Abstract
We explored whether geriatric assessment variables predicted mortality in addition to known prognostic factors in 101 patients aged ≥65 with newly diagnosed AML. Baseline comorbidity score (HR=1.92; 95%CI 1.18–3.11), difficulty with strenuous activity (HR=2.18; 95%CI 1.19–4.00), and pain (HR=2.17; 95%CI 1.19–3.97) were independent prognostic factors for greater risk of death in a multivariable model that included cytogenetic risk group. They remained independent predictors in the subset of patients with baseline ECOG PS 0–1. Our results support the use of geriatric assessment to better predict prognosis in older patients with AML, even among those with excellent functional status.
Keywords: Geriatric Assessment, Leukemia, Acute, Myeloid, Prognosis, Performance Status, Comorbidity
Introduction
Acute myeloid leukemia (AML) is a disease of older adults whose incidence will increase dramatically in coming decades due to population aging.[1] AML patients over age 65 have much worse prognosis than younger patients, with a five-year disease-specific survival of only 5%.[2] These poor outcomes are due to a combination of age-related changes in disease biology and clinical factors such as decreased physiologic reserve, functional impairment and frailty.[3–5] Previous work has identified age, performance status, comorbidity, and cytogenetic risk group as important prognostic factors in older patients with AML.[6] However, few studies have explored the relationship between geriatric assessment and AML outcomes.
Comprehensive geriatric assessment (CGA) is a systematic method of identifying multiple predictors of morbidity and mortality in older adults that may impact cancer treatment and is recommended for older cancer patients by NCCN guidelines.[7] This recommendation was in part based on a multicenter study demonstrating that a self-administered geriatric assessment identified important prognostic factors in cancer patients.[8] A geriatric evaluation includes assessment of multiple domains including comorbidity and physical, cognitive and social function. The feasibility of performing a modified CGA in older patients with AML has been demonstrated, but it is not yet known how this information predicts outcomes.[9]
Careful assessment of the potential benefits and risks of therapy is particularly vital in AML, as intensive chemotherapy with cytarabine and an anthracycline is the only treatment that gives hope of long-term survival. Response to induction is poor among older adults and toxicity is substantially higher than in younger individuals, but selected patients can achieve remission and cure.[10–12] Patients who are not candidates for induction may benefit from non-intensive treatments such as hypomethylating agents, and some are best served by purely palliative approaches.[13, 14] However, it can be difficult to predict which older patients will benefit from chemotherapy using routine clinical and biological factors alone. Growing evidence suggests that measures of comorbidity and functional status may also be valuable prognostic factors in elderly patients with AML.[15–18] We utilized prospectively collected quality of life data to evaluate the utility of geriatric factors as predictors of survival in older patients with AML across varying treatment intensities.
Methods
Data Collection
We performed a retrospective cohort study of consecutive patients ≥65 years of age that presented to Dana-Farber Cancer Institute (DFCI) between 2006–2011 for evaluation of a new diagnosis of AML. At the DFCI, all new patients with hematologic malignancies are asked to participate in a research protocol that involves a baseline questionnaire and prospective collection of clinical data into the Cancer Research Information System (CRIS) database. CRIS includes information collected by trained abstractors on patient demographics, initial treatment assignment, disease characteristics, pathology tests, hospitalizations, treatments and date and cause of death. We used CRIS to identify all patients ≥65 years of age who presented between January 2006 and December 2011 with a new diagnosis of AML. We excluded patients who filled out their survey after beginning chemotherapy for AML.
The survey includes items from the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ) for the evaluation of health-related quality of life of cancer patients (QLQ-C30) (Table 2). Responses to questions about function and symptoms are rated on a scale of 1 (not at all) to 4 (very much). Chart review was performed by a trained medical student (AS) and verified by a geriatric oncologist (JD). We validated all clinical data provided by CRIS. We gathered additional information on baseline diagnosis and pathology, laboratory tests, oncologist assigned Eastern Cooperative Oncology Group (ECOG) performance status (PS) and cytogenetic data. We recorded the course of treatment, number and length of hospitalizations, and survival. We considered inclusion of standard anthracycline and cytarabine regimens in initial treatment as induction. All patients provided written informed consent for their data to be included in the CRIS database. This study was approved by the DFCI Institutional Review Board.
Table 2.
EORTC QLQ-C30 questions by geriatric domain
| N (%) patients responding‡ | |||
|---|---|---|---|
| Domain | Question† | Less | More |
| Physical Functioning | Do you have any trouble doing strenuous activities, like carrying a heavy shopping bag or a suitcase? | 64 (66.0%) | 33 (34.0%) |
| Do you need help with eating, dressing, washing yourself or using the toilet? | 95 (96.9%) | 3 (3.1%) | |
| Were you limited in doing either your work or other daily activities? | 67 (67.0%) | 33 (33.0%) | |
| Social Functioning | Were you limited in pursuing your hobbies or other leisure time activities? | 67 (70.5%) | 28 (29.5%) |
| Has your physical condition or medical treatment interfered with your family life? | 87 (87.0%) | 13 (13.0%) | |
| Has your physical condition or medical treatment interfered with your social activities? | 77 (77.0%) | 23 (23.0%) | |
| Cognitive Functioning | Have you had difficulty remember things? | 92 (93.9%) | 6 (6.1%) |
| Have you had difficulty in concentrating on things, like reading a newspaper or watching television? | 94 (93.1%) | 7 (6.9%) | |
| Psychological State | Did you worry? | 77 (78.6%) | 21 (21.4%) |
| Did you feel depressed? | 52 (52.5%) | 47 (47.5%) | |
| Nutritional Status | Have you lacked appetite? | 79 (79.0%) | 21 (21.0%) |
| Pain Status | Have you had pain? | 85 (84.2%) | 16 (15.8%) |
Questions ask patient to consider the past week
More indicates “Quite a Bit” or “Very Much”; Less indicates “Not at All” or “A Little”
Definition of predictors and outcomes
To determine if geriatric assessment variables predict mortality in our population, we selected questions from the QLQ-C30 that correspond to geriatric domains, including physical function, social function, cognition, psychological state, nutritional status, and pain (Table 2). We divided survey responses into two categories: 1–2 (“not at all” or “a little”) vs. 3–4 (“quite a bit” or “very much”). We assessed comorbidities by means of the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), a tool designed to quantify the effect of comorbid conditions on mortality in patients with hematologic disease.[19] We defined a low albumin as < 3.5 mg/dL. We used median age at diagnosis as our age variable. We defined cytogenetic risk as favorable, intermediate, or adverse.[20] We defined overall survival (OS) as the time from the date of diagnosis of AML at DFCI to the date of death or the date of last follow-up. Disease-specific survival considered only deaths attributed to AML. Complete remission (CR) was defined according to the International Working Group.[21] There was no distinction made between those achieving CR after one or two cycles of induction chemotherapy.[22] We categorized initial treatment assignment into the following groups: induction chemotherapy, hypomethylating agents, and palliative/other therapies.
Statistical methods
We used Kaplan-Meier (KM) survival curves to describe the survival of the cohort, and to determine the univariate association between variables of interest and mortality. The log-rank test was employed to test the difference in KM curves between groups. Only variables that predicted mortality on univariate analyses (P < 0.05) were included in the multivariate analysis. We used multivariate Cox proportional hazard models to determine which factors were independently associated with mortality. We used Chi-squared tests to identify variables associated with reception of induction chemotherapy. We included these factors in a multivariate binary logistic regression model to determine independent predictors of receiving induction. A P-value < 0.05 was considered significant. Statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL).
Results
Between 2006 and 2011, 368 patients 65 and older presented to the DFCI with a diagnosis of AML. Of these, 163 (44.3%) did not complete the new patient survey prior to hospitalization for AML, 62 (16.8%) received previous chemotherapy for AML, and 42 were missing information on key variables, leaving 101 patients for the analysis. Baseline characteristics of the cohort are listed in Table 1. Overall, the cohort was white (98%), had a performance status ≤1 (79.3%), and had ≤1 comorbidity (72.4%).
Table 1.
Demographic and clinico-pathologic characteristics
| Characteristic | N (%) |
|---|---|
| Age at diagnosis (years) | |
| 65–70 | 41 (40.6%) |
| 71–75 | 24 (23.8%) |
| 75–80 | 20 (19.8%) |
| >80 | 16 (15.8%) |
| Male | 63 (62.4%) |
| White | 99 (98.0%) |
| BMI (kg/m2), mean ±SD | 27.9 ±4.9 |
| Physician-rated ECOG PS | |
| 0 | 25 (24.8%) |
| 1 | 55 (54.5%) |
| 2 | 17 (16.8%) |
| 3 | 4 (4.0%) |
| HCT-comorbidity index | |
| ≤1 | 64 (63.4%) |
| >1 | 37 (36.6%) |
| Number of medications, mean ±SD | 5 ±3 |
| History of tobacco use | 59 (59.6%) |
| Family history of hematologic malignancy | 15 (14.9%) |
| Origin of disease | |
| De novo | 55 (54.5%) |
| Secondary to MDS | 34 (33.7%) |
| Treatment-related | 12 (11.9%) |
| Cytogenetic risk group | |
| Favorable | 2 (2.0%) |
| Intermediate | 47 (46.5%) |
| Adverse | 32 (31.7%) |
| Unknown | 20 (19.8%) |
| Percent blasts in bone marrow, mean ±SD | 40.7 ±24.2 |
| Initial treatment Received | |
| Induction chemotherapy | 35 (35.0%) |
| Decitabine or Azacitidine | 34 (34.0%) |
| Other* | 7 (7.0%) |
| Palliative only | 24 (24.0%) |
| Consolidation chemotherapy | 20 (19.8%) |
| Stem cell therapy | 18 (17.8%) |
| Initial treatment on clinical trial | 23 (23.0%) |
| Patients achieving complete response by initial treatment | |
| Induction chemotherapy | 25 (71.4%) |
| Decitabine or Azacitidine | 2 (5.9%) |
| Other* | 0 (0.0%) |
| Palliative only | 0 (0.0%) |
| Relapse | 12 (11.9%) |
Other includes oral 6-mercaptopurine, Iressa clinical trial (CT), FLT3 inhibitor with mTOR inhibitor CT, CT with Revlimid and Velcade, histone deacetylase inhibitor CT, Cloretazine CT, and all-trans retinoic acid
Abbreviations: BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HCT, Hematopoietic Cell Transplant; MDS, myelodysplastic syndrome
About one-third (35.0%) of the patients underwent induction, 20 (19.8%) underwent consolidation chemotherapy, and 18 (17.8%) patients received stem cell therapy (SCT), most of which was non-myeloablative from a matched unrelated donor. 41.0% of patients received chemotherapy other than standard induction, and about a quarter (24%) of the cohort received only palliative or supportive care. 23% of patients received initial treatment in a clinical trial.
Self-reported geriatric assessment variables are displayed in Table 2. Answers to questions ranged from 1 (not at all) to 4 (very much) and asked patients to consider their condition in the past week. One-third of patients reported substantial (“quite a bit” or “very much”) difficulty doing strenuous activities or limitations in their work or daily activities, and only 3 patients reported requiring more help with activities of daily living (ADLs) including eating, dressing, washing, and toileting. Cognitive complaints were relatively uncommon, although nearly half (47.5%) of patients reported feeling more depressed in the past week. 15.8% of patients reported substantial pain during the past week.
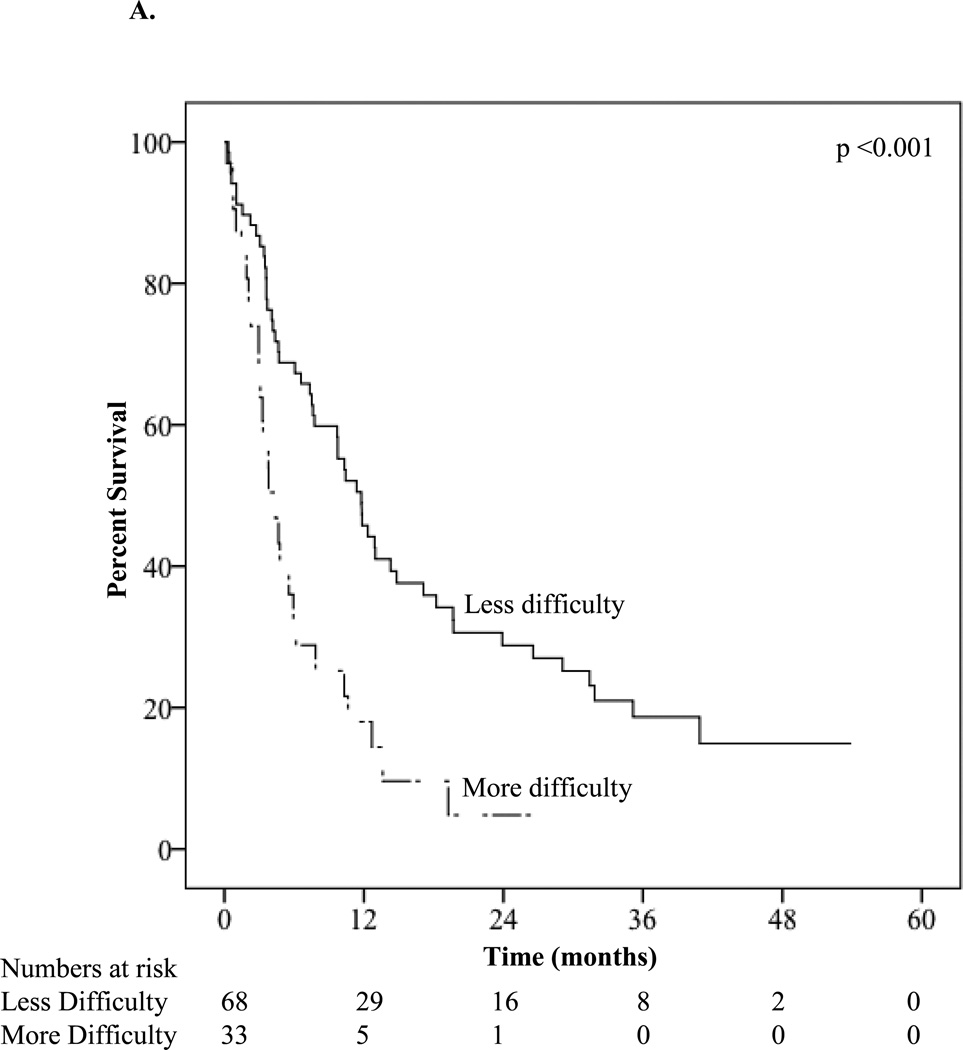
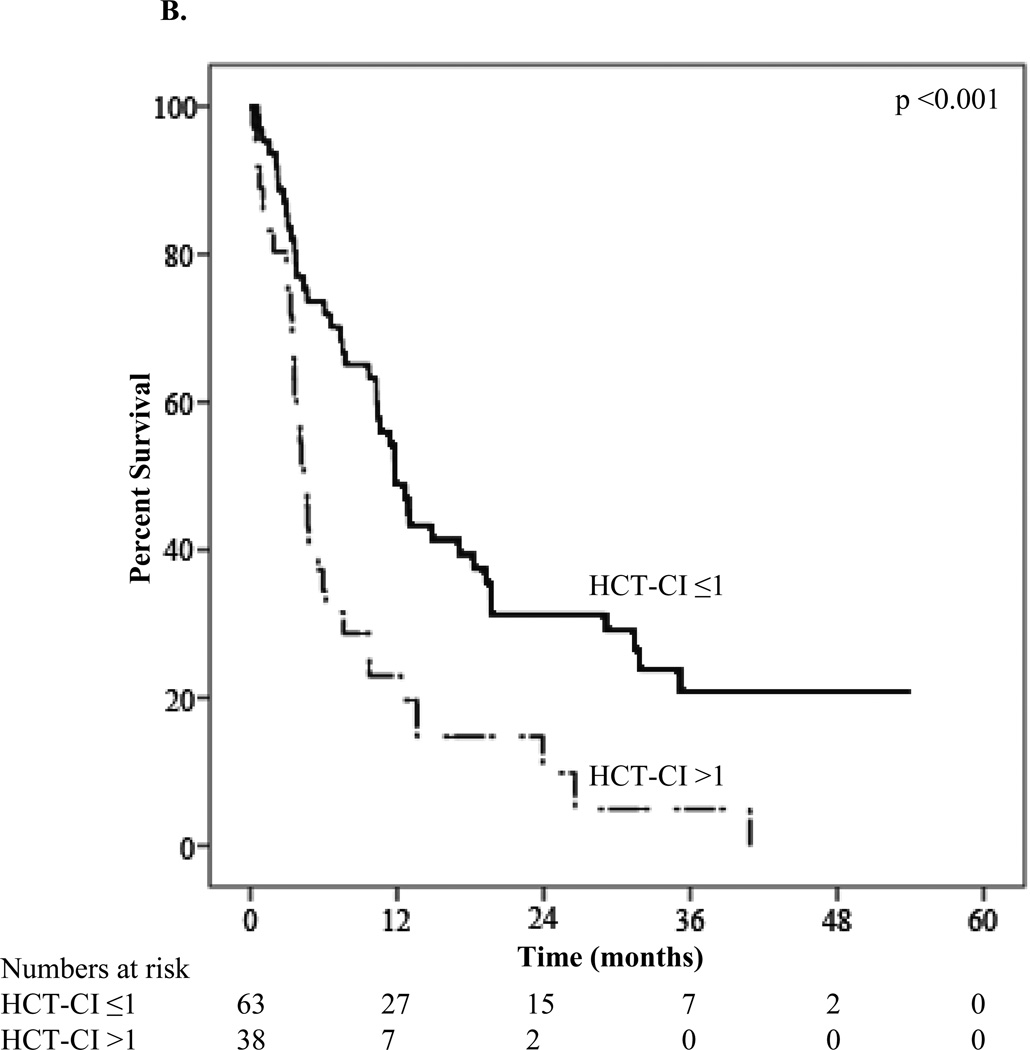
The median overall survival of the group was 7.8 months. The one-year OS was 37.4% and the one-year disease-specific survival was 39.0%. Survival based on demographic, tumor, treatment, and survey characteristics is presented in Table 3. As expected, median OS differed substantially by initial treatment (induction chemotherapy: 14.8 ±4.4 months versus decitabine or azacitidine: 11.4 ±1.9 months versus other therapy: 3.1 ±1.1 months versus palliative only: 3.4 ±2.4 months; p<0.001). A number of study variables predicted survival, in addition to the known prognostic factors. Patients reporting less difficulty with strenuous activity had increased survival compared to patients reporting more difficulty (11.8 versus 4.4 months; P<0.001) (Figure 1A). Less pain in the week prior to baseline was also associated with better survival (10.3 versus 4.1 months; P<0.002) as was HCT-CI score ≤1 versus >1 (11.8 versus 4.4 months; P<0.001) (Figure 1B).
Table 3.
Kaplan-Meier survival estimates based on patient and tumor characteristics
| Characteristic | Median OS* (months) |
1-year OS (% survival) |
2-year OS (% survival) |
P-value* |
|---|---|---|---|---|
| Age at diagnosis (years) | <0.001 | |||
| ≤72 | 12.3 ±1.9 | 51.4 ±7.2 | 32.8 ±7.0 | |
| >72 | 4.7 ±1.2 | 22.2 ±6.2 | 10.3 ±4.7 | |
| BMI (kg/m2) | 0.095 | |||
| <28 | 4.8 ±1.1 | 31.6 ±6.6 | 20.4 ±5.9 | |
| ≥28 | 11.8 ±1.1 | 47.2 ±7.8 | 24.9 ±7.1 | |
| Albumin | 0.062 | |||
| Normal | 9.7 ±1.9 | 40.3 ±5.4 | 23.6 ±4.9 | |
| Low (<3.5g/dL) | 3.0 ±1.2 | 18.8 ±11.9 | 9.4 ±8.9 | |
| HCT-comorbidity index | <0.001 | |||
| ≤1 | 11.8 ±1.2 | 48.8 ±6.6 | 31.3 ±6.3 | |
| >1 | 4.4 ±0.5 | 23.0 ±7.1 | 9.8 ±6.0 | |
| ECOG PS | 0.015 | |||
| ≤1 | 10.3 ±1.0 | 40.8 ±5.8 | 28.8 ±5.6 | |
| >1 | 4.4 ±0.7 | 33.3±10.3 | 5.6 ±5.4 | |
| Origin of AML | 0.022 | |||
| De novo | 11.8 ±2.0 | 46.9 ±7.0 | 31.1 ±6.8 | |
| Secondary | 6.6 ±1.3 | 29.3 ±7.1 | 14.2 ±5.9 | |
| Cytogenetic risk group | 0.001 | |||
| Favorable | ** | ** | ** | |
| Intermediate | 12.9 ±1.7 | 53.5 ±7.6 | 37.2 ±7.7 | |
| Adverse | 6.6 ±2.0 | 26.9 ±8.0 | 3.4 ±3.3 | |
| Peripheral blast percent | 0.022 | |||
| ≤8% | 11.4 ±1.0 | 44.5 ±7.2 | 34.1 ±7.2 | |
| >8% | 6.1 ±2.0 | 32.6 ±7.1 | 12.9 ±5.2 | |
| Initial Treatment | <0.001 | |||
| Induction chemotherapy | 14.8 ±4.4 | 60.1 ±8.6 | 39.1 ±8.9 | |
| Decitabine or Azacitidine | 11.4 ±1.9 | 45.0 ±9.0 | 18.2 ±7.6 | |
| Other | 3.1 ±1.1 | 14.3 ±13.2 | ** | |
| Palliative only | 3.4 ±2.4 | 4.2 ±4.1 | ** | |
| Complete response achieved | <0.001 | |||
| No | 4.7 ±1.0 | 24.4 ±5.4 | 14.4 ±4.5 | |
| Yes | 23.9 ±6.7 | 76.8 ±8.3 | 48.0 ±10.7 | |
| Stem cell therapy (SCT) | <0.001 | |||
| No SCT | 5.9 ±1.4 | 25.9 ±5.1 | 15.6 ±4.3 | |
| Yes SCT | 29.1 ±9.1 | 88.9 ±7.4 | 50.8 ±12.5 | |
| Strenuous activity difficulty | <0.001 | |||
| Less difficulty | 11.8 ±1.4 | 45.8 ±6.1 | 28.8 ±5.8 | |
| More difficulty | 4.4 ±0.9 | 18.0 ±7.2 | 4.8 ±4.5 | |
| Pain in last week | 0.022 | |||
| Less often | 10.3 ±2.0 | 42.5 ±5.6 | 27.6 ±5.3 | |
| More often | 4.1 ±1.0 | 20.0 ±11.3 | ** |
OS=Overall survival
P-values calculated using log-rank test
Too few patients to calculate
Figure 1.
A. Kaplan-Meier survival curve stratified by difficulty with strenuous activity
B. Kaplan-Meier survival curve stratified by HCT-comorbidity index
On multivariate analysis (Table 4), adverse and unknown cytogenetic versus intermediate and favorable risk group (Hazard Ratio [HR], 2.61; 95%CI 1.60 to 4.25), HCT-CI score >1 versus ≤1 (HR, 1.92; 95%CI 1.18 to 3.11), more difficulty with strenuous activity versus less difficulty (HR, 2.18; 95%CI 1.19 to 4.00), and pain more versus less often (HR, 2.17; 95%CI 1.19 to 3.97) were independent prognostic factors for increased risk of death. We performed a second analysis to determine if the effect of the predictors was independent of initial treatment assignment. Both initial treatment with induction therapy (HR= 0.26; 95%CI 0.14 to 1.50) and hypomethylating agents (HR=0.39; 95%CI 0.22 to 0.68) were associated with a substantially decreased risk of mortality compared to those treated with palliative therapy. HCT-CI score (HR=1.49; 95%CI 1.36 to 3.84), difficulty with strenuous activity (HR=1.81; 95%CI 1.04 to 3.13) and pain (HR=2.55; 95%CI 1.41 to 4.64) retained significance, while cytogenetics was no longer significant (HR=2.29; 95%CI 0.90 to 2.45).
Table 4.
Multivariate analysis of prognostic factors for increased risk of death
| Factor | Hazard Ratio | 95% CI* |
|---|---|---|
| Cytogeneticsα | 2.61 | 1.60–4.25 |
| HCT-CI scoreβ | 1.92 | 1.18–3.11 |
| Difficulty with strenuous activityγ | 2.18 | 1.19–4.00 |
| Painδ | 2.17 | 1.19–3.97 |
| ECOG PSε | 0.96 | 0.50–1.83 |
| Origin of AMLδ | 1.18 | 0.74–1.89 |
Hazard Ratios and 95% confidence intervals estimated by Cox proportional hazards models.
Adverse and unknown vs. Intermediate and favorable
HCT-CI score >1 vs. ≤1
More difficulty vs. less difficulty
Pain more often vs. less often
ECOG PS >1 vs. ≤1
Secondary vs. de novo
We performed a sub-analysis among the 80 patients with the best ECOG PS (0 or 1) to determine if the study variables in the final model would predict mortality in the healthiest patients. More vs. less difficulty with strenuous activities (median OS =11.8 versus 3.1 months; P<0.001), pain more vs. less often (median OS =10.4 versus 3.4 months; P=0.036), and comorbidity score >1 versus ≤1 (median OS =11.8 versus 4.8 months; P=0.008) remained predictors of increased risk of death.
Compared to patients who received non-intensive or supportive treatments, those who received induction were younger (p < 0.001), on fewer medications (p=0.29), had lower comorbidity scores (p=0.002), and less difficulty with strenuous activities (p=0.025). On binary logistic regression, only age at diagnosis >72 versus ≤72 (Odds ratio=23.8; 95%CI 6.30 to 90.19) and HCT-CI score >1 versus ≤1 (HR, 4.56; 95%CI 1.41 to 14.72) remained as independent predictors of not receiving IC.
Discussion
In this retrospective study of older patients with AML, we found that baseline geriatric assessment variables added valuable prognostic information to conventional clinical and pathological predictors of mortality. The model that best predicted survival in our cohort included a disease-specific comorbidity score and self-reported measures of strenuous activity and pain in addition to cytogenetic risk group. Geriatric assessment variables remained independent predictors of mortality even among patients with the best functional status. Our study represents one of the first to use self-assessed variables to predict survival in older patients with AML, and suggests that more comprehensive risk assessment tools for this population are needed.
Although the need for geriatric assessment in oncology is well recognized, there is as yet no widely validated tool for use in oncology settings, and no instrument specific to hematologic malignancies. Well-conducted studies in general oncology populations have demonstrated that geriatric assessment in combination with conventional clinical and disease-specific factors can accurately predict vulnerability to treatment toxicity. In one prospective multicenter study, factors that independently predicted toxicity included poor hearing, falls in the past six months, difficulty managing medications, difficulty walking one block and decreased social activity, but few patients with hematologic malignancies were included. [8] A score to predict CR and early death in patients ≥ age 60 with AML who are candidates for induction has recently been developed, and is a major step forward in the effort to individualize treatment. [23] However, the tool is based on standard biological and clinical factors and does not include any measure of functional status or comorbidity. In our cohort, age itself was not an independent predictor of overall survival when other study variables were added to the model. This illustrates the need to better individualize prognosis using a multi-dimensional evaluation such as CGA.
Our findings agree with those of other studies showing that age and conventional prognostic factors do not sufficiently account for differences in survival of older AML patients [24–26], and therefore lack the necessary resolution to discriminate between good or poor candidates for intensive therapy.[3, 27] For example, while we found that self-reported physical function was associated with survival, in our cohort only a question focused on strenuous activities was predictive. This question provided more information than ECOG PS, as it was still predictive of survival even in patients with the best PS. Thus, in planning prospective geriatric assessment of candidates for induction, a more challenging functional test such as 6 minute walking speed will likely be a more helpful predictor than a standard “up and go” test. [28] On the other hand, patients who are being considered for non-intensive chemotherapy would benefit more from an assessment targeted at detecting the geriatric syndromes and frailty that would make them vulnerable to toxicity from these regimens.
Comorbidity is an important predictor of outcome in any cancer, and AML is no exception.[29] Similar to other studies, we found that a higher HCT-CI score was associated with decreased survival.[18, 30] The HCT-CI is a validated index of comorbidity found to be more sensitive than the Charlson comorbidity index for predicting non-relapse mortality and overall survival in patients with hematologic malignancies in a general AML population.[19] However, it does not contain the entire range of comorbidities found in the Charlson Index, and determining which comorbidity score is preferable in older AML patients is an important area for future work. In our study, comorbidity predicted whether patients would receive induction therapy as well as survival. It is an important and easily quantifiable factor that should be included in the risk-stratification of older patients with AML.
Pain is a highly prevalent symptom in the elderly that is intimately linked to multiple geriatric domains and health-related quality of life. Self-reported frequent pain in the week prior to AML diagnosis was a powerful prognostic indicator for worsened survival in our model. Pain has been shown to predict mortality in a general population, but the mechanisms for this relationship are unclear. [31] Bone pain from AML is not a frequent symptom at presentation in older adults, and on medical record review we found that the majority of pain seemed to be due to non-cancer comorbidities or related to bone marrow biopsy. It is possible that pain is functioning as a marker of comorbid conditions not included in the HCT-CI score. This finding should be further explored in larger cohorts of patients with AML.
It is important to emphasize that the older patients who present to our regional cancer center are a select group with fewer comorbidities and functional limitations than expected for their age. Thus, questions in a number of geriatric domains were not predictive. Cognitive function and dependence in daily activities are usually powerful prognostic factors, but only 6.1% of our patients reported significant problems with memory and only 3% required help with activities of daily living. This also underscores the fact that geriatric oncology tools designed to detect markers of frailty in a general population may have substantial “ceiling” effects in older patients who qualify for intensive therapies or clinical trials.
As expected, initial treatment regimen was an important determinant of mortality. This reflects both the benefit of treatment, and the skill of clinicians in selecting it based on patient factors such as comorbidity and functional status. We found that comorbidity score and self-reported measures of physical function and pain were predictors of outcome independent of treatment assignment, suggesting that they are useful across a wide range of patient characteristics. Previous studies in older adults with AML have shown that patients with better PS and fewer comorbidities have increased survival after induction.[32] A study of AML patients ≥ age 70 suggested that induction may only be beneficial for survival in a small subset of patients based on age, PS, tumor karyotype, and creatinine level, but [33] this study did not include geriatric assessment. In our cohort, age and comorbidity were the only independent prognostic factors for reception of induction chemotherapy, but as only 35 patients received induction, the power for this analysis was limited. Ongoing studies incorporating geriatric assessment in the care of older patients with AML will help develop tools to improve selection of older patients for aggressive treatment. [9]
Our work has a number of limitations. As a single-institution study, the population seen at DFCI for treatment of AML is highly select. The patients that agreed to complete our surveys were likely the least sick, and they were seen in the outpatient setting prior to hospitalization. Furthermore, patients with AML admitted first as inpatients were excluded from the study as they completed the new patient survey after initiating chemotherapy. While these factors limit generalizability, the fact that geriatric assessment variables predict outcome even in this population suggest their value in a more general one, as this selection bias would influence our results toward the null. In this exploratory analysis, we used self-reported variables from a validated quality of life survey that was not designed for geriatric patients. True geriatric assessment involves the use of validated instruments for evaluation of specific domains such as mood and cognition, for which objective analysis is critical. Nevertheless, we found that self-reported variables on physical function and pain were helpful predictors of outcome, suggesting that true geriatric assessment would provide even more valuable information. Finally, we did not have information on molecular genetics, a new and important prognostic indicator in AML.
In conclusion, our study provides evidence that assessment of geriatric domains adds important prognostic information over and above that of established laboratory and clinical factors in older patients with AML, even among those with an excellent performance status and relatively few comorbidities. Our data suggest that in a highly select population such as patients being considered for induction therapy, assessments must be targeted at higher levels of function. Further studies in larger cohorts of elderly patients with AML are needed to better define the geriatric domains that are most valuable in predicting prognosis in combination with known prognostic factors and new molecular genetic techniques.
Acknowledgements
Funding Source: Dr. Driver is funded by a Veterans’ Administration Career Development award (JAD). Alexander Sherman is funded by an American Federation for Aging Research Grant #1T35AG038027-02 (AES).
Footnotes
Author contributions: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data (AES, GM, KRF, DJD, GAA, DS, MW, RMS, JAD), (2) drafting the article or revising it critically for important intellectual content (AES, GM, KRF, DJD, GAA, DS, MW, RMS, JAD), (3) final approval of the version to be submitted (AES, GM, KRF, DJD, GAA, DS, MW, RMS, JAD)
Conflict of Interest Disclosures: The authors report no conflicts of interest or disclosures that could inappropriately influence their work on this project. Dr. Richard M Stone has served in a Consultant or Advisory Role for Genzyme © and has received investigator-initiated research funding from Novartis ©. Dr. Daniel J DeAngelo has served in a consultant or advisory role for Novartis ©.
References
- 1.U.S.C. Bureau, editor. U.S. Interim Projections by Age, Sex, Race and Hispanic Origin. 2004
- 2.Howlader N, N.A, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review. 2012. [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedding U, Rohrig B, Klippstein A, et al. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132(10):665–671. doi: 10.1007/s00432-006-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60(2):120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 6.Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18(4):809–816. doi: 10.1038/sj.leu.2403289. [DOI] [PubMed] [Google Scholar]
- 7.Balducci L, Colloca G, Cesari M, et al. Assessment and treatment of elderly patients with cancer. Surg Oncol. 2010;19(3):117–123. doi: 10.1016/j.suronc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 9.Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc. 2011;59(10):1837–1846. doi: 10.1111/j.1532-5415.2011.03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behringer B, Pitako JA, Kunzmann R, et al. Prognosis of older patients with acute myeloid leukemia receiving either induction or noncurative treatment: a single-center retrospective study. Ann Hematol. 2003;82(7):381–389. doi: 10.1007/s00277-003-0650-0. [DOI] [PubMed] [Google Scholar]
- 11.Lengfelder E, Hanfstein B, Haferlach C, et al. Outcome of elderly patients with acute promyelocytic leukemia: results of the German Acute Myeloid Leukemia Cooperative Group. Ann Hematol. 2012 doi: 10.1007/s00277-012-1597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauncey TR, Gundacker H, Shadman M, et al. Sequential phase II Southwest Oncology Group studies (S0112 and S0301) of daunorubicin and cytarabine by continuous infusion, without and with ciclosporin, in older patients with previously untreated acute myeloid leukaemia. Br J Haematol. 2010;148(1):48–58. doi: 10.1111/j.1365-2141.2009.07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Donnell MR, Abboud CN, Altman J, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9(3):280–317. doi: 10.6004/jnccn.2011.0027. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, openlabel, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pigneux A, Harousseau JL, Witz F, et al. Addition of lomustine to idarubicin and cytarabine improves the outcome of elderly patients with de novo acute myeloid leukemia: a report from the GOELAMS. J Clin Oncol. 2010;28(18):3028–3034. doi: 10.1200/JCO.2009.26.4648. [DOI] [PubMed] [Google Scholar]
- 16.Spataro V, Kovacsovics T, Bach S, et al. Acute myeloid leukemia in the elderly: results of an individualized approach in two centres. Leuk Lymphoma. 2000;39(5–6):521–530. doi: 10.3109/10428190009113382. [DOI] [PubMed] [Google Scholar]
- 17.Djunic I, Virijevic M, Novkovic A, et al. Pretreatment risk factors and importance of comorbidity for overall survival, complete remission, and early death in patients with acute myeloid leukemia. Hematology. 2012;17(2):53–58. doi: 10.1179/102453312X13221316477651. [DOI] [PubMed] [Google Scholar]
- 18.Savic A, Kvrgic V, Rajic N, et al. The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leuk Res. 2012;36(4):479–482. doi: 10.1016/j.leukres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Rowe JM, Kim HT, Cassileth PA, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116(21):5012–5021. doi: 10.1002/cncr.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug U, Rollig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376(9757):2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 24.Wahlin A, Markevarn B, Golovleva I, et al. Prognostic significance of risk group stratification in elderly patients with acute myeloid leukaemia. Br J Haematol. 2001;115(1):25–33. doi: 10.1046/j.1365-2141.2001.03043.x. [DOI] [PubMed] [Google Scholar]
- 25.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 26.Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–4423. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 28.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etienne A, Esterni B, Charbonnier A, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109(7):1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 30.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136(4):624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 31.Feeny D, Huguet N, McFarland BH, et al. Hearing, mobility, and pain predict mortality: a longitudinal population-based study. J Clin Epidemiol. 2012;65(7):764–777. doi: 10.1016/j.jclinepi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colovic M, Colovic N, Radojkovic M, et al. Induction chemotherapy versus palliative treatment for acute myeloid leukemia in a consecutive cohort of elderly patients. Ann Hematol. 2012 doi: 10.1007/s00277-012-1478-2. [DOI] [PubMed] [Google Scholar]
- 33.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]