Abstract
Background
Purinergic signaling provides regulation of colonic motility. Smooth muscle cells (SMC), interstitial cells of Cajal (ICC) and platelet-derived growth factor receptor α–positive (PDGFRα+) cells are electrically coupled and form a functional (SIP) syncytium that constitutes the receptive field for motor neurotransmitters in the tunica muscularis. Each cell type in the SIP syncytium has specialized functions in mediating motor neurotransmission. We compared gene transcripts for purinergic receptors and membrane-bound enzymes for purine degradation expressed by each cell type of the SIP syncytium.
Methods
Fluorescence-activated cell sorting (FACS) was used to purify SMC, ICC and PDGFRα+ cells from mixed cell populations of colonic muscles dispersed from reporter strains of mice with constitutive expression of green fluorescent proteins. Differential expression of functional groups of genes related to purinergic signaling was determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Key Results
We detected marked phenotypic differences between SMC, ICC and PDGFRα+ cells. Substantial numbers of genes of importance in purinergic neurotransmission were enriched in PDGFRα+ cells in relation to SMC and ICC. Notably, genes related to mediating effects and extracellular biotransformation of enteric purinergic inhibitory neurotransmitters were strongly expressed by PDGFRα+ cells.
Conclusions & Inferences
Our results demonstrate differential expression of genes for proteins involved in purinergic signaling in the SIP syncytium. These results may further clarify the specific functions of each cell type, identify novel biomarkers for postjunctional cells and provide hypotheses for further studies to understand the physiological roles of cells of the SIP syncytium.
Keywords: purine genes, interstitial cells, PDGFRα, colon, ATP, NAD
INTRODUCTION
Extracellular purine, pyridine and pyrimidine substances (also referred to as extracellular nucleotides and nucleosides) are known to elicit cell surface receptor-mediated signals in mammalian cells and to function as neurotransmitters, neuromodulators and autocrine and paracrine mediators1–4. In the distal gastrointestinal (GI) tract, including the colon, purinergic signaling regulates excitability and motility, and adenosine 5′-triphosphate (ATP), nicotinamide adenine dinucleotide (NAD+) and adenosine 5′-diphosphate ribose (ADPR) have been proposed to be neurotransmitters5–9. Extracellular nucleotides are rapidly metabolized by cell surface enzymes to biologically active and inactive metabolites10. In some cases a substance can be produced from multiple extracellular substrates. For example, adenosine 5′-monophosphate (AMP) and adenosine can be formed by multiple-step enzymatic degradation of either ATP, NAD+ or ADPR8,10. The biological effects of extracellular nucleotides and their metabolites are mediated by P1 and P2 purine receptors on effector cells, that are selectively activated by adenosine (P1 receptors) or adenine and pyrimidine nucleotides (P2 receptors). Human diseases of the GI tract display distinct purine gene dysregulation profiles11, and such changes can result in functional effects but also provide novel pathways for diagnostics and possible therapeutics.
Smooth muscle cells (SMC) are traditionally considered to be the primary effectors in neuromuscular transmission in visceral smooth muscles12. In the GI tract however, SMC do not appear to be exclusive or even suitable targets for neurogenic purines, because these compounds elicit predominantly small inward currents in GI SMC7. This response contrasts with the P2Y1 receptor-mediated activation of robust outward K+ currents and hyperpolarization in intact colonic muscles in response to purine neurotransmitter(s). The outward current is generated by activation of small-conductance Ca2+-activated K+ channels (SK3) and possibly additional K+ conductances5,7,13. Recently, a new class of interstitial cells, immunoreactive for platelet-derived growth factor receptor-α (PDGFRα+ cells), have been shown to respond to extracellular purines by binding of P2Y1 receptors and activation of SK3 currents14.
Morphological studies show that varicose enteric nerve fibers are closely associated with PDGFRα+ cells and interstitial cells of Cajal (ICC), and both classes of interstitial cells form gap-junctions with SMC15,16. Myogenic regulation of motility and post-junctional neurogenic responses result from the integrated activity of the SMC/ICC/PDGFRα+ cell (SIP) syncytium17. However, a detailed description of the role of each cell type of the SIP syncytium in extracellular purinergic signaling is not yet available. The topology of the various purinergic components at the cell surface not only organizes the signal transduction machinery, but also controls the final cellular response. One way to discover the potential cellular targets of extracellular nucleotides is to compare cell-specific expression profiles of genes or proteins involved in signaling and metabolism. In the present study we have isolated cells from strains of mice with cell-specific expression of fluorescent reporters in SMC, ICC, and PDGFRα+ cells. The cells were purified by fluorescence activated cell sorting (FACS), and comparisons of the purine effectorsomes (i.e. P1 and P2 purinergic receptors and membrane-bound (ecto-) enzymes that degrade extracellular nucleotides) were made using quantitative PCR.
MATERIALS AND METHODS
Mice and tissue preparation
Transgenic mice with green fluorescence proteins (eGFP or copGFP) tagged to cell-specific promoters (e.g. smMHC for SMC, Kit for ICC and PDGFRα for PDGFRα+ cells) were used to isolate each cell type and determine purine gene expression. C57BL/6 mice and Pdgfratm11(EGFP)Sor/J heterozygote mice, 3–6 weeks of age (both from Jackson Laboratory, Bar Harbor, ME, USA), smMHC/Cre/eGFP mice, 3–6 weeks of age (donated by Dr. Michael Kotlikoff, Cornell University), and Kit+/copGFP, 3–6 weeks of age18 were anaesthetized with isoflurane (AErrane; Baxter, Deerfield, IL, USA) and killed by cervical dislocation. After longitudinal laparotomy the colons were removed and opened along the mesentery and rinsed free of content with ice-cold Krebs-Ringer solution (mM): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4. The mucosa and submucosa were peeled away and cells were isolated from tunica muscularis of the entire colon. Mice were maintained and experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Use and Care Committee (IUCUC) at the University of Nevada.
Tissue dispersion and cell purification
Colonic muscles (25±5 mg tissue weight) were equilibrated in Ca2+-free Hanks’ solution and cells were dispersed as described previously19,18. eGFP-PDGFRα cells, eGFP-SMC, and CopGFP-ICC were purified by fluorescence-activated cell sorting (FACS) (Becton Dickinson FACSAriaII) using the blue laser (488 nm) and the GFP emission detector (530/30 nm). Expression of genes in each sorted cell type was compared against expression in the total cell population (TCP) of colonic tunica muscularis. TCP represents all cells dispersed from tunica muscularis. Each experiment with sorted cells was performed with colonic muscles from three mice of each reporter strain. TCP were prepared from 6 mice (2 for each cell specific reporter strain).
RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from PDGFRα+ cells, SMC, and ICC using illustra RNAspin Mini RNA Isolation kit (GE Healthcare, Little Chalfont, UK). Concentration and purity of RNA was measured using a ND-1000 Nanodrop Spectrophotometer (Nanodrop, Wilmington, DE), comparative amounts of RNA were used for first-strand cDNA synthesized using SuperScript III (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. PCR was performed with specific primers (Table 1) using Go-Taq Green Master Mix (Promega Corp., Madison, WI, USA). PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. qRT-PCR was performed with the same primers as PCR using Fast Sybr green chemistry (Applied Biosystems, Foster City, CA, USA) on the 7900HT Real Time PCR System (Applied Biosystems).
Table 1.
Primer sequences used for qRT-PCR [Gene Name, Primer Sequences, Product length (bp), Accession Number]
| Primer Name | Primer Sequence | Expected Size | Accession # |
|---|---|---|---|
| Gapdh-F | GCCGATGCCCCCATGTTTGTGA | 178 bp | NM_008084 |
| Gapdh-R | GGGTGGCAGTGATGGCATGGAC | ||
| Pdgfra-F | ATGACAGCAGGCAGGGCTTCAACG | 195 bp | NM_011058 |
| Pdgfra-R | CGGCACAGGTCACCACGATCGTTT | ||
| Kit-F | CGCCTGCCGAAATGTATGACG | 162 bp | NM_021099 |
| Kit-R | GGTTCTCTGGGTTGGGGTTGC | ||
| Myh11-F | CAGCTGGAAGAGGCAGAGGAGG | 198 bp | NM_013607 |
| Myh11-R | AACAAATGAAGCCTCGTTTCCTCTC | ||
| Uchl1-F | CGATGGAGATTAACCCCGAGATG | 169 bp | NM_011670 |
| Uchl1-R | TTTTCATGCTGGGCCGTGAG | ||
| Adora1-F | AACATTGGGCCACAGACCTA | 144 bp | NM_001008533 |
| Adora1-R | TGTCTTGTACCGGAGAGGGA | ||
| Adora2a-F | CCATTCGCCATCACCATCAG | 165 bp | NM_009630 |
| Adora2a-R | CAAGCCATTGTACCGGAGTG | ||
| Adora2b-F | CCCTTTGCCATCACCATCAG | 159 bp | NM_007413 |
| Adora2b-R | TTTATACCTGAGCGGGACGC | ||
| Adora3-F | GATGCACTTCTATGCCTGCC | 139 bp | NM_009631 |
| Adora3-R | AGTGGTAACCGTTCTATATCTGACT | ||
| P2ry1-F | ACCGAGGTGCCTTGGTCGGT | 140 bp | NM_008772 |
| P2ry1-R | CCGGTCTTGGTCAGGGCACA | ||
| P2ry2-F | AGCACCATCAATGGCACCTGGGA | 132 bp | NM_008773 |
| P2ry2-R | CACGACGTTCAGGCACAACCC | ||
| P2ry4-F | GGCCCTCAATGCCCCAACCC | 169 bp | NM_020621 |
| P2ry4-R | GCCAGTGCCAAAGGGCCAGT | ||
| P2ry6-F | ACCGCACTGTGTGCTACGAC | 136 bp | NM_183168 |
| P2ry6-R | CGGCGGGCCATGCGACAATA | ||
| P2ry12-F | GGCTGACGTCACTGAACGCCTG | 162 bp | NM_027571 |
| P2ry12-R | TCTCTTCGCTTGGTTCGCCACC | ||
| P2ry13-F | CAACACCACTGGGATGCAGGGCT | 157 bp | NM_028808 |
| P2ry13-R | GCTGGGGATGTGGACGAACACC | ||
| P2ry14-F | ACCCTCCAAACCAGCCCTGC | 197 bp | NM_133200 |
| P2ry14-R | AGGCCCATGAGAAAGTCAGCCA | ||
| P2rx1-F | CCACGCTTCAAGGTCAACAGGC | 180 bp | NM_008771 |
| P2rx1-R | GATACCAACCACCCCGCCCTTC | ||
| P2rx2-F | TTGTGCATGGACAGGCAGGGAAA | 185 bp | NM_153400 |
| P2rx2-R | ACTTGAGGGGTGCCTTGGGGT | ||
| P2rx3-F | CTTCCGTGGGAGTGGGGACTGTT | 186 bp | NM_145526 |
| P2rx3-R | GCCCCCGAGTCTGTGGACTGC | ||
| P2rx4-F | TGACGCTGGTGTGCCAACGC | 176 bp | NM_011026 |
| P2rx4-R | GGCAGAAGGGATCCGTCCGC | ||
| P2rx5-F | TCCGTCTCAGGGGGAGAACGT | 138 bp | NM_033321 |
| P2rx5-R | TCCCCAGCGTGACAGTCGGT | ||
| P2rx6-F | GAGAGTGGTGCTGTGCCCAGGAAG | 188 bp | NM_011028 |
| P2rx6-R | CGGAACACTGGGCAGTACGGA | ||
| P2rx7-F | CAGACTACACCTTCCCTTTGCAG | 179 bp | NM_011027 |
| P2rx7-R | GCCAGTCTGGATTCCTTTGCTC | ||
| Cd38-F | CAAGAACCCTTGCAACATCACAAGAG | 147 bp | NM_007646 |
| Cd38-R | GTGAACATCTTTCCCTGGATCCAAG | ||
| Bst1-F | GAGATCTGGGTTATGCATGACGTTG | 127 bp | NM_009763 |
| Bst1-R | GTCGGTAGTCATTGATACAGCTGTG | ||
| Entpd1-F | GTACCTGAGTGAGTACTGCTTCTC | 168 bp | NM_009848 |
| Entpd1-R | GCTGGGATCATGTTGGTCAAGTTC | ||
| Nt5e-F | AACGGTGGAGATGGATTCCAGATG | 151 bp | NM_011851 |
| Nt5e-R | GACTTGCTGCAGAGAACTTGATCC | ||
| Enpp1-F | AAATCTTTCTTGCCGGGAGC | 110 bp | NM_008813 |
| Enpp1-R | AGAAGGTCAGGGGCTCAATC | ||
| Enpp2-F | TTTGGTCTGTGAGCATCCCT | 179 bp | NM_015744 |
| Enpp2-R | CGGAGTAAGAGGTGAGCCAT | ||
| Enpp3-F | GCACTACAGAATACGCCTGG | 183 bp | NM_134005 |
| Enpp3-R | CATAGCTTTGCCGTACCCAC | ||
| Art1-F | AGGGATGGAGTTCAGGTTCAA | 181 bp | NM_009710 |
| Art1-R | TCCGCGTATACTTTGTTGGC | ||
| Art2.1-F | ATACTCATGAAGAGGAGGTGTT | 188 bp | NM_007490 |
| Art2.1-R | ACAGCTCTCTCTAGATCCTAAGC | ||
| Art2.2-F | CCTCGTGAAGAGGAGGTGTT | 189 bp | NM_019915 |
| Art2.2-R | GCTCTCTCTGGATCCTGATATACTG | ||
| Art3-F | ATTCCTCGGGGGTCTGAAAA | 142 bp | NM_181728 |
| Art3-R | TTCTGGCATTCTGTCAGGGT | ||
| Art4-F | CACTGAGGCTCCTCTTAAGGT | 184 bp | NM_026639 |
| Art4-R | CTGGTTCAGCCAGGTTAGGT | ||
| Art5-F | TTTTGGGGCTCCTATCCAGG | 180bp | NM_007491 |
| Art5-R | TTCTCCCCACCCAAATAGGC | ||
| Kcnn1-F | GTTGTTGGTCTTCAGCGTCTCCTC | 150 bp | NM_032397 |
| Kcnn1-R | GTCTCCATAGCCAATGGACAAGAAG | ||
| Kcnn2-F | ACTATCTGCCCAGGAACTGTGCTC | 128 bp | NM_080465 |
| Kcnn2-R | CATTGCTCCAAGGAAGTTGCTAGTG | ||
| Kcnn3-F | CTGCTGGTGTTCACCATCTCTCTG | 138 bp | NM_080466 |
| Kcnn3-R | GTCCCCATAGCCAATGGAAAGGAAC | ||
| Kcnn4-F | AAGATGCTGGCCGCCATCCACA | 157 bp | NM_008433 |
| Kcnn4-R | TCTTCTCCAGGGCACGGTGCGA |
Statistical analyses
Gene expression was compared between eGFP-SMC, eGFP-PDGFRα+and CopGFP-ICC, and each cell type was also compared with TCP of colonic tunica muscularis of corresponding control animals. Regression analysis of the mean values of three multiplex qPCRs for the log10 diluted cDNA was used to generate standard curves. Unknown amounts of messenger RNA (mRNA) were plotted relative to the standard curve for each set of primers and graphically plotted using Microsoft Excel. Primer efficiencies of 90–110% were only accepted for analysis. This gave transcriptional quantification of each gene relative to the endogenous Gapdh standard after log transformation of the corresponding raw data. In pilot studies Gapdh was tested on all three cell types used in the present study and represents an appropriate control for qPCR analyses. All data were expressed as means ± S.E.M. Student’s t-test was used where appropriate to evaluate differences in the data, and P < 0.05 taken to indicate statistically significant differences.
RESULTS
1. Cell markers in sorted SMC, PDGFRα+ cells and ICC
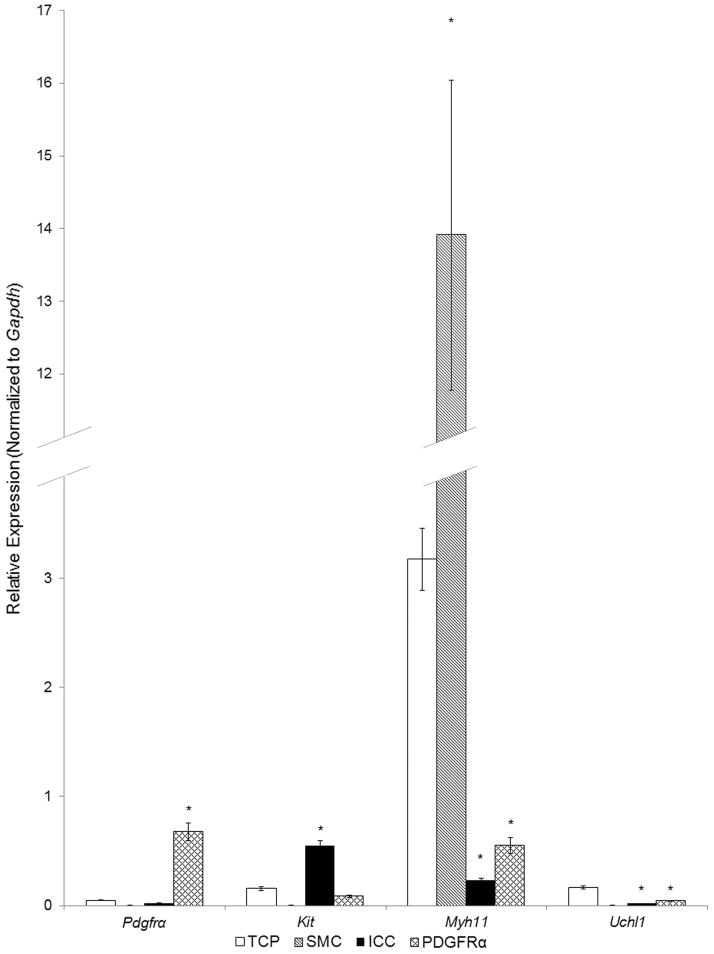
The qRT-PCR analyses demonstrated that the FACS-sorted cells were highly enriched with cell specific markers: Kit, Pdgfra and Myh11 were enriched in sorted CopGFP-ICC, eGFP-PDGFRα+ cells, and eGFP-SMC, respectively. The Kit-enriched cells had negligible expression of either Pdgfra or Myh11 whereas the Pdgfra-enriched cells had negligible expression of either Kit or Myh11. Likewise, the Myh11-enriched cells showed negligible expression of either Pdgfra or Kit. Each purified cell population had negligible expression of Uchl1, the gene encoding PGP9.5, indicating that the cell populations examined were relatively free of neurons. These data demonstrate the relative purity of the cell populations analyzed by qRT-PCR (Fig. 1).
Fig. 1. Expression of cell type markers in SMC, ICC, and PDGFRα+ cells of the murine colon by qRT-PCR analysis.
The expression of cell type markers Pdgfra (PDGFRα+ cells), Myh11 (SMC), Kit (ICC) were compared with the expression of these genes in total cell population (TCP) of dispersed tunica muscularis, in which isolation of mRNA was performed without cell sorting (n=6). Sorted SMC (n=3) were minimally positive for Pdgfra and Kit, but demonstrated strong enrichment in transcripts of Myh11. Sorted PDGFRα+ cells (n=3) were minimally positive for Myh11 and Kit, but were enriched with Pdgfra. Sorted ICC (n=3) were minimally positive for Myh11 and Pdgfra, but enriched with transcripts of Kit. After sorting, all three populations of purified cells displayed negligible transcripts of UChl1, the gene encoding the neural protein PGP9.5. The results were normalized to expression of the housekeeping gene Gapdh. Asterisks indicate P<0.05 when compared to the transcript isolated from TCP.
2. Purinergic Receptors
2.1. P1 Receptors
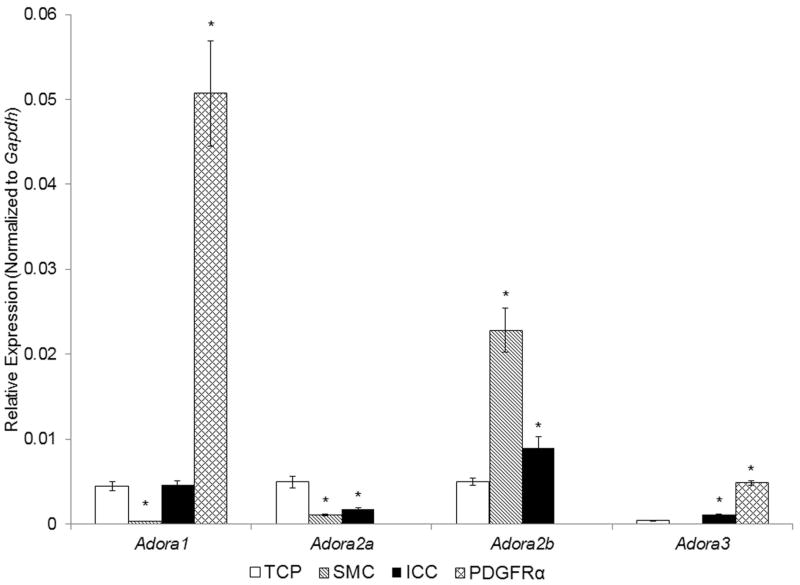
Among genes encoding the four types of adenosine receptors (P1 receptors) PDGFRα+ cells were enriched with Adora1 and Adora3 and transcripts for Adora2a and Adora2b were not resolved (Fig. 2). Expression of Adora1 and Adora3 was stronger in PDGFRα+ cells than in SMC, ICC or TCP. In contrast, Adora2b was expressed more in SMC than in ICC or in TCP. Adora2a was modestly expressed in SMC and ICC but less than in TCP, suggesting that this receptor is mainly expressed on neurons or other non-SIP cells.
Fig. 2. Expression of genes for P1 receptors in SMC, ICC, and PDGFRα+ cells of the murine colon by qRT-PCR analysis.
Note that SMC and ICC were enriched with Adora2b, whereas PDGFRα+ cells were enriched with Adora1 and Adora3. TCP, total cell population (n=6), Sorted SMC, ICC and PDGFRα+ cells (each n=3). Asterisks indicate P<0.05 when compared to the transcript isolated from TCP.
2.2. P2 Purinergic Receptors
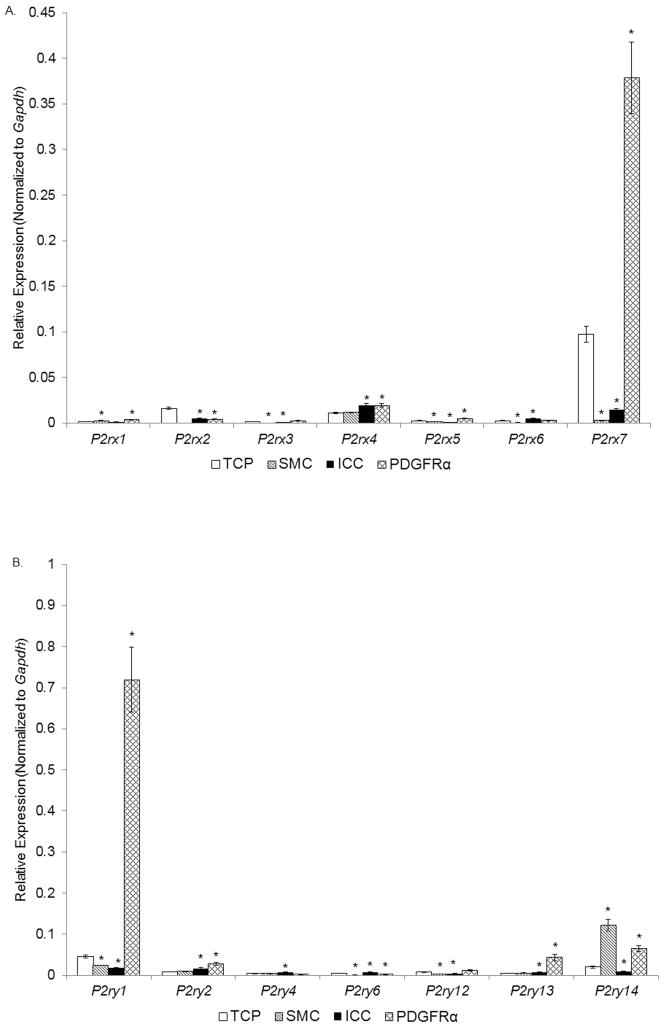
All P2X receptor genes were expressed more highly in the PDGFRα cells than SMC. PDGFRα+ cells were enriched (i.e. showed greater expression than in the TCP) with P2rx1, P2rx3, P2rx4, P2rx5, P2rx6, and particularly with P2rx7 (Fig. 3A, Table 2). Therefore, PDGFRα+ cells might be a target for extracellular ATP acting on ionotropic P2X receptors. ICC expressed P2rx4 and P2rx6 more than the TCP. ICC also expressed a low level of P2rx7 that was significantly less than in PDGFRα+ cells or the TCP. Among the genes for P2Y receptors SMC were enriched with P2yr14 (Fig. 3B, Table 2). The gene for P2yr14 was also enriched in PDGFRα+ cells, but the expression was lower than in SMC. PDGFRα cells showed higher expression of P2ry1, P2ry2, P2ry12, and P2ry13, than TCP or SMC. ICC were enriched with P2ry2, P2ry4, and P2ry6 suggesting that these cells might be targeted by extracellular pyrimidine substances rather than purines.
Fig. 3. Expression of genes for P2X receptors (panel A) and P2Y receptors (panel B) in SMC, ICC, and PDGFRα+ cells of the murine colon by qRT-PCR analysis.
Note that PDGFRα+ cells are enriched with P2rx7 and P2rx4 as well as P2ry1, P2ry2, P2ry12, P2ry14, and P2ry14, whereas SMC were enriched with P2ry14. ICC showed strong expression of P2rx4, P2ry2, P2ry4, and P2ry6. TCP, total cell population (n=6), Sorted SMC, ICC and PDGFRα+ cells (each n=3). Asterisks indicate P<0.05 when compared to the transcript isolated from TCP.
Table 2.
Genes for P2 purinergic receptors: fold differences for the comparison of expression in smooth muscle cells (SMC, n=3), interstitial cells of Cajal (ICC, n=3) and PDGFRα+ cells (n=3) vs. total cell population (TCP, n=6).
| Gene Symbol | Gene Name | Fold Difference SMC vs TCP | P-Value | Fold Difference ICC vs TCP | P-Value | Fold Difference PDGFRα+ vs TCP | P-Value |
|---|---|---|---|---|---|---|---|
| P2rx1 | purinergic receptor P2X, ligand-gated ion channel, 1 | 1.45 | 0.0059 | −1.16 | 0.5143 | 2 | 0.0316 |
| P2rx2 | purinergic receptor P2X, ligand-gated ion channel, 2 | 0 | 0.0000 | −3.25 | 0.0012 | −3.94 | 0.0003 |
| P2rx3 | purinergic receptor P2X, ligand-gated ion channel, 3 | −55.8 | 0.0055 | −3.66 | 0.0158 | 1.53 | 0.0844 |
| P2rx4 | purinergic receptor P2X, ligand-gated ion channel, 4 | 1.05 | 0.7840 | 1.79 | 0.0035 | 1.77 | 0.0342 |
| P2rx5 | purinergic receptor P2X, ligand-gated ion channel, 5 | −1.91 | 0.0258 | −5.07 | 0.0038 | 1.85 | 0.0249 |
| P2rx6 | purinergic receptor P2X, ligand-gated ion channel, 6 | −5.09 | 0.0008 | 2.07 | 0.0009 | 1.1 | 0.3311 |
| P2rx7 | purinergic receptor P2X, ligand-gated ion channel, 7 | −33.61 | 0.0079 | −6.78 | 0.0124 | 3.88 | 0.0008 |
| P2ry1 | purinergic receptor P2Y, G-protein coupled 1 | −1.87 | 0.0087 | −2.51 | 0.0035 | 15.98 | 0.0000 |
| P2ry2 2 | purinergic receptor P2Y, G-protein coupled | 1.08 | 0.5929 | 1.94 | 0.0110 | 3.3 | 0.0033 |
| P2ry4 | purinergic receptor P2Y, G-protein coupled 4 | 1.02 | 0.9396 | 1.99 | 0.0167 | −1.67 | 0.1645 |
| P2ry6 | purinergic receptor P2Y, G-protein coupled 6 | −4.02 | 0.0000 | 1.53 | 0.0159 | −2.22 | 0.0002 |
| P2ry12 | purinergic receptor P2Y, G-protein coupled 12 | −2.48 | 0.0015 | −1.62 | 0.0076 | 1.53 | 0.1043 |
| P2ry13 | purinergic receptor P2Y, G-protein coupled 13 | 1.07 | 0.5779 | 1.5 | 0.0083 | 8.78 | 0.0004 |
| P2ry14 | purinergic receptor P2Y, G-protein coupled 14 | 6.01 | 0.0009 | −2.24 | 0.0107 | 3.19 | 0.0004 |
3. Cell surface nucleotide-metabolizing enzymes
Degradation of extracellular ATP and NAD+ is accomplished in multiple steps by several enzymes (Fig. 4). Therefore, we next sought to determine the relative expression in SIP syncytium of key enzymes involved in extracellular purine biotransformation.
Fig. 4.
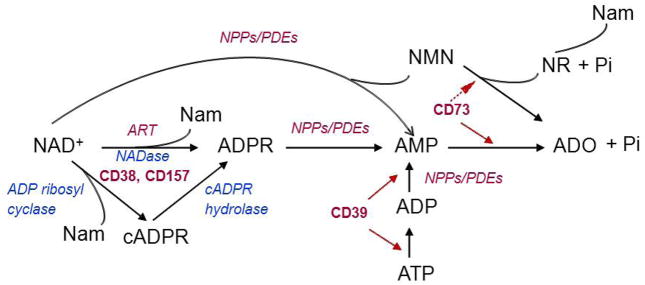
Biotransformation pathways for extracellular purines.
3.1. CD39/Ecto-nucleoside triphosphate diphosphohydrolase 1 (Entpd1) and CD73/ecto-5′-nucleotidase (Nt5e)
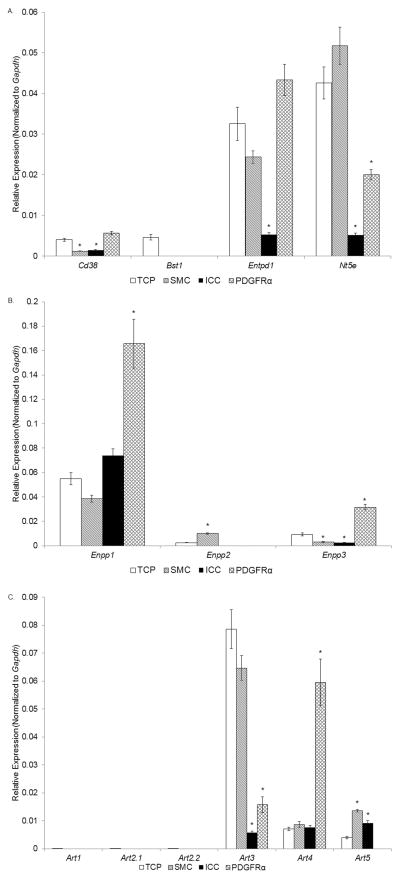
Entpd1, the gene that encodes CD39, was expressed more in PDGFRα cells than in SMC or ICC (Fig. 5A). In fact, Entpd1 expression in PDGFRα cells appeared higher than in TCP, but this did not reach statistical significance. The expression of Entpd1 in SMC was less than in the TCP. ICC showed negligible expression of Entpd1. Therefore, PDGFRα+ cells appear to possess the machinery necessary for catabolism of extracellular ATP. We also found that SMC are enriched with Nt5e, the gene that encodes the ecto-5′ nucleotidase CD73, whereas PDGFRα+ cells and ICC expressed much less Nt5e (Fig. 5A). Therefore, adenosine generated by extracellular nucleotides that are autocrine and paracrine mediators in the vicinity of the SIP syncytium may have more rapid and possibly greater effects on SMC than on other cells.
Fig. 5. Expression of genes for ecto-nucleotidases (panel A), ecto-nucleotide pyrophosphatases/phosphodiasterases (panel B), and mono-ADP ribosyl transferases (panle C) in SMC, ICC, and PDGFRα+ cells of the murine colon by qRT-PCR analysis.
Note that PDGFRα+ cells show relatively higher expression of Cd38 and Entpd1 and low expression of Nt5e. SMC showed relatively strong expression of Entpd1. Bst1 was resolved in neither cell type. TCP, total cell population (n=6), Sorted SMC, ICC and PDGFRα+ cells (each n=3). Asterisks indicate P<0.05 when compared to the transcript isolated from TCP.
3.2. NAD glycohydrolases CD38 and CD157/Bst1
Colonic tunica muscularis express Cd38 and Cd157/Bst1 since both genes were found expressed in TCP (Fig. 5A). None of the cells comprising the SIP syncytium expressed levels of Cd157/Bst1. However, Cd38 was expressed in all three cell types: interestingly, Cd38 showed stronger expression in PDGFRα+ cells than in the TCP, whereas Cd38 expression in SMC or ICC was less than in TCP. This might be an important observation considering the neurotransmitter role of NAD+, reported recently, and the observation that PDGFRα+ cells express the proper machinery required for mediating purinergic motor neurotransmission in the colon5,7,9. SMC or ICC, on the other hand, do not appear to express significant amounts of Cd38, suggesting that CD38-mediated catabolism of NAD+ does not primarily occur at the SMC or ICC cell membranes.
3.3. Ecto-nucleotide pyrophosphatase/phosphodiasterases (Enpp)
PDGFRα+ cells highly expressed Ennp1 and Enpp3 whereas Enpp2 was not resolved in these cells (Fig. 5B). In contrast, SMC showed strong expression of Enpp2 and modest expression (less than TCP) of Enpp1 and Enpp3 (Fig. 5B). ICC were somewhat enriched with Enpp1 (but much less than the PDGFRα+ cells), expressed negligible amounts of Enpp3, and expressed unresolvable levels of Enpp2 (Fig. 5B).
3.4. Mono-ADP ribosyl transferases (ARTs, Art1-5)
Here we demonstrate that the TCP of the muscularis expresses no Art1, Art2.1 and Art2.2, but Art3, Art4, and Art5 transcripts were resolved (Fig. 5C). PDGFRα+ cells showed strong expression of Art4, low expression of Art3, and unresolvable expression of other Art genes. SMC and ICC showed stronger expression of Art5 than the TCP. Art3 expression was greater in SMC than in PDGFRα+ cells and ICC. Therefore, Art4 appears to be characteristic for PDGFRα+ cells, whereas Art5 is characteristic for SMC and ICC.
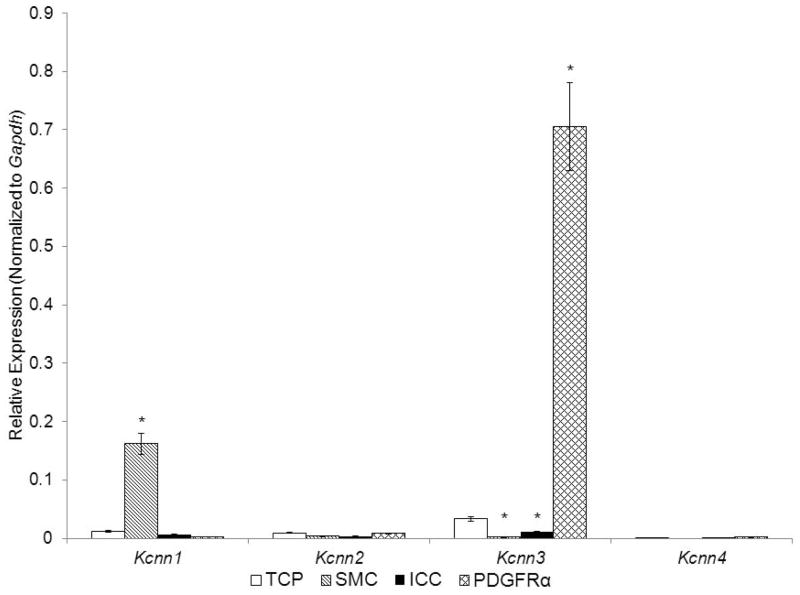
4. Small-conductance Ca2+-activated K+ channels (SK channels, Kcnn1-3)
Consistent with previous reports14 we confirmed that Kcnn3 is strongly enriched in PDGFRα+ cells vs. the TCP and, importantly, the expression of Kcnn3 in these cells far exceeded the expression of Kcnn3 in either SMC or ICC. Other members of the Kcnn family of genes were expressed at very low levels in cells of the SIP syncytium. Therefore, PDGFRα+ cells are distinguished as the cells in the SIP syncytium that have an enriched transcriptional and protein expression of Kcnn314, 20. Kcnn2 and Kcnn4 showed relatively low expression in all SIP cells (Fig. 6), and Kcnn1 (which encodes SK1 channels that are less sensitive to apamin than SK2 and SK3) was more highly expressed in SMC than in other SIP cells.
Fig. 6. Expression of genes for small-conductance Ca2+-activated K+ channels in SMC, ICC, and PDGFRα+ cells of the murine colon by qRT-PCR analysis.
Note that PDGFRα+ cells demonstrate strong expression of Kcnn3, whereas SMC show strong expression of Kcnn1. TCP, total cell population (n=6), Sorted SMC, ICC and PDGFRα+ cells (each n=3). Asterisks indicate P<0.05 when compared to the transcript isolated from TCP.
DISCUSSION
In this study we compared relative levels of gene transcripts that encode proteins involved in extracellular purinergic signaling in the 3 cell types of the SIP syncytium in murine colonic tunica muscularis. Each cell type displayed specific profiles of gene transcripts, suggesting cell-specific functions within the SIP syncytium in terms of mediating the effects of purinergic neurotransmitters and paracrine mediators and in achieving deactivation of these signals.
Adora1,2a,2b,3
In the GI tract, adenosine is involved in the regulation of gut sensory and secretomotor functions21, in suppression of enteric nervous system excitability22,23, and in immune and inflammatory responses11,22–24. Thus, knowing the preferential distribution of adenosine receptor genes in the functional SIP syncytium might facilitate identification of novel therapeutic targets for adenosine receptor agonists and antagonists. SMC were enriched with Adora2b, whereas PDGFRα+ cells were enriched with Adora1 and Adora3, but lacked Adora2a or Adora2b. Adora2a was expressed in colon muscularis, but apparently in cell types different from those of the SIP syncytium. Therefore, we demonstrate here the molecular basis for differential effects of adenosine on cells in the SIP syncytium, and these will need further characterization by physiological experiments.
P2rx1-7 and P2ry1,2,4,6,12-14
P2X1-7 receptors are ligand-gated ion channels that are activated by ATP25, whereas P2Y receptors are G-protein coupled receptors that are activated by adenine, pyridine and pyrimidine nucleotides26. NAD+ can activate P2X7 receptor through mono-ADP-ribosylation on the cell membrane27. NAD+5,7 and ADPR8,28,29 are also ligands for the P2Y1 receptor. The latter findings may be particularly important for the functions of the GI tract because the effects of the enteric purinergic inhibitory neurotransmitter(s) appear to be mediated exclusively by P2Y1 receptors (coupled to inhibitory responses mainly through SK3 channels) and NAD+ and ADPR, but not ATP, mimic the enteric inhibitory purinergic neurotransmitter5,7,8,13,29. Therefore, the observation that P2ry1 was far more highly expressed by PDGFRα+ cells than by SMC, ICC or TCP is of importance for the integrated response of the SIP syncytium to purinergic neural regulation. The present study supports the notion that SMC is not the primary target for the purinergic inhibitory motor neurotransmitter in the colon. In fact, our data confirm previous findings14,30,31 that PDGFRα+ cells have the molecular machinery (i.e., P2Y1 receptors and SK3 channels) to receive and transduce neurotransmitters released from enteric inhibitory nerve terminals. ICC may mediate effects of extracellular UTP and UDP and derivatives since ICC demonstrated expression of P2ry2, P2ry4 and P2ry6. PDGFRα+ cells also expressed more P2ry2 and P2ry4 than SMC. SMC and PDGFRα+ cells might be targets for UDP-sugars26 as the two cell types expressed P2ry14.
Entpd1 and Nt5e
Extracellular nucleotide metabolism can regulate ligand concentrations at receptor sites, limit membrane receptor desensitization, terminate receptor activation by agonists, or generate intermediate products with similar or different signaling properties than the released nucleotide32. ENTPDase1 (CD39) is the primary ecto-nucleotidase involved in the degradation of extracellular ATP. It is anchored to cell membranes32 and this restricts the effectiveness of this enzyme to cell-surface localized catalysis. Genetic polymorphisms of CD39 have been linked to Crohn’s disease33 and CD39 gene deletion in mice exacerbates dextran sodium sulphate-induced colitis34. In the present study we found that Entpd1 was expressed more in PDGFRα +cells than in SMC or ICC or TCP, suggesting that ATP added to or released in muscles can result in production of ADP and AMP at the surface of PDGFRα +cells.
Ecto-5′-nucleotidase (CD73/Nt5e) is a membrane-bound glycoprotein that catalyzes the extracellular dephosphorylation of AMP to adenosine35,36. CD73-mediated hydrolysis of AMP to adenosine is a step in the degradation not only of ATP (via formation of ADP and AMP), but also of NAD+ (via formation of ADPR and AMP)32. Human CD73 is also an orthologue of a bacterial enzyme that recognizes NAD+ and nicotinamide mononucleotide (NMN) as substrates37. The physiological relevance of these catalytic activities of CD73 in the GI tract needs further investigation. The acute phase response in Crohn’s disease and ulcerative colitis is associated with Nt5e gene dysregulation in mucosal biopsies11 but the expression of this gene in muscle layers has not been determined. Nt5e was more highly expressed by SMC than by ICC or PDGFRα+ cells, and therefore, adenosine would be formed primarily near SMC receptors.
Cd38 and Bst1/Cd157
CD38 is a type II transmembrane protein that degrades NAD+ to cyclic ADP-ribose via its ADP-ribosyl cyclase activity and NAD+ to ADPR and nicotinamide via its NAD+ hydrolase activity38. Its role in the colon has not been determined. In colonic muscles isolated from CD38−/− mice we found that the effects of the endogenous purine neurotransmitter and the degradation of NAD+, were intact8, suggesting that either other enzymes that degrade NAD+ were overexpressed in CD38−/−mice, or other enzymes are normally responsible for degradation of NAD+. CD157 is the only other enzyme in mammals that shares homology with CD38 and NAD-glycohydrolase and cyclase activities. In the present study, expression of Bst1/Cd157 was not resolved in any of the cells in the SIP syncytium, although expression of this gene was noted in the TCP. However, Cd38 was expressed in all three cell types. It is important to note that PDGFRα+ cells showed the strongest expression of Cd38, suggesting that the first steps in the degradation of NAD+ that is released from motor nerve terminals may occur at PDGFRα+ cells.
Enpp1-3
Three of the seven members of the ecto-nucleotide pyrophosphatase/phosphodiasterase (E-NPP) family -ENPP1, ENPP2, and ENPP3 – are known to hydrolyze nucleotides39. NPPs hydrolize ATP to ADP and AMP as well as ADP to AMP39. NPPs also degrade NAD+ to AMP and NMN as well as ADPR to AMP32. All substrates for NPPs are present within the interstitium of colonic muscles5,7,8, but the role of NPPs in degradation of these nucleotides in the GI tract is unknown. We found that PDGFRα+ cells highly expressed Enpp1 and Enpp3 whereas SMC highly expressed Enpp2. This is an interesting distinction, because NPP1 and NPP3 are type II membrane proteins with a large extracellular domain, whereas NPP2 is a secreted protein and exists only in soluble form and is synthesized as a pre-pro-enzyme32. Further studies are warranted to understand the functional relevance of such distinction in the SIP syncytium in the colon.
Art1-5
Mammalian ARTs constitute a family of structurally-related proteins that transfer ADP-ribose from NAD+ to target proteins. Therefore, ART activity in the extracellular compartment provides sophisticated regulatory mechanisms for cell communication40. In mice, ADP-ribosylation by ART2.1 and ART2.2 causes activation of the P2X7 receptor on cell surface41. ART3 and ART4 show deviations from the consensus motif of arginine-specific ARTs, and enzyme activity has not been demonstrated for either of these proteins42. ART3 and ART4 may have acquired novel substrate specificity, or they may have lost enzyme activity and acquired new function(s). ART5 is predicted to be a secretory enzyme43. We demonstrate here that expression of Art1, Art2.1 and Art2.2 could not be resolved in murine colon muscularis. PDGFRα+ cells highly expressed Art4, whereas Art5 was abundant in SMC and ICC. The functional relevance of Art expression in the SIP syncytium remains to be determined.
Kcnn1-3
Responses to purinergic inhibitory neurotransmitter(s) in the large intestine are mediated specifically by P2Y1 receptors5,7,13,29 and activation of K+ conductances that are sensitive to apamin5,7,44. Four closely related genes, Kcnn1-4, encode SK and IK channels. SK2 and SK3 channels are very sensitive to apamin45 and may, therefore, mediate part of the inhibitory response to the enteric purinergic neurotransmitter. Previous studies reported SK3 channel immunoreactivity in Kit-negative fibroblast-like cells30 and Kcnn3 expression in PDGFRα+ cells14. PDGFRα+ cells were shown to have the ultrastructure of cells referred to as ‘fibroblast-like’31. In our studies PDGFRα+ cells were highly enriched with transcripts of Kcnn3, whereas Kcnn1 was most highly expressed in SMC. The expression of Kcnn3 far exceeded the expression of any other Kcnn family genes in the SIP syncytium. Therefore, these observations support the notion that PDGFRα+ cells are a primary effector in purinergic neurotransmission.
While this study provides the most comprehensive investigation of the purinergic ‘effectorsome’ in colonic muscles, it is incomplete in fully describing the molecular apparatus responsible for activation and inactivation of purine motor neurotransmission. This is because we have not yet quantified cell-specific protein expression resulting from gene translation, and tests have not been performed to determine whether the proteins encoded by expressed genes are functional in the SIP syncytium. We also know little about the proteins expressed by motor nerve terminals, glia, or immune cells that might also participate in purinergic signaling, generation of active metabolites, or deactivation of signals. Even if motor neurons could be purified, we have no definitive method for determining the extent to which genes expressed encode proteins trafficked to nerve terminals. Immunohistochemistry can be a useful method for determining cellular localization of proteins, but it is notoriously inadequate as a means of quantifying protein expression and absence of labeling can signify poor immunoreactivity as well as lack of expression. Full understanding of the molecular and functional milieu of the SIP syncytium must await more definitive assays of functional proteins in situ.
Summary
The three cell types composing the SIP syncytium in the murine colon (i.e. SMC, ICC and PDGFRα+ cells) display different expression profiles of key genes involved in purine signaling. Therefore, each cell type may have very specific roles in mediating and terminating purinergic signals. SMC demonstrate strong expression of Adora2b, Art5, Enpp2, Nt5e and P2ry14, modest expression of P2rx1, Art3, Art4, and lack Adora1, Adora3, Art1, Art2.1, Art2.2, and Cd157/Bst1. PDGFRα+ cells demonstrate strong expression of Adora1, Adora3, Art4, Enpp1, Enpp3, P2rx7, P2rx1, P2rx4, P2rx5, P2ry1, P2ry2, P2ry12, P2ry13, and P2ry14, modest expression of P2rx3, P2rx6, Cd38 and Entpd1and lack Adora2a, Adora2b, Art1, Art2.1, Art2.2, Art5, Enpp2, and Cd157/Bst1. ICC show strong expression of P2ry2, P2ry4, P2ry6, Art5 and modest expression of Adora2b and P2rx4. These data can be used to examine targets of specific purine effects, to examine (patho)physiological consequences of extracellular purines on cells of the SIP syncytium, and identify cells of the SIP syncytium.
Key Messages.
The present study demonstrates that smooth muscle cells (SMC), interstitial cells of Cajal (ICC) and platelet-derived growth factor receptor α positive (PDGFRα+) cells (SIP syncytium) in the tunica muscularis demonstrate differential expression of genes related to purinergic signaling, suggesting cell-specific functions within the SIP syncytium in mediating enteric purinergic signals.
Motility of the colon is regulated by purinergic neurotransmitters and paracrine mediators that may target specific cell types. We compared gene transcripts for purinergic receptors and membrane-bound enzymes for purine degradation expressed by each cell type of the SIP syncytium.
SMC, ICC and PDGFRα+ cells were isolated from colonic muscles of reporter strains of mice with cell-specific expression of green florescent proteins and were purified by fluorescence-activated cell sorting. Expression of genes was evaluated and compared by quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Our results demonstrate marked phenotypic differences between SMC, ICC and PDGFRα+ cells. Notably, genes related to mediating effects and biotransformation of enteric purinergic inhibitory neurotransmitters were highly expressed by PDGFRα+ cells.
Acknowledgments
The authors would like to acknowledge the technical assistance of Jared Townsend and Nancy Horowitz in tissue dissection, cell dispersion and cell sorting.
FUNDING
This work was supported by a NIH grant PO1 DK41315.
Abbreviations
- ADP
adenosine 5′-diphosphate
- ADPR
adenosine 5′-diphosphate ribose
- AMP
adenosine 5′-monophosphate
- ATP
adenosine 5′-triphosphate
- GI
gastrointestinal
- GFP
green fluorescence protein
- ICC
interstitial cells of Cajal
- NAD+
nicotinamide adenine dinucleotide
- NADP
nicotinamide adenine dinucleotide phosphate
- NAADP
nicotinic acid adenine dinucleotide phosphate
- PDGFR
platelet-derived growth factor receptor
- SK
small-conductance K+ channel
- SMC
smooth muscle cells
- qRT-PCR
quantitative polymerase chain reaction
- RNA
ribonucleic acid
- RT-PCR
reverse transcription-polymerase chain reaction
- TCP
total cell population
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-diphosphate
Footnotes
DISCLOSURE
No competing interests declared.
AUTHOR CONTRIBUTION
LP performed the qRT-PCR analysis, analyzed results and participated in drafting the paper. KMS and VMY designed the experiments, interpreted results and wrote the paper. All work was performed in the Department of Physiology and Cell Biology of the University of Nevada School of Medicine.
Reference List
- 1.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87 :659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 2.Durnin L, Dai Y, Aiba I, Shuttleworth CW, Yamboliev IA, Mutafova-Yambolieva VN. Release, neuronal effects and removal of extracellular beta-nicotinamide adenine dinucleotide (beta-NAD(+) ) in the rat brain. Eur J Neurosci. 2012;35:423–435. doi: 10.1111/j.1460-9568.2011.07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts JA, Lukewich MK, Sharkey KA, Furness JB, Mawe GM, Lomax AE. The roles of purinergic signaling during gastrointestinal inflammation. Curr Opin Pharmacol. 2012;12:659–666. doi: 10.1016/j.coph.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. PNAS. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20 (Suppl 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. beta-Nicotinamide Adenine Dinucleotide Is an Enteric Inhibitory Neurotransmitter in Human and Nonhuman Primate Colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5′-diphosphate-ribose (ADPR) is a neural regulator in primate and murine large intestine along with beta-NAD+ J Physiol. 2012;590(Pt8):1921–1941. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of beta-NAD(+) and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol Motil. 2013;25(3):e194–e204. doi: 10.1111/nmo.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 11.Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis. 2009;15 :971–984. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- 13.Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 14.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair PJ, Bayguinov Y, Sanders KM, Ward SM. Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol Motil. 2012;24:e437–e449. doi: 10.1111/j.1365-2982.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 17.Koh SD, Ward SM, Sanders KM. Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol Motil. 2012;24:705–718. doi: 10.1111/j.1365-2982.2012.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca(2+)-activated Cl(−) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh SD, Dick GM, Sanders KM. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol Cell Physiol. 1997;273:C2010–C2021. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- 20.Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397–1404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–236. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del TM, Blandizzi C. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233–253. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Ren T, Grants I, Alhaj M, McKiernan M, Jacobson M, Hassanain HH, Frankel W, Wunderlich J, Christofi FL. Impact of disrupting adenosine A(3) receptors (A(3)(-)/(-) AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2011;17:1698–1713. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christofi FL. Unlocking mysteries of gut sensory transmission: is adenosine the key? News Physiol Sci. 2001;16:201–207. doi: 10.1152/physiologyonline.2001.16.5.201. [DOI] [PubMed] [Google Scholar]
- 25.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 26.Harden TK, Sesma JI, Fricks IP, Lazarowski ER. Signalling and pharmacological properties of the P2Y receptor. Acta Physiol (Oxf) 2010;199:149–160. doi: 10.1111/j.1748-1716.2010.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/s1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson AJ, Muraro L, Dahlberg C, Migaud M, Chevallier O, Khanh HN, Krishnan K, Li N, Islam MS. ADP ribose is an endogenous ligand for the purinergic P2Y1 receptor. Mol Cell Endocrinol. 2011;333:8–19. doi: 10.1016/j.mce.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanderwinden JM, Rumessen JJ, de Kerchove dA, Jr, Gillard K, Panthier JJ, de Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–358. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- 31.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman DJ, Kunzli BM, Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunzli BM, Berberat PO, Dwyer K, Deaglio S, Csizmadia E, Cowan P, d’Apice A, Moore G, Enjyoji K, Friess H, Robson SC. Variable impact of CD39 in experimental murine colitis. Dig Dis Sci. 2011;56:1393–1403. doi: 10.1007/s10620-010-1425-9. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garavaglia S, Bruzzone S, Cassani C, Canella L, Allegrone G, Sturla L, Mannino E, Millo E, De FA, Rizzi M. The high-resolution crystal structure of periplasmic Haemophilus influenzae NAD nucleotidase reveals a novel enzymatic function of human CD73 related to NAD metabolism. Biochem J. 2012;441:131–141. doi: 10.1042/BJ20111263. [DOI] [PubMed] [Google Scholar]
- 38.De Flora A, Zocchi E, Guidal L, Franco L, Bruzzone S. Autocrine and Paracrine Calcium Signaling by the CD38/NAD+/Cyclic ADP-Ribose System. Ann NY Acad Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- 39.Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal. 2006;2:361–370. doi: 10.1007/s11302-005-5303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seman M, Adriouch S, Haag F, Koch-Nolte F. Ecto-ADP-ribosyltransferases (ARTs): emerging actors in cell communication and signaling. Curr Med Chem. 2004;11:857–872. doi: 10.2174/0929867043455611. [DOI] [PubMed] [Google Scholar]
- 41.Adriouch S, Hubert S, Pechberty S, Koch-Nolte F, Haag F, Seman M. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol. 2007;179:186–194. doi: 10.4049/jimmunol.179.1.186. [DOI] [PubMed] [Google Scholar]
- 42.Glowacki G, Braren R, Firner K, Nissen M, Kuhl M, Reche P, Bazan F, Cetkovic-Cvrlje M, Leiter E, Haag F, Koch-Nolte F. The family of toxin-related ecto-ADP-ribosyltransferases in humans and the mouse. Protein Sci. 2002;11:1657–1670. doi: 10.1110/ps.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glowacki G, Braren R, Cetkovic-Cvrlje M, Leiter EH, Haag F, Koch-Nolte F. Structure, chromosomal localization, and expression of the gene for mouse ecto-mono(ADP-ribosyl)transferase ART5. Gene. 2001;275:267–277. doi: 10.1016/s0378-1119(01)00608-4. [DOI] [PubMed] [Google Scholar]
- 44.Costa M, Furness JB, Humphreys CM. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- 45.Finlayson K, McLuckie J, Hern J, Aramori I, Olverman HJ, Kelly JS. Characterisation of [(125)I]-apamin binding sites in rat brain membranes with HE293 cells transfected with SK channel subtypes. Neuropharmacology. 2001;41:341–350. doi: 10.1016/s0028-3908(01)00067-3. [DOI] [PubMed] [Google Scholar]