanisms including protein biosynthesis, cytoskeleton organization, signal transduction, regulation of apoptosis and protein degradation were largely enriched in and contributed to the functionality of A4-P cells. These were suggested to have a cross-talk with other pathways such as protein and energy metabolism, RNA metabolism, cellular differentiation and redox reactions.
Conversely, functional grouping of the proteins in A4-T cells comprised pathways associated with the classical hallmarks of cancer cells viz. resistance to apoptosis, energy metabolism, cell proliferation, angiogenesis and invasion and metastases (Fig. 1D.ii). Thus, molecules involved in associated with protein biosynthesis, folding and transport control the dynamic process of protein metabolism in transformed cells towards matching its proliferative activities.
Towards confirming levels of some of the identified proteins, their expressions were validated between A4-P and A4-T cells through immunoblotting (Fig. 1E; Fig. 2A,B,C,D). SeOvCa is unique in that, transformation is associated with expression of epithelial markers [21]. Enhanced vimentin expression in A4-P cells suggest mesenchymal while elevated levels of Cytokeratin 8 and 18 in A4-T cells correlate with epithelial features respectively (Fig. 1E), besides being in concordance with their cell morphology and the prevalent hypothesis.
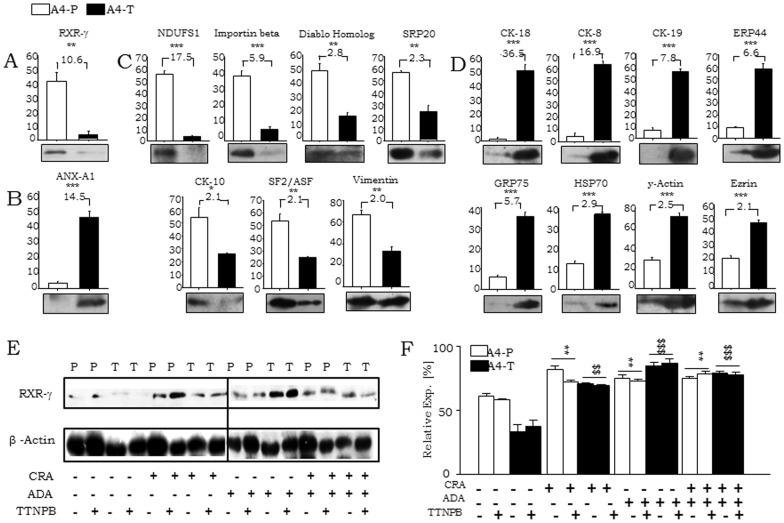
Figure 2. Validation of differentially expressed proteins and induction of RXR-γ levels on retinoid treatments.
Quantitative validation of the expression and fold change of some proteins, identified in both groups; Group I proteins, A, exclusively expressed in A4-P and B, in A4-T cell respectively; whereas D, group-II proteins quantitatively up-regulated in A4-P cells and E, in A4-T. E. Relative expression of RXR-γ and β-actin in CRA, ADA or TTNPB retinoids treated A4-P (P) and A4-T (T) cells validated through immunoblotting. F. Quantitation of relative RXR-γ expression in A4-P and A4-T cells. Statistical analysis showing test of significance (*-control A4-P and retinoids treated cells; $- control A4-P and retinoids treated cells).The data shown are representative of three separate experiments and depicted as mean ± SEM *p<0.05, **p<0.01, ***p<0.001.
Functional characterization of RXR-γ an exclusive Group I protein
Ten Group I proteins were expressed exclusively in the A4-P cells (EEx proteins). Literature-based functional annotation led to their categorizat ion into four functional groups viz.
Cell differentiation and apoptosis – RXR-γ, PRDX4;
Cell proliferation – GNA11, PIBF-1, ANXA5, Cathepsin D (CTSD);
Mitosis and cytokinesis – KLH9;
Epithelial-mesenchymal transition (EMT) – TPM1, ANXA5.
While all the above functions are relevant in the process of transformation, we focused on studying the functionality of cellular differentiation and apoptosis that is critical in maintaining tissue homeostasis and known to be regulated by RXR-γ at the transcriptional level by dimerizing with retinoic acid or retinoic acid X receptors (RAR or RXR respectively) or other permissive heterodimer partners like PPAR-γ [22], [23], [24]. We thus decided to investigate the role of RXR-γ in our epithelial ovarian cancer progression model.
RXR-γ interactions with nuclear receptors and modulation of cellular differentiation in A4-P cells upon retinoids treatment
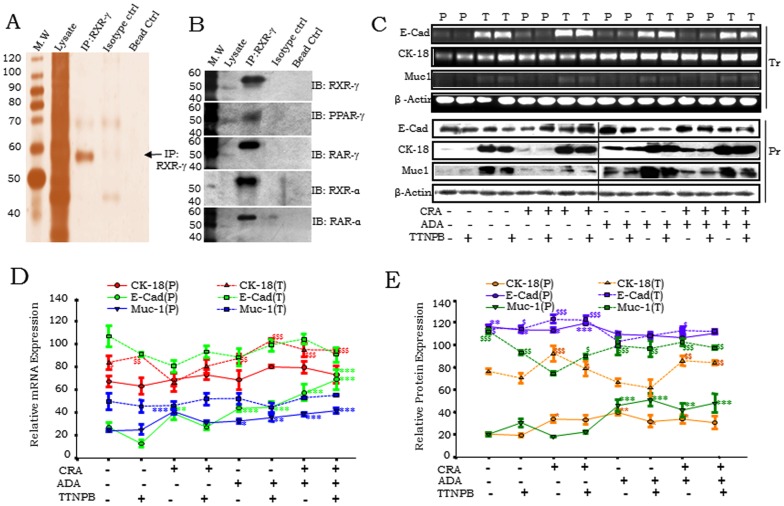
Retinoid treatment enhanced RXR-γ levels in A4-P cells; and interestingly, resumed significant expression in A4-T cells as well (Fig. 2E,F). CRA and ADA individual treatment elevated RXR-γ levels in both cell types, though this induction was less effective in combination with TTNPB. Towards validation of RXR-γ interactions with other nuclear receptors, co-immunoprecipitation and immunoblotting affirmed interactions with PPAR-γ, RAR-γ, RXR-α and RAR-α in pre-transformed cells (Fig. 3A,B). Evaluation of RXR-γ involvement in cellular differentiation was achieved through profiling epithelial markers E-cadherin (E-cad), Cytokeratin 18 (CK-18) and Mucin-1 (Muc-1) at gene expression and protein levels, in steady state and on exposure to natural viz. CRA and synthetic retinoids (ADA and TTNPB) (Fig. 3C). At steady state, lower expression of E-Cad was observed in A4-P cells. Expression of E-Cad further increased with CRA and also with ADA; CRA was given alone or in combination with ADA and TTNPB. Levels of E-Cad, CK18 and Muc-1 were endogenously higher in A4-T cells. Synthetic retinoid ADA alone or in combination with CRA upregulated CK18 expression in both cell types. Although, specific role of TTNPB in cellular differentiation is unknown, TTNPB treatment resulted in minor upregulation of differentiation markers. All three markers were enhanced in response to retinoid exposure in A4-P cells thereby affirming the involvement of RXR-γ in modulation of cellular differentiation. Retinoid treatment in the A4-T cells resulted in induction of RXR-γ without any significant alterations in the levels of these epithelial differentiation marker at gene expression and protein levels (Figs. 3D,3E).
Figure 3. RXR-γ interacts with a number of nuclear receptors and modulates cellular differentiation.
A. Co-Immunoprecipitation (Co-IP) with RXR-γ showing eluted Immunocomplex by silver staining. B. Validation of RXR-γ indicating interaction in Co-IP with RXR-γ with PPAR-γ, RAR-γ, RXR-α and RAR-α in A4-P cells validated through immunoblotting. C. Expression profiling of CK-18, Muc-1 and E-Cadherin at transcriptional (Tr) and protein (Pr) performed by semi-quantitative RT-PCR and immunoblotting in CRA, ADA or TTNPB retinoids treated A4-P (P) and A4-T (T) cells. D. Quantitation of mRNA expression of E-Cad, CK18 and MUC1 epithelial differentiation markers in A4-P (P; line) and A4-T cells (T; dashed line) upon retinoids treatment validated through RT-PCR. E. Quantitation of protein expression of E-Cad, CK18 and MUC1 makers in A4-P (P; line) and A4-T cells (T; dashed line) upon retinoids treatment validated through immunoblotting. Data shown are representative of three separate experiments depicted as mean ± SEM *p<0.05, **p<0.01, ***p<0.001.
Retinoid induced RXR-γ levels sensitize transformed cells towards apoptosis via intrinsic pathway
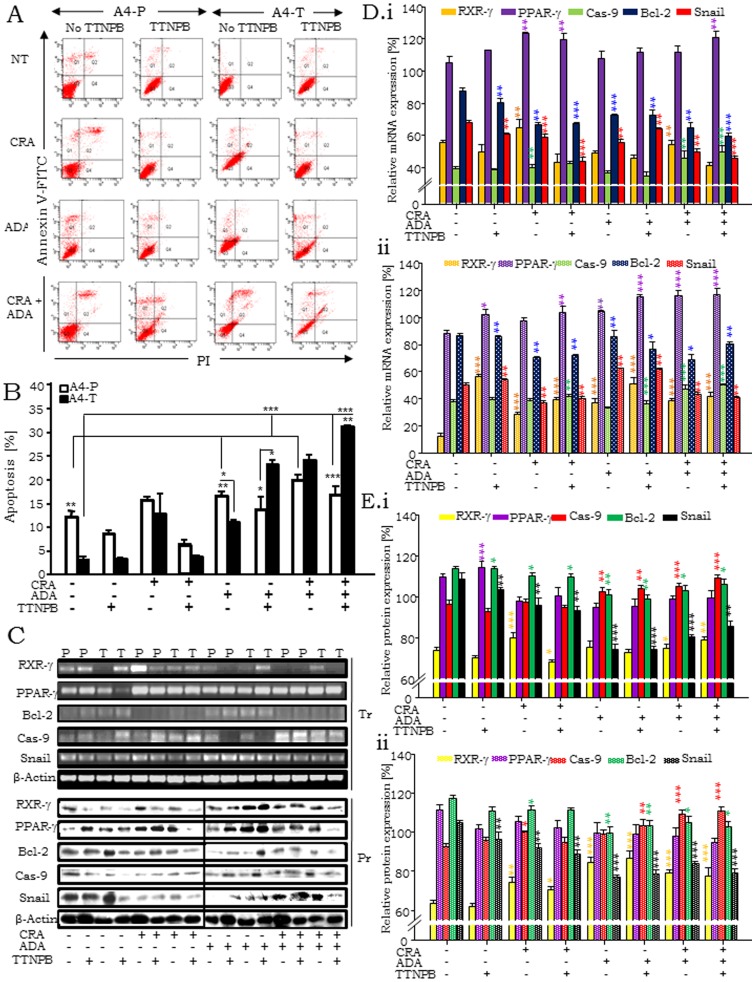
Role of RXR-γ in mediating apoptosis in response to natural and synthetic retinoids was evaluated (Fig. 4A). At steady state, apoptosis was significantly lower in A4-T cells as compared to A4-P cells indicating acquisition of resistance to apoptosis during the transformation process. Apoptosis was enhanced in both cell types on exposure to CRA and ADA – either alone or in combination (Fig.4B). While TTNPB by itself failed to induce cell death, in combination with ADA and CRA it sensitized A4-T cells to apoptosis. Retinoid mediated activation of RXR-γ expression was also found to correlate directly with higher levels of apoptosis (Fig. 2E,F). We profiled expression of the transcription factor Snail (that antagonizes p53-mediated pro-survival signaling through active repression of the pro-apoptotic molecules PUMA/BBC3, ATM and PTEN in ovarian cancer cells under stress; [19]) to evaluate the effect of RXR-γ led apoptosis on it. Caspase 9, a marker of intrinsic apoptosis pathway, upregulated during RXR-γ and PPAR-γ induction and Bcl-2 as markers of apoptosis [25]. Snail and Bcl-2 expression were reduced, while significantly elevated expression of RXR-γ, PPAR-γ and Caspase 9 were evident on retinoid treatment (Fig. 4C, 4D, 4E). The expression of these molecules was further enhanced on combinatorial retinoid treatment. We further probed the effects of retinoids on cell cycle profiles (Fig. S1A,1B). As expected, steady state A4-T cells possess higher S & G2/M populations than A4-P cells indicating active proliferation. CRA treatment enhances apoptosis along with G2/M arrest in A4-P cells while ADA and TTNPB induce only G2/M arrest. In the combinatorial treatments, CRA with ADA or presence of all three retinoids induced a G1/S arrest in transformed cells.
Figure 4. RXR-γ levels sensitize cellular apoptosis in A4-T cells upon retinoid treatment.
A. Annexin V-FITC assay data showing apoptosis in A4-P and A4-T cells on different retinoids treatment regimes; where i. having no retinoid treatment, ii. treated with CRA, iii. with ADA and iv. with both having alternative treatment of another synthetic retinoid i.e. TTNPB. B. Statistical analysis of apoptosis assay showing significant apoptosis among both cell types in different sets of retinoid treatment. C. Expression analyses of RXR-γ, PPAR-γ, Bcl-2, Caspase 9 and snail at transcriptional (Tr.) and protein level (Pr.) through RT-PCR and immunoblotting respectively. D. Quantitation of mRNA expression, i. expression of RXR-γ, PPAR-γ, Caspase 9, Bcl-2 and snail upon retinoids treatment in A4-P cells whereas, ii. shows their levels in A4-T cells on validation through RT-PCR. E. Quantitation of protein expression, i. expressions of RXR-γ, PPAR-γ, Caspase 9, Bcl-2 and snail in A4-P cells whereas, ii. shows their levels in A4-T cells upon retinoids treatment validated through immunoblotting.
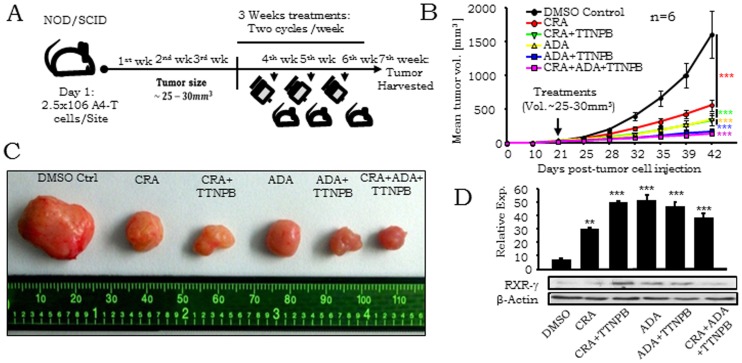
In vivo retinoid treatment significantly reduce xenograft growth in NOD-SCID mice through RXR-γ mediated sensitization of transformed cells towards apoptosis
We further extended the above re-sensitization of RXR-γ levels in A4-T cells effects obtained in vitro to experimental tumors (Fig. 5A). Mean tumor volume (Figs. 5B,5C) at each treatment point along with mean tumor volume and weight at 7th week (Figs. S1C, S1D) showed significant reduction in retinoid treated mice tumors vs. those in DMSO treated controls. Overall, the combinatorial retinoid treatment was most effective. The distinctly upregulated RXR-γ expressions in the retinoid-treated tumors strongly suggest sensitization of transformed A4 cells to apoptosis. Combined effect of retinoids was found to be most lethal for tumor growth through resumed RXR-γ mediated apoptosis of tumor cells in vivo. RXR-γ levels were found significantly higher in all 5 sets including CRA, CRA & TTNPB, ADA, ADA & TTNPB and CRA, ADA & TTNPB; in comparison to DMSO vehicle control. This is a definitive correlation with RXR-γ stimulation and induction of apoptosis in these cells in vitro (Fig.5D).
Figure 5. In vivo retinoid treatment resumes RXR-γ levels and reduces xenograft growth.
A. Experimental procedure illustrating retinoids treatment regime in NOD-SCID mice. Mice were observed upto 3 week until tumor size grows 25–30 mm3, treatment of DMSO, CRA, CRA+TTNPB, ADA, ADA+TTNBP and CRA+ADA+TTNBP started on 4th week and proceeded upto 7th week. B. Graphical representation showing tumor volumes of control and retinoids treated NOD-SCID mice at different time points. C. Comparative tumor sizes of control and retinoids treated tumors. D. Quantitative expression of RXR-γ in control and retinoids treated tumors validated through immunoblotting. Data shown are representative of three separate experiments (n = 6 for in vivo experiments) and depicted as mean ± SEM *p<0.05, **p<0.01, ***p<0.001.
Discussion
The existence of several histological sub-types that correlate with different cell(s)-of-origin in ovarian cancer [26] remains a hurdle in the establishment of representative progression models in this disease. This is in contrast to other malignancies such as prostate cancer in which such models have been applied over the last two decades in elucidating molecular mechanisms of disease [1], [27], [28], [29]. In the present study, detailed exclusive and differential protein profiling of a progression model established earlier in our lab provided novel insights into altered molecular patterns during SeOvCa progression. A4-P cells with replicative immortality represent a pre-neoplastic stage while A4-T cells with aggressive and metastatic characteristics are representative of transformation and disease progression.
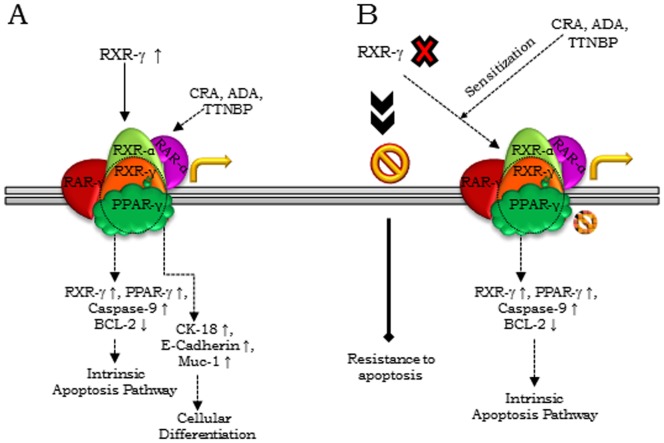
Our data affirms that the two functional states of the model are associated with distinct protein profiles. Within the group of proteins exclusive to the A4-P cells, characterization of the role of RXR-γ revealed a sensitivity of the pre-transformed cells to apoptosis and differentiation as described earlier [30]. Compromised RXR-γ levels are also reported in several malignancies including non-small cell lung cancer [31]; where it also has been reported that epigenetic silencing of RXR-γ correlated with decreased overall survival of patients [32]. In our pre-transformed cells, RXR-γ cooperates with PPAR-γ, RAR-γ, RAR-α and RXR-α to form functional heterodimeric complexes, where RXR-γ with PPAR-γ coordinates cellular apoptosis through the intrinsic pathway confirmed with elevated Caspase-9 levels. Further, we observed that RXRγ activation in transformed cells re-sensitizes them to apoptosis as a synergistic effect of agonists that mediate cytotoxic effects in vitro as well as in experimental tumors (Fig. 6). This is an important identification towards application of retinoid-based therapies. In this study, we characterized the pleotropic nature of RXR-γ signaling in our SeOvCa-progression model system. Loss of RXR-γ levels indicated to facilitate mechanistic benefits to transformed cells towards acquisition of resistance to apoptosis; consequently, retinoid-sensitized tumor cells upregulate RXR-γ levels leading to significant cell death.
Figure 6. Schematic model showing modulation of cellular differentiation and apoptosis by RXR-γ during the progression of epithelial ovarian cancer.
A. RXR-γ modulation at steady state in pre-transformed cells; retinoids treatment enhances RXR-γ levels and scale up apoptosis (upon RXR-γ interaction with PPAR-γ) and expression of epithelial differentiation specific markers (upon RXR-γ interactions with RAR-γ, RXR-α and RAR-α). B. Deficiency of RXR-γ providing benefits of resistance to apoptosis to transformed cells; retinoid treatment induced RXR-γ levels sensitize these cells towards significant apoptosis.
The present proteomics approach is a first account of changes in SeOvCa that reflect on various transformation–associated functional pathways. Significantly, RXR-γ signaling could be a potential gateway in preventing disease progression. The elucidation of RXR-γ signaling extends contemporary approaches of cellular transformation in SeOvCa that can now be exploited further in development and evaluation of new therapeutic modalities.
Material and Methods
Ethics statement
All animal work was conducted with the National Centre for Cell Science (NCCS) Institutional Animal Ethics Committee (IAEC) approval of experiments in the NCCS Experimental Animal House (EAH) Facility, and was performed as per the norms, laws and policies laid down by the committee.
Cell culture, treatments and transfections
Derivation of the A4 progression model of pre-transformed and transformed SeOvCa cells (A4-P and A4-T cells) is described earlier [14], [18]. Retinoid (RXR-γ ligand) treatment was carried out using either natural retinoid viz. 9 Cis Retinoic acid (CRA;10 µM) or synthetic retinoids Adapalene (ADA; 2 µM; RAR agonist) or 4-[(E)-2-(5,6,7,8 –Tetrahydro - 5,5,8,8 –tetramethyl – 2 naphthalenyl) – 1 -propenyl] benzoic acid Arotinoid acid (TTBPB; 10µM; RXR and RAR agonist) for 48h.
Sample preparation, 2-Dimensional gel electrophoresis (2DE) and image analyses
Cell pellets (107) of A4-P and A4-T were suspended in 500 µl ml of urea lysis buffer containing 8 M Urea, 2 M Thiourea,100 mM DTT, 2% CHAPS and 0.2% ampholytes with protease-inhibitor cocktail (Amersham USB Guideline). Cell extract was allowed to be mixed for at least 15 minutes and incubated for 30 minutes at room temperature to facilitate proper protein solublisation. Protein samples were further centrifuged (110,000g for 1 hour at 4°C) and suspension was collected. Protein concentration was estimated with 2DE quant kit (GE healthcare) at 480 nm (Bekman Coulter). Prepared samples were run on first dimension (pI) followed by of second dimension in denaturing SDS-PAGE (Mw). A total of 350 µg whole cell protein lysate was taken on 18 cm immobilized pH gradient (IPG) strip (pH 4–7) and rehydrated overnight. A three step IEF voltage program was prepared to the strips on a Protean IEF cell (Bio-Rad): 50 V for 20 mins, 10,000 V for 2 hours minutes and 10,000 V for 45,000 V-hr. Strips were further reduced by incubation in the equilibration/reduction buffer (6 M Urea, 0.375 M Tris pH 8.8, 2% SDS, 20% glycerol, 2% (w/v) DTT (Sigma)) and then alkylated the same buffer but containing 2.5% (w/v) Iodoacetamide (Sigma) instead of DTT. The second dimension was accomplished by running the IPG strips on 1.0 mm-thick of 10%-(w/v)- SDS-PAGE. Gels were stained with mass spectrometry compatible modified coomassie blue (Pierce, Thermo-Fisher) and silver stain. Image acquisition of protein gel was accomplished using Quantity One® software of VersaDocTM system (Bio-Rad Laboratories, USA) with equal parametric values. Image analysis and spot detection were performed on PDQuest 2-DE analysis software advanced version 8.0 (Bio-Rad). For quantitative and qualitative spot comparison across both gels, match-sets (Master set) of six replicates of A4-P and A4-T 2DE-gels were prepared and analysed. Software facilitates annotation of each and individual spot, unique identities were provided to every protein spots of replicate gel. An analysis set of proteins have been prepared to identify spots that are significantly different. Analysis set were made based on identified proteins spots unique to A4-P and A4-T and common spots among two replicate groups with a 2.0 fold quantity variation threshold. A total estimate of matched and unmatched spots was prepared to final images and differential proteins were identified in-between two cell types representative gels.
In-gel digestion and protein identification using MALDI-TOF/TOF
Protein spots in 2DE showing differential expression and satisfying the statistical criteria were selected and excised for in-gel digestion and further MS analyses. Spot excision was performed manually with the help of sterile sharp spot cutter. Briefly, the gel slices were destained in 25 mM ammonium bicarbonate, subsequent dehydrated with a 2∶1 mixture of 50 mM ammonium bicarbonate:100% acetonitrile (ACN) for repeated 3 times each 5 minutes. Gel slices were reduced with 10 mM DTT at 60°C for 1 hour. After cooling, gel slices were incubated for 15 min at room temperature with 50 mM iodoacetic acid. After washing and dehydrating the gel slices with 25 mM ammonium bicarbonate and ACN for 10 min, they were vacuum dried and tryptic digestion performed with 50 mM ammonium bicarbonate containing 20 ug/mL modified proteomic grade trypsin (Sigma-Aldrich) according to the manufacturer's instructions and kept on ice for 30 min. Additional 25 mM ammonium bicarbonate was added and digestion was continued overnight at 37°C. Extracted peptides were completely dried using a speedvac and re-suspended in 10 µl of 20% Ammonium Bicarbonate and 1% formic acid solution.
After processing through the Zip-Tip pipette tips (Millipore, USA), peptide mixtures were dissolved with matrix solution. The matrix used for MALDI analysis was a-cyano-4-hydroxycinnamic acid (Sigma) at 20 mg/ml in 50% acetonitrile, 0.1% trifluoroacetic acid. Equal volumes of peptide and matrix solution were mixed, and 1 µl of the resulting solution was spotted on a stainless steel MALDI sample plate. Spectra of digested peptides were acquired on a 4800 MALDI-TOF/ TOF mass spectrometer (AB Sciex, Framingham, MA) linked to 4000 series explorer software (version 3.5.3). Produced mass spectra were recorded in a reflector mode within a mass range from 800 to 4000 Da, using a Nd:YAG 355nm laser. The acceleration voltage and extraction voltage were set on 20 kV and 18 kV respectively. Six point calibration of the instrument was performed with peptide standard kit (AB Sciex). All of the MS spectra were obtained from accumulation of 900 shots. MS/MS spectra were acquired with a total accumulation of 1500 laser shots and collision energy of 1Kv. At completion of MS survey scans, the data was processed to generate a list of precursor ions for interrogation by MS/MS. The combined MS and MS/ MS peak lists were explored using the GPSTM Explorer software version 3.6 (AB Sciex). Protein identification was performed by MS/MS ion search using MASCOT (version 2.1) (http://www.martixscience.com) search engine against the SwissProt database. The search parameters were set as follows: all entries and human taxonomy, trypsin digestion and one missed cleavage, fixed modifications: carbamidomethylation of cysteine residues, mass tolerance: 150 ppm for MS and 0.4 Da for MS/MS. Identified proteins having at least two unique matched peptides were selected with an identification confidence interval threshold of ≥95%.
Co-immunoprecipitation and Immunoblotting
1mg cellular protein extracted in RIPA buffer (1M Tris pH 7.4, 4M NaCl, 0.5M EDTA, NP-40, 10% SDS) was incubated with 5 µg RXR-γ antibody for 2 h at 4°C. This was followed with overnight incubation with 20 µl protein-A agarose (Amersham, GE Healthcare). Complex-bound bead were collected through centrifugation at 12,000 g for 1 min, was washed with TBS (50 mM Tris–HCl, 150 mM NaCl, PMSF), resuspended in 2×SDS buffer and heated at 95°C for 5 min. Eluted proteins were resolved on 2–4% denaturing SDS-PAGE at 80 V followed by immunoblotting, that was performed as described earlier [19]. Details of antibodies used in the study will be made available on request.
Semi-quantitative reverse transcription-PCR
Semi-quantitative reverse transcription-PCR was performed under standard conditions as described earlier [19] and amplified products resolved on a 1.5% agarose gel; β-actin was used as internal control.
Cell cycle and apoptosis assay
Cell cycle analysis of transfected and retinoid-treated cells was done with PI (Propidium-Iodide) staining using standard protocols [20]. Data acquisition and analysis was performed on FACSCalibur (Becton Dickinson, San Diego, CA, http:// www.bdbiosciences.com) using ModFit analytical software. Annexin V–FITC apoptosis assay was performed as described earlier [20] using FACSCanto II (Becton Dickinson); DiVa software (Becton Dickinson) was used for data analysis.
In vivo studies
In vivo experimentation was performed in NOD/ SCID mice bred and maintained at Experimental Animal Facility, NCCS; and carried out as per the norms, laws and policies of the institutional ethical committee. A4-T cells (2.5×106) were injected subcutaneously (SC) in thighs of 4–6 week-old male mice and observed every 48 h till 3 weeks for tumor formation. Injections of retinoids i.e. 9Cis RA, ADA and TTNPB as well in combination started while tumor size reaches 25–30 mm3 in volume, where DMSO given to vehicle control mice. Treatments of DMSO, 25 µM 9Cis RA, 25 µM 9Cis RA+10 µM TTNPB, 5 µM ADA, 5 µM ADA +10 µM TTNPB and 25 µM 9Cis RA+5 µM ADA +10 µM TTNPB retinoids injections were given twice per week into the tumor of each mouse. Tumor size was monitored in two perpendicular directions using Vernier's calliper; individual tumor weights and sizes were more precisely quantified in the seventh week after sacrificing mice to harvest tumors.
Statistical analysis
All experiments were carried out at least in triplicate; data are expressed as mean ± SEM of three independent experiments. The significance of difference in the mean values was determined using two–tailed Student's t test; p<0.05 was considered significant. ANOVA test was performed to compare gene and protein expression and tumor volume over time between treatment groups at a significance level of <0.05. Student-Bonferroni test was used to evaluate sub-comparisons to control the test-wise error rate.
Supporting Information
A. PI based FACS analysis of cell cycle in A4-P and A4-T cells on different treatment regime of no retinoid treatment, treatment with CRA, ADA and with both having alternative treatment of another synthetic retinoid i.e. TTNPB, showing percentage of relative populations in different cell cycle phases. B. Quantitation of different cell cycle phases of A4-P and A4-T cells on different retinoid treatments. C. Graphical representation showing tumor volumes of retinoids treated NOD-SCID mice. D. Graphical representation showing tumor weight of retinoids treated NOD-SCID mice. The data shown are representative of three separate experiments and depicted as mean ± SEM *p<0.05, **p<0.01, ***p<0.001.
(DOC)
Summary of total numbers of proteins identified between A4-P and A4-T cells through 2DE analyses followed by MALDI-TOF-TOF (MS/MS) identification.
(DOC)
Acknowledgments
We thank Dr. S.C. Mande, Director, NCCS (Pune, India) for encouragement and support. RSK received a research fellowship from the Department of Biotechnology, New Delhi. We also thank Dr. (Mrs.) Surekha Zingde (ACTREC, Kharghar, Navi-Mumbai) and Dr. R. Srikanth (Proteomics facility) for providing support in our proteomics work. Technical assistance by Mr. Avinash Mali, Mihir Metkar, Manish Kumar and support from the Proteomics, Experimental Animal House, FACS and Confocal facilities at NCCS are gratefully acknowledged.
Funding Statement
This work was supported by the Department of Biotechnology, Government of India, New Delhi to SAB [Grant no. BT/PR7186/MED/14/965/2006]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Xie BX, Zhang H, Wang J, Pang B, Wu RQ, et al. (2011) Analysis of differentially expressed genes in LNCaP prostate cancer progression model. J Androl 32: 170–182. [DOI] [PubMed] [Google Scholar]
- 2. Dumont N, Crawford YG, Sigaroudinia M, Nagrani SS, Wilson MB, et al. (2009) Human mammary cancer progression model recapitulates methylation events associated with breast premalignancy. Breast Can Res 11: R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hruban RH, Wilentz RE, Kern SE (2000) Genetic progression in the pancreatic ducts. Am J Pathol 156: 1821–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA (2000) The hallmark of cancers. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 6.Am Can So, Inc. (2012) Society American Cancer: Cancer facts and figures 2012.
- 7. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J. Clin 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 8. Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, et al. (2010) Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene 29: 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell DA (2005) Origins and molecular pathology of ovarian cancer. Mod Pathol 18: S19–S32. [DOI] [PubMed] [Google Scholar]
- 10. Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, et al. (2001) Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Gen Dev 15: 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kislinger T, Cox B, Kannan A, Chung C, Hu P, et al. (2006) Global survey of organ and organelle protein expression in mouse: combined proteomic and transcriptomic profiling. Cell 125: 173–86. [DOI] [PubMed] [Google Scholar]
- 12. Hanash SM, Pitteri SJ, Faca VM (2008) Mining the plasma proteome for cancer biomarkers. Nat 452: 571–9. [DOI] [PubMed] [Google Scholar]
- 13. Kulasingam V, Diamandis EP (2008) Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract 5: 588–99. [DOI] [PubMed] [Google Scholar]
- 14. Bapat SA, Mali AM, Koppikar CB, Kurrey NK (2005) Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Can Res 65: 3025–9. [DOI] [PubMed] [Google Scholar]
- 15. Kuppumbatti YS, Bleiweiss IJ, Mandeli JP, Waxman S, Mira YLR (2000) Cellular retinol-binding protein expression and breast cancer. J Natl Can Inst 92: 475–480. [DOI] [PubMed] [Google Scholar]
- 16. Roberts D, Williams SJ, Cvetkovic D, Weinstein JK, Godwin AK, et al. (2002) Decreased expression of retinol-binding proteins is associated with malignant transformation of the ovarian surface epithelium. DNA Cell Biol 21: 11–19. [DOI] [PubMed] [Google Scholar]
- 17. Cvetkovic D, Williams SJ, Hamilton TC (2003) Loss of cellular retinol-binding protein 1 gene expression in microdissected human ovarian cancer. Clin Can Res 9: 1013–1020. [PubMed] [Google Scholar]
- 18. Wani AA, Sharma N, Shouche YS, Bapat SA (2006) Nuclear–mitochondrial genomic profiling reveals a pattern of evolution in epithelial ovarian tumor stem cells. Oncogene 25: 6336–6344. [DOI] [PubMed] [Google Scholar]
- 19. Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, et al. (2009) Snail and Slug mediate radio- and chemo-resistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27: 2059–2068. [DOI] [PubMed] [Google Scholar]
- 20. Sharma N, Jadhav SP, Bapat SA (2010) CREBBP re-arrangements affect protein function and lead to aberrant neuronal differentiation. Differentiation 79: 218–31. [DOI] [PubMed] [Google Scholar]
- 21. Auersperg N, Maines-Bandiera SL, Dyck HG (1997) Ovarian carcinogenesis and the biology of ovarian surface epithelium. J Cell Physiol 173: 261–265. [DOI] [PubMed] [Google Scholar]
- 22. Mangelsdorf DJ, Evans RM (1995) The RXR heterodimers and orphan receptors. Cell 83: 841–850. [DOI] [PubMed] [Google Scholar]
- 23. Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, et al. (2004) Peroxisome proliferator–activated receptor and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Can Therap 3: 1249–1262. [PubMed] [Google Scholar]
- 24. de Lera AR, Bourguet W, Altucci L, Gronemeyer H (2007) Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 6: 811–820. [DOI] [PubMed] [Google Scholar]
- 25. Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25: 4798–811. [DOI] [PubMed] [Google Scholar]
- 26. Auersperg N (2010) The Origin of Ovarian Carcinomas: A Unifying Hypothesis. Int J of Gynec Path 30: 12–21. [DOI] [PubMed] [Google Scholar]
- 27. Simoneau AR, Jones PA (1994) Bladder cancer: the molecular progression to invasive disease. World J Urol 12: 89–95. [DOI] [PubMed] [Google Scholar]
- 28. Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, et al. (1996) Genetic progression model for head and neck cancer: implications for field cancerization. Can Res 56: 2488. [PubMed] [Google Scholar]
- 29. Garnis C, Buys TP, Lam WL (2004) Genetic alteration and gene expression modulation during cancer progression. Mol Can 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gudas LJ, Wagner JA (2011) Retinoids regulate stem cell differentiation. J Cell Physiol 226: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brabender J, Metzger R, Salonga D, Danenberg KD, Danenberg PV, et al. (2005) Comprehensive expression analysis of retinoic acid receptors and retinoid X receptors in non-small cell lung cancer: implications for tumor development and prognosis. Carcinogenesis 26: 525–530. [DOI] [PubMed] [Google Scholar]
- 32. Lee SM, Lee JY, Choi JE, Lee SY, Park JY, et al. (2010) Epigenetic inactivation of retinoid X receptor genes in non-small cell lung cancer and the relationship with clinicopathologic features. Can Gen Cytogenet 197: 39–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. PI based FACS analysis of cell cycle in A4-P and A4-T cells on different treatment regime of no retinoid treatment, treatment with CRA, ADA and with both having alternative treatment of another synthetic retinoid i.e. TTNPB, showing percentage of relative populations in different cell cycle phases. B. Quantitation of different cell cycle phases of A4-P and A4-T cells on different retinoid treatments. C. Graphical representation showing tumor volumes of retinoids treated NOD-SCID mice. D. Graphical representation showing tumor weight of retinoids treated NOD-SCID mice. The data shown are representative of three separate experiments and depicted as mean ± SEM *p<0.05, **p<0.01, ***p<0.001.
(DOC)
Summary of total numbers of proteins identified between A4-P and A4-T cells through 2DE analyses followed by MALDI-TOF-TOF (MS/MS) identification.
(DOC)