Abstract
Quercetin is a common flavonoid polyphenol which has been shown to exert neuroprotective actions in vitro and in vivo. Though quercetin has antioxidant properties, it has been suggested that neuroprotection may be ascribed to its ability of inducing the cell’s own defense mechanisms. The present study investigated whether quercetin could increase the levels of paraoxonase 2 (PON2), a mitochondrial enzyme expressed in brain cells, which has been shown to have potent antioxidant properties. PON2 protein, mRNA, and lactonase activity were highest in mouse striatal astrocytes. Quercetin increased PON2 levels, possibly by activating the JNK/AP-1 pathway. The increased PON2 levels induced by quercetin resulted in decreased oxidative stress and ensuing toxicity induced by two oxidants. The neuroprotective effect of quercetin was significantly diminished in cells from PON2 knockout mice. These findings suggest that induction of PON2 by quercetin represents an important mechanism by which this polyphenol may exert its neuroprotective action.
Keywords: Paraoxonase 2, quercetin, polyphenols, neuroprotection
Introduction
A large number of molecules with a polyphenol structure have been shown to exert protective actions in pathological conditions such as cardiovascular disease, metabolic disorders, obesity, diabetes, infections, cancer and neurotoxic / neurodegenerative processes [1–3]. In particular, there has been increasing attention devoted to the possibility that polyphenols may act as neuroprotective agents; polyphenols have an alleged ability to antagonize oxidative stress which is recognized as an important factor in a variety of neurodegenerative diseases, in the adverse effects of a number of neurotoxicants, and as a mechanism for age-related degenerative processes [4–6].
Quercetin is an important flavonoid polyphenol found in many common fruits and vegetables, such as apples, berries, onions and capers [7]; its estimated dietary intake ranges from 4 to 68 mg/day [8], but can increase to 200–500 mg/day in individuals who consume high quantities of fruits and vegetables rich in flavonols. Furthermore, quercetin is also sold as a dietary supplement, with a recommended dosage of 1 g/day [9].
Evidence exists of a neuroprotective effect of quercetin [10]. In vitro studies have shown that quercetin antagonizes cell toxicity induced by various oxidants and other neurotoxic molecules believed to act by inducing oxidative stress [11–15]. In addition, several studies show that quercetin can exert neuroprotection and antagonize oxidative stress when administered in vivo. Indeed, oral quercetin has been shown to protect rodents from oxidative stress and neurotoxicity induced by a variety of agents [16–22].
The exact mechanism(s) of quercetin neuroprotective effects have not been elucidated. Though quercetin is a potent scavenger of reactive oxygen and nitrogen species (ROS, RNS) [23], it has been argued that the concentration of quercetin expected to be present in the brain may not be sufficient to exert an appreciable direct antioxidant effect, compared for example to glutathione and vitamin C, which are present at millimolar concentrations [24]. Rather, it has been suggested that quercetin may exert its neuroprotective effects by other mechanisms, particularly by modulating the cell’s own anti-oxidant defense systems [25–28]. For example, quercetin has been shown to affect anti-oxidant factors including the Nrf2 pathway, heme oxygenase, and glutamate cysteine ligase (GCL) [14, 29–31]. The present study investigated whether quercetin could modulate paraoxonase 2 (PON2), which has potent antioxidant activity [32–36].
PON2 is the oldest member of the paraoxonase family of three genes (PON1, PON2, PON3) which are clustered in tandem on the long arm of human chromosome 7q21–22, and on mouse chromosome 6 [37, 38]. PON2, which is expressed in most tissues including the brain, has been shown to exert an antioxidant effect in cells [32–36, 39]. Sub-cellular distribution studies have shown that PON2 is localized primarily in the mitochondria, a major source of free radical-related oxidative stress [38, 40, 41]. PON2 has been shown to bind to Coenzyme Q10 that associates with Complex III in mitochondria, and PON2 deficiency causes mitochondrial dysfunction [41]. PON2 protein, mRNA and lactonase activity were found in all mouse brain regions, with the highest levels in three dopaminergic regions (striatum, substantia nigra, nucleus accumbens). PON2 levels were higher in astrocytes than in neurons, and in both cell types the ability of two oxidants [hydrogen peroxide (H2O2) and 2,3-dimethoxy-1,4-naphthoquinone (DMNQ)] to induce the formation of reactive oxygen species and to decrease cell viability was significantly enhanced in cells from PON2 knockout (PON2−/−) mice, despite similar glutathione levels [38].
As higher PON2 levels are associated with increased resistance to oxidative stress-induced toxicity, the possibility of modulating PON2 levels in cells may represent a neuroprotective strategy of much interest. While there is a substantial amount of work on the modulation of PON1 [42], only limited research has been carried out on PON2. In macrophages, PON2 expression was increased by oxidative stress [43], and by urokinase plasminogen activator [44, 45], while arachidonic acid [46], unesterified cholesterol [47], the licorice phytoestrogen glabidrin [48], and the hypocholesterolemic drug atorvastatin [49] have been reported to up-regulate PON2 expression in various cell types. With regard to polyphenols, pomegranate juice was found to increase PON2 in macrophages through activation of the PPAR- and AP-1 pathways [50], while extracts of Yerba mate (Ilex paraguariensis) have recently been reported to increase PON2 mRNA and lactonase activity in macrophages in vitro and after in vivo administration to healthy women [51]. A single study with quercetin reported that this compound increased PON2 mRNA and protein in macrophages in vitro [52]. Results of the present study indicate that quercetin increases levels of PON2 in mouse brain cells, and that the neuroprotective effects of this polyphenol are greatly diminished in the absence of PON2.
Materials and Methods
Materials
DMEM medium, RPMI 1640 medium, fetal bovine serum (FBS), and Hank’s balanced salt solution (HBSS) were from Invitrogen (Carlsbad, CA). Anti PON2, PON1, PON3 antibodies were from Abcam (Cambridge, MA, USA). Dimethylsulfoxide (DMSO), hydrogen peroxide (H2O2), 2,3-dimethoxy-1,4-naphthoquinone (DMNQ), mouse anti-β-actin antibody, dihydrocoumarin (3,4-dihydro-2H,1-benzopyran-2-one), 3-(4,5-dimethyltiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT), 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one (quercetin; >95%), and 2-chloro-5-nitro-N-phenylbenzamide (GW9662) were from Sigma-Aldrich (St. Louis, MO). Anthra[1,9–cd]pyrazol-6(2H)-one (SP600125) was obtained from Cayman Chemical (Ann Arbor, MI), while rosiglitazone was from Enzo Life Sciences (Farmingdale, NY).
Animals
Wild-type and PON2 knockout mice, kindly provided by Drs. A.J. Lusis, D.M. Shih and S. Reddy (UCLA) were used in this study. Mice were sacrificed at postnatal day (PND) 0.5 for preparation of primary striatal astrocytes and neurons. Mice were housed in a specific pathogen-free facility with ad libitum access to food and water and a 12-h light cycle. All procedures for animal use were in accordance with the National Institute of Health Guide for the Use and Care of Laboratory Animals, and were approved by the University of Washington Institutional Animal Care and Use Committee.
Cell cultures
Primary striatal astrocytes and neurons were obtained from PND 0.5 mice, as previously described [36, 39]. For astrocyte preparation, the striatum was dissected, mechanically dissociated and incubated with trypsin, followed by trituration, repeated washing, and filtration. After counting, cells were plated at a density of 107 cells per flask in 75 cm2 tissue culture flasks pre-coated with poly-D-lysine and grown in DMEM containing 10% FBS, 100 U/ml penicillin and 100 ug/ml streptomycin at 37°C in 5% CO2 / 95% air. After 10 days in culture, cells were plated in 6-well plates for experiments at a density of 6×105 astrocytes/well, or in 48-well plates at a density of 5×104 astrocytes/well. Cultures of striatal neurons were prepared from 0.5 day-old mice, as described by Giordano et al. [36, 39]. The striatum was collected in HBSS medium containing 0.02% BSA and 10 mM HEPES. The tissue was digested for 25 min in HBSS containing papain (1 mg/ml) and DNAse (40 ug/ml) and centrifuged at 300 × g for 5 min at room temperature. The supernatant (containing papain) was removed and the pellet was gently triturated in Neurobasal A medium with B27 supplement (Invitrogen), using a Pasteur pipette to dissociate larger aggregates. Cells were centrifuged at 200 × g for 10 min and the cell pellet was gently resuspended. Neurons were then counted, seeded on poly-D-lysine coated 6-well plates at a density of 6×105 neurons/well, and cultured in neurobasal medium supplemented with B27 (minus antioxidant). Neurons were cultured for eight days before experiments. Mouse RAW264.7 macrophages were grown in RPMI containing 10% FBS, 100 u/ml penicillin and 100ug/ml streptomycin at 37°C in 5% CO2/95% air.
Western blots
Immunoblots were carried out as described by Giordano et al. [36, 39]. Twenty-five ug of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted using antibodies against PON1, PON2, PON3 and β-actin. After electrophoresis, proteins were transferred to polyvinylidenedifluoride membranes that were incubated with antibodies using the following dilutions: 1:250 (for PON1, PON2, and PON3), and 1:750 (for β-actin). After the transfer blots were rinsed in Tris-buffered saline (pH=7.5), blocked for one hour, and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody a dilution of 1:750 (for PON2 and PON3), or incubated with horseradish peroxidase-conjugated anti-mouse secondary antibody at the dilutions of 1:1000 for β-actin, or 1:750 for PON1. Band intensity was measured by densitometry using ImageJ (provided by the National Institutes of Health), and the intensity of the bands was normalized to β-actin content.
RT-PCR
Reverse transcription was performed according to the manufacturer's established protocol using total RNA and the SuperScript® III First-Strand Synthesis System. For gene expression measurements, 4 uL of cDNA were included in a PCR reaction (25 uL final volume) that also consisted of the appropriate forward (FP) and reverse (RP) primers at 360 nM each, 80 nMTaqMan probe, and TaqMan Gene Expression Master Mix. The PCR primers and the dual-labeled probes [6–carboxyfluorescein (FAM) and 6-carboxy-tetramethyl-rhodamine (TAMRA)] for all genes were designed using ABI Primer Express v.1.5 software (Applied Biosystems Inc., Foster City, CA). Amplification and detection of PCR amplicons were performed with the ABI PRISM 7900 system (Applied Biosystems Inc., Foster City, CA) with the following PCR reaction profile: 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 30 sec and 62°C for 1 min. β-actin amplification plots derived from serial dilutions of an established reference sample were used to create a linear regression formula in order to calculate expression levels, and β-actin gene expression levels were utilized as an internal control to normalize the data.
PON2 activity assay
PON2 lactonase activity was measured as described earlier [36, 39, 43]. Briefly, cells were washed and resuspended in 400 uL Tris buffer containing 25 mM Tris/HCl, pH 7.6 / 1 mM CaCl2. The homogenates were sonicated twice for 10 seconds on ice. A 50 uL aliquot was saved for the measurement of protein concentration. Enzyme activity was measured using 200 to 300 ug of protein per mL assay mixture. After 10 minutes of incubation, the lactonase activity was measured kinetically using dihydrocoumarin (DHC) as substrate, by monitoring the absorbance at 270 nm in a NanoDrop 2000 (Thermo Scientific).
Cytotoxicity assay
Cell viability was quantified colorimetrically using the metabolic dye MTT, as previously described [36, 39]. Cells in 24-well plates were pre-treated for 24 h with 20 uM quercetin or solvent control and then washed with PBS and treated for 24 h at 37°C with either H2O2 or DMNQ, dissolved in distilled water and DMSO, respectively. At the end of exposure, medium was removed and cells were incubated with 0.50 ml/well of Locke’s buffer solution containing 5 ug/ml MTT for 30 min. MTT was removed and the reaction product was dissolved in 0.25 ml DMSO/well. The absorbance was read at 570 nm in a SpectraMax 190 microplate reader, and results expressed as percentage of viable cells relative to controls. Values of IC50 were determined from concentration-response curves using 4–5 concentrations of each compound.
Assay of reactive oxygen species (ROS) formation
ROS formation was determined by fluorescence using 2’,7’-dichlorofluorescin diacetate (DCFH2-DA), as previously described [36]. DCFH2-DA is readily taken up by cells and is subsequently de-esterified to DCFH2, which can be oxidized to dichlorofluorescein (DCF) by hydrogen peroxide, peroxynitrite, and other ROS or reactive nitrogen species. Cells were pre-treated with 20 uM quercetin for 24 hours or left untreated as controls. Cells were first washed with Locke's solution and then pre-incubated for 30 min at 37°C with DCFH2-DA in Locke's solution. DCFH2 was added from a stock solution prepared in DMSO which was also added to the blank. Cells were then washed with Locke’s solution to remove extracellular DCFH2-DA before treatments with H2O2 or DMNQ. After treatments (at 37°C), cells were washed twice with Locke's buffer and lysed with 0.1 M KH2PO4 and 0.5% Triton X-100, pH 7.2 for 5 min. Cell lysates were then scraped from the dishes, and the supernatant was collected. The fluorescence (EX = 488 nm and EM = 525 nm) was read immediately, using a SpectraMax 190 spectrophotometric plate reader. ROS formation was expressed as the amount of fluorescence per mg of protein measured using the bicinchoninic acid protein assay kit.
Statistical analysis
Data are expressed as the mean ± S.D. of at least three independent experiments. Statistical analysis was performed using one-way or two-way ANOVA followed by a Bonferroni test for multiple comparisons.
Results
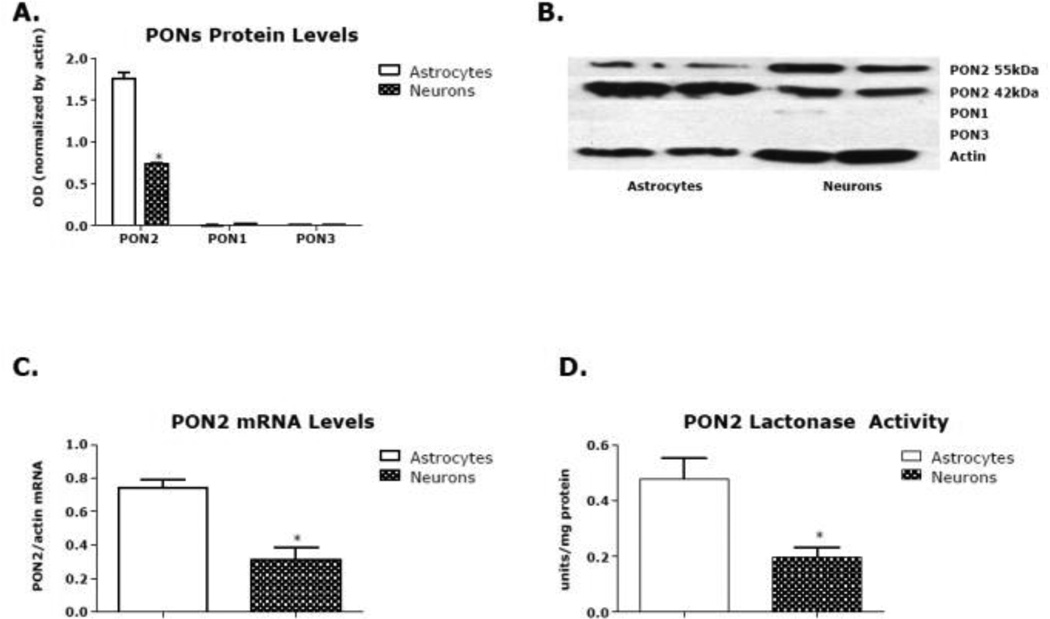
We had previously shown that PON2 levels in mouse brain were highest in the striatum [36]. We thus chose to carry out the present studies in astrocytes and neurons from this brain region. Fig. 1A shows that levels of PON2 protein are higher in mixed-gender striatal astrocytes than neurons, confirming previous observations [36, 39]. Data presented in Fig. 1A refer to the 42 kDa band, corresponding to the molecular weight of PON2, though a second band (at 55 kDa) was also evident (Fig. 1B). This second band, found by several but not all investigators, may represent a splice variant of PON2; however, its exact nature has not been determined. Levels of PON2 mRNA were also higher in striatal astrocytes than in neurons (Fig. 1C), as was lactonase activity, measured by dihydrocoumarin hydrolysis (Fig. 1D). All PONs have lactonase activity [53]; however, given the virtual absence of PON1 and PON3 in mouse striatal astrocytes and neurons (Fig. 1A), the measured lactonase activity can be ascribed solely to PON2.
Fig. 1. Levels of PONs in mouse striatal astrocytes and neurons.
(A) PON2, PON1, and PON3 protein level in primary mouse striatal astrocytes and neurons. (B) Example of blot of data of A. (C) PON2 mRNA levels in primary mouse striatal astrocytes and neurons. (D) PON2 lactonase activity levels in primary mouse striatal astrocytes and neurons. Results are the mean (± SD) of three independent experiments. *Significantly different from astrocytes, p<0.05.
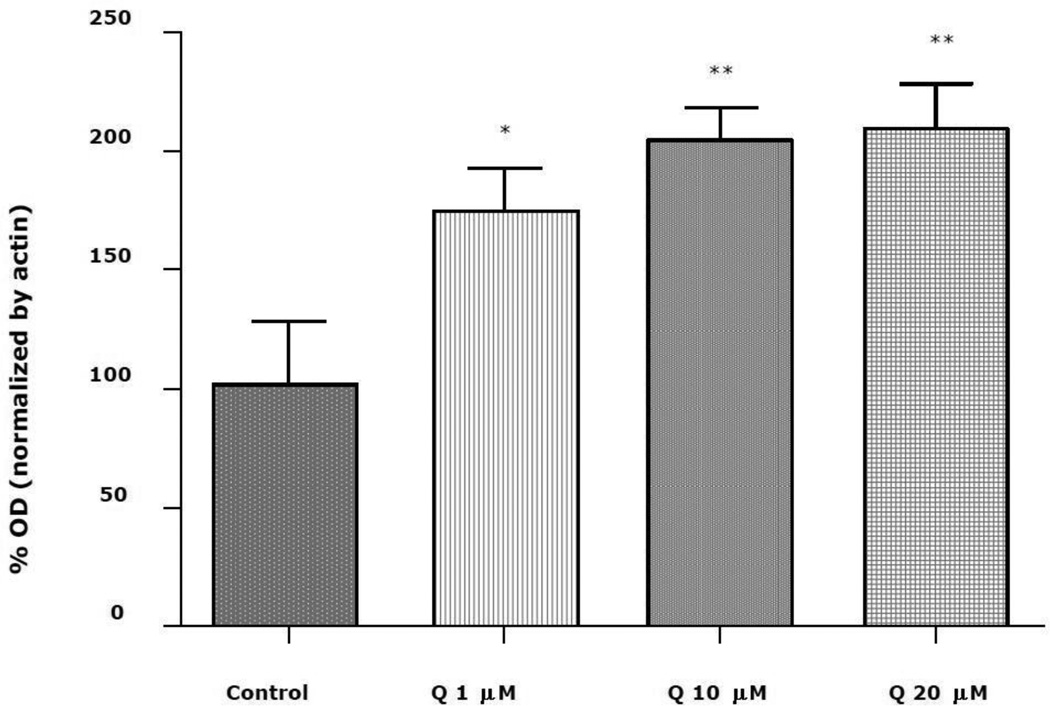
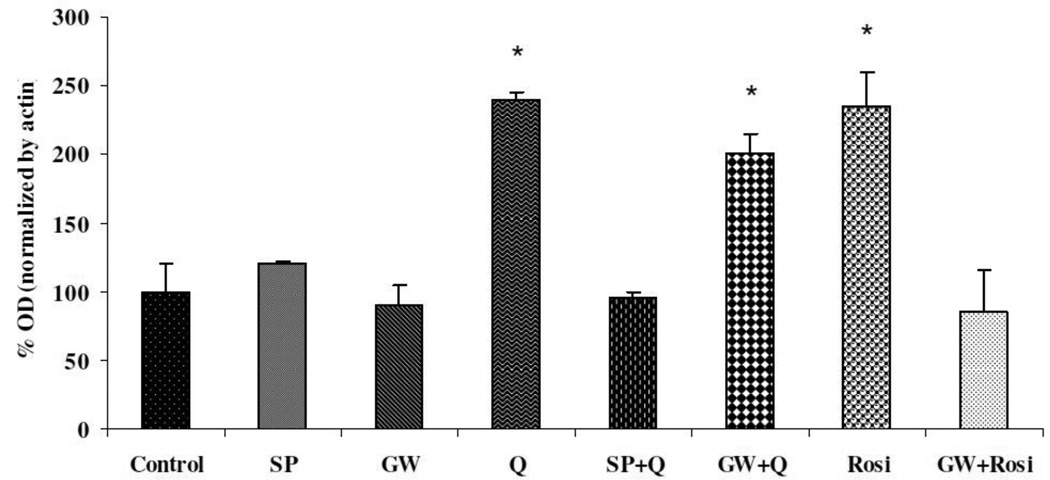
Quercetin (1, 10, or 20 uM) increased PON2 expression in striatal astrocytes in a concentration-dependent manner, with a maximum increase of about two-fold (Fig. 2). No cytotoxicity (assessed by the MTT assay) was observed at these concentrations (not shown). Similar increases of PON2 were also found in mouse striatal neurons (180 ± 6% of control at 20 uM quercetin; n=3; p<0.05), and in mouse RAW264.7 macrophages (168 ± 12% of control at 20 uM quercetin; n=3; p<0.05). The increase of PON2 by quercetin in astrocytes was antagonized by SP600125, an inhibitor of the JNK (c-Jun-N-terminal kinase, also known as SAPK, stress-activated protein kinase)/AP-1 pathway, but not by GW9662, a PPARγ inhibitor (Fig. 3). The PPAR agonist rosiglitazone also increased PON2 expression by about two-fold, and in contrast to quercetin, the increase in PON2 expression by rosiglitazone was abrogated by incubating the astrocytes with the PPAR inhibitor GW9662 (Fig. 3).
Fig. 2. Concentration-response effect of quercetin on PON2 protein level in mouse striatal astrocytes.
PON2 protein expression in mixed-gender mouse striatal astrocytes following 24 hours exposure to 1, 10, or 20 uM quercetin (Q). Graph shows the quantification of the 42 kDa PON2 alloform. Results are the mean (± SD) of at least three independent experiments. Significantly different from control, *p<0.05, **p<0.01
Fig. 3. Effect of JNK/AP-1 and PPAR inhibition on induction of PON2 by quercetin in mouse striatal astrocytes.
Mouse striatal astrocytes (mixed gender) were exposed for 24 h to 20 uM quercetin (Q) or 50 uM rosiglitazone (Rosi), with or without 1 hour pre-treatment with 20 uM of the JNK/AP-1 inhibitor SP600125 (SP), or 50 uM of the PPARγ inhibitor GW9662 (GW). Results are the mean (± SD) of three independent experiments. Significantly different from control, *p<0.05.
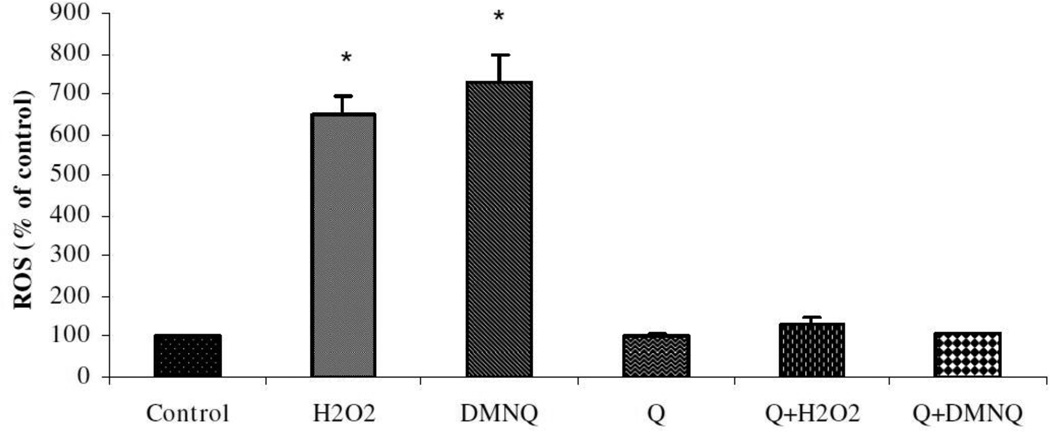
To assess the functional consequences of PON2 induction by quercetin, striatal astrocytes (mixed gender) were pre-treated with quercetin (20 uM for 24 h, which increased PON2 protein levels by ~2.5-fold) followed by washout, and the ability of two oxidants, H2O2 and DMNQ, to induce oxidative stress was determined. Fig. 4 shows that quercetin pre-treatment significantly decreased ROS production induced by both oxidants. The relevance of PON2 induction by quercetin as a mechanism of neuroprotection was further probed by assessing cytotoxicity induced by H2O2 and DMNQ in striatal astrocytes (Table 1). As seen previously (Giordano et al. 2011), striatal astrocytes from PON2−/− mice were much more sensitive than astrocytes from wild-type mice to the toxicity of the two oxidants (~6-fold decrease in their IC50 values; Table 1). In wild-type astrocytes, pre-treatment with quercetin significantly decreased the toxicity of the two oxidants, as evidenced by a 4-fold increase in their IC50 value (Table 1). In contrast, the same treatment with quercetin in striatal astrocytes from PON2−/− mice afforded a much lower degree of protection (<2-fold; Table 1).
Fig. 4. Oxidative stress in mouse striatal astrocytes treated with quercetin.
Mouse striatal astrocytes (mixed gender) were pre-treated for 24 hours with 20 uM quercetin (Q) or solvent control. After wash-out, cells were exposed to 50 uM H2O2 or 50 uM DMNQ. ROS formation was measured by fluorescence of dichlorofluorescein (DCF). Results are the mean (± SD) of at least three independent experiments. Significantly different from control, *p<0.01.
Table 1.
Quercetin protection against oxidative stress-induced toxicity requires the presence of PON2
| Cell type/Oxidant | IC50 (uM) | Ratio | |
|---|---|---|---|
| Control | Quercetin | ||
| Wild-type striatal astrocytes | |||
| H2O2 | 38.9 ± 4.5 | 157.0 ± 8.1* | 4.0 |
| DMNQ | 37.5 ± 5.6 | 131.3 ± 9.2* | 3.5 |
| PON2−/− striatal astrocytes | |||
| H2O2 | 6.3 ± 1.3 | 11.9 ± 1.2 | 1.9 |
| DMNQ | 6.1 ± 1.0 | 8.3 ± 1.1 | 1.4 |
Mixed gender striatal astrocytes were exposed for 24 h to 20 uM quercetin. After washout, cells were treated for 24 h with 4–5 concentrations of oxidants and cytotoxicity as measured by the MTT assay. Values are the mean (± SD) of 3 separate experiments.
Significantly different from wild-type control, p<0.01.
Discussion
Quercetin is a common flavonoid polyphenol shown to have neuroprotective actions in vitro and in vivo [11–22]. Mechanism(s) of such neuroprotection remain elusive, though it has been suggested that quercetin, as well as other polyphenols, may induce the cell’s own ability to counteract oxidative stress-induced toxicity [25–28]. We have recently characterized PON2 in brain, and have found that this enzyme confers protection against oxidative stress-induced neurotoxicity [36, 39]. As quercetin had been shown to increase PON2 levels in macrophages [52], we sought to investigate whether a similar effect would be seen in brain cells, and to determine its functional consequences. Our findings indicate that quercetin increases PON2 expression in mouse striatal astrocytes (chosen because they express the highest levels of PON2 among brain regions and cell types; [36]), and in mouse striatal neurons, in addition to macrophages [52]. Such induction of PON2 by quercetin was paralleled by an increased ability of the cells to scavenge ROS and to antagonize oxidant-induced toxicity. Sub-cellular distribution studies have shown that PON2 is localized primarily in the mitochondria [36, 41]. Mitochondria are a major contributor of cellular ROS; ROS produced in the mitochondria can also target the electron transport chain (e.g. complex I), resulting in a cycle generating more ROS, followed by ATP depletion and ultimately cell death [2, 54]. Oxidative stress is recognized as an important factor in a variety of neurodegenerative diseases, in the adverse effects of a number of neurotoxicants, and as a mechanism for age-related degenerative processes [4–6]. The preponderant localization of PON2 in mitochondria would support a role for this enzyme in protecting cells from oxidative damage. In HeLa cells, PON2 has been shown to bind to Coenzyme Q10 that associates with Complex III in mitochondria, and PON2 deficiency causes mitochondrial dysfunction [41]. In human endothelial cells PON2 has been shown to reduce, indirectly but specifically, the release of superoxide from the inner mitochondrial membrane, without affecting levels of other radicals such as hydrogen peroxide and peroxynitrite [55]. Of relevance is the fact that mitochondria, together with the cytoplasm and the nucleus, are also preferential accumulation sites for quercetin in cells [56–58].
Shiner et al. [50] reported that polyphenols in pomegranate juice could increase PON2 levels through the PPAR and JNK/AP-1 pathways. In astrocytes, inhibition of PPAR did not affect quercetin’s induction of PON2; in contrast, the effect of quercetin was completely antagonized by SP600125, a compound which inhibits JNK by inhibiting the ATP binding site of the kinase [59]. This would suggest an involvement of JNK in the effect of quercetin. JNK is usually activated by a variety of stimuli, including oxidative stress, and phosphorylates various transcription factors such as c-Jun, Elk-1, and AP-1, leading to changes in gene expression [60]. As recently suggested [61, 62], quercetin may thus induce a very low-level of oxidative stress (as shown for example, in hepatoma cells; [63]), which in turn would activate the JNK/AP-1 pathway [64], causing an increased expression of PON2.
PON2 levels have been recently shown to be modulated by estrogens through activation of estrogen receptor (ER)-alpha [39]. Though quercetin has been shown to exert estrogenic effects, its classification as a phytoestrogen is a matter of debate, as contrasting findings have been reported [65, 66] particularly with respect to the involvement of ER-alpha and ER-beta [57, 67, 68]. Thus, the potential contribution of ERs in quercetin’s effects on PON2 remains to be investigated.
Independent of the underlying cellular/molecular mechanisms, the ability of quercetin to increase intracellular levels of PON2 has important functional consequences, as shown by the results of Fig. 4 and of Table 1. An increase of PON2 by 2–2.5 fold protects cells against oxidative stress induced by two oxidants (H2O2 and DMNQ), and significantly diminishes their toxicity. We had previously shown that a 2–3-fold differential expression of PON2 levels, as observed between brain cells from male and female mice, similarly caused a 3–4-fold difference in susceptibility to the toxicity of these two oxidants [39]. Other intracellular pathways such as Nrf2, heme oxygenase, and GCL [14, 29–31] have been shown to be affected by quercetin and may also contribute to its neuroprotective action. However, experiments done in cells from PON2−/− mice clearly show that the neuroprotective effect of quercetin is significantly reduced, though not completely abolished, by the absence of PON2 (Table 1). This finding provides strong support for an important role of PON2 induction in neuroprotection afforded by quercetin.
A limitation of the present studies is that they show an in vitro effect of quercetin, which will need to be confirmed in vivo. Quercetin is present in foods as glycosides that can be efficiently absorbed [69]. Total quercetin derived from the diet is normally present in plasma in the nanomolar range (<100 nM), but can be increased in the micromolar range after supplementation [70, 71]. Furthermore, the half-life of quercetin ranges between 11 and 28 h, suggesting the possibility of significantly increasing plasma concentration upon supplementation [71]. In vitro studies with blood-brain barrier models consistently indicate that quercetin enters the brain [72–74]. Upon administration of quercetin in vivo, nanomolar levels are found in brain tissue [74–77], though bioavailability of quercetin can be increased [78]. In particular, the formulation of quercetin in lipid nanoparticles significantly increases its penetration in the brain [79–81]. Above all, however, oral quercetin in vivo has been shown to protect rodents from oxidative stress and neurotoxicity induced by a variety of agents [16–22].
An additional limitation of the present study is that only the aglycone, i.e. quercetin itself, was tested. Quercetin is known to be metabolized to various conjugated metabolites, such as 3’-O-methyl-quercetin (isorhamnetin), quercetin-3-O-glucuronide, 3’-O-methylquercetin-O-glucuronide, and quercetin-3’-O-sulfate [9, 82]. Interestingly, conjugated quercetin has been shown to enter the cell where it can be converted to its non-conjugated form [57, 83]. Nevertheless, the ability of such metabolites to affect intracellular levels of PON2 would be an important avenue of investigation. It is relevant that various other polyphenol catabolites have been shown to exert strong biological activity, particularly in protecting neuronal cells against DMNQ-induced oxidative stress and toxicity [84].
In summary, the common flavonoid polyphenol quercetin, which had been reported to exert neuroprotection in vitro and in vivo, has been shown to increase the expression of PON2 in brain cells. The mechanism of such induction appears to involve activation of the JNK/AP-1 pathway, but alternative pathways need to be further investigated. The neuroprotective action of quercetin in vitro was significantly reduced in cells lacking PON2, thereby suggesting that PON2 induction by quercetin represents an important component of its neuroprotective action, possibly shared by other polyphenols.
Acknowledgments
This study was supported in part by grants P42ES04696 and P30ES07033 from the National Institute of Environmental Health Sciences, and P30HD02274 from the National Institute of Child Health and Human Development. We thank Dr. Fred Farin and Jasmine Wilkerson from the Functional Genomics and Proteomics Facility in the Dept. of Environmental and Occupational Health Sciences at the University of Washington for measuring PON2 mRNA levels.
References
- 1.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiological studies. Am. J. Clin. Nutr. 2005;81(Suppl.):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scalbert A, Andres-Lacueva C, Arita M, Kroon P, Manach C, Urpi-Sarda M, Wishart D. Databases on food phytochemicals and their health-promoting effects. J. Agr. Food Chem. 2011;59:4331–4348. doi: 10.1021/jf200591d. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 6.Martin I, Grotewiel MS. Oxidative damage and age-related functional declines. Mech. Aging Dev. 2006;127:411–423. doi: 10.1016/j.mad.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 7.USDA (United States Department of Agriculture) Beltsville, MD: USDA, Beltsville Human Nutrition Research Center; 2003. USDA Database for the Flavonoid Content of Selected Foods; p. 77. [Google Scholar]
- 8.Chen C, Zhou J, Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sci. 2010;87:333–338. doi: 10.1016/j.lfs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Ossola B, Kaariainen TM, Mannisto PT. The multiple faces of quercetin in neuroprotection. Expert Op. Drug Saf. 2009;8:397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- 11.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis; investigations in primary rat mesencephalic cultures. Biochem. Pharmacol. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Bournival J, Quessy, Martinoli MG. Protective effects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 2009;29:1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bournival J, Plouffe M, Renaud J, Provencher C, Martinoli MG. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidat. Med. Cell. Longev. 2012:11. doi: 10.1155/2012/921941. ID 921941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antunez K, Jones DP, Go YM, Liang YL, Dajas F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Rad. Biol. Med. 2010;49:738–747. doi: 10.1016/j.freeradbiomed.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZJ, Cheang LCV, Wang MW, Lee SMY. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and proinflammatory gene expression in PC-12 cells and in zebrafish. Int. J. Mol. Med. 2011;27:195–203. doi: 10.3892/ijmm.2010.571. [DOI] [PubMed] [Google Scholar]
- 16.Hu P, Wang M, Chen WH, Liu J, Chen L, Yin ST, Yong W, Chen JT, Wang HL, Ruan DY. Quercetin relieves chronic lead exposure-induced impairment of synaptic plasticity in rat dentate gyrus in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008;378:43–51. doi: 10.1007/s00210-008-0301-z. [DOI] [PubMed] [Google Scholar]
- 17.Barcelos GRM, Grotto D, Serpeloni JM, Angeli JPF, Rocha BA, Souza VVO, Vicentini JT, Emanuelli T, Bastos JK, Antunes LMG, Knasmuller S, Barbosa F., Jr Protective properties of quercetin against DNA damage and oxidative stress induced by methylmercury in rats. Arch. Toxicol. 2011;85:1151–1157. doi: 10.1007/s00204-011-0652-y. [DOI] [PubMed] [Google Scholar]
- 18.Selvakumar K, Bavithra S, Suganthi M, Benson CS, Elumalai P, Arunkumar R, Krishnamoorthy G, Venkataraman P, Arunakaran J. Protective role of quercetin on PCBs-induced oxidative stress and apoptosis in hippocampus of adult rats. Neurochem. Res. 2012;37:708–721. doi: 10.1007/s11064-011-0661-5. [DOI] [PubMed] [Google Scholar]
- 19.Lv C, Hong T, yang Z, Zhang Y, Wang L, Dong M, Zhao J, Mu J, Meng Y. Effect of quercetin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Evid. Based Complem. Altern. Med. 2012:6. doi: 10.1155/2012/928643. ID 928643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao RQ, Qi DS, YU HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- 21.Keddy PGW, Dunlop K, Warford J, Samson ML, Jones QRD, Vasantha Rupasinghe HP, Roberstson GS. Neuroprotective and anti-inflammatory effects of the flavonoid-enriched fraction AF4 in a mouse model of hypoxic-ischemic brain injury. PLoS One. 2012;7(12):e52324. doi: 10.1371/journal.pone.0051324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer S, Halliwell B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012;7:99–109. doi: 10.1007/s12263-011-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005;81(Suppl.):268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 26.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspec. Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Kay CD. The future of flavonoid research. Br. J. Nutr. 2010;104:S91–S95. doi: 10.1017/S000711451000396X. [DOI] [PubMed] [Google Scholar]
- 29.Kang JH, Chang SY, Jang HJ, Cho JM, Kim DB, Lee SS, Ko SH, Park YM, Needs PW, Jo YH, Kim MJ. Quercetin-induced upregulation of human GCLC gene is mediated by cis-regulatory element for early growth response protein-1 (EGR1) in INS-1 beta-cells. J. Cell. Biochem. 2009;108:1346–1355. doi: 10.1002/jcb.22365. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi Y, Matsushima M, Nakamura T, Shibasaki M, Hashimoto N, Imaizumi K, Shimokata K, Hasegawa Y, Kawabe T. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem. Biophys. Res. Commun. 2012;417:169–174. doi: 10.1016/j.bbrc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 31.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem. Biol. Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Ng CJ, Wadleigh DJ, Gangopadhyyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 33.Horke S, Witte I, Wilgenbus P, Kruger M, Starnd D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 34.Horke S, Witte I, Altenhoffer S, Wilgenbus P, Goldeck M, Forstermann U, Xiao J, Kramer GL, Haines DC, Chowdhary PK, Haley RW, Teiber JF. Paraoxonase-2 is down regulated by the Pesudomonas aeruginosa quorum-sensing signal N-(3-oxododecanyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem. J. 2010;426:73–83. doi: 10.1042/BJ20091414. [DOI] [PubMed] [Google Scholar]
- 35.Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie JC, Precourt LP, Amre D, Sinnett D. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of human and rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1252–G1261. doi: 10.1152/ajpgi.00369.2007. [DOI] [PubMed] [Google Scholar]
- 36.Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicol. Appl. Pharmacol. 2011;256:369–378. doi: 10.1016/j.taap.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 38.Draganov DI, La Du BN. Pharmacogenetics of paraoxonase: a brief review. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 39.Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, Costa LG. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Rad. Biol. Med. 2013;58:98–108. doi: 10.1016/j.freeradbiomed.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J. Alzheim. Dis. 2010;20(Suppl. 2):S453–S473. doi: 10.3233/JAD-2010-100321. [DOI] [PubMed] [Google Scholar]
- 41.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke C, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antiox. Redox Signal. 2011;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem. Pharmacol. 2011;81:337–344. doi: 10.1016/j.bcp.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase-2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
- 44.Fuhrman B, Khateeb J, Shiner M, Nitzan O, Karry R, Volkova N, Aviram M. Urokinase plasminogen activator upregulates paraoxonase 2 expression in macrophages via an NADPH oxidase-dependent mechanism. Arterioscler. Thromb. Vasc. Res. 2008;28:1361–1367. doi: 10.1161/ATVBAHA.108.166041. [DOI] [PubMed] [Google Scholar]
- 45.Fuhrman B, Gantman A, Khateeb J, Volkova N, Horke S, Kiyan J, Dumler I, Aviram M. Urokinase activates macrophage PON2 gene transcription via the PI3K/ROS/MEK/SREBP-2 signalling cascade mediated by the PDGFR-beta. Cardiovasc. Res. 2009;84:145–154. doi: 10.1093/cvr/cvp184. [DOI] [PubMed] [Google Scholar]
- 46.Rosenblat M, Volkova N, Roqueta-Rovera M, Nakamura MT, Aviram M. Increased macrophage cholesterol biosysnthesis and decreased cellular paraoxonase 2 (PON2) expression in 6-desaturase knockout (6-DS-KO) mice: beneficial effects of arachidonic acid. Atherosclerosis. 2010;210:414–421. doi: 10.1016/j.atherosclerosis.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 47.Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is upregulated by unesterified cholesterol through activation of the phosphatidylinositol 3-kinase (PI3K) pathway. Biol. Chem. 2007a;388:1353–1358. doi: 10.1515/BC.2007.145. [DOI] [PubMed] [Google Scholar]
- 48.Yehuda I, Madar Z, Szuchman-Sapir A, Tamir S. Glabridin, a phytoestrogen from licorice root, up-regulates manganese superoxide dismutase, catalase and paraoxonase 2 under glucose stress. Phytother. Res. 2011;25:659–667. doi: 10.1002/ptr.3318. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblat M, Hayek T, Hussein K, Aviram M. Decreased macrophage paraoxonase 2 expression in patients with hypercholesterolemia is the result of their increased cellular cholesterol content: effect of atorvastatin therapy. Arterioscler. Thromb. Vasc. Biol. 2004;24:175–180. doi: 10.1161/01.ATV.0000104011.88939.06. [DOI] [PubMed] [Google Scholar]
- 50.Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR and AP-1 pathway activation. Atherosclerosis. 2007b;195:313–321. doi: 10.1016/j.atherosclerosis.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Stucker Fernandez E, de Oliveira Machado M, Minuzzi Becker A, de Andrade F, Maraschin M, Luiz da Silva E. Yerba mate (Ilex paraguariensis) enhances the gene modulation and activity of paraoxonase-2: in vitro and in vivo studies. Nutrition. 2012;28:1157–1164. doi: 10.1016/j.nut.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Boesch-Saadatmandi C, Pospissil RT, Graeser AC, Canali R, Boomgaarden I, Doering F, Wolffram S, Egert S, Mueller MJ, Rimbach G. Effect of quercetin on paraoxonase 2 levels in RAW264.7 macrophages and in human monocytes - role of quercetin metabolism. Int. J. Mol. Sci. 2009;10:4168–4177. doi: 10.3390/ijms10094168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2 and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 55.Altenhofer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Forstermann U, Horke S. One enzyme, two functions. PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J. Biol. Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiorani M, Guidarelli A, Blasa M, Azzolini C, Candiracci M, Piatti E, Cantoni O. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010;21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Notas G, Nifli AP, Kampa M, Pelekanou V, Alexaki VI, Theodoropoulos P, Vercauteren J, Castanas E. Quercetin accumulates in nuclear structures and triggers specific gene expression in epithelial cells. J. Nutr. Biochem. 2012;23:656–666. doi: 10.1016/j.jnutbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Jones QRD, Warford J, Vasantha Rupasinghe HP, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012;33:602–610. doi: 10.1016/j.tips.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Mehan S, Meena H, Sharma D, Sankhla R. JNK: a stress-activated protein kinase therapeutic strategies and involvement in Alzheimer’s and various neurodegenerative abnormalities. J. Mol. Neurosci. 2011;43:376–390. doi: 10.1007/s12031-010-9454-6. [DOI] [PubMed] [Google Scholar]
- 60.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr. Op. Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr. Op. Biotechnol. 2008;19:73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008;476:107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 63.Chang YF, Hsu YC, Hung HF, Lee HJ, Lui WY, Chi CW, Wang JJ. Quercetin induces oxidative stress and potentiates the apoptotic action of 2-methoxyestradiol in human hepatoma cells. Nutr. Cancer. 2009;61:735–745. doi: 10.1080/01635580902825571. [DOI] [PubMed] [Google Scholar]
- 64.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nf-kB and AP-1/JNK pathways to induce cell death in human hepatoma cells. Nutr. Cancer. 2010;62:390–401. doi: 10.1080/01635580903441196. [DOI] [PubMed] [Google Scholar]
- 65.Miodini P, Fioravanti L, Di Fronzo G, Cappelletti V. The two phyto-estrogens genistein and quercetin exert different effects on oestrogen receptor function. Br. J. cancer. 1999;80:1150–1155. doi: 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Woude H, ter Veld MGR, Jacobs N, van der Saag PT, Murk AJ, Rietjens IMCM. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005;49:763–771. doi: 10.1002/mnfr.200500036. [DOI] [PubMed] [Google Scholar]
- 67.Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, Marino M. Quercetin-induced apoptotic cascade in cancer cells: antioxidant versus estrogen receptor alpha-dependent mechanisms. Mol. Nutr. Food Res. 2009;53:699–708. doi: 10.1002/mnfr.200800239. [DOI] [PubMed] [Google Scholar]
- 68.Bulzomi P, Galluzzo P, Bolli A, Leone S, Acconcia F, Marino M. The pro-apoptotic effect of quercetin in cancer cell lines requires ER-dependent signals. J. Cell. Physiol. 2011;227:1891–1898. doi: 10.1002/jcp.22917. [DOI] [PubMed] [Google Scholar]
- 69.Hollman PC, deVries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995;62:1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 70.Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- 71.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81(Suppl.):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 72.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Rad. Biol. Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 73.Faria A, Pestana D, Teixera D, Azevedo J, De Freitas V, Mateus N, Calhau C. Flavonoid transport across RBE4 cells: a blood-brain barrier model. Cell. Mol. Biol. Lett. 2010;15:234–241. doi: 10.2478/s11658-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto K, Tsuji A, Kawai Y, Terao J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Rad. Biol. Med. 2011;51:329–336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 75.deBoer VCJ, Dihal AA, van der Woude H, Arts ICW, Wolffram A, Alink GM, Rietjens IMCM, Keijer J, Hollma PCH. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005;135:1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- 76.Bieger J, Cermak R, Blank R, deBoer VCJ, Hollman PCH, Kamphues J, Wolffram S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008;138:1417–1420. doi: 10.1093/jn/138.8.1417. [DOI] [PubMed] [Google Scholar]
- 77.Huebbe P, Wagner AE, Boesch-Saadatmandi C, Sellmer F, Wolffram S, Rimbach G. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res. 2010;61:242–246. doi: 10.1016/j.phrs.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GM. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem. Pharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Dajas F, Rivera-Megret F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, Echeverry C, Lafon L, Heizen H, Ferreira M, Morquio A. Neuroprotection by flavonoids. Braz. J. Med. Biol. Res. 2003;36:1613–1620. doi: 10.1590/s0100-879x2003001200002. [DOI] [PubMed] [Google Scholar]
- 80.Das S, Mandal AK, Ghosh A, Panda S, Das N, Sarkar S. Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr. Aging Sci. 2008;1:169–174. doi: 10.2174/1874609810801030169. [DOI] [PubMed] [Google Scholar]
- 81.Dhavan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011;63:342–351. doi: 10.1111/j.2042-7158.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 82.Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MRA, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Rad. Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 83.Fiorani M, Accorsi A, Cantoni O. Human red blood cells as a natural flavonoid reservoir. Free Rad. Res. 2003;37:1331–1338. doi: 10.1080/10715760310001615998. [DOI] [PubMed] [Google Scholar]
- 84.Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, Brighenti F, Borges G, Crozier A, Conte A, Del Rio D. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011;55:1–9. doi: 10.1002/mnfr.201000525. [DOI] [PubMed] [Google Scholar]