Abstract
Systemic inhibition of Notch signaling was previously shown to attenuate experimental autoimmune encephalomyelitis (EAE), a disease model of multiple sclerosis in mice. Different studies attributed these effects to decreased T-bet and IFNγ expression, enhanced regulatory T cell function, reduced T cell chemotaxis to the central nervous system (CNS) or impaired Th9 cell differentiation. Interpretation of these heterogeneous findings is difficult, since past experimental strategies did not ensure complete Notch inhibition in T cells and since many cell populations could be affected by systemic Notch blockade. To resolve the role of Notch in T cells during EAE, we used the pan-Notch inhibitor dominant negative form of Mastermind-like 1 (DNMAML), as well as several complementary loss-of-function approaches specifically in myelin-reactive T cells. Notch inhibition in T cells profoundly decreased EAE incidence and severity. Notch-deprived myelin-reactive T cells had preserved activation and effector differentiation in secondary lymphoid tissues. However, Notch-deprived T cells failed to accumulate in the CNS post-immunization. Parking wild type and DNMAML T cells together in bone marrow chimeras increased accumulation of Notch-deprived T cells in the CNS post-immunization but did not prevent EAE, indicating the absence of dominant suppression by DNMAML T cells. Analysis of CNS-infiltrating DNMAML T cells revealed markedly defective IL-17A and IFNγ production, despite preserved T-bet expression. Altogether, our findings capture the profound overall effects of Notch signaling in myelin-reactive T cells and demonstrate that Notch controls the accumulation and pathogenic functions of CD4+ T cells within their target organ but not in lymphoid tissues during EAE.
Introduction
Notch signaling plays multiple roles in health and disease (1, 2). Notch ligands of the Delta-like (Dll) or Jagged family interact with Notch receptors, resulting in sequential proteolysis and release of intracellular Notch (ICN). In the nucleus, ICN interacts with CSL/RBP-Jk (encoded by Rbpj) and Mastermind-like coactivators (MAML) to activate target genes. In the hematopoietic system, Notch regulates development of early T cell progenitors and several other innate and adaptive immune system lineages (3–9). In addition, mounting evidence indicates a context-dependent role for Notch in T cell differentiation and function (10, 11).
Prior studies showed that systemic Notch blockade could attenuate experimental autoimmune encephalomyelitis (EAE, a mouse model of multiple sclerosis), but with conflicting information about the intensity and mechanisms of this effect. Using gamma-secretase inhibitors (GSIs) to ubiquitously inhibit Notch signaling as well as Notch1 activation and a Notch1 antisense strategy, Osborne's group reported that Notch directly regulates expression of Tbx21 (encoding T-bet) in peripheral T cells during EAE (12). GSIs were also observed to enhance remyelination and axonal survival in EAE, indicating the existence of non-immune effects of these drugs (13, 14). Another study using GSIs and anti-Notch3 neutralizing antibodies described Notch3 as a dominant receptor influencing EAE via PKCtheta expression in Th1/Th17 CD4+ T cells (15). Systemic blockade of the Notch ligand Dll4 was shown to bolster T regulatory cell (Treg) function during EAE, while others using a similar approach reported altered T cell differentiation or chemotaxis (16–18). Jagged2 activation was reported to reduce IL-17A in secondary lymphoid organs and increase Treg responses (19). Finally, Notch was linked to Th9 differentiation in EAE (19). These discrepant results might reflect the use of heterogeneous experimental systems based on systemic Notch modulation or gain-of-function, which can trigger unintended off- and on-target effects and hinder accurate conclusions about Notch function specifically in T cells. This is particularly important in EAE since Notch affects many immune and non-immune cells that contribute to disease pathogenesis (11, 20). In addition, experimental strategies that focus on individual Notch ligands or receptors may fail to completely block Notch signaling in myelin-reactive T cells, thus underestimating the impact of Notch inhibition or leading to misleading effects on the immune system
To resolve these conflicting results, we investigated Notch function specifically in mature T cells during EAE using several complementary loss-of-function approaches, including expression of the pan-Notch inhibitor DNMAML and inactivation of Notch receptor genes. In addition, we evaluated the effects of Notch inhibition in TCR transgenic mice that are sensitized to EAE by a dominant population of myelin-reactive T cells. T cell-specific Notch inhibition resulted in near complete protection from EAE, independent of T cell activation and effector differentiation effects in secondary lymphoid organs. Notch-deprived CD4+ T cells failed to accumulate in the CNS post-immunization despite preserved in vitro migration. Parking WT and DNMAML CD4+ T cells together in BM chimeras increased accumulation of Notch-deprived CD4+ T cells in the CNS but did not suppress disease. In the CNS, Notch-deprived myelin-reactive CD4+ T cells failed to produce IL-17A and IFNγ, despite preserved expression of the master transcription factor, T-bet. Our findings reveal the overall effects of Notch in T cells during EAE, as complete T cell-specific Notch inhibition led to significantly more protection than reported with other methods of Notch blockade. Moreover, we demonstrate that Notch specifically regulates the secondary response of myelin-reactive CD4+ T cells in the CNS independently of effects on T-bet and Tregs during the primary response in lymphoid organs.
Materials and Methods
Mice
C57BL/6.Ptprca (B6-SJL, CD45.1+) were from the NCI (Frederick, MD); C57BL/6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J (2D2) T cell receptor transgenic were provided by Dr. Segal (University of Michigan) (21); Rbpjf/f mice by Dr. Honjo (Kyoto, Japan) (6); Notch1f/f mice by Dr. Kopan (St. Louis, MO) (5); and Notch2f/f by Dr. Gridley (Scarborough, ME) (22). ROSA26DNMAMLf mice (DNMAML) contain a Cre-inducible cassette encoding the DNMAML-GFP pan-Notch inhibitor (23). DNMAML, Rbpjf/f, Notch1f/f, and Notch1f/fNotch2f/f mice were crossed to Cd4-Cre mice to achieve Cre-mediated excision in CD4+CD8+ double positive thymocytes, and thus in all mature T cells, without interference with Notch signaling in early T cell development (abbreviated DN, RB KO, N1 KO, N1/2 KO). ROSA26DNMAMLf x Cd4-cre mice were crossed to 2D2 mice (abbreviated 2D2/DN). All mice were backcrossed to the B6 background (>8 generations). The University of Michigan's Committee on Use and Care of Animals approved all experiments.
EAE induction
On day 0, age-matched (6–14 weeks) and sex-matched mice were immunized with Complete Freund's Adjuvant containing heat-killed Mycobacterium tuberculosis (Fisher, Pittsburg, PA) and myelin oligodendrocyte glycoprotein (MOG35-55) peptide (MEVGWYRSPFSRVVHLYRNGK; 0.25 μg/site; Biosynthesis, Lewisville, TN). On day 0 and 2, mice received pertussis toxin (Fisher) (300 ng i.p.). Mice were scored for disease severity according to the following scale: 1= limp tail; 2=inability to right oneself; 3=hind limb weakness; 4=hind limb paralysis; 5=moribund. All analyses of T cell function were done at peak disease (score 3–5). For disease incidence calculations, a mouse that had reached a clinical score of ≥2 was indicated as having EAE.
Isolation of CNS-infiltrating cells
After anesthesia, mice were perfused with PBS. Brains and spinal cords were digested with Collagenase (2.125 mg/mL; Invitrogen, Grand Island, NY) and DNase I (1mg/mL; Roche. Indianapolis, IN) followed by purification on a 30/70% Percoll (Sigma, St. Louis, MO) gradient.
ELISpot
Draining lymph node cells (axial, brachial, inguinal) from immunized mice at peak disease were restimulated in MultiScreen HTS Filter plates (Millipore, Billerica, MA) +/− 50 μg/mL MOG35-55 for 18 hours. Antibodies used for cytokine detection were from eBioscience (anti-IFNγ and IL-17A). Streptavidin-Horseradish peroxidase (HRP) was from Southern Biotech (Birmingham, AL). HRP substrate was from Vector Laboratories (Burlingame, CA).
Quantitative reverse transcription PCR
RNA from CD4+Vα3.2+Vβ11+CD44+ T cells was isolated using TRIzol (Invitrogen, Carlsbad, CA). cDNA was prepared with Superscript II (Invitrogen). qPCR was performed with TaqMan (Applied Biosystems, Carlsbad, CA) on Mastercycler realplex (Eppendorf, Westbury, NY). Primers were from Applied Biosystems. Relative expression was calculated using the ΔΔCt method.
Transwell migration assays
The following chemokine ligands were from R&D Systems (Minneapolis, MN): Ccl20/Mip-3a, Ccl2/Mcp-1, Cxcl10/Crg-2, Ccl3/Mip-1a, and Ccl21/6Ckine. CD4+ T cells from 2D2 and 2D2/DNMAML immunized mice at peak disease were purified by Miltenyi Magnetic Bead technology (Auburn, CA). Purified CD4+ T cells were warmed to 37°C, plated in a NeuroProbe ChemoTx System (Gaithersburg, MD) and allowed to migrate for 4 hours before analysis of migrated CD4+Vα3.2+Vβ11+CD44+ T cells in bottom wells by flow cytometry. Number of cells migrated was normalized using a standard curve of known numbers of T cells and a fixed number of counting beads (Bangs Laboratories, Fishers, IN) by flow cytometry. Specificity of migration was determined by enumerating the number of activated 2D2 CD4+Vα3.2+Vβ11+CD44+ T cells that migrated to the bottom well in the absence of chemokines.
Flow cytometry
The following antibodies were from BioLegend, eBiosciences (both San Diego, CA) or BD Biosciences (San Jose, CA): anti-CD4, CD8α, CD44, CD45.1, CD45.2, TCRβ, CD49d (α1), CD29 (β4), IFNγ, IL-17A, Vβ11, Vα3.2, Foxp3, and T-bet (4B10). For T cell restimulation, we used plate-bound anti-CD3 (145-2C11) and anti-CD28 (37.51) (Biolegend, 2.5 μg/ml). Intracellular flow cytometry was performed per manufacturer's instructions after addition of Brefeldin A (>2 hours) (BD). Analysis/sorting were on FACSCanto or FACSAria II/III (BD). Dead cells were excluded with 4′6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO). Files were analyzed in FlowJo (Tree Star, San Carlos, CA).
Generation of mixed bone marrow chimeras
Lethally irradiated (900 rads) B6-CD45.1 mice received B6 CD45.2-WT and B6-CD45.1, or B6 CD45.2-DNMAML and B6-CD45.1 bone marrow (mixed at 1:1 or 7:3 ratio). Mice were allowed to reconstitute for 8–12 weeks before EAE induction.
Statistical analysis
Comparison of two means was performed with 2-tailed unpaired Student t test or nonparametric Mann-Whitney U-test (GraphPad-Prism, LaJolla, CA). For differences in disease incidence, significance was determined by Chi-square and Fisher's exact test (GraphPad-Prism). P values are indicated as follows throughout the manuscript: p<0.05*; p<0.01**; p<0.001***.
Results
Notch inhibition in myelin-reactive CD4+ T cells prevents EAE
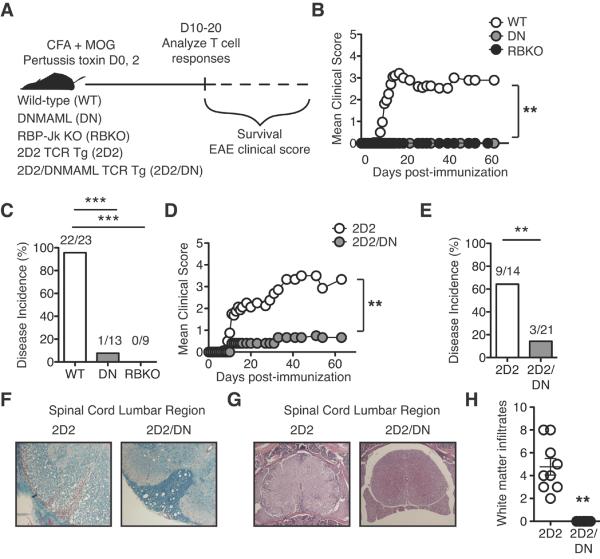
To overcome limitations of past studies, we used in vivo loss-of-function models to block Notch signaling specifically in T cells during EAE (Fig. 1A). We inhibited the Notch transcriptional activation complex downstream of all Notch receptors in mature T cells by expressing a dominant negative Mastermind-like mutant (DNMAML) or by inactivating Rbpj, encoding CSL/RBP-Jk (6, 23). In selected experiments, DNMAML was introduced into 2D2 TCR transgenic T cells, recognizing MOG35-55 (21). Notch blockade efficiently prevented EAE, with <8% of mice developing symptoms, as compared to ~95% of controls (Fig. 1B, C). When DNMAML was expressed in 2D2 T cells, disease incidence was also markedly reduced (Fig. 1D, E). Decreased EAE in 2D2/DNMAML mice correlated with reduced demyelination (Fig. 1F) and CNS cellular infiltrates (Fig. 1G, H). Previous reports showed significantly less protection, perhaps because of incomplete Notch blockade or family redundancy when only Notch1, Notch3 or Dll4 was inhibited (12, 15–19). Thus, Notch inhibition in T cells markedly reduced EAE, even in the presence of a high frequency of myelin-reactive T cells in TCR transgenic mice.
Figure 1. Inhibition of Notch signaling in myelin-reactive CD4+ T cells markedly attenuates EAE.
(A) Experimental design; (B) Mean clinical EAE score (≥2 experiments); (C) Percent disease incidence (score ≥2) of immunized wild-type (WT), DNMAML (DN) or CSL/RBP-Jk-deficient (RBKO) mice (pooled results from ≥2 experiments for each strain). In all models, Cd4-Cre-mediated Notch inactivation was achieved specifically in mature T cells; (D) Mean clinical score in TCR transgenic 2D2 or 2D2/DN mice (expressing DNMAML in T cells) (≥3 experiments); (E) Percent disease incidence (score ≥2) of immunized 2D2 and 2D2/DN mice (≥2 experiments); (F) Luxol fast blue and (G) H&E staining of spinal cord lumbar sections from 2D2 and 2D2/DN mice (representative of n=3 mice/group; 2 experiments); (H) Number of white matter infiltrates per H&E section (counted blindly). p<0.01**.
Preserved effector differentiation of myelin-reactive Notch-deprived CD4+ T cells in lymphoid tissues
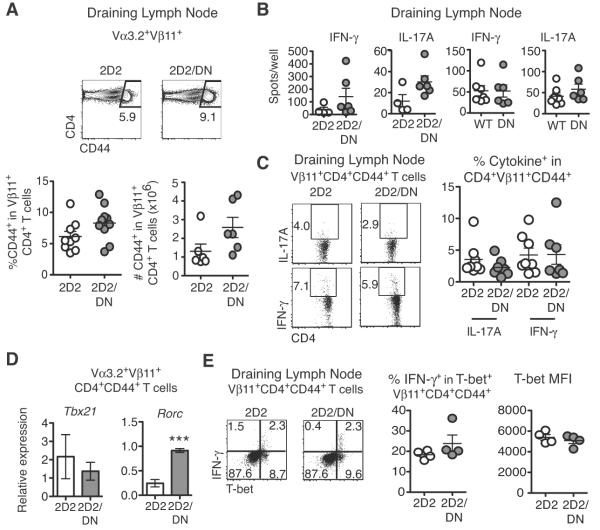
Previous studies in EAE suggested that Notch modulates effector T cell differentiation in secondary lymphoid tissues (12, 16, 19). We assessed T cell responses at peak disease using 2D2 transgenic mice, in which myelin-reactive T cells can be tracked based on expression of a Vα3.2+Vβ11+ TCR (21). DNMAML expression preserved 2D2 CD4+ T cell activation in draining lymph nodes (DLN) as measured by increased CD44 expression (Fig. 2A). Analysis of DLN IFNγ and IL-17A-producing cells, two important EAE drivers, revealed no significant difference between WT and Notch-inhibited CD4+ T cells by ELISpot (Fig. 2B)(24). Intracellular IL-17A and IFNγ expression by activated 2D2/DNMAML T cells was also largely preserved (Fig. 2C). These data suggest that Notch inhibition in myelin-reactive CD4+ T cells did not significantly impact IFNγ and IL-17A production in DLN. These results are similar to data showing that anti-Dll4 treatment during EAE did not alter cytokine production by proteolipid protein-specific T cells (17).
Figure 2. Notch inhibition in myelin-reactive CD4+ T cells does not alter initial activation or effector T cell differentiation.
(A) Percent and absolute number of Vβ11+CD4+CD44+ T cells in the draining lymph node (DLN) at peak disease (n=3–4 mice/group; ≥2 experiments); (B) Number of IFNγ and IL-17A-secreting cells as assessed by ELISpot in DLN from immunized WT, DN, 2D2, and 2D2/DN (n=3–4 mice/group; ≥2 experiments); (C) Frequency of IFNγ and IL-17A-producing DLN Vβ11+CD4+CD44+ T cells after restimulation with anti-CD3/CD28 and staining for intracellular cytokines (n=3–4 mice/group; 2 experiments); (D) Tbx21 and Rorc mRNA in activated CD44+ 2D2/DN and 2D2 Vα3+Vβ11+CD4+ T cells (n=3–4 mice/group; 2 experiments); (E) Intracellular T-bet and IFNγ in Vβ11+CD4+CD44+ T cells as assessed by intracellular flow cytometry (2 experiments; n=3–4 mice/group). Representative flow cytometry plots are shown. MFI: mean fluorescence intensity. p<0.001***.
Prior studies suggested that Notch1 regulates expression of Tbx21 (encoding T-bet) in Th1 cells, while Dll4-mediated signaling can increase Rorc mRNA (encoding Rorγt) in Th17 cells (12, 25). However, we found no significant change in Tbx21 transcripts and a trend for increased Rorc mRNA in activated 2D2/DNMAML CD4+ T cells post-immunization (Fig. 2D). We next studied T-bet expression after verifying antibody specificity in Tbx21−/− mice during EAE (Fig. S1) (26). We observed a preserved frequency of T-bet+ cells and normal staining intensity among 2D2/DNMAML CD4+ T cells (Fig. 2E). Thus, complete inhibition of CSL/RBP-Jk- and MAML-dependent canonical Notch signals in T cells blocked the induction of EAE, but preserved Tbx21 mRNA and T-bet protein in MOG-reactive T cells.
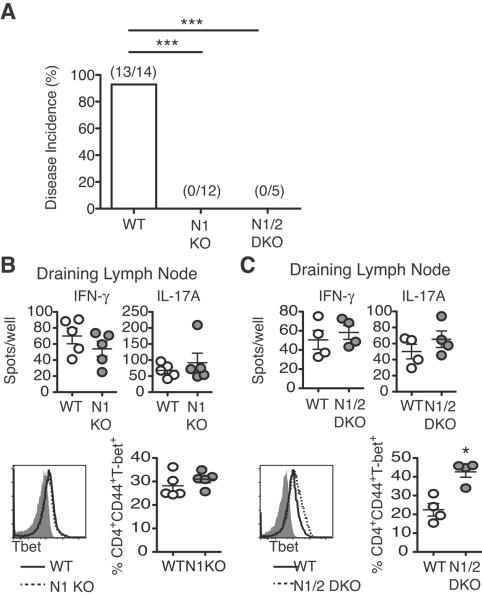
Past work suggested that Notch receptors, such as Notch1, can enhance NFkB activation in T cells by CSL/RBP-Jk and MAML-independent pathways (27). To assess a possible role for these pathways, we studied IFNγ production and T-bet expression in T cells lacking Notch1 or both Notch1/Notch2 during EAE. This strategy should capture any effect of the Notch receptors that does not require CSL/RBP-Jk and MAML-dependent signals. Disease incidence was drastically reduced in the absence of Notch1 or Notch1/2 (Fig. 3A), consistent with dominance of these receptors in T cells. We observed a normal frequency of IL-17A and IFNγ+ cells in Notch1- (Fig. 3B) and Notch1/2-deficient T cells (Fig. 3C), as well as preserved or even slightly increased T-bet levels in Notch1- (Fig. 3B) and Notch1/2-deficient (Fig. 3C) T cells, respectively. Thus, blockade of the Notch transcriptional complex or Notch1/2 inactivation did not impact T-bet and IFNγ production by DLN T cells in EAE. The discrepancy between our findings and previous results may reflect differences between systemic Notch modulation and our complete T cell-specific Notch inhibition.
Figure 3. Inhibition of Notch1 or Notch1/Notch2 protects from EAE independently of changes in Th1 and Th17 differentiation.
(A) Percent disease incidence (score ≥2) of immunized WT, Notch1-deficient (N1KO) and Notch1/2-deficient (N1/2DKO) mice (2 experiments); (B) Number of IFNγ and IL-17A-secreting cells by ELISpot in the DLN from immunized WT and N1KO (n=2–3 mice/group; ≥2 experiments). Frequency of T-bet+CD44+CD4+ N1KO T cells as assessed by intracellular flow cytometry (2 experiments; n=3–4 mice/group); (C) Number of IFNγ and IL-17A-secreting cells by ELISpot in DLN from immunized WT and N1/2DKO (n=2–3 mice/group; ≥2 experiments). Frequency of T-bet+CD44+CD4+ N1/2DKO T cells as assessed by intracellular flow cytometry (2 experiments; n=3–4 mice/group). p<0.05*; p<0.001***.
Notch-inhibited CD4+ T cells fail to accumulate in the CNS despite preserved in vitro migration
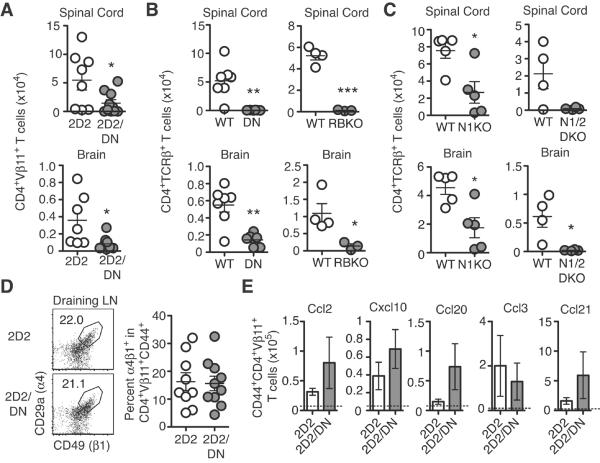
Since T cell responses were preserved in lymphoid tissues, we investigated the role of Notch signaling in CNS-infiltrating T cells at peak disease. Prior reports using Dll4 blockade showed significantly reduced accumulation of T cells in the CNS (17). Similarly, 2D2/DNMAML CD4+ T cells showed markedly reduced accumulation in the CNS post-immunization (Fig. 4A). Similar findings were observed with polyclonal T cells expressing DNMAML or lacking CSL/RBP-Jk (Fig. 4B), as well as in the absence of Notch1/2 and to a slightly lesser extent Notch1 (Fig. 4C). To investigate T cell characteristics that are required for trafficking to the CNS, α4β1 expression and in vitro migration of Notch-deprived CD4+ T cells were assessed. The α4β1 integrin mediates T cell adhesion to the endothelium, a prerequisite for T cell entry into the CNS (28). We found no difference in α4β1 expression between 2D2 and 2D2/DNMAML CD4+ T cells post-immunization (Fig. 4D), although these data do not rule out a defect in integrin conformation or function. Prior work reported that anti-Dll4 inhibits chemotaxis due to decreased Ccr2, Ccr5 and Ccr6 expression (17). In addition, Notch can regulate Ccr7 expression in CNS-infiltrating leukemic T cells (29). Other chemokine receptors have been linked to CD4+ T cell infiltration into the inflamed CNS, such as Cxcr3 (30). However, we found no significant change in expression of these chemokine receptors by 2D2/DNMAML T cells during EAE (data not shown). Next, we assessed responses of Notch-deprived myelin-reactive CD4+ T cell to candidate chemokines in vitro (Fig. 4E). After immunization, activated 2D2/DNMAML CD4+ T cells migrated as well as 2D2 T cells in response to chemokines that interact with Ccr2, 5, 6 and 7 and Cxcr3. These data indicate that Notch-deprived myelin-reactive CD4+ T cells can migrate towards chemotactic signals at least in vitro, although they do not rule out defective migration in vivo. Our conclusions differ from results with Dll4 blockade (17). These differences could reflect specific effects of individual Notch ligands or incomplete Notch inhibition in past studies, as well as effects on T cell migration induced by bystander cells.
Figure 4. Notch-deprived myelin-reactive CD4+ T cells fail to accumulate in the CNS in vivo but have preserved trafficking in vitro.
Spinal cord and brain infiltrates from immunized (A) 2D2, 2D2/DN; (B) WT, DN, RBKO; (C) WT, N1KO, N1/2KO mice. Numbers of infiltrating CD4+ T cells were measured by flow cytometry (n=2–4 mice/group; ≥2 experiments); (D) Expression of α4β1 in 2D2/DN Vα3.2+Vβ11+CD4+CD44+ T cells as assessed by flow cytometry (n=3–4 mice/group; 3 experiments); (E) Transwell migration of 2D2/DN Vβ11+CD4+CD44+ T cells towards indicated chemokines. Dotted line shows background number of primed 2D2 Vα3.2+Vβ11+CD4+CD44+ T cells migrating without chemokine (n=3–4 mice/group; 3 experiments). p<0.05*; p<0.01**; p<0.001***.
Myelin-reactive Notch-deprived CD4+ T cells do not suppress EAE induced by WT CD4+ T cells
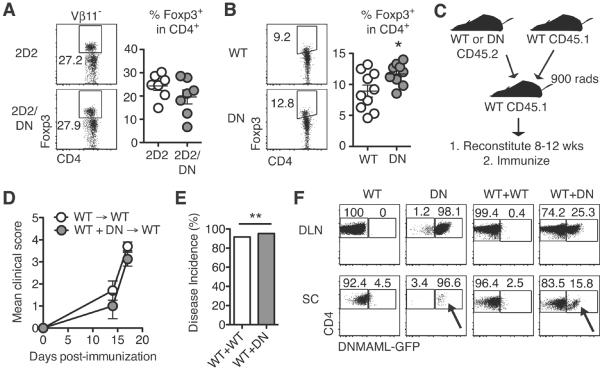
Past studies relying on systemic Notch ligand fusion proteins and antibodies suggested that Notch modulation increased Treg frequency (16). To assess if this contributed to protection from EAE in our T cell-specific genetic model of Notch inhibition, we assessed Foxp3 expression, focusing on non-transgenic T cells that have the most Treg activity in TCR transgenic mice (31). No change in Treg frequency was observed in 2D2/DNMAML mice post-immunization (Fig. 5A). We observed a slight but significant increase in Treg frequency in DNMAML compared to WT CD4+ T cells post-immunization (Fig. 5B). To assess the overall suppressive capacity of DNMAML T cells post-immunization, we generated mixed BM chimeras to park WT and DNMAML T cells in the same recipients (Fig. 5C). WT/DNMAML chimeras succumbed to EAE at the same frequency as mice containing only WT T cells, suggesting the absence of dominant suppressor function in DNMAML T cells (Fig. 5D–E).
Figure 5. CNS accumulation of DN CD4+ T cells is enhanced in the presence of WT CD4+ T cells, but Notch-deprived CD4+ T cells fail to suppress disease.
Foxp3 expression in (A) Vβ11−CD4+ 2D2/DN and (B) CD4+ DN T cells as assessed by intracellular flow cytometry (n=3–4 mice/group; ≥2 experiments); (C) Experimental design using mixed bone marrow chimeras; (D) Mean clinical score (representative of 5 experiments); (E) Percent disease incidence (score ≥2) of immunized BM chimeras (3 experiments); (F) Representative flow cytometry plots from BM chimeras. T cells were tracked by CD45.1 and CD45.2 expression at peak disease (n=4–5 mice/group; 3 experiments). DLN: draining lymph node; SC: spinal cord. Arrows indicate DNMAML T cells in the CNS, with partial rescue of their accumulation in the presence of wild-type T cells (WT+DN: mixed bone marrow chimeras). p<0.05*; p<0.01**.
The very low abundance of DNMAML T cells in the CNS prevented accurate assessment of their effector function. It was previously shown that Ccr6-deficient T cells failed to traffic to the CNS during EAE (32). However, bystander T cells induced Ccr6-independent T cell migration into the CNS. To determine if WT T cells could overcome the inability of Notch-deprived CD4+ T cells to accumulate in the CNS, we measured T cell numbers in the CNS of WT/DNMAML bone marrow chimeras at peak disease. DNMAML CD4+ T cells partially regained their ability to accumulate in the CNS in the presence of WT CD4+ T cells (Fig. 5F). These data suggest that bystander WT T cells can induce Notch-deprived T cells to accumulate in the CNS.
Notch inhibition in CNS-infiltrating CD4+ T cells blocks IL-17A and IFNγ expression independently of T-bet
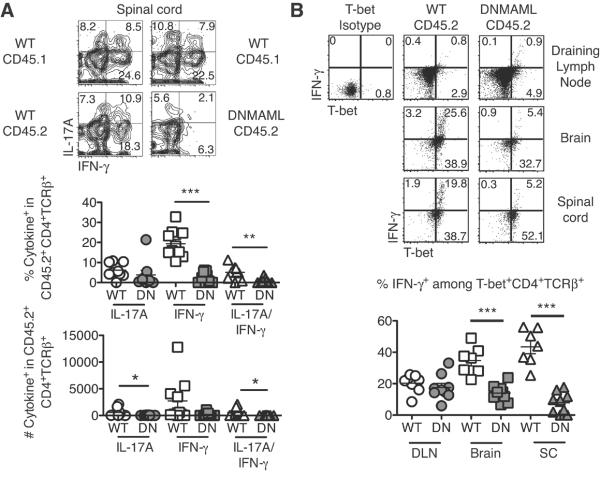
Decreased T cell reactivation in the CNS can result in reduced EAE severity (33, 34). Since DNMAML CD4+ T cells accumulate in the CNS in the presence of WT T cells (Fig. 5F), we could study the impact of Notch inhibition on T cell effector differentiation in the CNS. In immunized mixed BM chimeras, WT CD45.1+ competitor T cells functioned as an internal positive control. While DNMAML CD4+ T cells produced IFNγ and IL-17A in DLN (Fig. 2B, C, F, 6B), they had markedly reduced production of IFNγ and IL-17A in the CNS (Fig. 6A). The blunted cytokine response occurred without defect in T-bet expression, as evidenced by the presence of many T-bet+IFNγ− cells (Fig. 6B). Our results suggest that Notch-mediated regulation of IFNγ production is an important feature of its effects in EAE, but through mechanisms that are T-bet-independent and CNS-restricted. Moreover, decreased T cell migration into the CNS could account for some of the effects of Notch inhibition. However, it cannot fully explain protection from EAE, as Notch-inhibited CD4+ T cells that enter the CNS in the presence of bystander WT T cells have markedly reduced effector function.
Figure 6. Notch-deprived CD4+ T cells have markedly reduced IL-17A and IFNγ production in the CNS despite preserved T-bet expression.
Spinal cord infiltrates from immunized BM chimeras restimulated for 6 hours with anti-CD3/CD28. (A) Frequency and number of IFNγ and IL-17A-producing DN CD4+ T cells in the DLN or CNS by intracellular flow cytometry (n=4–5 mice/group; 2 experiments). Markedly decreased production of IFNγ and reduced production of IL-17 was observed in CD45.2+ DNMAML T cells in the CNS; (B) Concomitant expression of T-bet and IFNγ by DN CD4+ T cells in the DLN or CNS as assessed by intracellular flow cytometry (n=4–5 mice/group; 2 experiments). T-bet induction was preserved in all compartments. IFNγ production by Notch-deprived DN T cells was reduced in the CNS but not in the DLN (as revealed by the %T-bet+ cells expressing IFNγ). p<0.05*; p<0.01**; p<0.001***.
Discussion
Our findings highlight an essential role for Notch signaling in CD4+ T cells mediating experimental autoimmune encephalomyelitis (EAE). We used genetic pan-Notch inhibition as well as inactivation of individual Notch receptor genes specifically in T cells to fully determine the impact of Notch signaling in CD4+ T cells during EAE. We found that complete Notch inhibition in MOG-transgenic T cells or polyclonal T cells nearly completely protected mice from EAE. This protection was not due to an effect on myelin-reactive T cell activation or differentiation in secondary lymphoid tissues, nor to a dominant suppressor function of DNMAML T cells. In contrast, Notch-deprived T cells failed to accumulate in the CNS, despite preserved in vitro chemotaxis. Parking WT and DNMAML T cells together in bone marrow chimeras resulted in enhanced accumulation of DNMAML T cells in the CNS. Functional analysis of DNMAML T cells in the CNS of bone marrow chimeras revealed a significant defect in IL-17A and IFNγ production, despite preserved T-bet expression. Collectively, Notch signaling regulates the accumulation and effector function of myelin-reactive T cells in their target organ during EAE. These findings are reminiscent of past observations with Dll4 blockade (17). However, decreased T cell accumulation cannot by itself explain all the consequences of Notch blockade in EAE, as additional effects on cytokine production by T cells were detected specifically in the CNS.
Past work suggested that Notch regulates the differentiation of myelin-reactive T cells in secondary lymphoid organs through effects on T-bet expression, IFNγ and IL-17A production, or Treg function (12, 16, 19, 35). In contrast, our work reveals a function for Notch signaling that predominates in myelin-reactive T cells infiltrating the CNS, and not in secondary lymphoid tissues. The reasons for these differences are unclear, but could reflect the use of heterogeneous methods to modulate Notch signaling in previous studies, with no capacity to assess the role of all Notch receptors specifically in T cells. For instance, several past reports have relied on systemic pharmacological modulation of Notch signaling with GSIs, blocking antibodies, or agonistic fusion proteins. Many cell populations in the immune system require Notch signaling (11, 20). Thus, using approaches that exceed physiological levels of Notch signaling or systemic modulation of Notch signaling may result in bystander effects on other cell types that impact T cells, independently of cell-autonomous effects of the pathway in T cells. Of note, preserved T cell differentiation in secondary lymphoid tissues was also reported with Dll4 blockade during EAE (17). However, this report described defects in in vitro T cell chemotaxis that we did not replicate using stringent genetic loss-of-function approaches in T cells.
Notch signaling has previously been suggested to regulate T-bet expression (12). Despite drastically decreased IFNγ production in the CNS by Notch-inhibited T cells, myelin-reactive Notch-deprived T cells had preserved expression of T-bet. This is similar to previous work published by our laboratory using models of graft-versus-host disease in which allogeneic Notch-deprived T cells had preserved expression of T-bet, but failed to produce IFNγ (36–38). Other signaling cascades such as IL-12 and IL-27 have been shown to elicit T-bet expression and could account for preserved T-bet expression in Notch-deprived T cells (39, 40). Overall, our findings suggest that Notch can control the production of inflammatory cytokines without directly controlling T helper lineage determination, but rather by influencing the responsiveness of antigen-specific T cells in vivo (38, 41).
Chemotaxis to the CNS is regulated by the cooperative effects of many signaling cascades (42). Using systemic inhibition of the Notch ligand Dll4, Notch has been suggested to regulate Ccr1, Ccr2, Ccr5, and Ccr6 expression during EAE, while another study described the ability of Notch to regulate Ccr7 expression in CNS-homing leukemia cells (17, 29). In contrast, we found that genetic pan-Notch inhibition in myelin-reactive T cells did not affect expression of these chemokine receptors or migration in response to their chemokine ligands, at least in vitro. However, our work does not rule out defective migration in vivo in response to chemotactic signals. These discrepant results may reflect differences in the effects of systemic Dll4 blockade as opposed to specific genetic Notch inhibition in T cells. For example, inhibition of Dll4 in other cell populations in secondary lymphoid organs could elicit chemotaxis changes in T cells that do not result from direct cell-autonomous effects in T cells.
Notch-inhibited myelin-reactive T cells failed to suppress disease induced by WT T cells in mixed bone marrow chimeras. This is in contrast to prior work suggesting that Dll4 blockade expanded Tregs, which resulted in slightly reduced EAE severity (16). These discrepancies could reflect differences in experimental strategy. In our observations, Notch-inhibited T cells were mixed with WT T cells in bone marrow chimeras. In this context, a mild increase in the suppressive capacity of Notch-deprived T cells may have been overcome by the large population of WT T cells in the same animal. However, our findings remain inconsistent with a dominant suppressive effect as the sole explanation for the protective effects of Notch inhibition in EAE.
If Notch signaling does not directly affect chemokine expression or signaling in myelin-reactive T cells, then what accounts for the markedly decreased accumulation of Notch-deficient T cells in the CNS during EAE? One possibility is that Notch signaling modulates integrin expression and/or function. For example, the integrin α4β1 is required for T cell chemotaxis to the CNS during EAE and MS (28, 43). Although Notch-deprived T cells expressed similar levels of surface α4β1, impaired integrin activation or defective downstream signaling could account for their decreased accumulation in the CNS. Another possibility is that Notch-deprived T cells fail to produce inflammatory cytokines such as IFNγ when infiltrating the CNS, and thus fail to induce expression or activation of integrins (e.g. the α4β1 ligand VCAM-1) in endothelial cells of the CNS (44, 45). This scenario would be consistent with the rescue of DNMAML T cell accumulation in the CNS that we observed in the presence of bystander wild-type T cells. Alternatively, Notch-deprived T cells may have preserved migration into the CNS, but fail to survive or proliferate in their target organ during local reactivation, either immediately after crossing the blood-brain barrier or during subsequent exposure to tissue antigens. Of note, failure of myelin-reactive T cells to be locally reactivated can ultimately result in decreased T cell accumulation in the CNS, even after successful initial migration (33, 34). This scenario could account for our observation that Notch-deprived CNS-infiltrating T cells in mixed bone marrow chimeras had markedly decreased production of inflammatory cytokines, which cannot be explained solely by a migration defect. Instead, defective T cell accumulation and cytokine production in the CNS could be linked to a role of Notch in enhancing T cell reactivation that becomes apparent predominantly in the target organ and not in secondary lymphoid tissues. We speculate that myelin-reactive T cells get exposed to a unique source or density of Notch ligands in the CNS to which they do not have access in lymph nodes. Alternatively, cytokines or other signaling pathways could compensate for the effects of Notch deprivation in lymph nodes but be missing in the CNS during EAE, resulting in a functional defect that becomes apparent only in the brain and spinal cord.
In conclusion, our work provides definitive experimental evidence to understand the overall effects of Notch signaling in CD4+ T cells during EAE. By using multiple loss-of-function approaches restricted to T cells, we demonstrate a profound requirement for Notch in CD4+ T cells to elicit EAE. The degree of protection observed in our study is markedly higher than reported in all past studies in the field, most likely because we were able to achieve complete inhibition of signaling downstream of all Notch receptors in myelin-reactive T cells. The constellation of mechanisms largely differed from previous reports and was independent of the master transcription factors of T helper lineages. Of note, these data in EAE are reminiscent of our findings in graft-versus-host disease, as markedly defective IFNγ production by Notch-deprived alloreactive T cells was observed despite preserved T-bet expression (36–38). In EAE, our results suggest a function for Notch in T cells that is CNS-restricted, possibly due to local exposure to Notch ligands during T cell restimulation in the CNS. Because effects on both EAE and GVHD outcome were observed upon interfering with the Notch transcriptional activation complex, future studies will work to elucidate direct transcriptional Notch targets in mature T cells that regulate T cell-mediated immune disorders.
Supplementary Material
Acknowledgments
1This work was supported by a Damon Runyon-Rachleff award (DRR-05A-09), the American Society of Hematology and the National Institutes of Health (RO1-AI091627) (IM). Individual support included T32 training grants (AI007413 to AS) and a Miller Award for innovative immunology research (AS). Flow cytometry was partially supported by P30-CA46592.
References
- 1.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louvi A, Artavanis-Tsakonas S. Notch and disease: a growing field. Sem Cell Dev Biol. 2012;23:473–480. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, Walle I.v.D., Cathelin S, Trimarchi T, Araldi E, Liu C, Ibrahim S, Beran M, Zavadil J, Efstratiadis A, Taghon T, Michor F, Levine RL, Aifantis I. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230–233. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radtke F, Wilson A, Stark G, Bauer M, Meerwijk J.v., MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 6.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 7.Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, Golub R. Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nat Immunol. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- 8.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9:116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 11.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Minter LM, Turley DM, Das P, Shin HM, Joshi I, Lawlor RG, Cho OH, Palaga T, Gottipati S, Telfer JC, Kostura L, Fauq AH, Simpson K, Such KA, Miele L, Golde TE, Miller SD, Osborne BA. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6:680–688. [PubMed] [Google Scholar]
- 13.Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Overcoming failure to repair demyelination in EAE: gamma-secretase inhibition of Notch signaling. J Neurol Sci. 2008;265:5–11. doi: 10.1016/j.jns.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Inhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosis. J Neuroimmunol. 2005;170:3–10. doi: 10.1016/j.jneuroim.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Jurynczyk M, Jurewicz A, Raine CS, Selmaj K. Notch3 inhibition in myelin-reactive T cells down-regulates protein kinase C theta and attenuates experimental autoimmune encephalomyelitis. J Immunol. 2008;180:2634–2640. doi: 10.4049/jimmunol.180.4.2634. [DOI] [PubMed] [Google Scholar]
- 16.Bassil R, Zhu B, Lahoud Y, Riella LV, Yagita H, Elyaman W, Khoury SJ. Notch ligand delta-like 4 blockade alleviates experimental autoimmune encephalomyelitis by promoting regulatory T cell development. J Immunol. 2011;187:2322–2328. doi: 10.4049/jimmunol.1100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds ND, Lukacs NW, Long N, Karpus WJ. Delta-like ligand 4 regulates central nervous system T cell accumulation during experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2803–2813. doi: 10.4049/jimmunol.1100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eixarch H, Mansilla MJ, Costa C, Kunkel SL, Montalban X, Godessart N, Espejo C. Inhibition of delta-like ligand 4 decreases Th1/Th17 response in a mouse model of multiple sclerosis. Neurosci Lett. 2013;541:161–166. doi: 10.1016/j.neulet.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Elyaman W, Bassil R, Bradshaw EM, Orent W, Lahoud Y, Zhu B, Radtke F, Yagita H, Khoury SJ. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity. 2012;36:623–634. doi: 10.1016/j.immuni.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Ann Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 21.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- 23.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 27.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. Embo J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Invest. 1998;102:2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen F, Gehrie E, Li M, Newcomb E, Zavadil J, Meruelo D, Lipp M, Ibrahim S, Efstratiadis A, Zagzag D, Bromberg JS, Dustin ML, Aifantis I. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459:1000–1004. doi: 10.1038/nature08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 31.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 33.Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami N, Lassmann S, Li Z, Odoardi F, Ritter T, Ziemssen T, Klinkert WE, Ellwart JW, Bradl M, Krivacic K, Lassmann H, Ransohoff RM, Volk HD, Wekerle H, Linington C, Flugel A. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, Yagita H, Khoury SJ. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, Friedman A, Kato K, He S, Cui S, Hexner E, Frank DM, Emerson SG, Pear WS, Maillard I. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran I, Sandy AR, Carulli A, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson L, Yan M, Siebel C, Maillard I. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandy AR, Chung J, Toubai T, Shan GT, Tran IT, Friedman A, Blackwell TS, Reddy P, King PD, Maillard I. T Cell-Specific Notch Inhibition Blocks Graft-versus-Host Disease by Inducing a Hyporesponsive Program in Alloreactive CD4+ and CD8+ T Cells. J Immunol. 2013;190:5818–5828. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 40.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 41.Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS ONE. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallusto F, Impellizzieri D, Basso C, Laroni A, Uccelli A, Lanzavecchia A, Engelhardt B. T-cell trafficking in the central nervous system. Immunol Rev. 2012;248:216–227. doi: 10.1111/j.1600-065X.2012.01140.x. [DOI] [PubMed] [Google Scholar]
- 43.Sheremata WA, Vollmer TL, Stone LA, Willmer-Hulme AJ, Koller M. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology. 1999;52:1072–1074. doi: 10.1212/wnl.52.5.1072. [DOI] [PubMed] [Google Scholar]
- 44.Tudor KS, Hess KL, Cook-Mills JM. Cytokines modulate endothelial cell intracellular signal transduction required for VCAM-1-dependent lymphocyte transendothelial migration. Cytokine. 2001;15:196–211. doi: 10.1006/cyto.2001.0922. [DOI] [PubMed] [Google Scholar]
- 45.Olsson T. Critical influences of the cytokine orchestration on the outcome of myelin antigen-specific T-cell autoimmunity in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev. 1995;144:245–268. doi: 10.1111/j.1600-065x.1995.tb00072.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.