Abstract
Objective
To evaluate cerebral hemodynamics during dopamine treatment in hypotensive (mean arterial blood pressure [MABP] < gestational age) ELBW infants.
Study Design
Continuous monitoring of cerebral blood flow velocity (CBFv), MABP, and PCO2 was performed in hypotensive ELBW infants on the first day of life, beginning with ~15 min baseline reading and continued during advancing dopamine infusion until MABP was optimized. Physiologic variables and CBFv reactivity were compared before and after MABP was optimized.
Result
Fifteen hypotensive ELBW infants (625 ± 174 g; 24 [23 to 24.8] weeks) were studied. Mean CBFv increased from 10.9 ± 3.7 to 15.7 ± 5.7 cm/s (P=0.001) simultaneously as MABP increased from 22.3 ± 2.8 to 35.2 ± 9.7 mm Hg (P<0.001). Mean CBFv reactivity (95% CI) was 3.9 (1.6 to 6.2) %∙mm Hg−1. Nine infants died and/or developed severe intraventricular hemorrhage (IVH).
Conclusion
Mean CBFv increased pressure-passively as MABP was optimized by dopamine treatment in very sick hypotensive ELBW infants on the first day of life.
Keywords: hypotension, mean arterial blood pressure, intraventricular hemorrhage, dopamine, cerebral autoregulation, extremely low birth weight
Despite the lack of evidence that treating hypotension improves outcomes, ~ 40% of extremely low birth weight (ELBW, birth weight ≤1000 g) infants receive therapy for hypotension during early life.1 Neonatologists treat hypotension with the intent of promoting adequate perfusion to vital organs, especially to the brain. Unfortunately, current hypotensive treatments, by overshooting intended optimal blood pressure, may play a role in the development of adverse associated outcomes such as intraventricular hemorrhage (IVH), periventricular leukomalacia, developmental delay, hearing loss, and death that are usually attributed simply to the presence of hypotension.1–6 Dempsey et al7 reported that untreated hypotensive ELBW infants with signs of good perfusion had similar outcomes to normotensive ELBW infants. Moreover, we previously reported that non-septic normovolemic hypotensive ELBW infants have similar baseline cerebral blood flow velocity (CBFv) compared to matched normotensive controls.8 Thus, not all ELBW infants with hypotension require therapy or even have reduced cerebral perfusion. It is plausible that treatment, by abruptly increasing blood pressure (BP) in hypotensive premature infants with impaired cerebral autoregulation, could pressure-passively increase CBF and lead to IVH.
Dopamine is frequently used for the treatment of hypotension in premature infants.9, 10 Despite its widespread use, there have only been a few studies addressing dopamine’s effects on cerebral hemodynamics in hypotensive ELBW11 or very low birth weight (VLBW, birth weight ≤1500 g) infants.12–17 This prospective observational study was therefore conducted to evaluate alterations of cerebral hemodynamics during the treatment of hypotension with dopamine in hypotensive ELBW infants. We hypothesized that mean CBFv, assessed by Doppler ultrasound, would increase significantly from baseline while optimal BP was reached during dopamine treatment in hypotensive ELBW infants on the first day of life.
Methods
The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board. Infants were enrolled after written informed consent was obtained from parents.
Study Population
ELBW infants born at the University of Arkansas for Medical Sciences between January 2006 and May 2007 were eligible for this study if they received mechanical ventilation for respiratory distress syndrome, had umbilical arterial and venous catheters placed during newborn stabilization, developed hypotension on the first day of life, and were already involved in a physiological study evaluating cerebral autoregulation capacity. Infants with major congenital anomalies, obvious hypovolemia, early-onset sepsis, or air leaks and infants who received fluid boluses, vasopressors, or hydrocortisone (before study procedures) were excluded. Routine intensive care procedures were left to the discretion of the attending neonatologists.
Study Design
When an ELBW infant became hypotensive, at any time (including the middle of the night) of any day (including weekends and holidays), one of two research assistants initiated continuous monitoring of BP, CBFv, and PCO2. The monitoring sessions often lasted 2–3 hrs, beginning with a ~15 min baseline period (before dopamine treatment). The dopamine infusion was begun at 5 µg/kg/min (in D5W at 0.2 ml/hr) and increased stepwise by 2.5 µg/kg/min (0.1 ml/hr) increments (7.5, 10, 12.5, 15, 17.5, and 20) every 20 min until MABP was optimized. The dopamine was always infused with maintenance intravenous fluids through the umbilical venous catheter using specialized infusion pumps (Baxter AS50, Baxter Healthcare Corporation, Deerfield, IL). Other interventions were not typically performed during the monitoring periods.
Monitoring Equipment
Continuous BP monitoring was performed with an umbilical arterial catheter (Diametrics, St. Paul, MN; or Argyle/Tyco Healthcare/Kendall, Mansfield, MA) attached to a BP transducer (Transpac IV, Abbott Critical Care Systems, North Chicago, IL).
CBFv in the right middle cerebral artery was continuously monitored using transcranial Doppler ultrasound (Nicolet Biomedical Pioneer, Madison, WI). A lightweight 2-MHz pulsed-wave button transducer was placed transtemporally, anterior to the ear and above the zygomatic arch, and held in place by an appropriately sized crocheted hat (courtesy of the Arkansas Homemakers Extension Council). A depth of 16–22 mm was used to study the proximal portion of the middle cerebral artery.18–20 A 100-Hz low-pass filter was used to dampen “noise” from the vessel wall. To obtain reliable CBFv measurements, the transducer was placed with the minimum angle of insonation, accomplished when the highest intensity acoustic signal was perceived and the highest intensity Doppler spectrum was visualized. The ultrasound intensity was 5–21 mW/cm2. CBFv tracings were consistent with minimal to no drift in signal intensity, as the transducer did not move or need replacement during the monitoring periods.
Continuous PCO2 monitoring was performed with a Neotrend system (Diametrics Medical Ltd., St. Paul, MN) or a transcutaneous blood gas monitor (MicroGas 7650 rapid, Radiometer, Westlake, OH). Neotrend was a system that continuously monitored arterial blood gases and temperatures, using fiber optic technology, without the need for blood withdrawal; it is no longer being produced. Briefly, for transcutaneous PCO2 monitoring, the skin on either side of the chest was prepped with a small amount of Aquaphor Emollient Ointment (Beiersdorf, Norwalk, CT). A double-sided adhesive ring of tape was applied to the skin, and one drop of an electrolyte solution (Radiometer) was placed into the center of the adhesive ring. The probe was then affixed to the adhesive ring. After 5 min of continuous monitoring (by either system), an arterial blood gas measurement was obtained from the umbilical arterial catheter and processed by the NICU laboratory. The Neotrend and/or transcutaneous blood gas monitor was then calibrated with the PCO2 laboratory result (the change was rarely >3 mm Hg).
Analog signals from the BP monitor (112 Hz), transcranial Doppler (100 Hz), and the blood gas monitors (1 Hz) were simultaneously collected with a data acquisition system (PowerLab 8 channel, ADInstruments, Mountain View, CA). Cyclic waveform analyses were performed to calculate MABP (average amplitude of the BP waveform over one cycle) and systolic and diastolic BPs (maximum and minimum values of the BP cycle, respectively). Fast Fourier analysis was performed on the CBFv signal to determine the systolic, diastolic, and mean CBF velocities.
Data Collection and Definitions
For each infant, time and date of birth, whether or not their mother received antenatal steroids, birth weight, gestational age, race, gender, Apgar scores at 1 and 5 min, presence of severe IVH, survival to hospital discharge, and postnatal age at the time of physiological monitoring was obtained from the research database. Gestational age was estimated based on obstetrical criteria and neonatal criteria; the neonatal estimate was used if the two estimates differed by >2 weeks. Severe (grades III and IV) IVH was determined using the staging criteria of Papile et al,21 based on any cranial ultrasound during the hospitalization.
Hypotension was defined as MABP in mm Hg < gestational age in weeks22 for ≥ 30 min. Optimal BP was defined as MABP in mm Hg ≥15% above gestational age in weeks15 for ≥30 min. CBFv reactivity was used to determine cerebral autoregulatory capacity during dopamine treatment. Briefly, CBFv reactivity is expressed as the percent change of CBFv that varies per mm Hg change in MABP during some time period.14, 23 When the 95% confidence interval (CI) for mean CBFv reactivity includes 0, i.e., no statistically significant change in CBFv compared to changes in MABP, intact cerebral autoregulation is implied.14, 23 In contrast, if the mean percent change in CBFv/mm Hg change in MABP >0, and the 95% CI does not include 0, then this implies impaired cerebral autoregulation.14, 23 Resistive index (RI) was defined as: ([systolic CBFv-diastolic CBFv]/systolic CBFv)·100.24
Statistics
Physiological variables for the hypotensive infants were analyzed before and after treatment with dopamine (Table II). Baseline values for MABP, mean CBFv, PCO2, and RI were determined from ~15 min of continuous monitoring that preceded treatment with dopamine. After optimal MABP was reached, MABP, mean CBFv, PCO2, and RI were again determined. Comparisons of physiological variables before and after dopamine were performed by paired t-test.
Table 2.
Physiological data before and after optimal MABP
| Study Group | Before dopamine | After optimal MABP | P |
|---|---|---|---|
| MABP (mm Hg) | 22.3 ± 2.8 | 35.2 ± 9.7 | < 0.001 |
| Mean CBFv (cm/s) | 10.9 ± 3.7 | 15.7 ± 5.7 | 0.001 |
| PCO2 (mm Hg) | 48.9 ± 12.1 | 44.0 ± 9.2 | 0.189 |
| RI (%) | 74.8 ± 11.3 | 59.7 ± 7.4 | 0.0001 |
Mean ± SD
CBF reactivity was determined for each infant by comparing baseline and optimal MABP values after dopamine for mean CBFv and MABP, respectively, and then determined for the group of hypotensive infants.
Results
Fifteen hypotensive ELBW infants (625 ± 174 g, 24 (23 to 24.8 weeks) were studied. Nine infants died and/or developed severe IVH. Eight infants died (5 had severe IVH; 3 died before having a cranial ultrasound). One infant with a severe IVH survived. Other characteristics of the study population are shown in Table I. There were significant increases in MABP after treatment with dopamine (P<0.001), which was expected (Table 2).
Table I.
Patient Characteristics
| Characteristics | (n =15) |
|---|---|
| Birth weight (g)* | 625 ± 174 |
| Gestational age (weeks)† | 24 (23–24.8) |
| Black (n) | 5 |
| Female (n) | 8 |
| Postnatal age at study (hrs)† | 6 (4.1–8.3) |
| Apgar 1 min † | 3 (2–4) |
| Apgar 5 min † | 5 (4–6) |
| First arterial pH* | 7.26 ± 0.16 |
| Severe IVH (n) | 6 |
| Death (n) | 8 |
| Antenatal steroids (n) | 11 |
Mean ± SD,
Median (25th–75th percentile)
With the exception of one infant who died secondary to complications from unremitting bilateral pneumothoraces, all other infants responded to dopamine with an increase in MABP. None of the infants received fluid boluses, other vasopressors, or hydrocortisone before dopamine treatment. The increased MABP was accompanied by significant increases in mean CBFv (10.9 ± 3.7 at baseline vs 15.7 ± 5.7 cm/s after optimal MABP was reached; P=0.001). PCO2 was not different before and after dopamine treatment. Dopamine-treated infants also displayed significant decreases in RI after optimal MABP was reached, from 74.4% to 59.7% (P=0.001). The mean CBF reactivity (95% CI) was 3.9 (1.6 to 6.2) %∙mm Hg−1.
The median (25th, 75th percentile) dose of dopamine was 10 (7.5, 14.4) µg/kg/min to get to the optimal MABP. The dose of dopamine to obtain the optimal MABP was 5 (n = 1), 7.5 (n = 4), 10 (n = 3), 12.5 (n = 2), 15 (n = 2), and 17.5 µg/kg/min (n = 2), respectively. One infant did not reach optimal MABP, and did not have dopamine increased beyond 5 µg/kg/min.
An example of the effects of dopamine treatment on mean CBFv, PaCO2, and MABP from a hypotensive premature infant is shown in the Figure. Dopamine treatment (5 µg/kg/min) was initiated 20 min after beginning monitoring. MABP and mean CBFv suddenly and simultaneously surged soon after dopamine administration, possibly due to the infant’s response to dopamine once the drug traversed through the medication tubing reaching the infant. MABP and mean CBFv subsequently returned to baseline, and dopamine was increased to 7.5 µg/kg/min (at about 40 min). The dopamine was increased to 10 µg/kg/min (at 66 min), with a resultant smaller surge in MABP that was again mirrored by a simultaneous surge in CBFv. MABP then returned to the hypotensive baseline, and dopamine was increased to 12.5 µg/kg/min (at 83 min). At this point, there was an abrupt, prolonged increase in MABP, reaching hypertensive levels; this was mirrored by increases in mean CBFv above the 90th percentile.25 Due to the hypertension, dopamine was reduced to 10 µg/kg/min. During the study period, PaCO2 varied slightly between 48 and 53 mm Hg.
Figure.
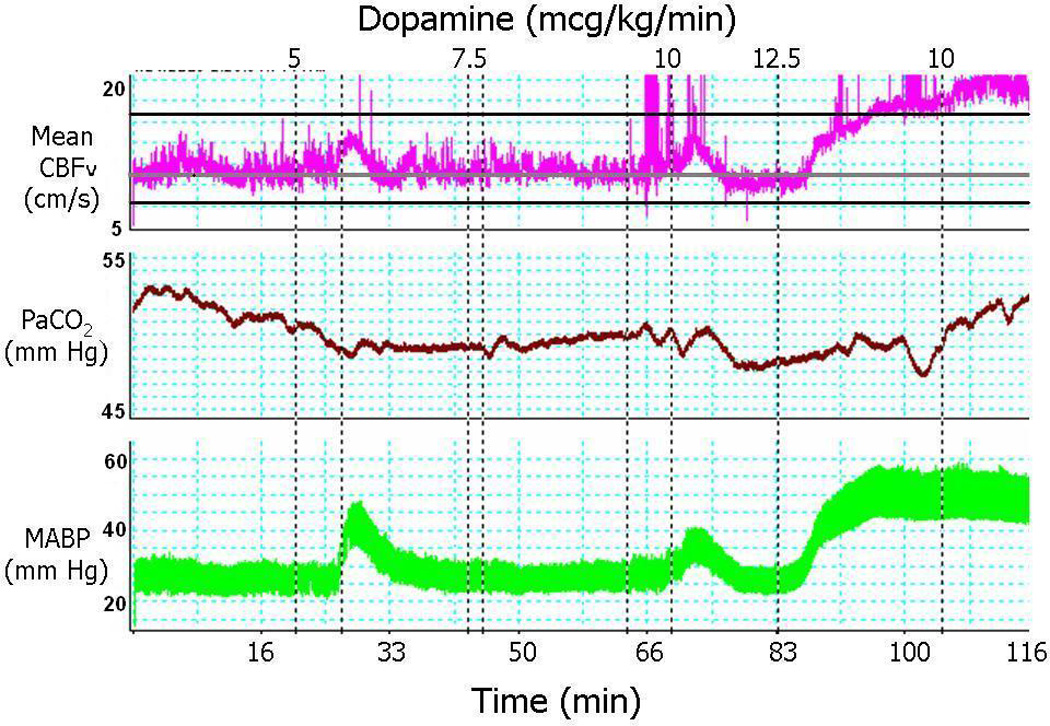
Example of the effects of dopamine on MABP, PCO2, and mean CBFv in a hypotensive ELBW infant.
Discussion
The major aim of our study was to examine cerebral hemodynamics during dopamine infusion in hypotensive ventilated ELBW infants on the first day of life. We observed a simultaneous significant increase in mean CBFv as MABP increased to an optimal level during dopamine infusion. The increase in mean CBFv could be because the cerebral vasculature remained pressure-passive, or that the increased MABP was above the upper limit of autoregulation, or alternatively because dopamine could have changed cerebral vessel diameter. Mean CBF reactivity for these infants was consistent with ~2 to 4%∙mm Hg−1 previously reported for hypotensive VLBW infants14, 26 and in premature infants with severe intracranial hemorrhage.23 Since the 95% CI for mean CBF reactivity did not include 0, we inferred that these ELBW infants had a pressure-passive cerebral circulation and impaired cerebral autoregulation during dopamine infusion. Although determination of vascular resistance from changes in RIs are controversial, significant decreases in RI were noted after optimal MABP was reached; our findings are consistent with previous studies.12, 16 Thus, hypotensive ELBW infants who have their BP optimized during dopamine treatment likely have impaired cerebral autoregulation and increased CBF.12, 24, 27
In all, 9 infants died and/or developed severe IVH. We recognize that the rates of mortality and morbidity observed in our study are quite high. Perhaps these hypotensive infants represented a sicker population of ELBW premature infants who were inherently at higher risk for IVH and death. Treated hypotension has previously been reported to be associated with poor outcomes, however, it is not clear whether hypotension, the treatment for hypotension, or some other unrecognized variables were to blame for the adverse outcomes.1, 4
Our observations are consistent with previous reports showing that increasing MABP in hypotensive premature infants does not restore intact cerebral autoregulation.11, 12, 14–16 In the first comparable study of dopamine’s effects on cerebral hemodynamics in 5 hypotensive premature infants on the first day of life (6 weeks more mature and more than double the birth weight of our infants), CBFv increased but returned to baseline by 50–90 min after MABP was increased with dopamine.12 Thus, it was concluded that while cerebral autoregulation was impaired during dopamine treatment, it was not totally ineffective. The next study included a heterogeneous sample of 11 hypotensive infants (birth weight 540–2100 g and gestational age 24–32 weeks) monitored at 13 hrs of age.14 They concluded that dopamine treatment for hypotension did not restore intact autoregulation; however, since treatment included fluid boluses, dopamine, and dobutamine, it is unclear exactly how the results relate directly to dopamine’s effect on cerebral hemodynamics. In a subsequent study using higher initial and maximum dopamine doses than ours, it was observed that 12 hypotensive ELBW infants on the first day of life had impaired cerebral autoregulation because CBF correlated with MABP before and after dopamine (10–30 µg/kg/min) treatment.11 Since cerebral autoregulation remained impaired after increasing MABP with dopamine into an adequate range, the authors concluded that careful physiological monitoring was necessary during dopamine infusions.11 In a later study, 28 hypotensive VLBW infants treated with dopamine to an optimal MABP had changes in cerebral intravascular oxygenation (HbD, reflecting cerebral perfusion changes) that were significantly correlated with increases in MABP, i.e., they had impaired cerebral autoregulation.15 Lastly, it was observed in 17 hypotensive premature infants (<32 weeks gestation) with patent ductus arteriosus on day of life 5 (range 1–25 days) that RI decreased significantly after dopamine treatment.16 Alternatively, one dopamine-cerebral hemodynamic study, however, failed to find a simultaneous increase in CBFv in hypotensive VLBW infants when MABP was raised to a normal range.13 The discrepancy between this study13 and the others11, 12, 14–16 is unclear, but could be due to the use of intermittent CBFv monitoring where an increase could have been missed.
There are some possible limitations to our study. First, this is an observational study evaluating CBFv in hypotensive ELBW infants during treatment using dopamine, with a somewhat small sample size. While it is quite difficult to accrue and enroll sufficient numbers of hypotensive ELBW infants into studies,28 as shown by the similar low sample sizes in other studies (that mostly included more mature and heavier infants),11–15 we evaluated cerebral hemodynamics during dopamine therapy in the largest number of hypotensive ELBW infants on the first day of life ever studied. In addition, we used transcranial Doppler ultrasound measurements of CBFv instead of more direct measures of CBF. Despite concerns that CBFv measurements may not be a reliable proxy for CBF due to possible vessel diameter changes, good correlations have been observed between relative changes of CBFv and near-infrared spectroscopy measures of cerebral hemodynamics.29–31 Thirdly, the first-line treatment for non-hypovolemic and non-septic hypotensive ELBW infants in our neonatal intensive care unit (NICU) was dopamine infusions beginning at 5 µg/kg/min and incrementally increasing the dose by 2.5 µg/kg/min to get to an optimal MABP. Many clinicians, however, use fluid boluses first, and thus our observations may not be generalizable to other NICUs. It is also possible that our stepwise doses of dopamine were too high and may have contributed to excessively high CBFv in some infants. It may be prudent, for example, to start dopamine at 5 µg/kg/min and increase by 1 µg/kg/min increments to prevent overshooting of CBFv. Further, while it would have been illuminating to determine autoregulatory capacity during hypotension (prior to receiving dopamine), individual MABP varied little during baseline periods making the calculation of CBFv reactivity unfeasible. We also did not assess whether there was a patent ductus arteriosus, or the degree and direction of blood flow through the patent ductus arteriosus, or how a patent ductus arteriosus would affect RI. Bouissou et al16 observed that infants with a significant PDA had a similar decrease in cerebral arterial RI following dopamine therapy, and presumably, most ELBW infants have a patent ductus arteriosus on the first day.32 A final limitation is that we did not evaluate the effects of hypotension on other organs, such as the kidneys or heart. While hemodynamics to other organs will be important for future studies, we focused on the brain because disturbances of CBFv have been associated with brain injuries in premature infants.33
In conclusion, in hypotensive ELBW infants on the first day of life with baseline gestational age-appropriate CBFv24 and RI, CBFv increased pressure-passively as MABP was optimized during treatment with dopamine. Some infants demonstrated abrupt increases in CBFv to >90th percentile during dopamine infusion. It is plausible that increasing BP in this manner with the use of dopamine in ELBW infants could potentially place them at risk for brain injury. Close monitoring and careful titration of dopamine infusions is essential. Further research is needed to evaluate the appropriate indications for the use of dopamine in hypotensive ELBW infants, and investigations comparing permissive hypotension vs treated hypotension in ELBW infants are warranted.
Acknowledgements
This research was presented in part at the Pediatric Academic Society, Society for Pediatric Research meeting in Vancouver, British Columbia, Canada, May 2010. Dr. Lightburn was supported by the University of Arkansas for Medical Sciences Children’s University Medical Group. Dr Kaiser was supported by the National Institutes of Health (1K23NS43185, RR20146, and 1R01NS060674) and the University of Arkansas for Medical Sciences Translational Research Institute (1UL1RR029884).
The technical assistance of Natalie C. Sikes and Melanie J. Mason, and the support of the University of Arkansas for Medical Sciences neonatologists, NICU nurses, respiratory therapists, and ultrasound technicians, are gratefully appreciated.
Abbreviations
- ELBW
extremely low birth weight
- IVH
intraventricular hemorrhage
- CBFv
cerebral blood flow velocity
- MABP
mean arterial blood pressure
- RI
resistive index
- NICU
neonatal intensive care unit
- CI
confidence interval
- VLBW
very low birth weight
Footnotes
Conflict of interest
The authors declare no potential, perceived, or real conflicts of interest. The authors also declare there are no competing financial interests
References
- 1.Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117:1131–1135. doi: 10.1542/peds.2005-1230. [DOI] [PubMed] [Google Scholar]
- 2.Kuint J, Barak M, Morag I, Maayan-Metzger A. Early treated hypotension and outcome in very low birth weight infants. Neonatology. 2009;95:311–316. doi: 10.1159/000180113. [DOI] [PubMed] [Google Scholar]
- 3.Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19:103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 4.Martens SE, Rijken M, Stoelhorst GMSJ, van Zwieten PHT, Zwinderman AH, Wit JM, et al. Is hypotension a major risk factor for neurological morbidity at term age in very preterm infants? Early Hum Dev. 2003;75:79–89. doi: 10.1016/j.earlhumdev.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Barrington KJ, Dempsey EM. Cardiovascular support in the preterm: Treatments in search of indications. J Pediatr. 2006;148:289–291. doi: 10.1016/j.jpeds.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 6.Batton B, Zhu X, Fanaroff J, Kirchner HL, Berlin S, Wilson-Costello D, et al. Blood pressure, anti-hypotensive therapy, and neurodevelopment in extremely preterm infants. J Pediatr. 2009;154:351–357. doi: 10.1016/j.jpeds.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birth weight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94:F241–F244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 8.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr. 2009;154:824–828. doi: 10.1016/j.jpeds.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seri I. Cardiovascular, renal, and endocrine actions of dopamine in neonates and children. J Pediatr. 1995;126:333–344. doi: 10.1016/s0022-3476(95)70445-0. [DOI] [PubMed] [Google Scholar]
- 10.Sassano-Higgins S, Friedlich P, Seri I. A meta-analysis of dopamine use in hypotensive preterm infants: blood pressure and cerebral hemodynamics. J Perinatol. 2011;31:647–655. doi: 10.1038/jp.2011.2. [DOI] [PubMed] [Google Scholar]
- 11.Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;114:1591–1596. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 12.Seri I, Rudas G, Bors Z, Kanyicska B, Tulassay T. Effects of low-dose dopamine infusion on cardiovascular and renal functions, cerebral blood flow, and plasma catecholamine levels in sick preterm neonates. Pediatr Res. 1993;34:742–749. doi: 10.1203/00006450-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Penny DJ, Kim NS, Yu VYH, Smolich JJ. Mechanisms of blood pressure increase induced by dopamine in hypotensive preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81:F99–F104. doi: 10.1136/fn.81.2.f99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasinghe D, Gill AB, Levene MI. CBF reactivity in hypotensive and normotensive preterm infants. Pediatr Res. 2003;54:848–853. doi: 10.1203/01.PDR.0000088071.30873.DA. [DOI] [PubMed] [Google Scholar]
- 15.Pellicer A, Valverde E, Elorza MD, Madero R, Gayá F, Quero J, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: A randomized, blinded, clinical trial. Pediatrics. 2005;115:1501–1512. doi: 10.1542/peds.2004-1396. [DOI] [PubMed] [Google Scholar]
- 16.Bouissou A, Rakza T, Klosowski S, Tourneux P, Vanderborght M, Storme L. Hypotension in preterm infants with significant patent ductus arteriosus: Effects of dopamine. J Pediatr. 2008;153:790–794. doi: 10.1016/j.jpeds.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Wong F, Barfield C, Horne R, Walker A. Dopamine therapy promotes cerebral flow-metabolism coupling in preterm infants. Intensive Care Med. 2009;35:1777–1782. doi: 10.1007/s00134-009-1602-5. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very low birth weight infants. J Pediatr. 2004;144:809–814. doi: 10.1016/j.jpeds.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58:931–935. doi: 10.1203/01.pdr.0000182180.80645.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser JR, Gauss CH, Williams DK. Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J Perinatol. 2008;28:34–41. doi: 10.1038/sj.jp.7211848. [DOI] [PubMed] [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 22.Joint Working Group of the British Association of Perinatal Medicine and the Research Unit of the Royal College of Physicians. Development of audit measures and guidelines for good practice in the management of neonatal respiratory distress syndrome. Arch Dis Child. 1992;67:1221–1227. doi: 10.1136/adc.67.10_spec_no.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J Pediatr. 1989;115:638–645. doi: 10.1016/s0022-3476(89)80301-4. [DOI] [PubMed] [Google Scholar]
- 24.Bada HS, Hajjar W, Chua C, Sumner DS. Noninvasive diagnosis of neonatal asphyxia and intraventricular hemorrhage by Doppler ultrasound. Pediatrics. 1979;5:775–779. doi: 10.1016/s0022-3476(79)80735-0. [DOI] [PubMed] [Google Scholar]
- 25.Romagnoli C, Giannantonio C, De Carolis MP, Gallini F, Zecca E, Papacci P. Neonatal color Doppler US study: Normal values of cerebral blood flow velocities in preterm infants in the first month of life. Ultrasound Med Biol. 2006;32:321–331. doi: 10.1016/j.ultrasmedbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Jorch G, Jorch N. Failure of autoregulation of cerebral blood flow in neonates studied by pulsed Doppler ultrasound of the internal carotid artery. Eur J Pediatr. 1987;146:468–472. doi: 10.1007/BF00441596. [DOI] [PubMed] [Google Scholar]
- 27.Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979;94:118–121. doi: 10.1016/s0022-3476(79)80373-x. [DOI] [PubMed] [Google Scholar]
- 28.Batton BJ, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Feasibility study of early blood pressure management in extremely preterm infants. J Pediatr. 2012;161:65–69.e61. doi: 10.1016/j.jpeds.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 30.Pellicer A, Valverde E, Gayá F, Quero J, Cabañas F. Postnatal adaptation of brain circulation in preterm infants. Pediatr Neurol. 2001;24:103–109. doi: 10.1016/s0887-8994(00)00239-3. [DOI] [PubMed] [Google Scholar]
- 31.Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr. 1997;131:549–554. doi: 10.1016/s0022-3476(97)70060-x. [DOI] [PubMed] [Google Scholar]
- 32.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–1121. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]
- 33.Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 1983;309:204–209. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]


