Abstract
Objective
Hypo- and hyperphosphatemia have each been associated with increased mortality in maintenance hemodialysis (MHD) patients. There has not been previous evaluation of a differential relationship between serum phosphorus level and death risk across varying age groups in MHD patients.
Design and Settings
In a 6-year cohort of 107,817 MHD patients treated in a large dialysis organization, we examined the association between serum phosphorus levels with all-cause and cardiovascular mortality within 5 age categories (15-<45, 45-<65, 65-<70, 70-<75 and ≥75 years old) using Cox proportional hazards model adjusted for case-mix covariates and malnutrition inflammation complex syndrome (MICS) surrogates.
Main outcome measure
all-cause and cardiovascular mortality.
Results
The overall mean age of the cohort was 60±16 years, among whom there were 45% women, 35% Blacks and 58% diabetics. The time averaged serum phosphorus level (mean ± SD) within each age category was 6.26±1.4, 5.65±1.2, 5.26±1.1, 5.11±1.0 and 4.88±1.0 mg/dl, respectively (P for trend <0.001). Hyperphosphatemia (>5.5 mg/dl) was consistently associated with increased all-cause and cardiovascular mortality risks across all age categories including after adjustment for case-mix and MICS-related covariates. In fully adjusted models, a low serum phosphorus level (<3.5 mg/dl) was associated with increased all-cause mortality only in elderly MHD patients ≥65 years old (hazard ratio [HR] (95% confidence interval[CI]): 1.21(1.07-1.37), 1.13(1.02-1.25), and 1.28(1.2-1.37) for patients 65-<70, 70-<75, and ≥75 years old, respectively], but not in younger patients (<65 years old). A similar differential for cardiovascular mortality of serum phosphorus levels between old and young age groups was observed.
Conclusions
The association between hyperphosphatemia and mortality is similar across all age groups of MHD patients, whereas hypophosphatemia is associated with increased mortality only in elderly MHD patients. Preventing very low serum phosphorus levels in elderly dialysis patients may be associated with better outcomes, which needs to be examined in future studies.
Keywords: hemodialysis, mortality, phosphorus, elderly
Introduction
Dialysis patients have a higher risk of morbidity and mortality than that of the general population.1,2 Cardiovascular disease accounts for approximately 50% of these deaths1 and accelerated atherosclerosis is a major cause of cardiovascular mortality in dialysis patients.3 The heightened cardiovascular risk among dialysis patients may be due to traditional risk factors and uremia-specific risk factors which include mineral and bone disorders (MBD).4,5 A 25%-30% decline in kidney function may result in phosphorus retention and hyperphosphatemia, which subsequently give rise to abnormalities in vitamin D metabolism and alterations in parathyroid gland function (eg, secondary hyperparathyroidism and mixed renal osteodystrophy).6 Moreover, hyperphosphatemia in dialysis patients contribute to elevations in fibroblast growth factor-23 (FGF-23) levels7,8 and vascular calcification9-14 which are both independently associated with adverse cardiovascular outcomes and mortality.15-19 An experimental study demonstrated that increased serum phosphorus concentrations stimulate the transformation of vascular smooth muscle cells into osteoblast-like cells, which predispose to vascular calcification.20 Studies of dialysis patients in North America, Latin America, Europe and Asia have consistently shown that hyperphosphatemia as well as increases in serum phosphorus levels over time, are associated with increased risk of all-cause mortality, cardiovascular morbidity and mortality, and increased hospitalization rates.15,16,18,21-32 Hypophosphatemia, as well as declines in serum phosphorus levels over time are also associated with increased risk of all-cause and cardiovascular mortality in dialysis patients.22,23,25,31
The elderly comprise the fastest growing group of dialysis patients worldwide, and account for 25%-30% of dialysis patients in end-stage renal disease (ESRD) registries.1,33 From 2000 to 2007, US elderly patients ≥65 years old had a greater incidence in ESRD than younger age groups. During this period, the prevalence rate of ESRD cases in patients 65-74 and ≥75 years old rose by 24% and 28%, respectively, which was markedly higher than the 17.5% growth rate observed in all age groups.1 Similarly, in European countries, there has been a more rapid rise in elderly patients age ≥65 years initiating dialysis treatments compared with patients 18-65 years old.34,35 Elderly dialysis patients experience a higher risk of death compared to their younger counterparts.36,37 Malnutrition, inflammation and cardiovascular comorbidities are independent predictors of mortality among hemodialysis patients >75 years old.38,39 Furthermore, elderly dialysis patients are more likely to be manifest worse nutritional parameters indicative of protein energy malnutrition.36,40,41 We therefore hypothesized that a differential association between serum phosphorus concentrations and mortality in elderly versus non-elderly MHD patients exists.
Subjects and Methods
Study Population
We extracted and examined data from all individuals with CKD stage 5 who underwent hemodialysis treatment from July 2001 to June 2006 in any one of the 580 outpatient dialysis facilities of DaVita, a large dialysis organization in USA. The study was approved by the institutional review committees of both the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and DaVita Clinical Research; a requirement for a written consent was waived due to the large sample size, patient anonymity, and nonintrusive nature of the research. The first (baseline) studied quarter for each patient was the calendar quarter in which the patient's hemodialysis vintage was greater than 90 days. Patients who were ≥15 years old, received hemodialysis treatments in the baseline quarter, and who underwent serum phosphorus level measurements were included in the present study. Among the 127,034 patients who were ≥15 years old and received hemodialysis treatment greater than 90 days during the study period, we excluded 19,487 MHD patients who had missing serum phosphorus levels and follow-up periods. A total of 107,817 MHD patients were included in the present analysis.
Baseline Demographic and Comorbidity Measures
The creation of the DaVita maintenance hemodialysis patient cohort has been previously described.42-46 To minimize measurement variability, all repeated measures for each patient during any given calendar quarter (i.e. over a 13-week interval) were averaged to create a summary estimate that was used in all models. Average values were obtained from up to 20 calendar quarters (from July 1, 2001 through June 30, 2006) for each laboratory parameter and clinical measure for each patient during the study cohort period. Patients were followed for outcome events until June 30, 2007. Dialysis vintage was defined as the duration of time between the first day of hemodialysis treatment and the day that the patient entered the cohort study.
Information on race/ethnicity, primary insurance, marital status and the presence of diabetes at baseline were obtained from the DaVita database. Data on pre-existing comorbidities and history of tobacco smoking were obtained by linking the DaVita database to the data from Medical Evidence Form 2728 from the US Renal Data System (USRDS) and were categorized into 9 comorbid conditions: atherosclerotic heart disease (ischemic heart disease, myocardial infarction, or cardiac arrest), congestive heart failure, other cardiac diseases (pericarditis and cardiac arrhythmia), hypertension, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer and non-ambulatory state. The recorded causes of death were obtained from the USRDS, and cardiovascular death was defined as death due to myocardial infarction, cardiac arrest, heart failure, cerebrovascular accident and other cardiac diseases. The study population was stratified into 5 age categories (15-<45, 45-<65, 65-<70, 70-<75 and ≥75 years old) for data analysis.
Laboratory Measures
Blood samples were drawn using standardized techniques in all DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, FL, typically within 24 hours. All laboratory values were measured using automated and standardized methods in the DaVita Laboratory. Most laboratory parameters were measured monthly, including urea nitrogen, creatinine, albumin, calcium, phosphorus, bicarbonate, and total-iron binding capacity (TIBC). Serum ferritin and intact parathyroid hormone were measured at least quarterly. Hemoglobin was measured at least monthly in all patients and weekly to biweekly in most patients. The normalized protein equivalent of total nitrogen appearance (nPNA), also known as normalized protein catabolic rate (nPCR), was measured monthly as an indicator of daily protein intake. Corrected calcium levels were calculated using the following equation: ‘corrected calcium (mg/dl) = {0.8 × [4 - serum albumin (g/dl)]} + serum calcium (mg/dl).’ Most blood samples were collected before dialysis, except for post-dialysis serum urea nitrogen to calculate urea kinetics. To mitigate measurement error, we averaged all monthly serum phosphorus values into a single time-averaged serum phosphorus value per patient. We divided time-averaged serum phosphorus levels into 6 categories defined a priori: <3.5, 3.5-5.5, >5.5-6.5, >6.5-7.5, >7.5-8.5 and >8.5 mg/dl. The phosphorus category of 3.5-5.5 mg/dl was designated as the reference group based on the Kidney Disease Outcomes Quality Initiative (KDOQI) recommended serum phosphorus target range.47
Statistical Methods
Descriptive statistics included proportions, means ± SD or medians (interquartile ranges [IQR]) as dictated by data type. We compared time-averaged serum phosphorus levels between different age categories using trend analysis and adjusted for demographics, dialysis dose, presence of comorbid conditions, residual renal function, serum calcium and intact parathyroid hormone concentrations. Logistic regression analyses with adjustment for case-mix covariates and malnutrition inflammation complex syndrome (MICS) surrogates (see below) were used to calculate the odds ratio of hypophosphatemia (defined as serum phosphorus level <3.5 mg/dl) and hyperphosphatemia (defined as serum phosphorus level ≥5.5 mg/dl) across the 5 age groups. We also tested the linearity of logistic regression models using restricted cubic splines.
We evaluated the association between serum phosphorous levels (main predictor) with all-cause mortality (separately) and cardiovascular mortality (outcomes) using Cox proportional hazard models within each age category. For each analysis, we created three models with incremental multivariable adjustment:
Unadjusted models (minimally adjusted models) included phosphorus categories and entry calendar quarter (q1 through q20).
Case-mix adjusted models included all of the variables in the minimally adjusted model plus age, gender, race/ethnicity (non-Hispanic Whites, Blacks, Hispanics and Asians), presence of diabetes, dialysis vintage (<6 months, 6-24 months, 2-5 years and ≥5 years), primary insurance (Medicare, Medicaid and others), marital status (married, single, widowed and divorced), 9 pre-existing comorbidities identified in the Medical Evidence Form 2728 (atherosclerotic heart disease, cancer, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, hypertension, other cardiac diseases, peripheral vascular disease and non-ambulatory state), history of tobacco smoking, type of vascular access (arteriovenous fistula, arteriovenous graft and catheter), dialysis dose as indicated by single pool Kt/V and residual renal function.
Case-mix plus MICS adjusted models included all of the covariates in the case-mix model plus 12 surrogates of nutritional status and inflammation which included body mass index (BMI), serum levels of albumin, TIBC, ferritin, creatinine, calcium, intact parathyroid hormone, bicarbonate, hemoglobin, peripheral white blood cell count (WBC), lymphocyte percentage and nPNA.
Patients who received a renal transplant, switched to peritoneal dialysis or left DaVita dialysis clinics were censored at the time of the event. Missing covariate data were imputed by the means or medians of the existing values as appropriate. All statistical analyses were conducted with Stata 10.0 (Stata Corp., College Station, TX).
Results
Cohort Description
The baseline demographics, comorbid conditions and laboratory characteristics of the 107,817 MHD patients, stratified by age category are summarized in Table 1. The overall mean age of the cohort was 60±16 years, among whom there were 45% women, 35% Blacks and 58% diabetics. Older MHD patients were more likely to be female, White, and diabetic and were more likely to have pre-existing comorbidities, dialysis catheter use, and shorter dialysis vintage. They were also more likely to have lower serum levels of albumin, creatinine, phosphorus, intact parathyroid hormone, nPNA, and lower BMI compared to younger MHD patients. A total of 57,851 MHD patients (54%) died during the follow-up period, and 23,743 (41%) deaths were attributable to cardiovascular diseases. The median (IQR) follow-up time for the cohort was 2.21 (1.18-3.68) years.
Table 1.
Baseline Patient characteristics of 107,817 maintenance hemodialysis patients according to 5 incremental age categories
| Age Range (years) | All | 15-<45 | 45-<65 | 65-<70 | 70-<75 | ≥75 | P-for trend |
|---|---|---|---|---|---|---|---|
| N (%) | 107,817 | 19,182 (18) | 41,770 (39) | 12,562 (12) | 12,240 (11) | 22,063 (20) | N/A |
| Age (years) (mean±SD) | 60±16 | 34.6± 7.3 | 55.4± 5.6 | 67± 1.4 | 72±1.4 | 80.4±4.3 | <0.001 |
| Gender (%female) | 45 | 40 | 44 | 49 | 49 | 47 | <0.001 |
| Race (%) | |||||||
| White | 46 | 29 | 38 | 48 | 56 | 67 | <0.001 |
| Black | 35 | 49 | 40 | 31 | 26 | 20 | <0.001 |
| Hispanic | 16 | 19 | 19 | 17 | 14 | 10 | <0.001 |
| Asian | 3 | 3 | 3 | 4 | 4 | 3 | 0.01 |
| DM (%) | 58 | 33 | 66 | 71 | 65 | 51 | <0.001 |
| Vintage (time on dialysis; %) | |||||||
| <6 mo | 58 | 46 | 56 | 59 | 61 | 70 | <0.001 |
| 6-24 mo | 17 | 15 | 17 | 17 | 18 | 17 | 0.002 |
| 2-5 yr | 16 | 18 | 18 | 17 | 16 | 11 | <0.001 |
| ≥ 5 yr | 9 | 21 | 9 | 7 | 5 | 2 | <0.001 |
| Primary insurance (%) | |||||||
| Medicare | 68 | 60 | 58 | 78 | 79 | 82 | <0.001 |
| Medicaid | 6 | 12 | 9 | 2 | 2 | 1 | <0.001 |
| Other | 26 | 28 | 33 | 20 | 19 | 17 | <0.001 |
| Marital status (%) | |||||||
| Married | 47 | 33 | 49 | 54 | 54 | 47 | <0.001 |
| Divorced | 9 | 7 | 12 | 8 | 7 | 4 | <0.001 |
| Single | 28 | 59 | 31 | 18 | 13 | 11 | <0.001 |
| Widowed | 16 | 1 | 8 | 20 | 26 | 38 | <0.001 |
| Kt/V (single pool; mean±SD) | 1.52±0.35 | 1.47±0.35 | 1.49±0.35 | 1.55±0.35 | 1.56±0.36 | 1.58±0.35 | <0.001 |
| BMI (kg/m2; mean±SD) | 26.7±6.7 | 26.8±7.4 | 28±7.2 | 26.8±6.2 | 26±5.8 | 24.3±4.9 | <0.001 |
| nPNA (g/kg/day; mean±SD) | 0.95±0.25 | 0.97±0.26 | 0.96±0.26 | 0.95±0.25 | 0.93±0.25 | 0.91±0.24 | <0.001 |
| Vascular access (%) | |||||||
| AVF | 25.7 | 33.5 | 26.1 | 23.3 | 23.3 | 20.8 | <0.001 |
| AVG | 29.5 | 26.2 | 30.6 | 33.4 | 31.8 | 26.9 | 0.88 |
| Catheter | 44.5 | 40 | 43 | 42.9 | 44.5 | 52 | <0.001 |
| Other/Missing | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 | 0.54 |
| Comorbid conditions (%) | |||||||
| Atherosclerotic heart disease | 22 | 4.4 | 18 | 28 | 31 | 33 | <0.001 |
| Cancer | 4.7 | 1 | 3.1 | 6 | 7 | 9 | <0.001 |
| Congestive heart failure | 28 | 12 | 26 | 34 | 35 | 38 | <0.001 |
| COPD | 6 | 1.1 | 5 | 8 | 9 | 9 | <0.001 |
| Cerebrovascular disease | 8 | 2.4 | 7 | 10 | 10 | 10 | <0.001 |
| Hypertension | 80 | 75 | 81 | 81 | 81 | 80 | <0.001 |
| Other cardiac diseases | 6 | 2.1 | 4.1 | 7 | 8 | 10 | <0.001 |
| Peripheral vascular disease | 12 | 3.4 | 10 | 15 | 16 | 16 | <0.001 |
| Non-Ambulatory states | 3.19 | 1.23 | 2.92 | 3.68 | 3.81 | 4.59 | <0.001 |
| Active smoking | 5 | 7 | 7 | 4.3 | 3.7 | 1.8 | <0.001 |
| Serum Levels (baseline) | |||||||
| Albumin (g/dL) | 3.67±0.5 | 3.82±0.5 | 3.68±0.5 | 3.65±0.4 | 3.62±0.4 | 3.57±0.4 | <0.001 |
| Creatinine (mg/dL) | 8.01±3.3 | 10.72±3.8 | 8.31±3.1 | 7.2±2.6 | 6.83±2.5 | 6.14±2.2 | <0.001 |
| TIBC (mg/dL) | 209±46 | 209±45 | 213±47 | 208±46 | 207±46 | 204±46 | <0.001 |
| Ferritin (ng/mL) | 380(181, 708) | 358(163, 688) | 387(185, 719) | 403(192, 723) | 393(190, 731) | 367(177, 680) | 0.065 |
| Bicarbonate (mg/dL) | 22.44±3.1 | 21.79±3.1 | 22.24±3 | 22.6±3 | 22.8±2.9 | 23.12±3 | <0.001 |
| Calcium (mg/dL) | 9.45±0.7 | 9.35±0.8 | 9.44±0.7 | 9.48±0.7 | 9.5±0.6 | 9.52±0.6 | <0.001 |
| Phosphorus (mg/dL) | 5.56±1.5 | 6.28±1.7 | 5.71±1.5 | 5.33±1.3 | 5.18±1.3 | 4.96±1.2 | <0.001 |
| Intact PTH (pg/mL) | 248(145, 422) | 341(185, 644) | 265(157, 442) | 228(134, 367) | 213(127, 341) | 200(121, 311) | <0.001 |
| Hemoglobin (g/dL) | 12.02±1.4 | 11.89±1.5 | 12.01±1.4 | 12.04±1.3 | 12.05±1.3 | 12.13±1.3 | <0.001 |
| WBC (×103/μL) | 7.45±2.5 | 7.17±2.4 | 7.43±2.4 | 7.49±2.4 | 7.53±2.5 | 7.63±2.8 | <0.001 |
| %Lymphocyte | 20.51±7.9 | 22.75±8.3 | 20.81±7.9 | 19.81±7.6 | 19.33±7.6 | 18.98±7.6 | <0.001 |
Note: Continuous variables expressed as mean ± SD; categorical variables expressed as number (percent); P-for trend shows the differences in each age categories.
Abbreviations: DM, diabetes mellitus; BMI, body mass index; nPNA, normalized protein nitrogen appearance; AVF, arteriovenous fistula; AVG, arteriovenous graft; COPD, chronic obstructive pulmonary disease; TIBC, total iron-binding capacity; Intact PTH, intact parathyroid hormone; WBC, white blood cells.
Time-Averaged Serum Phosphorus Concentrations across 5 Incremental Age Categories
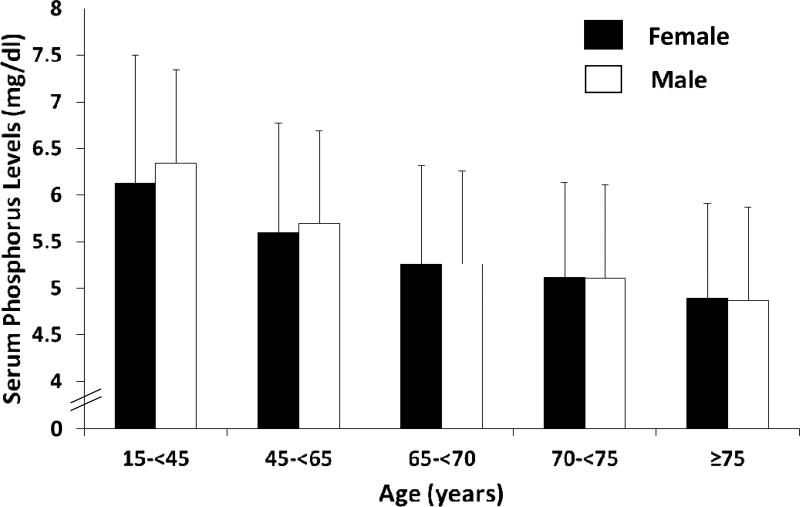
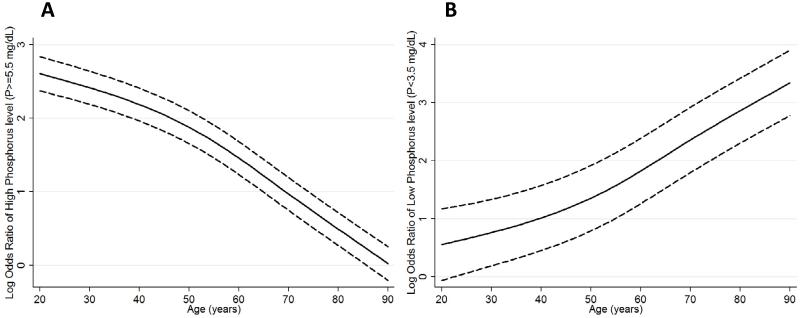
There was a graded decline in time-averaged serum phosphorus level (mean ± SD) with incrementally older age: 6.26±1.4, 5.65±1.2, 5.26±1.1, 5.11±1.0 and 4.88±1.0 mg/dl in patients 15-<45, 45-<65, 65-<70, 70-<75 and ≥75 years old, respectively (P for trend <0.001). In both sexes, older MHD patients had significantly lower time-averaged serum phosphorus levels than younger MHD patients (P for trend <0.001 in both sexes, Figure 1). This trend persisted after case-mix adjustment. In case-mix plus MICS adjusted logistic regression models, older MHD patients 65-<70, 70-<75 and ≥75 years old were 1.62, 1.96 and 2.32 times more likely to have serum phosphorous levels <3.5 mg/dl, compared with those 15<-45 years old (Table 2). Similarly, compared to the 15-<45 year old age group, patients 45-<65, 65-<70, 70-<75 and ≥75 years old were 26%, 46%, 52% and 63% less likely to have serum phosphorus levels >5.5 mg/dl in fully adjusted models, respectively (Table 3). Cubic spline models showed a similar trend for hyperphosphatemia (Figure 2, Panel A) and hypophosphatemia (Figure 2, Panel B) across the entire continuum of age.
Figure 1.
Time-averaged serum phosphorus levels in 107,817 maintenance hemodialysis patients according to age category and sex.
Table 2.
Odds ratio (95% confidence intervals) of low serum phosphorus levels (<3.5 mg/dl) stratified by age category
| Age Range (years) | 15-<45 | 45-<65 | 65-<70 | 70-<75 | ≥75 |
|---|---|---|---|---|---|
| Unadjusted | Reference | 1.67 (1.44-1.94) | 2.93 (2.48-3.45) | 3.93 (3.35-4.61) | 5.43 (4.7-6.28) |
| Case-mix | Reference | 1.94 (1.66-2.27) | 3.6 (3.02-4.29) | 4.94 (4.16-5.87) | 6.93 (5.89-8.15) |
| Case-mix and MICS | Reference | 1.14 (0.97-1.35)† | 1.62 (1.34-1.96) | 1.96 (1.63-2.36) | 2.32 (1.95-2.77) |
Note: P-values<0.001 except p-value
0.11; case-mix model was adjusted for gender, race/ethnicity, presence of diabetes mellitus, baseline comorbidities, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, types of vascular access, dialysis dose as indicated by single pool Kt/V and residual renal function; case-mix and MICS model was adjusted for all of the covariates in the case-mix model as well as body mass index, serum levels of albumin, total-iron binding capacity, ferritin, creatinine, calcium, intact parathyroid hormone, bicarbonate, hemoglobin, blood white blood cells, lymphocyte percentage and normalized protein nitrogen appearance.
Abbreviations: MICS, malnutrition-inflammation complex syndrome.
Table 3.
Odds ratio (95% confidence intervals) of high serum phosphorus levels (≥5.5 mg/dl) stratified by age category
| Age Range (years) | 15-<45 | 45-<65 | 65-<70 | 70-<75 | ≥75 |
|---|---|---|---|---|---|
| Unadjusted | Reference | 0.48 (0.46-0.49) | 0.27 (0.25-0.28) | 0.21 (0.2-0.22) | 0.14 (0.13-0.15) |
| Case-mix | Reference | 0.49 (0.47-0.51) | 0.28 (0.27-0.3) | 0.23 (0.21-0.24) | 0.15 (0.14-0.16) |
| Case-mix and MICS | Reference | 0.74 (0.71-0.77) | 0.54 (0.51-0.57) | 0.48 (0.45-0.51) | 0.37 (0.35-0.39) |
Note: All P-values<0.001; case-mix model was adjusted for gender, race/ethnicity, presence of diabetes mellitus, baseline comorbidities, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, types of vascular access, dialysis dose as indicated by single pool Kt/V and residual renal function; case-mix and MICS model was adjusted for all of the covariates in the case-mix model as well as body mass index, serum levels of albumin, total-iron binding capacity, ferritin, creatinine, bicarbonate, calcium, intact parathyroid hormone, hemoglobin, blood white blood cells, lymphocyte percentage and normalized protein nitrogen appearance.
Abbreviations: MICS, malnutrition-inflammation complex syndrome.
Figure 2.
Restricted cubic spline models adjusted for case-mix and malnutrition inflammation complex syndrome surrogates representing logistic regression odds ratio of high serum phosphorus levels (phosphorus≥5.5 mg/dL) (A) and low serum phosphorus levels (phosphorus<3.5 mg/dL) (B).
Fully adjusted cubic spline models include adjustment for age, gender, race/ethnicity, presence of diabetes mellitus, baseline comorbid conditions, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, residual renal function, dialysis dose as indicated by single pool Kt/V, serum intact parathyroid hormone and calcium concentrations.
All-Cause Mortality associated with Serum Phosphorus Levels in 5 Incremental Age Categories
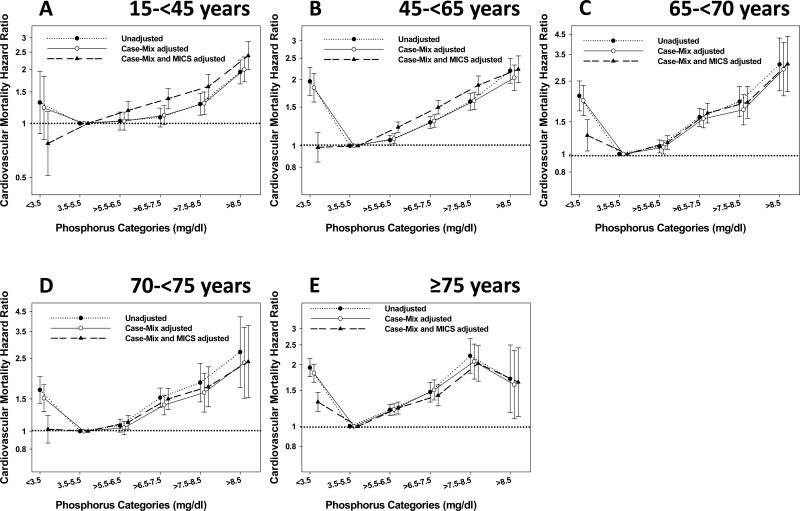
In the unadjusted and case-mix adjusted Cox proportional hazard models, there was a U-shaped association between time-averaged serum phosphorus levels and all-cause mortality across all age categories (Figure 3). After adjustment for MICS surrogates, a U-shaped association between serum phosphorus level and all-cause death risk persisted only in MHD patients ≥65 years, whereas patients < 65 years had a monotonic increase in mortality with increasing serum phosphorus level (Figure 3). The case-mix and MICS adjusted all-cause mortality hazard ratios (HRs) for each time-averaged serum phosphorus category stratified by age group are shown in Table 4. In comparison to serum phosphorus levels of 3.5-5.5 mg/dl (referent group), hyperphosphatemia was associated with increased all-cause death across all age categories in fully adjusted models (Figure 3 and Table 4). However, in comparison to the referent group, serum phosphorus levels <3.5 mg/dl were associated with increased all-cause mortality in older age groups (≥65 years old), but not in younger age groups (aged 15-<65 years old). Fully adjusted all-cause mortality HRs (95% CI) associated with serum phosphorus levels <3.5 mg/dl were 1.21 (1.07-1.37), 1.13 (1.02-1.25), and 1.28 (1.2-1.37) for patients 65-<70, 70-<75, and ≥75 years old, respectively (Figure 3 and Table 4).
Figure 3.
Association between time-averaged serum phosphorus levels and all-cause mortality using Cox proportional hazard models in patients aged 15-<45 years (A), 45-<65 years (B), 65-<70 years (C), 70-<75 years (D) and ≥75 years (E).
Case-mix model was adjusted for age, gender, race/ethnicity, presence of diabetes mellitus, baseline comorbidities, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, types of vascular access, dialysis dose as indicated by single pool Kt/V and residual renal function. Case-mix and MICS model was adjusted for all case-mix covariates plus body mass index, serum levels of albumin, total-iron binding capacity, ferritin, creatinine, calcium, intact parathyroid hormone, bicarbonate, hemoglobin, blood white blood cells, lymphocyte percentage and normalized protein nitrogen appearance. Abbreviation: MICS, malnutrition-inflammation complex syndrome.
Table 4.
All-cause mortality hazard ratio and 95% confidence intervals for serum phosphorus levels stratified by age category case-mix and MICS adjusted Cox proportional hazard models
| Phosphorus (mg/dl) | 15-<45 yo (N=19,182) | 45-<65 yo (N=41,770) | 65-<70 yo (N=12,562) | 70-<75 yo (N=12,240) | ≥75 yo (N=22,063) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | |
| <3.5 | 1.16 (0.95-1.42) | 0.15 | 0.95 (0.87-1.04) | 0.297 | 1.21 (1.07-1.37) | 0.002 | 1.13 (1.02-1.25) | 0.021 | 1.28 (1.2-1.37) | <0.001 |
| 3.5-5.5 (Reference) | 1 | N/A | 1 | N/A | 1 | N/A | 1 | N/A | 1 | N/A |
| >5.5-6.5 | 1.09 (1.01-1.17) | 0.027 | 1.11 (1.07-1.15) | <0.001 | 1.11 (1.05-1.18) | <0.001 | 1.13 (1.07-1.19) | <0.001 | 1.16 (1.11-1.21) | <0.001 |
| >6.5-7.5 | 1.26 (1.16-1.37) | <0.001 | 1.36 (1.3-1.42) | <0.001 | 1.49 (1.37-1.63) | <0.001 | 1.41 (1.29-1.54) | <0.001 | 1.34 (1.25-1.45) | <0.001 |
| >7.5-8.5 | 1.44 (1.3-1.59) | <0.001 | 1.64 (1.54-1.75) | <0.001 | 1.66 (1.45-1.91) | <0.001 | 1.91 (1.63-2.24) | <0.001 | 1.73 (1.51-1.98) | <0.001 |
| >8.5 | 1.94 (1.73-2.18) | <0.001 | 1.95 (1.77-2.14) | <0.001 | 2.22 (1.71-2.87) | <0.001 | 2.77 (2.1-3.66) | <0.001 | 1.56 (1.22-1.98) | <0.001 |
Note: Case-mix and MICS model includes adjustment for age, gender, race/ethnicity, presence of diabetes mellitus, baseline comorbid conditions, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, types of vascular access, dialysis dose as indicated by single pool Kt/V, residual renal function, body mass index, serum levels of albumin, intact parathyroid hormone, calcium, total-iron binding capacity, ferritin, creatinine, bicarbonate, hemoglobin, blood white blood cells, lymphocyte percentage and normalized protein nitrogen appearance.
Abbreviations: MICS, malnutrition-inflammation complex syndrome; HR, hazard ratio; CI, confidence interval.
Cardiovascular Mortality associated with Serum Phosphorus Levels in 5 Incremental Age Categories
In case-mix plus MICS adjusted models, hyperphosphatemia was associated with increased cardiovascular mortality in comparison to serum phosphorus levels of 3.5-5.5 mg/dl (referent group) in all age categories (Figure 4 and Table 5). Nevertheless, in fully adjusted models, serum phosphorus level <3.5 mg/dl were significantly associated with increased cardiovascular death only in elderly MHD patients 65-<70 years old (HR (95% CI) 1.26 (1.04-1.54), P=0.019) and those ≥75 years old (HR (95% CI) 1.32 (1.18-1.46), P<0.001). In comparison to the referent group, serum phosphorus levels <3.5 mg/dl were not associated with cardiovascular death in MHD patients <65 years old in fully adjusted models (Figure 4 and Table 5).
Figure 4.
Association between time-averaged serum phosphorus levels and cardiovascular mortality using Cox proportional hazard models in patients aged 15-<45 years (A), 45-<65 years (B), 65-<70 years (C), 70-<75 years (D) and ≥75 years (E).
Case-mix model was adjusted for age, gender, race/ethnicity, presence of diabetes mellitus, baseline comorbidities, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, types of vascular access, dialysis dose as indicated by single pool Kt/V and residual renal function. Case-mix and MICS model was adjusted for all case-mix covariates plus body mass index, serum levels of albumin, total-iron binding capacity, ferritin, creatinine, calcium, intact parathyroid hormone, bicarbonate, hemoglobin, blood white blood cells, lymphocyte percentage and normalized protein nitrogen appearance. Abbreviation: MICS, malnutrition-inflammation complex syndrome.
Table 5.
Cardiovascular mortality hazard ratio and 95% confidence intervals for serum phosphorus levels stratified b age category based in case-mix and MICS adjusted Cox proportional hazard models
| Phosphorus (mg/dl) | 15-<45 yo (N=19,182) | 45-<65 yo (N=41,770) | 65-<70 yo (N=12,562) | 70-<75 yo (N=12,240) | ≥75 yo (N=22,063) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | |
| <3.5 | 0.77 (0.51-1.16) | 0.217 | 0.98 (0.85-1.15) | 0.837 | 1.26 (1.04-1.54) | 0.019 | 1.02 (0.86-1.22) | 0.782 | 1.32 (1.18-1.46) | <0.001 |
| 3.5-5.5 (Reference) | 1 | N/A | 1 | N/A | 1 | N/A | 1 | N/A | 1 | N/A |
| >5.5-6.5 | 1.18 (1.05-1.33) | 0.006 | 1.22 (1.15-1.28) | <0.001 | 1.16 (1.06-1.26) | 0.001 | 1.12 (1.03-1.22) | 0.012 | 1.23 (1.15-1.31) | <0.001 |
| >6.5-7.5 | 1.37 (1.21-1.57) | <0.001 | 1.49 (1.39-1.6) | <0.001 | 1.67 (1.48-1.89) | <0.001 | 1.5 (1.32-1.71) | <0.001 | 1.42 (1.26-1.59) | <0.001 |
| >7.5-8.5 | 1.6 (1.37-1.88) | <0.001 | 1.88 (1.71-2.07) | <0.001 | 1.92 (1.57-2.34) | <0.001 | 1.75 (1.36-2.24) | <0.001 | 2.03 (1.66-2.48) | <0.001 |
| >8.5 | 2.39 (1.99-2.86) | <0.001 | 2.22 (1.93-2.56) | <0.001 | 3.1 (2.19-4.39) | <0.001 | 2.39 (1.52-3.77) | <0.001 | 1.64 (1.12-2.42) | 0.011 |
Note: Case-mix and MICS model includes adjustment for age, gender, race/ethnicity, presence of diabetes mellitus, baseline comorbid conditions, history of tobacco smoking, dialysis vintage categories, primary insurance, marital status, types of vascular access, dialysis dose as indicated by single pool Kt/V, residual renal function, body mass index, serum levels of albumin, intact parathyroid hormone, calcium, total-iron binding capacity, ferritin, creatinine, bicarbonate, hemoglobin, blood white blood cells, lymphocyte percentage and normalized protein nitrogen appearance.
Abbreviations: MICS, malnutrition-inflammation complex syndrome; HR, hazard ratio; CI, confidence interval.
Discussion
In this large, contemporary, and nationally representative cohort of MHD patients, we found that higher time-averaged serum phosphorus levels were associated with increased mortality in all age groups. We observed that elderly MHD patients had a greater likelihood of hypophosphatemia, and that low serum phosphorus levels were associated with increased mortality in this subgroup; this association was not observed in younger patients however. A similar pattern of results was observed for both all-cause and cardiovascular mortality.
We observed that hyperphosphatemia was significantly associated with increased all-cause and cardiovascular mortality which is consistent with data from prior publications,15,16,18,21-23,25-31 but to our knowledge ours is the first to evaluate the hyperphosphatemia—mortality association across varying age categories. Several studies22,25,26 have reported a significant increase in all-cause and cardiovascular death among MHD patients with serum phosphorus values >5.5 mg/dl which is the upper limit of K/DOQI serum phosphorus targets.47 In addition, increased mortality has been observed in MHD patients with more modest elevations of serum phosphorus.15,27,29 The association between hyperphosphatemia and mortality in dialysis patients may in part be due to vascular calcification. In dialysis patients, there is a potent association between hyperphosphatemia and arterial calcification.10,48 Vascular calcification has been associated with adverse outcomes including ischemic heart disease, congestive heart failure, all-cause and cardiovascular mortality in hemodialysis patients.49-52 The mechanisms by which hyperphosphatemia contributes to the initiation and progression of vascular calcification include (1) transformation of vascular smooth muscle cells (VSMCs) from a contractile to an osteochondrogenic phenotype and mineralization of VSMC matrix through sodium-dependent phosphate co-transporters, (2) induction of VSMC apoptosis, (3) prevention of monocyte/macrophage differentiation into osteoclastic-like cells, (4) escalation of FGF-23 levels, and (5) reduction in klotho expression.53 Hyperphosphatemia has also been shown to contribute to endothelial dysfunction, increased arterial stiffness and cardiac fibrosis.54,55
We found that the low serum phosphorus level was associated with all-cause and cardiovascular mortality in elderly MHD patients ≥65 years old only. Previous clinical studies22,31 have shown that serum phosphorus levels <3.5 mg/dl were associated with increased mortality in MHD patients. Although the underlying mechanism of this association remains unclear, prior studies have shown that hypophosphatemia may be a marker of poor dietary protein intake,56 which is an important predictor of mortality in MHD patients.57,58 Although we accounted for a number of nutritional parameters including serum albumin, creatinine, TIBC, ferritin, nPNA, and BMI in multivariable-adjusted models, we cannot exclude the possibility of residual confounding. Dietary protein intake may have a greater impact on serum phosphorus levels than utilization of phosphorus binders or dialytic clearance of phosphorus particularly in elderly patients. Lorenzo et al56 have reported that the relatively low serum phosphorus concentrations seen in older MHD patients were associated with a spontaneous reduction of protein intake and the relatively low caloric and protein intake is common in elderly MHD patients.59,60 Poor appetite and low protein intake significantly correlated with increased mortality in MHD patients.57,58 In our cohort study, we found that serum phosphorus levels positively correlated with nPNA and serum albumin levels (r=0.26 and 0.19 for nPNA and serum albumin levels, respectively). This may explain why the association between hypophosphatemia and mortality in younger patients observed in unadjusted and case-mix adjusted models was attenuated after further adjustment for MICS surrogates. Our results suggest that a very low serum phosphorus level (<3.5 mg/dl) is a mortality predictor; such a profound hypophosphatemia is unlikely to reflect better phosphorus control, particularly in elderly MHD patients in whom it may reflect inadequate protein intake and its associated death risk. Clinical judgment in this regard could be supported by other nutritional surrogates.
The strengths of this study include its large sample size and generalizability to the US dialysis population. Patient data was obtained from DaVita dialysis facilities which were under uniform standards of medical care. All laboratory values were measured in a single laboratory with optimal quality assurance. We adjusted for a number of confounders such as baseline cardiovascular and other comorbid conditions as well as dialysis vintage in multivariable models. Moreover, we used time-averaged measures with all laboratory data, rather than a single baseline measure in our analyses. Several limitations of this study bear mention. Given that this was an observational study, causal relationships cannot be directly inferred. We did not have information on utilization of MBD treatments (activated vitamin D therapy and phosphorus-binding agents), which may have resulted in residual confounding. We also obtained information on baseline comorbidity status from the Medical Evidence Form 2728, in which comorbid conditions may have been underreported.61 We lacked the data on laboratory markers of inflammation such as C-reactive protein; however, our analyses were adjusted for serum levels of albumin, ferritin, TIBC, WBC and lymphocyte percentage, which are known to be associated with inflammation in dialysis patients.62
Conclusion
In summary, differential associations between serum phosphorus levels with mortality were observed among MHD patients of varying age groups. High serum levels of phosphorus were significantly associated with increased all-cause and cardiovascular mortality across all age groups, whereas low serum phosphorus concentrations were associated with mortality in elderly patients≥65 years old, in whom there was a greater likelihood of hypophosphatemia.
Practical Application
These data suggest that the association between hyperphosphatemia and mortality is similar across all age groups, whereas hypophosphatemia is associated with increased mortality only in elderly MHD patients. Preventing very low serum phosphorus levels in elderly dialysis patients, for instance by liberalizing dietary protein intake, may be associated with better outcomes. Future studies are needed to examine whether the death risk of hypophosphatemia can be mitigated by dietary modifications.
Supplementary Material
Acknowledgements
We thank DaVita Clinical Research® (DCR) for providing the clinical data, analysis and review for this research project and for advancing the knowledge and practice of kidney care.
Funding Source: The study was supported by a research grant from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R01 DK078106, K24 DK091419, a philanthropist grant from Mr. Harold Simmons and a research grant from DaVita Clinical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflict of Interest: KKZ has received honoraria from Genzyme/Sanofi and Shire, manufacturers of phosphorus binders.
References
- 1.Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55(1 Suppl 1):S1–S420. A426–A427. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.Shoji T, Maekawa K, Emoto M, et al. Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis. 2010;210(1):145–149. doi: 10.1016/j.atherosclerosis.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13(7):1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis. 2003;41(6 Suppl 5):11–17. doi: 10.1016/s0272-6386(03)00372-x. [DOI] [PubMed] [Google Scholar]
- 6.Goodman WG. Importance of hyperphosphataemia in the cardio-renal axis. Nephrol Dial Transplant. 2004;19((1)(suppl)):i4–i8. doi: 10.1093/ndt/gfh1001. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89(9):4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi Y, Inaba M, Nakatsuka K, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65(5):1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino M, Brancaccio D, Gallieni M, Slatopolsky E. Pathogenesis of vascular calcification in chronic kidney disease. Kidney Int. 2005;68(2):429–436. doi: 10.1111/j.1523-1755.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 11.Krasniak A, Drozdz M, Pasowicz M, et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol Dial Transplant. 2007;22(2):515–521. doi: 10.1093/ndt/gfl564. [DOI] [PubMed] [Google Scholar]
- 12.Lezaic V, Tirmenstajn-Jankovic B, Bukvic D, et al. Efficacy of hyperphosphatemia control in the progression of chronic renal failure and the prevalence of cardiovascular calcification. Clin Nephrol. 2009;71(1):21–29. doi: 10.5414/cnp71021. [DOI] [PubMed] [Google Scholar]
- 13.Qunibi WY, Nolan CA, Ayus JC. Cardiovascular calcification in patients with end-stage renal disease: a century-old phenomenon. Kidney Int Suppl. 2002;62((82)(suppl)):S73–S80. doi: 10.1046/j.1523-1755.62.s82.15.x. [DOI] [PubMed] [Google Scholar]
- 14.Roman-Garcia P, Carrillo-Lopez N, Fernandez-Martin JL, Naves-Diaz M, Ruiz-Torres MP, Cannata-Andia JB. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010;46(1):121–128. doi: 10.1016/j.bone.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 16.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marco MP, Craver L, Betriu A, Belart M, Fibla J, Fernandez E. Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney Int Suppl. 2003;63((85)(suppl)):S111–S114. doi: 10.1046/j.1523-1755.63.s85.26.x. [DOI] [PubMed] [Google Scholar]
- 19.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 21.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 22.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 24.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70(2):351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 25.Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26(6):1938–1947. doi: 10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 26.Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46(5):925–932. doi: 10.1053/j.ajkd.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Benot A, Martin-Malo A, Alvarez-Lara MA, Rodriguez M, Aljama P. Mild hyperphosphatemia and mortality in hemodialysis patients. Am J Kidney Dis. 2005;46(1):68–77. doi: 10.1053/j.ajkd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16(6):1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 29.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Wald R, Sarnak MJ, Tighiouart H, et al. Disordered mineral metabolism in hemodialysis patients: an analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis. 2008;52(3):531–540. doi: 10.1053/j.ajkd.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67(3):1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 32.Kimata N, Albert JM, Akiba T, et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11(3):340–348. doi: 10.1111/j.1542-4758.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 33.ERA-EDTA Registry 2005 Annual Report. Academic Medical Center, Department of Medical Informatics; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 34.Jager KJ, van Dijk PC, Dekker FW, Stengel B, Simpson K, Briggs JD. The epidemic of aging in renal replacement therapy: an update on elderly patients and their outcomes. Clin Nephrol. 2003;60(5):352–360. doi: 10.5414/cnp60352. [DOI] [PubMed] [Google Scholar]
- 35.Brown EA, Johansson L. Epidemiology and management of end-stage renal disease in the elderly. Nat Rev Nephrol. 2011;7(10):591–598. doi: 10.1038/nrneph.2011.113. [DOI] [PubMed] [Google Scholar]
- 36.Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2011;6(7):1651–1662. doi: 10.2215/CJN.03530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 38.Oliva JS, Roa LM, Lara A, et al. Survival and factors predicting mortality in hemodialysis patients over 75 years old. J Nephrol. 2013;26(1):129–135. doi: 10.5301/jn.5000117. [DOI] [PubMed] [Google Scholar]
- 39.Chauveau P, Combe C, Laville M, et al. Factors influencing survival in hemodialysis patients aged older than 75 years: 2.5-year outcome study. Am J Kidney Dis. 2001;37(5):997–1003. doi: 10.1016/s0272-6386(05)80016-2. [DOI] [PubMed] [Google Scholar]
- 40.Celik G, Oc B, Kara I, Yilmaz M, Yuceaktas A, Apiliogullari S. Comparison of nutritional parameters among adult and elderly hemodialysis patients. Int J Med Sci. 2011;8(7):628–634. doi: 10.7150/ijms.8.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cianciaruso B, Brunori G, Traverso G, et al. Nutritional status in the elderly patient with uraemia. Nephrol Dial Transplant. 1995;10((6)(suppl)):65–68. doi: 10.1093/ndt/10.supp6.65. [DOI] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18(5):1584–1593. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 43.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25(12):2724–2734. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molnar MZ, Streja E, Kovesdy CP, et al. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011;94(3):945–954. doi: 10.3945/ajcn.111.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricks J, Molnar MZ, Kovesdy CP, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58(4):574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streja E, Kovesdy CP, Molnar MZ, et al. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis. 2011;57(6):883–893. doi: 10.1053/j.ajkd.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 48.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 49.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 50.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 51.Okuno S, Ishimura E, Kitatani K, et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49(3):417–425. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Salgueira M, del Toro N, Moreno-Alba R, Jimenez E, Areste N, Palma A. Vascular calcification in the uremic patient: a cardiovascular risk? Kidney Int Suppl. 2003;63((85)(suppl)):S119–S121. doi: 10.1046/j.1523-1755.63.s85.28.x. [DOI] [PubMed] [Google Scholar]
- 53.Kendrick J, Chonchol M. The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis. 2011;58(5):826–834. doi: 10.1053/j.ajkd.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20(7):1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amann K, Tornig J, Kugel B, et al. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int. 2003;63(4):1296–1301. doi: 10.1046/j.1523-1755.2003.00864.x. [DOI] [PubMed] [Google Scholar]
- 56.Lorenzo V, Martin M, Rufino M, et al. Protein intake, control of serum phosphorus, and relatively low levels of parathyroid hormone in elderly hemodialysis patients. Am J Kidney Dis. 2001;37(6):1260–1266. doi: 10.1053/ajkd.2001.24532. [DOI] [PubMed] [Google Scholar]
- 57.Shinaberger CS, Kilpatrick RD, Regidor DL, et al. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis. 2006;48(1):37–49. doi: 10.1053/j.ajkd.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 58.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80(2):299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 59.Movilli E, Mombelloni S, Gaggiotti M, Maiorca R. Effect of age on protein catabolic rate, morbidity, and mortality in uraemic patients with adequate dialysis. Nephrol Dial Transplant. 1993;8(8):735–739. doi: 10.1093/ndt/8.8.735. [DOI] [PubMed] [Google Scholar]
- 60.Lorenzo V, de Bonis E, Rufino M, et al. Caloric rather than protein deficiency predominates in stable chronic haemodialysis patients. Nephrol Dial Transplant. 1995;10(10):1885–1889. [PubMed] [Google Scholar]
- 61.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11(3):520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 62.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.