Abstract
Recent behavioral reports suggest that repeated exposure to cannabis and synthetic cannabinoid agonists is linked with mental disorders associated with dysfunction of serotonin 2A (5-HT2A) receptor neurotransmission such as anxiety and depression. Here, we studied the effect of a nonselective cannabinoid agonist, CP55940, on the activity of 5-HT2A receptors in hypothalamic paraventricular nucleus (PVN). We detected that repeated exposure to CP55940 enhanced the prolactin and corticosterone neuroendocrine responses mediated by 5-HT2A receptors and increased the membrane-associated levels of 5-HT2A receptors in PVN. Importantly, we also detected increased anxiety-like behaviors in CP55940 treated rats compared to controls. The data presented here suggest that the mechanisms mediating the cannabinoid-induced upregulation of 5-HT2A receptors would be brain-region specific, as we were unable to detect a CP55940-induced upregulation of 5-HT2A mRNA. Our results might provide insight into the molecular mechanism by which repeated exposure to cannabinoids could be associated with the pathophysiology of neuropsychiatric disorders.
Keywords: marijuana, serotonin, hypothalamus, 5-HT2A receptor, G-proteins
1. Introduction
A number of recent behavioral studies suggest that administration of Δ9-Tetrahydrocannabiniol (Δ9-THC), the main psychoactive component of marijuana, or several synthetic cannabinoids can regulate the activity of serotonin 2A (5-HT2A) receptors [11;24]. While acute cannabinoid administration reduces 5-HT2A receptor-mediated behavioral responses [11]; repeated exposure to cannabinoids seems to be associated with increased behavioral responses to 5-HT2A receptor agonists in adult rats [24]. Accordingly, we have recently reported that repeated exposure to cannabinoid agonists upregulates and increases the activity of 5-HT2A receptors in rat prefrontal cortex (PFCx) and in two neuronal cell models [17–20].
Cannabinoids produce their responses by activating two cannabinoid receptors, CB1 and CB2 receptors, in the brain [8]. These receptors are expressed in different areas of the brain, including PVN, amygdala, cerebellum, hippocampus, and cortex [1;2;13;16;22]. CB1 and CB2 receptors couple to Gi/o class of G-proteins and to the extracellular kinase (ERK) signaling pathway [4;8]. In our previous studies, we have reported that the cannabinoid-induced upregulation of 5-HT2A receptors in two neuronal cell models is mediated by CB2 receptors and ERK1/2 activation as it is inhibited in cells treated with CB2, but not CB1, shRNA lentiviral particles and by ERK1/2 inhibitors [17–19].
Activity of 5-HT2A receptors in either PFCx or hypothalamic paraventricular nucleus (PVN) has been associated with several physiological functions and neuropsychiatric disorders such as stress response, anxiety & depression and schizophrenia [6;12]. Although the clinical manifestations of the cannabinoid -induced upregulation of 5-HT2A receptors are currently under discussion, it has been suggested that repeated exposure to nonselective cannabinoid agonists might fasten the onset of the neuropsychiatric disorders described above [6;23;26;27;34]. Of note, recent preclinical studies indicated that chronic, but not acute, exposure to non-selective [30;31] or selective CB2 receptor agonists induced anxiety-like behaviors in rodents [21].
Here, we investigated the effect of repeated exposure to CP55940 on the activity of 5-HT2A receptors in the hypothalamic PVN. CP55940 is a nonselective cannabinoid agonist (CB1 and CB2 agonist, Ki: 0.58 nM and 0.68 nM for CB1 and CB2 receptors, respectively) [35]. We measured [(−)-1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane HCl] (−)DOI, 5-HT2A/AC agonist, induced changes in neuroendocrine responses, corticosterone and prolactin plasma levels, as an index of activity of 5-HT2A receptors in PVN. We have previously demonstrated that the neuroendocrine effects of (−)DOI are mediated exclusively by activation of 5-HT2A receptors, but not 5-HT2C receptors, in rat PVN [39;41]. Here we reported a cannabinoid agonist-induced upregulation of 5-HT2A receptors in hypothalamic PVN and increased-anxiety like responses in rats treated with CP55940. We hypothesize that these studies will further our understanding of the neurobiological mechanisms associated with repeated cannabinoid exposure.
2. MATERIALS AND METHODS
2.1 Drugs
CP55940, a CB1/CB2 agonist, was purchased from Tocris (Ellisville, MO). A fresh CP55940 solution (0.05 mg/ml) was prepared in Tween-80/ethanol/saline (1:1:18) prior to each dosing. (−) DOI [(−)-1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane HCl] was purchased from Sigma-Aldrich Inc. (St. Louis, MO) and dissolved in 0.9% saline at one concentration (0.35 mg/kg, s.c.). All solutions were made fresh before administration and injected at a volume of 1 ml/kg.
2.2 Animal Experimental Protocol
Male Sprague-Dawley rats (225–275 g) were purchased from Harlan (Indianapolis, IN). The rats were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hrs light/dark cycle, lights on 7:00 AM–19:00 PM). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the University of Kansas Institutional Animal Care and Use Committee (IACUC).
After arrival, the rats were allowed to acclimate to their environment for at least 7 days prior to the start of the treatment period. Eight rats were randomly assigned to each group. Cage-mates were assigned to the same treatment group. Rats were injected with either vehicle (1ml/kg, i.p.) or CP55940 (0.05 mg/kg, i.p.) once a day for 7 days. Rats were sacrificed by decapitation 48 hrs after the last CP55940 injection. The rats were challenged with either saline (1ml/kg) or (−)DOI (0.35 mg/kg) 30 min prior to sacrifice. Trunk blood was collected for hormone assays and brain tissues were rapidly obtained and frozen in dry ice.
2.3 Radioimmunoassay
Plasma prolactin and corticosterone concentrations were determined by radioimmunoassays as previously described [7].
2.4 Western blots
Membrane-associated proteins were isolated using the ProteoExtract™ Native Membrane Protein Extraction kit (Calbiochem, La Jolla, CA) according to manufacturer’s instructions. Briefly, PVN tissue was homogenized in extraction buffer I containing protease inhibitor cocktail. Homogenates were incubated for 15 mins at 4°C under gentle agitation, centrifuged 16,000 × g for 15 mins at 4°C, and then the supernatant containing the cytosolic fraction was collected. The pellet was incubated with extraction buffer II with protease inhibitor cocktail for 30 mins at 4°C under gentle agitation and centrifuged 16,000 × g for 15 mins at 4°C to isolate the membrane fraction contained in the supernatant. Expression of membrane-associated 5-HT2A receptors in PVN was determined by Western blot as previously described [7]. Films were analyzed densitometrically using Scion Image software (Scion Corporation, Frederick, MD, USA) as previously described [7]. Each sample was measured on three independent gels. All samples were standardized to controls and normalized to their respective actin levels.
2.5 Quantitative Real-Time PCR
These reactions were prepared using QuantiFast SYBR Green PCR Kit (Qiagen, Valencia, CA), the ABI 7500 fast real time PCR system (Applied Biosystems, Foster City, CA) and then data was analyzed using the comparative cycle threshold (Ct) method as described [36]. The primers used in this manuscript were: 5-HT2A (F:5’-AACGGTCCATCCACAGAG-3’,R:5’-AACAGGAAGAACACGATGC-3’), Gαq (F:5’-AGTTCGAGTCCCCACCACAG-3’,R:5’-CCTCCTACATCGACCATTCTGAA-3’), and GAPDH (F:5’- TGGAGTCTACTGGCGTCTTCAC-3’,R:5’-GGCATGGACTGTGGTCATGA-3’). These primers have been previously validated [3;25;29].
2.6 Behavioral Tests
We used a separate group of rats to measure anxiety-like behaviors in rats injected with either vehicle (1ml/kg, i.p.) or CP55940 (0.05 mg/kg, i.p.) once a day for 7 days. 48 hrs after the last CP55940 injection, anxiety-like behaviors and locomotor activity were assessed in an elevated plus maze (Med Associates,St. Albans,VE) as previously described [32;37].
2.7 Statistics
All data are expressed as the mean ± S.E.M., where n indicates the number of rats per group. Data was analyzed by an unpaired Student’s t-test or ANOVA (Newman-Keuls post-hoc test). GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD, USA) was used for statistical analyses.
3. RESULTS
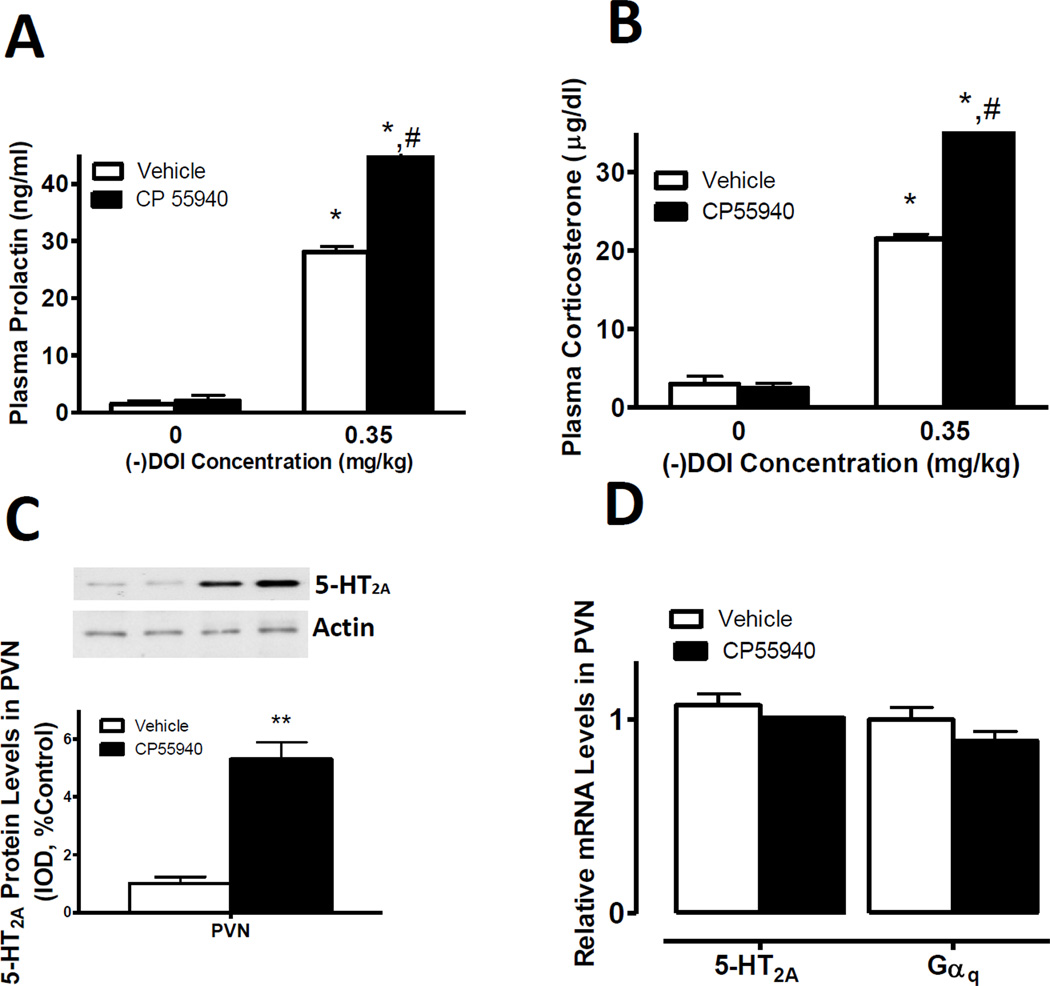
We first examined the effect of repeated administration of CP55940, a CB1/CB2 receptor agonist [4], on the activity and expression of 5-HT2A receptors in PVN. Rats were treated with CP55940 once a day for 7 days and then were challenged with (−)DOI (5-HT2A/2C receptor agonist) 30 mins prior to sacrifice. We used this challenge to measure activity of 5-HT2A receptors in PVN because the neuroendocrine effects of (−)DOI, are mediated exclusively by activation of 5-HT2A receptors, but not 5-HT2C receptors, in PVN [7]. In (−)DOI-challenged rats, we found significant (p<0.05) increases in 5-HT2A receptor-mediated prolactin (Fig.1A) and corticosterone (Fig.1B) plasma levels. The levels of prolactin increased from 28.1 ± 0.9 pg/ml in control rats challenged with (−)DOI to 44.60 ± 2.2 in CP55940-treated rats challenged with 0.35nM (−)DOI (Fig.1A). Plasma corticosterone levels increased from 21.5 ± 0.6 in control rats to 35.7 ± 0.7 in CP55940-treated rats (Fig.1B).
Figure 1. CP55940-induced enhanced activity and upregulation of 5-HT2A receptors in hypothalamic PVN.
(A and B) Increased 5-HT2A receptor-mediated prolactin and corticosterone neuroendocrine responses in CP55940 and (−)DOI treated rats compared to vehicle and (−)DOI treated controls. *p<0.05, significant effect of (−)DOI challenge in vehicle or CP55940 treated rats compared to vehicle-treated controls. #p<0.05, significant effect (−)DOI challenge in CP55940 treated rats compared to (−)DOI challenge in vehicle-treated rats. (C) Increased membrane-associated 5-HT2A receptor protein levels in the PVN of rats treated with CP55940 compared to vehicle treated controls. β-actin was used as a loading control and similar results were obtained in three separate experiments. (D) No significant changes in 5-HT2A or Gαq mRNA levels in PVN of CP55940 treated rats compared to vehicle treated controls. Data represent the mean ± SEM of 8 rats per group and were analyzed by one-way ANOVA or t-Student test. *p<0.05, significant effect of CP55940 compared with their respective vehicle-treated controls.
Upregulation of 5-HT2A receptors could mediate this cannabinoid-induced phenomenon in hypothalamic tissue. Interestingly, we found a significant (p<0.01) increase in the membrane-associated 5-HT2A receptor protein levels in PVN of rats treated with CP55940 compared to controls (approx. 5-fold increase in PVN, Fig.1C). Increased mRNA synthesis, decreased receptor degradation, and/or increased translocation of these receptors from the cytosol to the membrane are the plausible mechanisms that could underlie the increased membrane-associated levels of 5-HT2A receptors shown in Fig.1C. We then measured 5-HT2A mRNA levels in PVN samples of either controls or CP55940-treated rats. In PVN, we did not find any significant CP55940-mediated changes in 5-HT2A mRNA levels (Fig.1D) nor did we detect any modification in the levels of 5-HT2A receptor-associated G-protein, Gαq (Fig.1D).
Repeated cannabinoid administration has been linked to increased anxiety, depressed mood, irritability and restlessness [5;10]. Here we want to determine whether the increased cannabinoid-induced activity of hypothalamic 5-HT2A receptors is associated with anxiety-like behaviors in rats. We used a separate group of rats to measure anxiety-like behaviors in CP55940 treated animals 48 hours after the last cannabinoid administration. In the elevated plus maze, anxiety-related behavior is measured by the degree to which the rodent avoids the open (unenclosed) arms of the maze [32]. Since locomotor activity affects the interpretation of the elevated plus maze observations, we measured the number of transitions between the different arms of the maze as an index of locomotor activity as previously described [21].
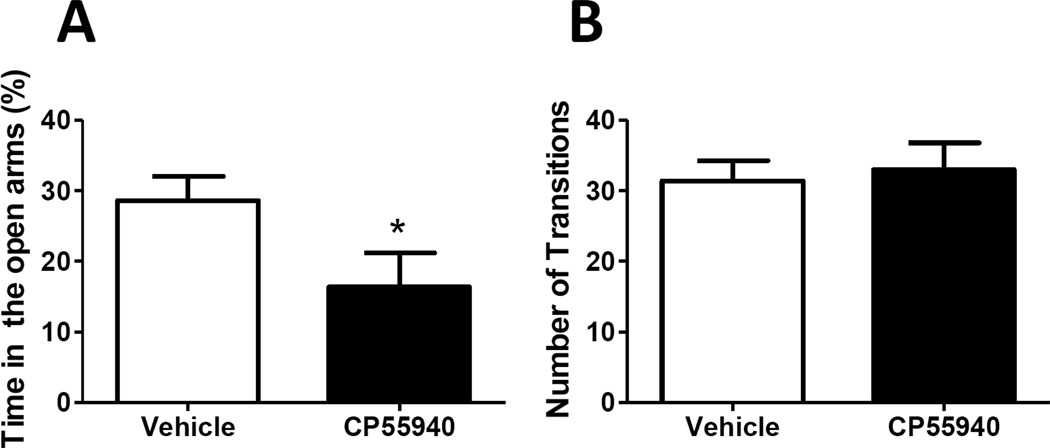
We detected significant (p<0.05) differences between vehicle and CP55940 treated rats when we measured the percent time spent in open arms of the plus maze (% open arms/total time, Fig.2A). Indeed, we detected that vehicle treated rats spent significantly (p<0.05) more time in the open arms of the maze compared to CP55940-treated rats (28.6 ± 3.2% and 16.4 ± 4.8% for vehicle and CP55940-treated rats, respectively). Interestingly, we did not detect any significant changes in the number of transitions between the different arms of the maze in vehicle or CP55940-treated rats (31.4 ± 2.9 and 33.0 ± 3.8 for vehicle and CP55940-treated rats, respectively) (Fig.2B).
Figure 2. Effect of repeated CP55940 treatment on anxiety-like behavior in the elevated plus maze test.
(A) Percent time spent in the open arms of the elevated plus maze was reported as a measure of anxiety-like behavior which was evaluated for a period of 5 minutes. (B) Transitions between the different arms of the maze was used as an index of locomotor activity. Mean ± SEM, n= 6–8 rats. *p<0.05, significant behavioral effect of CP55940 compared with their respective vehicle-treated control rats.
4. DISCUSSION
Our results identified a cannabinoid agonist-induced increase of 5-HT2A signaling in the hypothalamic PVN cells that was manifested by increased prolactin and corticosterone neuroendocrine responses to (−)DOI, a 5-HT2A/2C receptor agonist (Fig.1A & 1B). We have demonstrated that the neuroendocrine effects of (−)DOI on the secretion of these two stress hormones, are mediated by activation of 5-HT2A receptors in PVN [39;41]. We used (−)DOI (0.35 mg/kg), a submaximal dose (close to EC50) in these studies, since higher doses of (−)DOI may reach a ceiling effect that may prevent us from quantifying the cannabinoid-induced increases in 5-HT2A receptor activity in this brain area, as previously reported [24]. The upregulation of 5-HT2A receptors is a mechanism that could mediate the increased activity of the 5-HT2A receptor. Indeed, we found increased membrane-associated 5-HT2A receptor protein levels in PVN of CP55940-treated rats that were not associated with changes in 5-HT2A mRNA (Fig.1C & 1D). Since 5-HT2A receptors seem to be located mostly in the cytosol of neuronal cells [14;40], it is possible than repeated cannabinoid exposure may enhance the translocation of the receptor from the cytosol to the membrane and/or decrease the degradation of this receptor. Interestingly, we have reported that repeated exposure to cannabinoids is also associated with increased activity and protein expression of membrane-associated 5-HT2A receptors in rat PFCx [17;18]. However, this latter phenomenon was associated with increased 5-HT2A mRNA levels in rat PFCx suggesting that the mechanism by which CP55940 increase the protein expression and activity of 5-HT2A receptor in brain might be brain-region specific.
Enhanced activity of 5-HT2A receptors in hypothalamic PVN has been associated with several mood disorders including anxiety [6;15]. Repeated exposure to cannabinoids has been linked to increased anxiety, depressed mood, irritability, and restlessness [5;10]. When we used a separate group of rats to measure the effect of CP55940 on anxiety-like behaviors, we found that the time CP55940-treated rats spent in the open arms of the elevated plus maze was reduced compared to vehicle controls. This was not associated with changes in the number of transitions between vehicle and cannabinoid-treated rats, suggesting that repeated exposure to CP55940 enhances anxiety-like behaviors in rats without modifying locomotor activity. These studies are in line with preclinical reports that show chronic exposure to CP55940 and JWH133 (selective CB2 agonist) induce anxiety-like behaviors in young adult [21] and adolescent rodents [31], respectively. Noteworthy, clinical studies have reported that chronic cannabinoid users have higher levels of anxiety compared with control groups [33]. Furthermore, the severity of anxiety symptoms increased as the level of cannabinoid use increased [9;28].
Further studies are required to identify the cannabinoid receptor and the specific mechanism by which CP55940 upregulates 5-HT2A receptors in hypothalamic PVN. Our studies in two neuronal cell models suggest that CB2, but not CB1, receptors mediate this phenomenon [17–20]. Importantly, a 2011 report stated that Marijuana use among U.S. high school students has increased since 2008 [38]. The percentage of high school students who reported using marijuana or synthetic cannabinoids increased from 32% in 2008 to 39% in 2010 [38]. Despite this high incidence, the mechanism by which cannabinoid exposure produce some of its adverse effect is unknown. The significance of our studies is that they may be clinically relevant with respect to facilitating a better understanding of some mechanisms that may underlie the etiology of neuropsychiatric disorders in individuals repeatedly exposed to cannabinoids.
5. Conclusions
This study provides new insight into the cannabinoid agonist regulation of 5-HT2A receptors in rat hypothalamic PVN. Furthermore, to the best of our knowledge this study is the first to show that repeated CP55940 treatment enhances 5-HT2A receptor mediated increases in the neuroendocrine responses of two stress hormones, prolactin and corticosterone. We also detected increases in 5-HT2A activity and protein expression in PVN and increased anxiety-like behavior in CP55940 treated rats. Therefore, this cannabinoid-induced upregulation of 5-HT2A receptors might represent a potential adverse effect of long-term exposure to certain cannabinoid agonists.
Highlights.
Repeated CP55940 treatment enhances 5-HT2A-mediated neuroendocrine responses.
5-HT2A protein levels in PVN were increased after repeated CP55940 treatment.
5-HT2A and Gαq mRNA levels in PVN were not modified by CP55940 treatment.
CP55940 treatment increases anxiety-like behavior in rats.
Acknowledgments
Supported by: NIH/NIDA DA024329, University of Kansas Startup Funds, University of Kansas GRF #2301421 and NFGRF #2302213 Awards.
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HT2A
Serotonin 2A
- Δ9-THC
Δ9-Tetrahydrocannabinol
- ERK
extracellular kinase
- PVN
hypothalamic paraventricular nucleus
- CP 55,940
-
(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
(−)DOI [(−)-1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane HCl]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ashton JC, Appleton I, Darlington CL, Smith PF. Cannabinoid CB1 receptor protein expression in the rat choroid plexus: a possible involvement of cannabinoids in the regulation of cerebrospinal fluid. Neurosci. Lett. 2004;364:40–42. doi: 10.1016/j.neulet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Ashton JC, Darlington CL, Smith PF. Co-distribution of the cannabinoid CB1 receptor and the 5-HT transporter in the rat amygdale. Eur. J. Pharmacol. 2006;537:70–71. doi: 10.1016/j.ejphar.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson PJ, Young KW, Ennion SJ, Kew JN, Nahorski SR, Challiss RA. Altered expression of G(q/11alpha) protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca(2+) signaling. Mol. Pharmacol. 2006;69:174–184. doi: 10.1124/mol.105.014258. [DOI] [PubMed] [Google Scholar]
- 4.Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le FG, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur. J. Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 5.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am. J. Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco GA, Van de Kar LD, Sullivan NR, Landry M, Garcia F, Muma NA, Battaglia G. Cocaine-mediated supersensitivity of 5-HT2A receptors in hypothalamic paraventricular nucleus is a withdrawal-induced phenomenon. Neuroscience. 2006;143:7–13. doi: 10.1016/j.neuroscience.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS. J. 2006;8:E112–E117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clough AR, d'Abbs P, Cairney S, Gray D, Maruff P, Parker R, O'Reilly B. Adverse mental health effects of cannabis use in two indigenous communities in Arnhem Land, Northern Territory, Australia: exploratory study. Aust. N. Z. J. Psychiatry. 2005;39:612–620. doi: 10.1080/j.1440-1614.2005.01634.x. [DOI] [PubMed] [Google Scholar]
- 10.Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P. Cannabis and anxiety: a critical review of the evidence. Hum. Psychopharmacol. 2009;24:515–523. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- 11.Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol. Biochem. Behav. 2001;68:311–317. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- 12.de AJ, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog. Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- 13.den Boon FS, Chameau P, Schaafsma-Zhao Q, van AW, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3534–3539. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Research. 2000;864:176–185. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology. 2005;48:204–214. doi: 10.1016/j.neuropharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Felder CC, ckason-Chesterfield AK, Moore SA. Cannabinoids biology: the search for new therapeutic targets. Mol. Interv. 2006;6:149–161. doi: 10.1124/mi.6.3.6. [DOI] [PubMed] [Google Scholar]
- 17.Franklin JM, Carrasco GA. Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5-HT(2A)) receptor activity via ERK1/2 signaling. Synapse. 2012;67:145–159. doi: 10.1002/syn.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin JM, Carrasco GA. Cannabinoid-induced enhanced interaction and protein levels of serotonin 5-HT(2A) and dopamine D(2) receptors in rat prefrontal cortex. J. Psychopharmacol. 2012;26:1333–1347. doi: 10.1177/0269881112450786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid 2 receptor- and beta Arrestin 2-dependent upregulation of serotonin 2A receptors. Eur. Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.06.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid agonists increase the interaction between beta-Arrestin 2 and ERK1/2 and upregulate beta-Arrestin 2 and 5-HT(2A) receptors. Pharmacol. Res. 2012;68:46–58. doi: 10.1016/j.phrs.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br. J. Pharmacol. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Henquet C, Murray R, Linszen D, van OJ. The environment and schizophrenia: the role of cannabis use. Schizophr. Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 24.Hill MN, Sun JC, Tse MT, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int. J. Neuropsychopharmacol. 2006;9:277–286. doi: 10.1017/S1461145705005651. [DOI] [PubMed] [Google Scholar]
- 25.Kindlundh-Hogberg AM, Svenningsson P, Schioth HB. Quantitative mapping shows that serotonin rather than dopamine receptor mRNA expressions are affected after repeated intermittent administration of MDMA in rat brain. Neuropharmacology. 2006;51:838–847. doi: 10.1016/j.neuropharm.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysis. Arch. Gen. Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 28.Lee KS, Conigrave KM, Patton GC, Clough AR. Cannabis use in remote Indigenous communities in Australia: endemic yet neglected. Med. J. Aust. 2009;190:228–229. doi: 10.5694/j.1326-5377.2009.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 29.Mato S, Alberdi E, Ledent C, Watanabe M, Matute C. CB1 cannabinoid receptor-dependent and -independent inhibition of depolarization-induced calcium influx in oligodendrocytes. Glia. 2009;57:295–306. doi: 10.1002/glia.20757. [DOI] [PubMed] [Google Scholar]
- 30.O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J. Psychopharmacol. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- 31.O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J. Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 32.Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoS. ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: characteristics of users in an Australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- 34.Roth BL. Irving Page Lecture: 5-HT(2A) serotonin receptor biology: interacting proteins, kinases and paradoxical regulation. Neuropharmacology. 2011;61:348–354. doi: 10.1016/j.neuropharm.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- 36.Singh R, Jia C, Garcia F, Carrasco G, Battaglia G, Muma N. Activation of the JAK-STAT pathway by olanzapine is necessary for desensitization of serotonin2A receptor-stimulated phospholipase C signaling in rat frontal cortex but not serotonin2A receptor-stimulated hormone release. J. Psychopharmacol. 2009;12:651–665. doi: 10.1177/0269881109103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanford JA, Fowler SC. Similarities and differences between the subchronic and withdrawal effects of clozapine and olanzapine on forelimb force steadiness. Psychopharmacology (Berl) 1997;132:408–414. doi: 10.1007/s002130050363. [DOI] [PubMed] [Google Scholar]
- 38.University of Maryland. A Weekly FAX from the Center for Substance Abuse Research (CESAR FAX) 2011:1. [Google Scholar]
- 39.Van de Kar LD, Javed A, Zhang YH, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. Journal of Neuroscience. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT(2A) serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D'Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (−)DOI. J. Neurosci. 2002;22:9635–9642. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]