Abstract
This minireview is prompted by the recent report of Banas et al. (J Biol Inorg Chem 15:1147–1155, 2010), which purports to show and concludes that zinc levels are increased in prostate cancer. Such a conclusion conflicts with the overwhelming corroborating clinical and experimental evidence that has amassed from numerous reports over the past approximately 60 years; these consistently show that prostate zinc levels are decreased in the development and progression of prostate cancer. We submit that this is an established relationship in prostate cancer that must be considered and described in any studies that purport to identify results that are inconsistent with this established relationship. In support of this relationship, we provide a minireview of the information that has led to the establishment of this relationship. As with most established clinical relationships, exceptions and anomalies often exist. However, these must be described and explained in the context of the established relationship, and not in the context of refutation of the established relationship, at least not until sufficient corroborating evidence overwhelms the existing evidence. This provides a background to address and to critique the report of Banas et al. Of broader and more serious implications are the widespread recalcitrance and/or lack of knowledge within the clinical and biomedical research community for recognition that zinc decrease in prostate cancer is an established relationship. This leads to misinformation and misinterpretations regarding clinical, experimental, and epidemiological issues that do not serve the best interests of the scientific, medical, and public communities.
Keywords: Prostate zinc levels, Prostate cancer
Introduction
The need for this combined minireview and critique is prompted by and in response to the recent report of Banas et al. [1], which purports to demonstrate that zinc levels are increased in prostate malignancy as compared with normal prostate. Since this conclusion is in conflict with the massive overwhelming clinical and experimental evidence that zinc is markedly decreased in prostate cancer, the results and conclusion presented in the Banas et al. report must be challenged. For one to understand and appreciate the basis for the concerns that we will raise regarding the Banas et al. report, an accompanying review of the literature concerning zinc levels in normal prostate and prostate cancer is essential. This will be an abbreviated review since numerous and extensive reviews [2–6] already exist, and are available for the reader as well as the research and clinical community to obtain an expanded background regarding zinc and prostate cancer. Following this review, we will present and address the issues and our concerns regarding the Banas et al. report. Finally, we contend that the time has come for the scientific and medical community to recognize that the decrease in zinc that accompanies the development of prostate cancer is an established relationship.
Summary of the status and role of zinc in the normal prostate and prostate cancer
In this section, we will present a brief overview/summary of the status of zinc, which is based on the overwhelming and consistent clinical and experimental evidence that has been amassed over a period of approximately 60 years.
The functional role of zinc in normal human prostate
The human prostate gland is a complex composite of anatomic regions and tissues that have different embryo-logic origins, different cellular relationships, different functions, and different diseases. Three major regions are identified as the peripheral zone (about 70%), the central zone (about 25%), and the transition zone (about 5%). The peripheral zone is the principal region that is responsible for production and secretion of prostatic fluid; and the major and unique component of prostatic fluid is the extraordinarily high concentration of citrate. This constitutes the major function of the human prostate gland. Consequently, the peripheral zone glandular epithelial cells evolved as highly specialized and unique citrate-producing cells. This brings us to the role of zinc. The normal peripheral zone accumulates uniquely high zinc levels, being approximately tenfold greater than those of most other soft tissues (Table 1). This high accumulation of zinc is due to zinc-accumulating mechanisms; for example, the presence of the ZIP1 zinc-uptake transporter in the highly specialized citrate-producing glandular epithelial cells. The most relevant point is that the high level of cellular zinc is responsible for the high level of citrate production and secretion. This results from the fact that zinc inhibits mitochondrial aconitase activity, which prevents the oxidation of citrate via the Krebs cycle. Therefore, the normal peripheral zone glandular epithelial cells evolved as highly specialized zinc-accumulating, citrate-producing cells. This is the explanation for the parallel changes in zinc and in citrate as shown in Table 1.
Table 1.
Representative prostate levels
| (NMOLS/GRAM WWT) | CITRATE | ZINC |
|---|---|---|
| NORM PERIPH ZONE | 12,000–14,000 | 3,000–4,500 |
| MALIG PERIPH ZONE | 200–2000 | 400–800 |
| OTHER TISSUES | 250–450 | 200–400 |
| NORM PROS FLUID | 40,000–150,000 | 8,000–10,000 |
| PCA PROS FLUID | 500–3000 | 800–2000 |
| BLOOD PLASMA | 100–200 | 15 |
WWT, PCa prostate cancer
The status of zinc in prostate cancer
As shown in Table 1, the level of zinc is dramatically and significantly decreased in prostate cancer. Correspondingly, the level of citrate is also dramatically decreased in prostate cancer; which is a reflection of a decrease in zinc for the reason described above. In addition, the expression of ZIP1 transporter is downregulated in the malignant cells, and this prevents the uptake and accumulation of zinc so that the decrease in zinc is evident. Thus, in contrast to the normal glandular epithelial cells, the malignant cells in prostate cancer are characterized as citrate-oxidizing cells that have lost the ability to accumulate zinc. Whereas in normal prostate, high zinc has an important functional role, high zinc exhibits adverse tumor-suppressor effects in malignant prostate cells (for reviews see [2–6]). This is the reason why malignant prostate cells evolved with mechanisms such as down-regulation of ZIP1 that prevent the accumulation of zinc and its antitumor effects.
The literature and evidence that zinc is decreased in prostate cancer
Tissue analyses of zinc levels
In 1954, Mawson and Fischer [7] first described that zinc was markedly decreased in prostate cancer tissue samples versus normal and hyperplastic tissue samples. Since then, numerous reports have confirmed the decrease in zinc in malignant tissue, some of which are represented in Table 1. As compared with normal prostate, the mean decrease in zinc for the 16 reports is −62%, with a mean standard error of less than 5% This is an amazing statistical consistency when one considers the variables that exist among these studies; such as different populations, differing stages of cancer, differing mixtures of tissue components, differing zinc assay methods, and other variables. In contrast, we could find only one report [8] prior to the Banas et al. report that purports to show that zinc is increased in prostate cancer. In that report involving eight matched cancer and benign samples and no normal tissue samples, the authors state, “The samples were obtained in the formaldehyde solution from private pathology laboratories and the pathology laboratories of Firat University in Elazig, Turkey.” The reliability of such samples for zinc or other assays is questionable (Table 2).
Table 2.
Change in zinc levels in prostate cancer (PCa)
| Change in Zinc Levels in PCa | ||
|---|---|---|
| Reference | % Difference | |
| PCa vs Norm | PCa vs BPH* | |
| [7] Mawson and Fischer 1952 | −78 | |
| [22] Hoare et al 1956 | −63 | |
| [23] Sirawawa 1961 | −51 | |
| [24] Schrowdt 1964 | −49 | |
| [11] Gyorkey et al 1967 | −46 | |
| [25] Hienzach et al 1970 | −83 | |
| [26] Gyorkey 1973 | −70 | |
| [27] Wallace et al 1975 | --- | −65* |
| [28] Habib et al 1976 | −62 | |
| [29] Dunchik et al 1975 | −67 | |
| [30] Marezynska et al 1983 | −67 | |
| [31] Jafa et al 1980 | −71 | |
| [32] Feustal et al 1982 | −50 | |
| [33] Lahtonen 1985 | --- | −85* |
| [15] Feustal et al 1987 | −16 | |
| [34] Ogunlewe et al 1989 | −75 | |
| [9] Zaichick et al 1997 | −86 | |
| [35] Vartsky et al 2003 | --- | −52* |
| [36] Sapota et al 2009 | −48 | |
| (Table modified from [9]) [MEAN] | [−62] | [−67*] |
BPH benign prostatic hyperplasia
The consistency of zinc depletion in prostate cancer is importantly represented in Fig. 1. Zaichick et al. [9] showed the mean zinc level is decreased by 86% in malignant tissue samples; and most notable is that all 59 cancer subjects showed very low zinc levels, none of which exhibited a high zinc level that characterizes normal mean levels. Strikingly, Kurhanewicz et al. [10] with in situ magnetic resonance spectroscopy (MRS) measurements of citrate in peripheral zone showed an identical relationship. The reason for this identity is that zinc depletion accompanies and is responsible for the citrate decrease as we discussed above. There are now more than 40 MRS reports that confirm the in situ decrease of citrate in malignant loci, which translates into the decrease in zinc.
Fig. 1.
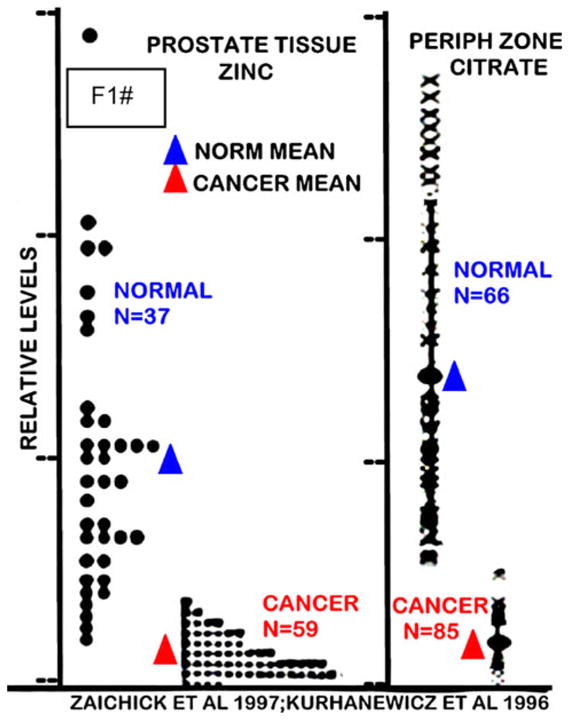
The comparative changes in normal versus cancer prostate gland zinc and citrate levels. Zinc levels were determined by energy dispersive x-ray fluorescence of tissue samples [9]. Citrate levels were determined by in situ magnetic resonance spectroscopy of the prostate gland in subjects [10]
In situ determination of zinc levels
In addition to the determination of zinc levels in tissue extracts, several reports have demonstrated loss of zinc in situ in prostate cells. For example, Györkey et al. [11] showed that in situ staining of prostate tissue sections reveals the absence of dithizone-detectable zinc in malignant acini as compared with high zinc staining in adjacent benign prostatic hyperplasia (BPH) glands (Fig. 2a). We obtained identical results as shown in Fig. 2b. This is also shown in Franklin et al. [12] in conjunction with the downregulation of ZIP1 that zinc levels are depleted in the malignant acini compared with high zinc detection in the normal acini (Fig. 3).
Fig. 2.
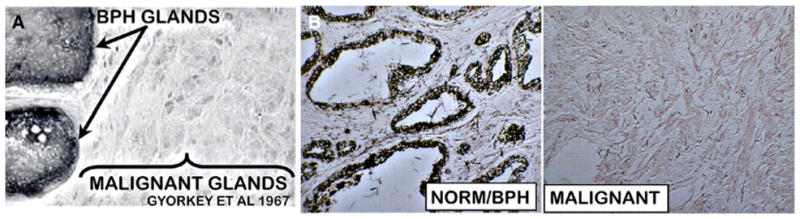
Dithizone zinc stain in prostate sections. a From [11]. b From our unpublished studies. BPH benign prostatic hyperplasia
Fig. 3.
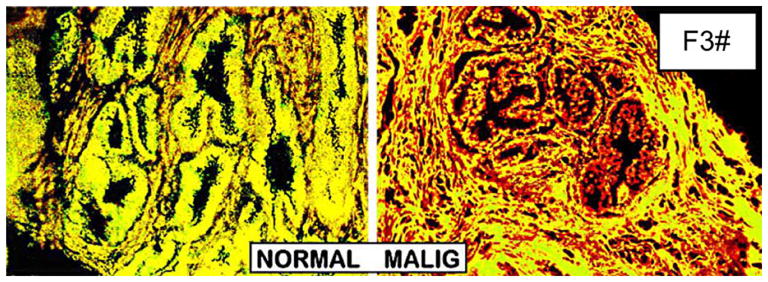
Zinc staining of prostate. Newport Green/6-methoxy-(8-p-toluenesulfonamido)quinoline (TSQ) stain. High zinc is stained yellow by excess Newport Green. Low zinc is stained red by TSQ. The cluster of yellow normal acini surrounded by slight red strain stroma that contains low zinc. Note that cluster of red malignant acini due to depletion of zinc. (From Franklin et al. [5])
It is extremely relevant to present the important extensive studies of Cortesi et al. [13, 14] which involved X-ray fluorescence (XRF) analysis of zinc levels in 8,323 noncancer segments and 669 cancer segments in biopsy cores from 440 noncancer and 158 cancer cases. The studies reveal a decrease in zinc in malignant loci, and that the decrease correlates with advancing stages of malignancy. Their following conclusions are focal to this discussion: “Zinc depletion in the prostate peripheral zone is the basis for a novel, non-invasive PCa [prostate cancer] detection, localization, volume evaluation and grading method… the zinc depletion occurs not only in the cancerous tissue segments but also, though less pronouncedly, in the non-cancer components surrounding the lesion, and in correlation with the Gleason score. This observation is consistent with the conclusions of Costello et al. that the zinc depletion is an early step in the cancer proliferation process and that zinc depletion precedes the transformation of cells from normal to cancerous type. It is well possible that although PCa has not been observed by the pathologist in these regions, the cellular precursor for its appearance is already present…”
Tumor suppressor effects of zinc
The evidence presented so far is derived from direct analyses of zinc levels. Contributing additional supporting evidence for a decrease in zinc in malignancy is provided by the effects of zinc. A key question is, “Why do malignant cells in situ in prostate cancer exhibit and require a decrease in cellular zinc levels?” Several reports from our laboratory and from others (see [2–6] for reviews and citations) have confirmed with in vitro and in vivo studies that zinc accumulation inhibits tumor activities and tumor growth by malignant prostate cells. To avoid this adversity, the malignant cells possess mechanisms such as ZIP1 downregulation that prevent the accumulation of zinc. Thus, high and increased cellular zinc levels would be incompatible with the development and progression of malignancy in prostate cancer.
In summary, abundant and consistent clinical and supporting experimental evidence amassed over a period of approximately 60 years (some of which was presented herein) demonstrates that zinc is markedly decreased in prostate malignancy. Conversely, there exists no sufficient clinical evidence in support of an increase in zinc levels in malignant cells in situ in prostate cancer. We submit that the decrease in zinc in association with prostate malignancy should be recognized as an irrefutable and established relationship of prostate cancer. This does not eliminate the possibilities of rare exceptions that “violate” this established relationship; but such possibilities must be presented in the context and recognition of being an “unusual condition” or an “anomaly” that needs explanation, rather than as evidence for an opposing prostate cancer relationship that zinc is increased in prostate malignancy.
Critique of the Banas et al. report of an increase in zinc in prostate malignancy
Banas et al. [1] reported that zinc levels are increased in prostate cancer tissue as compared with normal and BPH tissue, and purported to show a sixfold increase in zinc in Gleason grade 2, which returns to normal tissue levels during progression to Gleason grades 3 and 4. Their report also purports to show that zinc levels are not decreased in malignant compared with normal glands. It is not appropriate for us to question or refute the data that are presented. Data represent the outcome of all of the conditions and variables, good and bad, that exist in an experimental system. However, we do take serious issue and raise concern with the experimental design, context, interpretation, and conclusions presented by the authors, which leads us to seriously question the reliability of the study. The first obvious point is that their purported results are in contradiction to the overwhelming compilation of data and reports that we described earlier. The authors provided no meaningful review, representation, or citations of the voluminous studies that conflict with their observations. For example, reports exist [9, 13, 14] which show that all stages of primary site malignancy exhibit a marked decrease in zinc compared with normal prostate. Most relevant is the uncited report of Cortesi et al. [13], which demonstrated with XRF analysis that zinc is decreased in malignant cores and the decrease correlates with increasing Gleason grades. We are struck by the nearly complete omission of relevant reports regarding zinc in normal prostate and prostate cancer. Their literature discussion of the zinc changes in prostate cancer versus normal prostate is limited to the following: “According to works by Ho and Song [58] and Sapota et al. [59], the prostate contains the highest concentration of Zn of all the soft tissues; in prostate cancer this level decreases rapidly. Our studies reported earlier [15] showed that prostate cancer tissues contain elevated concentrations of Zn when compared with normal (healthy) prostate tissues.” (Note, the citations are from their report.) This is a misrepresentation of the state of the literature, and conveys to the reader that a balance of information exists in support of zinc decrease versus zinc increase in prostate cancer. Also, to explain their results, the authors stated, “The highest Zn concentration observed in Gleason grade 2 tissue… illustrates the fact that every highly proliferating cell system is dependent on sufficient availability of Zn….” This explanation is inconsistent with existing knowledge regarding the inhibitory effect of increased cellular zinc on growth/proliferation of malignant prostate cells.
The report of Banas et al. could be more informative and instructive if their presentation had been described in the context of results that are not consistent with the established relationship of decreased zinc in prostate malignancy. For example, they applied XRF analyses with a 15 μm-diameter beam which approximates the size of a glandular epithelial cell. As best that we could discern from their description, there is no identification of the tissue/cell composition that the beam is detecting. This raises questions such as the measurement including stroma, including luminal prostatic fluid, being secretory or basal cell, and other possibilities. Seemingly, a series of their measurements would include many variations of the biological components. In addition, the analyses in their report are based on samples from six patients; two for normal, hyperplastic, Gleason grade 3 and Gleason grade 4 samples and four patients for Gleason grade 2 samples. Thus although the application of the appropriate statistical analyses to a large number of assays performed on these tissues might validate the assay, such statistical analyses cannot validate the pathophysiological relevance of these observations on such a limited patient sample size. Any potential anomalies among these six patients will not be mitigated by increased sampling; on the contrary, such anomalies will be amplified. If these are not issues that might influence their results, one must ask what other factors or considerations might impact the absence of the “expected” established relationship of a decrease in zinc. Without this acknowledgement and explanation, one must seriously question the validity of their study.
Concluding remarks
Consideration of the overwhelming and consistent evidence amassed over approximately 60 years of reported clinical and experimental studies, and in the absence of any significant corroborated evidence to the contrary, should lead to the irrefutable and established relationship that a marked decrease in zinc levels is involved in the development and progression of prostate cancer. The appreciation and recognition of this relationship, as with other medical and physiological relationships, does not negate the possibility of the existence of anomalies or rare and special conditions in which the relationship is not applicable. It is only when an established relationship is recognized that anomalies can be identified as such and appropriately explained; and this adds to scientific/medical knowledge.
This relationship should be identified for the appropriate interpretation of clinical, biomedical, and epidemiology studies and their results. Otherwise, misrepresentations and disinformation will continue to be added to the scientific/medical literature and education. This has unfortunate and detrimental consequences. The direction and funding of research depends upon well-informed and accurately informed and knowledgeable scientists and clinicians who can provide a scientifically credible and objective assessment of the plausibility of new ideas and approaches relating to prostate cancer and other medical areas. Also, the public is dependent upon accurate and reliable information disseminated by the medical and scientific community, and inaccurate information does harm to the public interest.
We hope that this presentation is informative and perhaps educational regarding an important heath issue. Dealing with the problems of prostate cancer, its diagnosis, and its treatment is a critical issue. Zinc is an important factor that can be exploited in the development of bio-markers and chemotherapeutic agents to combat prostate cancer. An accurate understanding of the zinc relationships and its tumor suppressor activities is essential.
Acknowledgments
This review and the studies of L.C.C. and R.B.F. described in this review were supported in part by NIH grants CA71207, CA21097, CA79903, CA93443, and DK42839.
Footnotes
Subsequent to the submission of our manuscript and during its review and acceptance for publication by JBIC, the report of Johnson et al. (2010) appeared. Two co-authors of the Johnson et al. report (Dr. Kajdacsy-Balla and Dr. Bagasra) were co-authors on our Franklin et al. (2005) report, which we cite in this paper. We (Dr. Franklin and Dr. Costello) had no prior knowledge of the study and report of Johnson et al. before its publication and had no contact or discussion with any of the Johnson et al. co-authors concerning the Banas et al. report or the preparation of this report. Thus, no conflict of interest exists, and the Johnson et al. study and report are independent of and without prior knowledge of our paper.
Johnson et al. show, with in situ zinc staining of prostate tissue sections, the high cellular zinc level that characterizes normal acinar glandular epithelium. They further show that cellular zinc is markedly depleted in malignant acini and also in PIN, the latter considered by many to be a premalignant lesion. These results corroborate and extend the in situ observations reported by Franklin et al. (2005) and add to the in situ cellular loss of zinc also shown by Gyorkey et al. (1967), both of which we described in this paper. Thus reports of histological visualization of cellular zinc levels continue to demonstrate the loss of zinc in premalignant lesions and in various stages of malignancy as compared to high zinc levels in normal and benign prostate glandular epithelium.
Contributor Information
Leslie C. Costello, Email: lcostello@umaryland.edu, Department of Oncology and Diagnostic Sciences, Dental School, University of Maryland, 650 West Baltimore Street, Baltimore, MD 21201, USA. The Greenebaum Cancer Center, University of Maryland, 650 West Baltimore Street, Baltimore, MD 21201, USA
Renty B. Franklin, Department of Oncology and Diagnostic Sciences, Dental School, University of Maryland, 650 West Baltimore Street, Baltimore, MD 21201, USA. The Greenebaum Cancer Center, University of Maryland, 650 West Baltimore Street, Baltimore, MD 21201, USA
References
- Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Methods. 2010;4:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
References
- 1.Banas A, Kwiatek WM, Banas K, Gajda M, Pawlicki B, Cichocki T. J Biol Inorg Chem. 2010;15:1147–1155. doi: 10.1007/s00775-010-0675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello LC, Franklin RB. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello LC, Franklin RB. Oncology. 2001;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin RF, Costello LC. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin RB, Milon B, Feng P, Costello LC. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello LC, Feng P, Franklin RB. Mitochondrion. 2005;5:143–153. doi: 10.1016/j.mito.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mawson CA, Fischer MI. Can J Med Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- 8.Yaman M, Atici D, Bakirdere S, Akdeniz I. J Med Chem. 2005;48:630–634. doi: 10.1021/jm0494568. [DOI] [PubMed] [Google Scholar]
- 9.Zaichick VY, Sviridova TV, Zaichick S. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 10.Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 11.Györkey F, Min KW, Huff JA, Györkey P. Cancer Res. 1967;27:1348–1353. [PubMed] [Google Scholar]
- 12.Franklin RB, Feng P, Milon BC, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortesi M, Fridman E, Volkov A, Shilstein SSh, Chechik R, Breskin A, Vartsky D, Kleinman N, Kogan G, Moriel E, Gladysh V, Huszar M, Ramon J, Raviv G. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]
- 14.Cortesi M, Chechik R, Breskin A, Vartsky D, Ramon J, Raviv G, Volkov A, Fridman E. Phys Med Biol. 2009;54:781–796. doi: 10.1088/0031-9155/54/3/020. [DOI] [PubMed] [Google Scholar]


