Abstract
Differentiation of self from non-self is indispensable for maintaining B cell tolerance in peripheral tissues. CD22 and Siglec-G are two inhibitory co-receptors of the BCR that are implicated in maintenance of tolerance to self-antigens. Enforced ligation of CD22 and the BCR by a nanoparticle displaying both antigen and CD22 ligands induces a tolerogenic circuit resulting in apoptosis of the antigen reactive B cell. Whether Siglec-G also has this property has not be investigated in large part due to the lack of a selective Siglec-G ligand. Here, we report the development of a selective high-affinity ligand for Siglec-G and its application as a chemical tool to investigate the tolerogenic potential of Siglec-G. We find that liposomal nanoparticles decorated with antigen and Siglec-G ligand inhibit BCR signaling in both B1 and B2 B cells compared to liposomes displaying antigen alone. Not only is inhibition of B cell activation observed by ligating the BCR with Siglec-G, but robust tolerance towards T-independent and T-dependent antigens is also induced in mice. The ability of Siglec-G to inhibit B cell activation equally in both B1 and B2 subsets is consistent with our observation that Siglec-G is expressed at a relatively constant level throughout numerous B cell subsets. These results suggest that Siglec-G may contribute to maintenance of B cell tolerance towards self-antigens in various B cell compartments.
Keywords: B cells, Tolerance, Adaptive Immunity, B Cell Receptor, Sialic Acid, Siglecs
Introduction
B cells are activated by engagement of their cognate antigen with the B cell receptor (BCR) leading to a complex signal transduction cascade that can culminate in differentiation to antibody-secreting plasma cells (1). To avoid detrimental autoimmune responses, differentiation of self from non-self antigen is essential. This differential recognition can be aided by co-receptors that modulate BCR signalling to either promote an immune response to non-self or suppress a response to self (2). Two B cell inhibitory co-receptors are CD22 and Siglec-G, members of the siglec (sialic acid-binding immunoglobulin-like lectin) family, which recognize sialic acid terminated glycans on glycoproteins and glycolipids (3). Knock out mice deficient in both siglecs accumulate autoantibodies as they age, suggesting that they participate in maintaining B cell tolerance (4). However, the precise roles of these two siglecs in regulating activation of B cells to self-antigens are yet to be defined.
The siglec family of receptors are differentially expressed on white blood cells and regulate immune cell function (5). The expression of CD22 is primarily B cell restricted. On the other hand, a limited number of studies suggest that Siglec-G is more broadly expressed on B cells and myeloid cells (6, 7). Whereas CD22 is conserved between mouse and man, Siglec-G is the murine ortholog of human Siglec-10. No other siglecs have been detected on murine B cells to date (8). Both CD22 and Siglec-G contain at least one immunoreceptor tyrosine-based inhibitory motif (ITIM) on their cytoplasmic tail and are capable of inhibiting B-cell receptor mediated signaling (9). A leading model for CD22-mediated suppression of BCR signaling is that proximity to the BCR leads to phosphorylation of its ITIMs by Src kinases, which recruits inhibitory phosphatases such as SHP-1 to dampen BCR signaling (10, 11). Although Siglec-G has been less studied, Siglec-10 was shown to recruit SHP-1 in human B cells, suggesting that it may regulate BCR signaling by a mechanism similar to CD22 (12).
In addition to its function in B cells, Siglec-G has also been shown to play an important role in dendritic cells by discriminating danger- from pathogen-associated molecular patterns (DAMPs/PAMPs) (13). Much like the model for siglec-mediated inhibition in B cells, this inhibition is based on proximity to the receptor. Unlike PAMPs, DAMPs were shown to be capable of simultaneously binding TLR4 and CD24. By virtue of a stable interaction between Siglec-G and CD24, Siglec-G becomes recruited to the TLR, which leads to dampened activation compared to stimulation by PAMPs that cannot bind CD24. Interestingly, disruption of the sialic acid mediated interaction of CD24 and Siglec-G by microbial sialidases exacerbated inflammation in an intestinal perforation model of sepsis (14).
Insight into the role of Siglec-G on B cells has come from studying Siglec-G deficient mice (6, 7). These mice show an expansion of their B1 cell population in the pleural and peritoneal cavities (15). B1 cells are responsible for the production of natural IgM and play an important role in the immune response to T-independent antigen such as bacterial polysaccharides (16). After stimulation of the BCR by anti-IgM, B1 cells isolated from the peritoneal cavity of Siglec-G deficient mice show enhanced calcium signaling whereas no significant difference was observed in splenic B2 cells (7). This observation suggested a dominant role of Siglec-G in B1 cells, which appeared to be in line with a slightly higher expression on B1 cells as judged by Western blot analysis. Therefore, it is believed that Siglec-G plays a dominant role in peritoneal B1 cells. Mice deficient in both Siglec-G and CD22 have an even higher number of B1 cells and, in contrast to the single knock-out mice, develop spontaneous autoimmunity (4). Cumulatively, these mouse genetic studies strongly suggest that CD22 and Siglec-G act complimentary in suppressing B cell responses to self-antigens.
Recent studies have shown that a nanoparticle decorated with a B cell reactive antigen and a high affinity ligand for CD22 is able to induce antigen specific tolerance in mice (17, 18). Co-presentation of the antigen and CD22 ligand causes juxtaposition of CD22 with the BCR leading to a tolerogenic circuit that culminates in apoptosis of the antigen-reactive B cell. Based on the specificity of the ligand for CD22, tolerance induction was predominately dependent on CD22, although one report showed partial dependence on Siglec-G (17). Thus, while several studies suggest that CD22 and Siglec-G co-operatively suppress B cell responses, an independent role for Siglec-G in B cell tolerance has not been demonstrated (4, 17, 18).
To examine the potential of Siglec-G to induce B cell tolerance, we have developed a high affinity ligand for Siglec-G that does not cross-react with CD22 and show that siglec-engaging tolerance-inducing antigenic liposomes (STALs) displaying the Siglec-G ligand give rise to robust tolerance in mice toward both T-independent and T-dependent antigens. Analysis of the initial events following stimulation of B cells with liposomes indicates that both B1 and B2 cells are equally capable of being inhibited by recruitment of Siglec-G to the BCR. This latter finding is accordance with our finding that Siglec-G is expressed at equivalent levels in both B cell subtypes as assessed by using a newly developed anti-Siglec-G monoclonal antibody. We also show that Siglec-G is expressed throughout B cell development including pre-B cells before CD22 is expressed, suggesting that it may have a unique role in regulation of BCR signaling.
Materials and Methods
Animal Studies
The Institutional Animal Care and Use Committee of The Scripps Research Institute (TSRI) approved all experimental procedures involving mice. CD22KO and Siglec-GKO mice, on a C57BL/6J background, were obtained from L. Nitschke (University of Erlangen, Germany) and Y. Liu (University of Michigan), respectively. WT C57BL/6J mice were obtained from the TSRI rodent breeding colony. WT MD4 transgenic mice that express IgMHEL (C57BL/6J background) were obtained from Jackson laboratories and crossed onto the Siglecg−/− C57BL/6J and Cd22−/− C57BL/6J backgrounds. Moth eaten variable (Mev) mice were obtained from Jackson laboratories. The following antibodies were obtained from Biolegend and used for cell staining: B220 (RA3-6B2), TCRβ (H57-597), CD11c (N418), Gr-1 (RB6-8C5), NK1.1 (PK136), CD19 (6D5), CD5 (53-7.3), F4/80 (BM8), CD23 (B3B4), CD21 (7E9), IgM (RMM-1).
Siglec expressing cell lines
Myc-tagged Siglec-G expressing BW5147 cells (ATCC) were prepared as described previously (19), and were used herein as Siglec-G expressing cells unless otherwise specified. Myc-tagged Siglec-15 expressing BW5147 cells, and Myc-tagged Siglec-G expressing L929 cells (ATCC) were established by retro-viral transduction using the plasmids pMXs-IG-Siglec-15-CD3z and pMXs-IG-Siglec-G-CD3z as described previously (19). Myc-tagged Siglec-H expressing BW5147 cells were prepared as described previously (20). Murine CD22-expressing cells were prepared by stable transfection of CHO cells with a plasmid (pcDNA3.1-mCD22) encoding full-length mCD22. Cells were sorted for expression of mCD22high and maintained in F12/DMEM supplemented with 10% FBS and hygromycin as a selection marker. Other cell lines expressing murine siglecs in CHO cells have been reported previously (21).
Generation of a Siglec-G specific monoclonal antibody
Twenty million BW5147 cells expressing Myc-tagged Siglec-G were emulsified with complete Freund’s adjuvant (DIFCO LABORATORIES) (38:62, v/v) before immunization. Two female Lewis rats were immunized in the footpad with the immunogen (100 µL/footpad), followed by two boost injections of the cells emulsified with incomplete Freund’s adjuvant with 10-day intervals. Animals were sacrificed three days after the last injection and lymphocytes isolated from common iliac lymph nodes were washed three times with serum-free RPMI-1640 medium, and then fused at a 2:1 ratio with the mouse myeloma cell line P×63Ag.653 cells using polyethylene glycol 1500 (Roche). After the fusion, the cells were selected by hypoxanthine-aminopterin-thymidine (HAT) selection. The medium for hybridoma culture was RPMI supplemented with 10% FCS, 2 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 1 mM non-essential amino acid, 1 mM sodium pyruvate, and 50 µM 2-mercaptoethanol, and 2.5% Opticlone-II hybridoma cloning factor (MP biomedical). For the first screening, hybridoma culture supernatants were assayed for the binding to the L929 cells expressing Myc-tagged Siglec-G by flow cytometry in conjunction with an anti-rat IgG secondary antibody. For the second screening, 293T cells were transfected with pcDNA3.1-Myc-His-Siglec-G kindly provided from L. Nitschke (University of Erlangen, Germany). Hybridoma culture supernatants were assayed for binding to 293T cells transiently expressing Siglec-G by flow cytometry. Isotypes of the antibodies were determined by flow cytometry using biotinylated anti-Rat Ig antibodies (Biolegend) followed by streptavidin-PE. Clone 4A6 generated in this study is of the rat IgG2aκ isotype. For large scale preparation of the antibody, cell were grown one week post-confluence and the antibody in the culture supernatant was precipated by ammonium sulfate (291 g/L), dialyzed against PBS, and purified by affinity chromatography using Hitrap Protein G HP column (GE healthcare). Fractions containing the anti-Siglec-G antibody were dialyzed against PBS. Purified antibodies were quantified by monitoring the absorbance at 280 nm. For conjugation, five equivalents of NHS-activated AF-647 (Invitrogen) was reacted with the antibody for two hours at room temperature in sodium bicarbonate buffer (pH 8.5), followed by dialysis against PBS.
Cell preparation and flow cytometry
Single cell suspensions of the spleen, bone marrow and liver were prepared in HBSS containing 3% FCS. Spleen, bone marrow, and liver were ground and the resulting cell suspension was filtered through a cell strainer (40 µm). Hepatic lymphocytes were purified by centrifugation using a 44%/67% Percoll plus gradient (GE Healthcare). Peritoneal cells were obtained by peritoneal lavage in HBSS/3% FCS. After erythrocyte lysis, cells were stained for 20 min at 4 °C in HBSS containing, 0.1% BSA, and 2 mM EDTA (FACS buffer) with the respective antibodies (Biolegend) at 1:200 dilution. The Siglec-G antibody was used at 3 µg/ml. Prior to staining, Fc-receptors were blocked by anti-CD16/32 (Biolegend) at 1:200 dilution. Dead cells were gated out with 1 µg/mL of propidium iodide. Data was acquired at a LSRII flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star, Inc.).
Synthesis of siglec ligands
The CD22 ligand (6’-BPANeuGc) was prepared as reported earlier (22). The Siglec-G ligand (3’-BPANeuGc) and the natural α2-3-sialoside NeuGcα2-3Galβ1-4GlcAc-β-ethylamine (3’-NeuGc) were prepared using a similar strategy. Briefly, 9-amino-N-glycolylneuraminic acid and N-glycolylneuraminic acid were activated with CMP-synthetase from Neisseria meningitidis. Subsequently, without isolation, the resulting CMP-activated sialic acids were reacted with Galβ1-4GlcAc-β-ethlyazide using Pasteurella multocida α2-3-sialyltransferase 1 (PMST1) to give the respective trisaccharides (23). 9-Amino-NeuGcα2-3Galβ1-4GlcAc-β-ethyl azide was reacted with N-hydroxysuccinimide-activated biphenylacetyl acetic acid (BPA) using a solvent mixture of H2O and THF (1:1) and N,N-diisopropylamine (1.5 eq) as a base. Final hydrogenations with Pd/C (100 wt%) in H2O afforded 9-BPA-NeuGcα2-3Galβ1-4GlcAc-β-ethylamine (3’-BPANeuGc) and NeuGcα2-3Galβ1-4GlcAc-β-ethylamine (3’-NeuGc). The preparation of antigenic liposomes and immuno-liposomes containing siglec ligands was carried out as reported or in analogy (18). Conjugation of the siglec ligands, the nitrophenol hapten (NP) or Alexa-647 (invitrogen) to pegylated distearoylphosphoethanolamine (PEG2000-DSPE) was carried out by reaction of the respective compound with NHS-PEG2000-DSPE or NH2-PEG2000-DSPE (NOF). Hen egg lysozyme (HEL; Sigma) and the Fab-fragment of anti-IgM (Jackson Immunoresearch) were attached to PEG2000-DSPE by modifying lysine residues with a heterobifunctional crosslinker and subsequent coupling to maleimide-PEG2000-DSPE as described previously (18).
Preparation of liposomes
Liposomes were prepared by hydration of the lipids in PBS followed by sonication and extrusion through different filters down to a pore size of 100 nm as described previously (18). All liposomes were composed of a 60:35:5 molar ratio of distearoyl phosphatidylcholine (DSPC; Avanti Polar Lipids), cholesterol (Sigma), and the respective pegylated lipid. The total concentration of the liposomes as defined by the molarity of the lipids was 5 mM. Fluorescent liposomes for binding experiments contained 1 or 4% siglec ligand and 0.1% Alexa-647. Liposomes for functional assays contained 0.5% anti-IgM or 0.33-0.1% HEL and 1 or 4% siglec ligand.
Liposome binding assays
Murine splenocytes and siglec expressing cells were incubated with the respective fluorescent liposomes for 40 min at 4 °C in FACS buffer. Cells were washed, stained with anti-CD19, and binding was assessed by flow cytometry.
B cell purification
B cells were purified from splenocytes by negative selection using magnetic beads according to the manufacture’s protocol (Miltenyi). The purity of isolated cells was generally ≥ 99%.
Ca2+-flux
Following preparation of single cell suspensions of splenocytes for cells from the peritoneal cavity, cells were loaded with Indo-1 (invitrogen) as described previously (7). Cells were stimulated with liposomes and Indo-1 fluorescence was monitored by flow cytometry as described in our previous publication (18).
B-cell proliferation assay and western blotting
Purified IgMHEL B cells were stimulated with 5 uM liposomes for 3 min and their activation was analyzed by Western blotting. For analysis of B cell proliferation, cells were first labeled with 1 uM CellTrace Violet (CTV, Invitrogen) prior to stimulation with liposomes. For all in vitro assays, the media consisted of RPMI supplemented with 10% FCS, 2 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 1 mM non-essential amino acid, 1 mM sodium pyruvate, and 50 µM 2-mercaptoethanol.
Immunizations and ELISA
Mice were injected intraperitoneally or intravenously with liposomes displaying NP or HEL with or without siglec ligand. The liposomes were delivered in 200 µl PBS at 0.5 mM total lipid concentration. Whole blood (50 µl) was collected from mice via retro-orbital bleeds. NP- and HEL-specific antibodies were determined by ELISA with NP(1)-BSA-coated (20 µg/ml) and HEL-coated (10 µg/ml) maxisorp plates (Nunc) as described previously (18). NP(1)-BSA was prepared as described previously (24). The titer was defined as the endpoint titer, which was the dilution of serum that achieved an absorbance 2-fold above background.
Results
Siglec-G is expressed throughout B cell differentiation
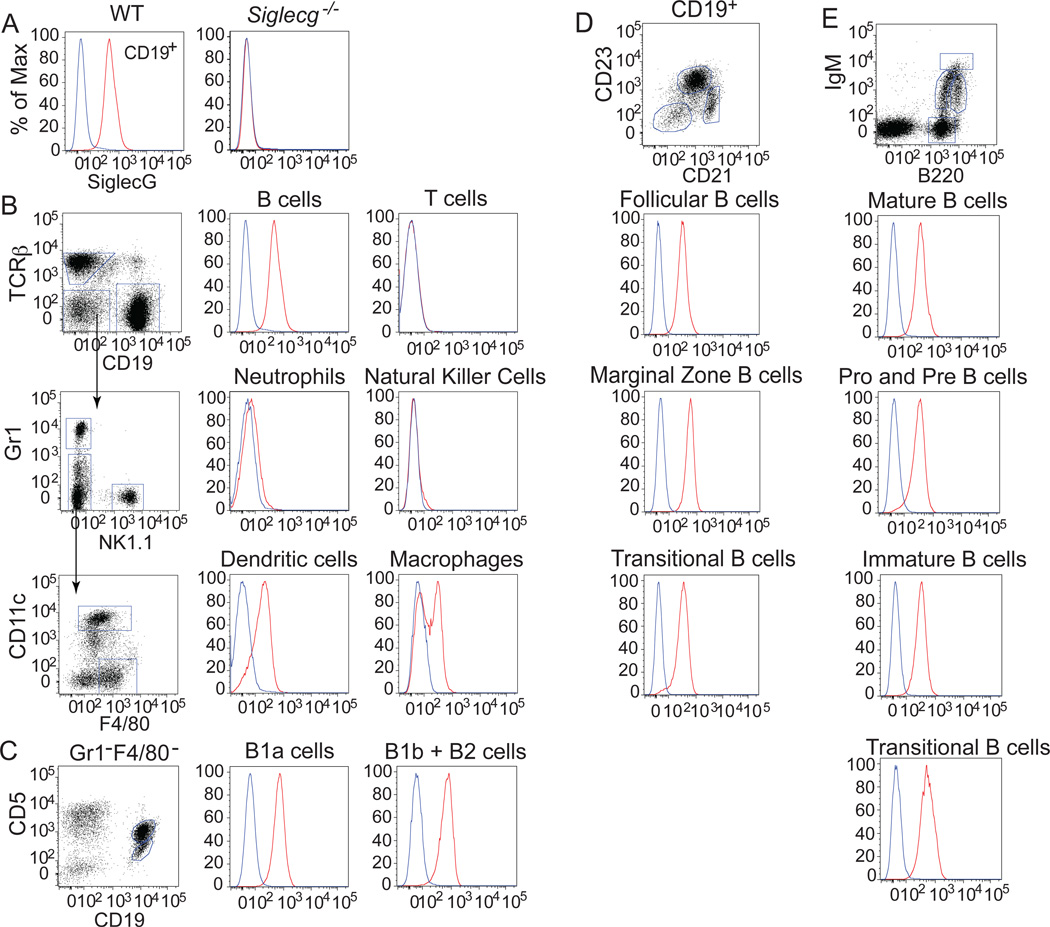
To quantitatively investigate cell surface expression of Siglec-G on murine leukocytes, we developed a monoclonal antibody toward the extracellular portion of Siglec-G. The antibody shows strong specificity for Siglec-G as highlighted by its lack of binding to cells from Siglec-G deficient mice (Fig. 1a). Among splenic leukocytes (Fig. 1b), Siglec-G is expressed at highest levels on B cells and, to a lesser extent, on dendritic cells and a subset of macrophages (Fig. 1b). Slight expression was also observed on neutrophils, and no expression was detected on T cells or natural killer cells. Interestingly, evaluation of B cell subsets from spleen, bone marrow, and the peritoneal cavity revealed a consistent expression of Siglec-G on all B cell subsets examined (Fig. 1c,d). Notably, significant Siglec-G expression appears early on in B cell development as early as pre-pro immature B cells. Siglec-G expression remains at a constant level on germinal center and memory B cells, while no expression is observed on plasma cells (Fig. SI1a). All subsets of splenic dendritic cells showed Siglec-G expression with the highest expression on CD11b+ myeloid dendritic cells (Fig. SI1b). In the liver, Siglec-G expression was found on B cells and macrophages (Fig. SI1c).
Figure 1. Analysis of cell surface expression of Siglec-G on leukocytes.
A, The anti-Siglec-G antibody stains splenic B cells from wild type but not Siglecg−/− mice (anti-Siglec-G in red, isotype control in blue). B, Siglec-G is expressed on B cells, dendritic cells and a subset of macrophages from the spleen. C, Siglec-G is expressed in equal amounts on CD5+ (B1a) and CD5− (B1b+B2) B cells in the peritoneal cavity. Siglec-G is expressed at a constant level in B cell subsets of the spleen (C) and bone marrow (D).
Specificity of a newly developed high affinity glycan ligand for Siglec-G
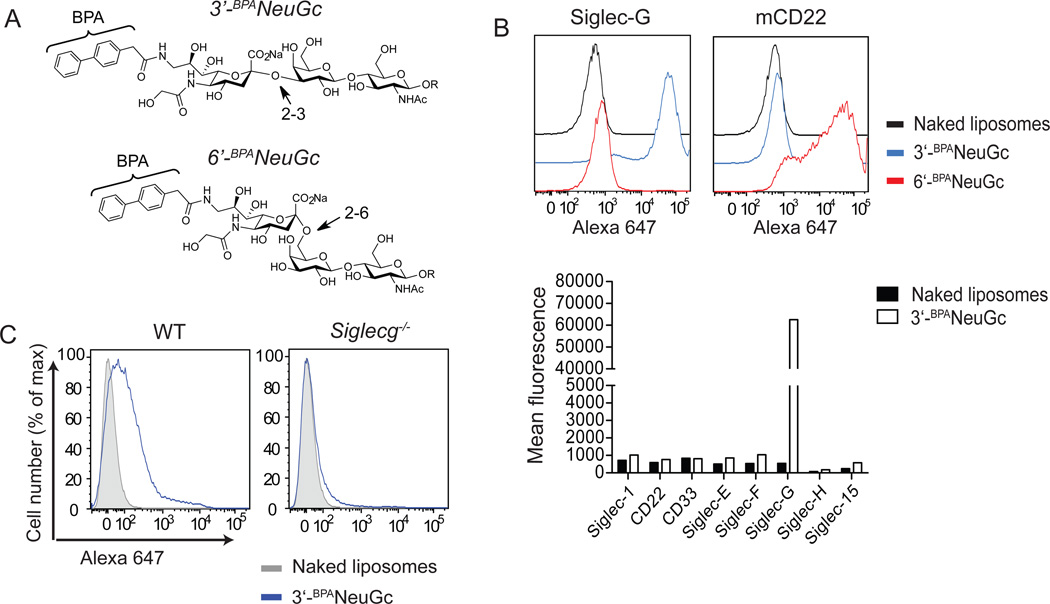
To evaluate the independent roles of CD22 and Siglec-G as regulators of BCR signaling, we set out to evaluate the ability of Siglec-G to induce tolerance by enforced co-ligation to the BCR using nanoparticles that expressed both an antigen and a siglec ligand, which we have described as siglec-engaging tolerance-inducing antigenic liposomes (STALs). To investigate the impact of the B cell siglecs on BCR signaling, selective ligands for either siglec were required. Previously, a high affinity and selective glycan ligand for CD22 (6’-BPANeuGc; Fig. 2a) was described, but no equivalent ligand has been reported for Siglec-G (22). α2-6-Sialosides carrying a biphenylacetyl-group (BPA) at the 9-position of the sialic acid (6’-BPANeuGc) show a 100-fold increase in affinity to CD22 compared to the natural unsubstituted ligand. Since Siglec-G is known to be capable of binding α2-3-sialosides, while CD22 exhibits strict preference for α2-6-sialosides, we tested and fortuitously found that addition of the BPA-substituent to an α2-3-sialosides results in a selective and high affinity ligand for Siglec-G. Accordingly, the ligand 3’-BPANeuGc was chemoenzymatically synthesized and incorporated into fluorescent liposomes for assessing binding to siglec-expressing cells. These 3’-BPANeuGc liposomes showed excellent targeting to Siglec-G expressing cells and did not bind to cells transfected with any of the other murine siglecs (Fig 2b). Furthermore, binding of the 3’-BPANeuGc liposomes to murine splenic B cells was completely dependent on Siglec-G as no binding was detected in splenocytes from Siglec-G deficient mice (Fig. 2c). Conversely, 6’-BPANeuGc liposomes bound exclusively to CD22-expressing cells (Fig. 2b).
Figure 2. Development of a high affinity glycan ligand specific for Siglec-G.
A, Chemical structure of high affinity glycan ligands for Siglec-G (3’-BPANeuGc) and CD22 (6’-BPANeuGc). B, Top: Liposomes displaying the ligands for Siglec-G and CD22 bind specifically to cultured cells expressing Siglec-G or CD22, respectively. The cells were incubated with Alexa 647-labeled naked liposomes (black line), 3’-BPANeuGc liposomes (blue line), and 6’-BPANeuGc liposomes (red line), washed, and analyzed by FACS. Bottom: Liposomes displaying 3’-BPANeuGc do not bind to cells expressing any other murine siglec except Siglec-G. Binding of liposomes is expressed as mean channel fluorescence (MCF). C, Liposomes displaying the Siglec-G ligand bind to WT but not Siglec-G deficient B cells.
Juxtaposition of Siglec-G and the BCR leads to inhibited Ca2+-signaling in both, B1 and B2 B cells
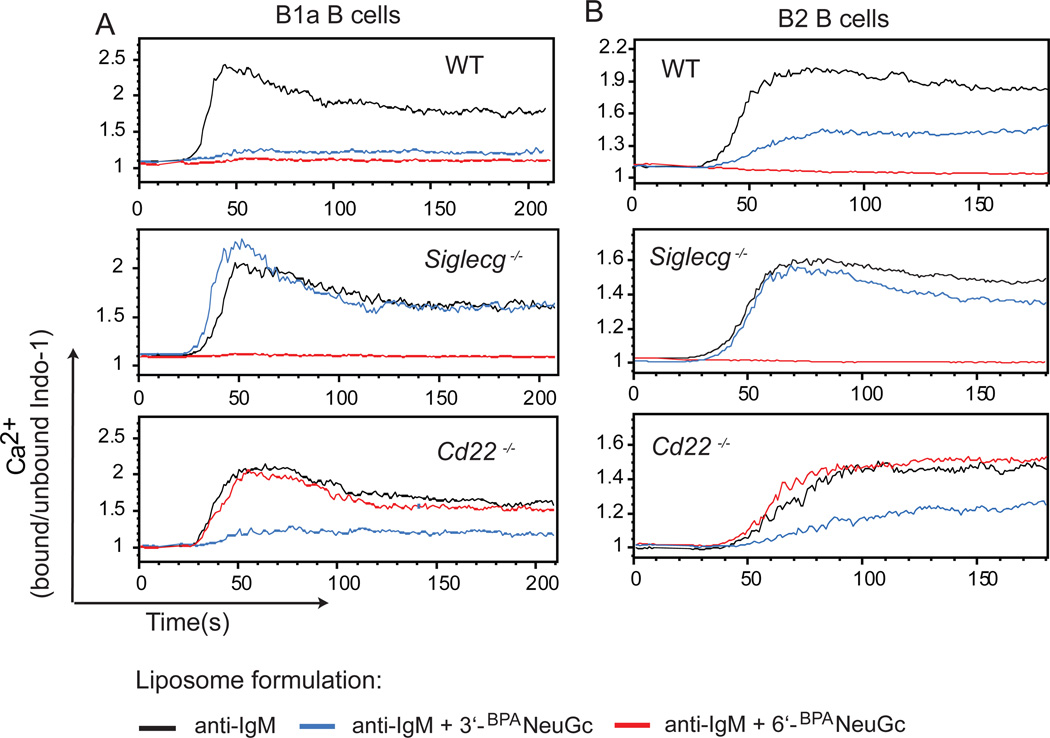
In previous studies, we and others have demonstrated that enforced co-localization of the BCR and CD22 using nanoparticles displaying both a cognate antigen and CD22 ligands, leads to strong inhibition of B cell activation (17, 18, 25). With a Siglec-G specific ligand in hand (3’-BPANeuGc), we set out to determine if the co-localization of BCR and Siglec-G also suppresses B cell activation, and determine the degree to which Siglec-G regulates BCR signaling in B1a cells and B2 cells. To this end, we prepared liposomes bearing an anti-IgM Fab-fragment that serves as a surrogate antigen, either with or without the Siglec-G ligand (3’-BPANeuGc) to assess the influence of co-ligation of the BCR with Siglec-G. Stimulation of either B1a or B2 B cells with liposomes displaying anti-IgM alone led to strong B cell activation as monitored by calcium flux. In contrast, stimulation with anti-IgM containing liposomes that also displayed 3’-BPANeuGc resulted in substantial suppression of Ca2+-flux in both B cell populations (Fig. 3 a,b). While B cells from Siglec-G deficient mice showed generally diminished responses after stimulation with the liposomes, there was no influence of the Siglec-G ligand on activation of the B cells from Siglec-G deficient mice. Most importantly, the inhibitory activity of the Siglec-G ligand was also prevented by pre-incubation of the cells with the anti-Siglec-G monoclonal antibody demonstrating that the 3’-BPANeuGc ligand exhibits its inhibitory function exclusively through Siglec-G (Fig. SI2). Moreover, anti-IgM liposomes displaying the CD22-specific ligand (6’-BPANeuGc) ligand suppressed activation compared to liposomes displaying anti-IgM alone in a CD22-dependent manner in both B2 and B1a B cells (Fig. 3). These results show that the 3’-BPANeuGc and 6’-BPANeuGc ligands are specific for Siglec-G and CD22, respectively, and that either siglec can independently suppress BCR activation when their ligands are incorporated into antigenic liposomes that enforce ligation of the siglec with the BCR.
Figure 3. Inhibition of Ca2+-flux in B cells by STALs displaying 3’-BPANeuGc.
(A,B) Calcium flux of peritoneal cavity B1a (B220lowCD5+) B cells (A) or splenic (B220+CD5−) B2 B cells (B) from WT, Siglecg−/− or Cd22−/− mice. Cells were stimulated at t=10 s with liposomes displaying anti-IgM (black), anti-IgM + 3’-BPANeuGc (blue), or anti-IgM + 6’-BPANeuGc (red) and the intracellular Ca2+-mobilization was measured by FACS.
Broad inhibition of B cell activation in an Shp-1 dependent manner
Although calcium flux is one hallmark of B cell activation, BCR signaling activates numerous signaling cascades that can initiate cell division and survival of B cells. To assess the impact of Siglec-G on the initial events in B cell activation, we analyzed cellular signaling pathways using phospho-specific antibodies in conjunction with Western blotting. These experiments were carried out using splenic B cells from MD4 mice (26), which are reactive toward hen egg lysozyme (HEL). We first verified in HEL-specific B cells that liposomes displaying HEL and Siglec-G ligand suppresses Ca2+-flux compared to liposomes displaying HEL alone in Siglec-G dependent manner (Fig. SI3). Relative to liposomes displaying HEL alone, liposomes that additionally display the Siglec-G ligand suppressed phosphorylation (or degradation) of multiple BCR signaling events including those that signal through MAP kinase, Erk, NFkB, and Akt (Fig. 4a). The inhibition of B cell signaling we observe is Shp-1 dependent since the activation of B cells from Shp-1 deficient (Moth eaten) mice is not inhibited by the Siglec-G ligand (Fig. 4b). B cells from Shp-1 deficient mice contain a mixture of B1 and B2 cells. Therefore, the results suggest that Siglec-G exhibits its inhibitory function through Shp-1 in both B cell subpopulations. These results demonstrate that ligand mediated recruitment of Siglec-G to the BCR strongly dampens both proximal and distal signaling components in the BCR signaling cascade and that this inhibition is dependent on Shp-1.
Figure 4. STALs displaying 3’-BPANeuGc broadly inhibit B cell receptor signaling in a SHP-1 dependant manner.
A, Liposomes displaying HEL + 3’-BPANeuGc inhibit phosphorylation or degradation of representative signaling components of B cell receptor signaling compared to liposomes displaying HEL alone. Purified splenic IgMHEL B cells were stimulated with the indicated liposomes for 3 min at 37 °C, lysed, and the cell lysates were analyzed by Western blotting. Results shown are representative of two independent experiments. B, Ca2+-flux in WT splenic B cells is inhibited after stimulation with liposomes displaying anti-IgM + 3’-BPANeuGc compared to liposomes displaying anti-IgM alone, while no inhibition is observed in SHP-1 deficient B-cells. Results are representative of two independent experiments.
Siglec-G ligands reduce B cell proliferation and survival in vitro
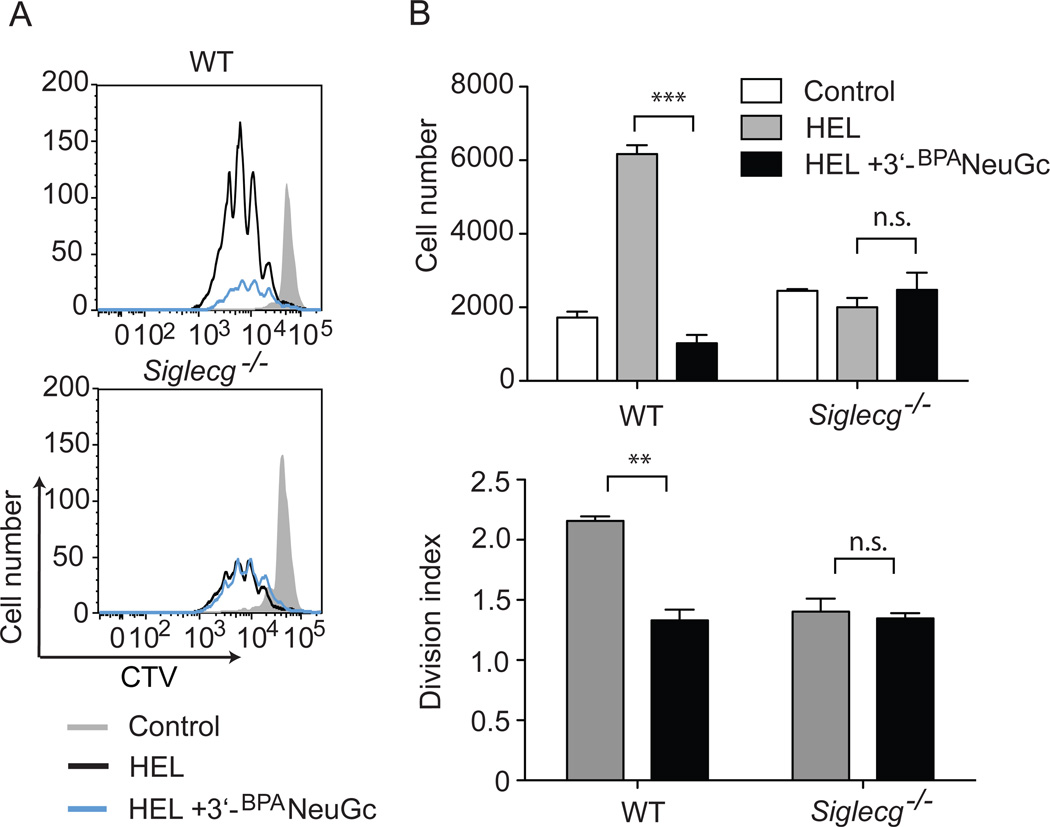
We next determined the longer-term fate of the HEL-reactive MD4 cells stimulated with STALs. Fluorescently labeled B cells were cultured in vitro with liposomes and proliferation of the cells was monitored by fluorescence dilution. Cells incubated with liposomes displaying HEL alone underwent robust proliferation as assessed at day 4 (Fig. 5a,b). Notably, all the cells exposed to liposomes displaying both HEL and 3’-BPANeuGc were activated and divided, but there was a pronounced diminishment in proliferation. Moreover, there was a significant reduction of live cells relative to the cells stimulated with HEL alone (Fig. 5b). These effects were dependent on Siglec-G as no difference was observed when Siglec-G deficient MD4 B cells were stimulated with liposomes displaying antigen and Siglec-G ligand. Therefore, co-ligation of the BCR and Siglec-G leads to inhibited B cell proliferation and decreased survival compared to stimulation by ligation of the BCR alone.
Figure 5. STALs displaying 3’-BPANeuGc inhibit proliferation and decrease survival of B cells in vitro.
A, Purified splenic IgMHEL B cells were incubated for 4 days with liposomes displaying HEL alone (black line) or liposomes displaying HEL + 3’-BPANeuGc-liposomes and their proliferation was determined by a fluorescence (CTV) dilution assay using FACS analysis. Unstimulated cells (grey) are shown as a control. B, Quantitation of living cells and cell proliferation. Statistical analyses were performed by Student’s t-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s. statistically not significant.
Liposomes displaying Siglec-G ligand and antigen induce tolerance in vivo
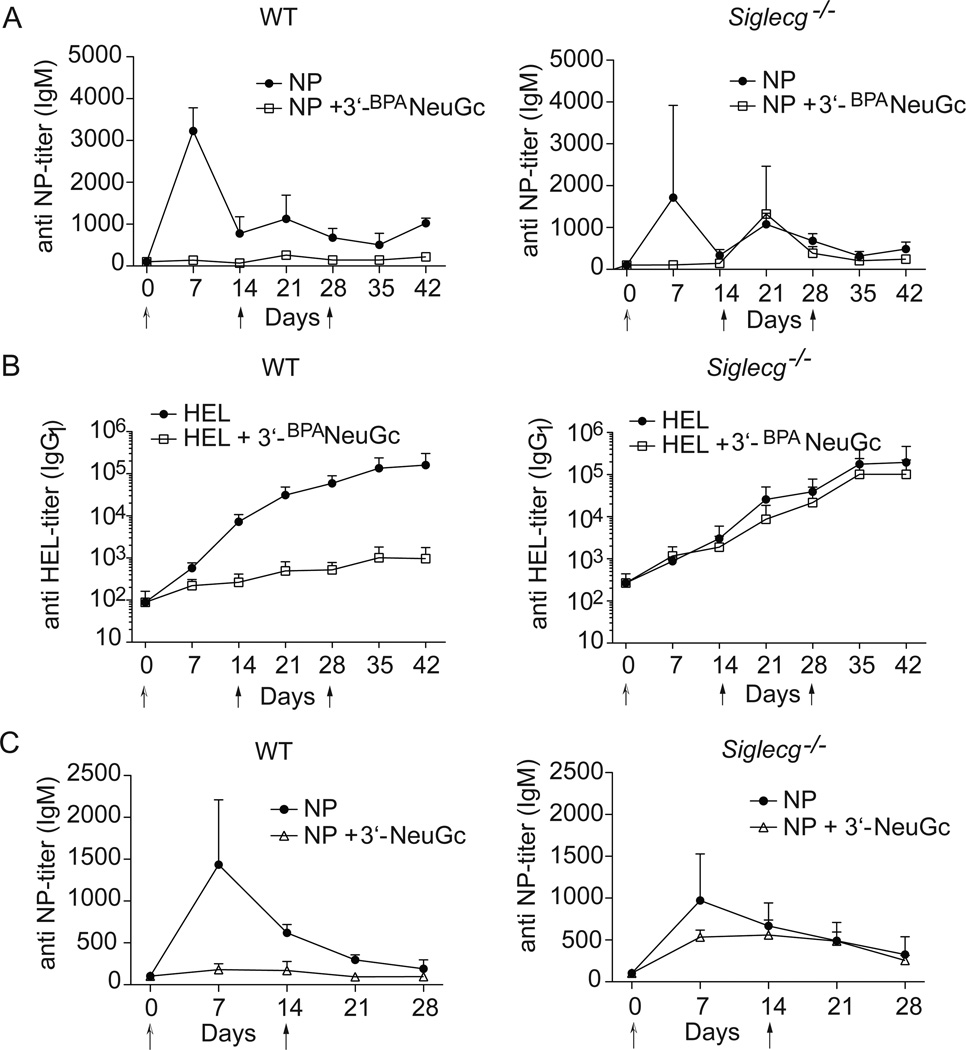
Co-presentation of antigen and CD22 ligands on nanoparticles has been documented to induce antigen-specific tolerance in mice (17, 18). Based on the ability of liposomes bearing the Siglec-G ligand to suppress BCR signaling in vitro, we set out to determine if Siglec-G could also independently induce tolerance in vivo. Accordingly, we investigated the ability of liposomal nanoparticles carrying the Siglec-G ligand to induce tolerance to exemplary T-independent and T-dependent antigens. Intraperitoneal injection of immunogenic liposomes displaying the T-independent antigen nitrophenol (NP) resulted in reproducible production of IgM and IgG antibodies, while liposomes that additionally displayed 3’-BPANeuGc had greatly reduced anti-NP titers, and failed to produce antibody upon subsequent challenge with the immunogenic liposomes two and four weeks later (Fig. 6a, Fig. SI4a). Similar results were obtained after intravenous administration of the liposomes (Supplementary Fig. 4b).
Figure 6. Induction of in vivo tolerance with STALs displaying 3’-BPANeuGc.
(A,B) Robust tolerance to A, T-independent (NP, n=4) and B, T-dependent antigen (HEL, n=8) in Siglec-G dependent manner. C, STALs displaying a natural α2-3-sialoside (3’-NeuGc) also induce tolerance to NP in a Siglec-G dependent manner (n=4). In all studies, mice were injected at day 0 with either liposomes displaying antigen alone or liposomes displaying antigen and Siglec-G ligand. On day 14 and 28 all mice received a challenge with liposomes displaying antigen alone. Antibody titers were assessed by ELISA. Data represents mean ± s.e.m.
Similarly, intravenous administration of immunogenic liposomes displaying the T-dependent antigen HEL induced a strong IgG1 response, while only negligible amounts of anti-HEL antibodies were produced after injection of liposomes with both HEL and 3’-BPANeuGc (Fig. 6b). Subsequent challenges with HEL-liposomes demonstrated that tolerance had been induced in the group of mice given the STALs in the initial injection. The identical experiment was repeated in Siglec-G deficient mice and no significant difference in antibody titers between the two groups was observed, demonstrating that the induction of tolerance is mediated by Siglec-G (Fig. 6B).
To assess the relevance of Siglec-G-mediated tolerance to its natural function on B cells, we investigated tolerance induction by antigenic liposomes displaying a natural sialoside. The sialoside chosen for this study is NeuGcα2-3Galβ1-4GlcNAc (3’-NeuGc), a terminal sequence commonly found on N-linked and O-linked glycans on many murine cell types. We find that even this natural ligand is capable of inducing tolerance and this occurs in a primarily Siglec-G dependent manner (Fig. 6c). Therefore, recruitment of Siglec-G to the immunological synapse in B cells can induce tolerance in mice and this may represent a natural tolerogenic mechanism that operates to maintain self-tolerance.
Discussion
We demonstrate here that the two murine B cell siglecs, Siglec-G and CD22, can independently regulate B cell receptor signaling, and can induce B cell tolerance when the antigen is presented in trans with siglec ligands that enforce ligation of the siglec with the BCR. An impediment for studying the role of trans ligands in regulating the function of Siglec-G has been the lack of a suitable high affinity and selective ligand. In this study, we circumvent this need through the development of such a high affinity glycan ligand for Siglec-G that does not cross-react with CD22 or any other murine siglec. Incorporation of this newly described Siglec-G ligand onto immunogenic liposomes clearly revealed that recruitment of Siglec-G to the immunological synapse inhibits B cell activation as revealed by calcium flux in an Shp-1 dependent manner, signaling components of the BCR, and proliferation. Therefore, we believe that enforced ligation of Siglec-G and the BCR, resulting from a membrane displaying both antigen and Siglec-G ligand, results in inhibition of B cell activation in a manner similar to that described previously for CD22.
In B cell proliferation assays, we noticed that not only was B cell activation inhibited by liposomes displaying antigen and 3’-BPANeuGc, but the number of live B cells was greatly reduced compared to B cells stimulated with antigen alone. This finding suggested that a tolerogenic circuit is induced resembling the one we previously described by liposomes decorated with antigen and CD22 ligand. Indeed, STALs were able to induce robust tolerance in vivo toward both T-independent and T-dependent antigen. In contrast to WT mice, Siglec-G deficient mice did not become tolerized, which clearly demonstrated the specificity of our observations.
Interestingly, we discovered that antigenic liposomes displaying a natural α2-3-sialoside could also induce tolerance in a Siglec-G dependent manner. We believe this may relate to a natural function for Siglec-G on B cells. Tolerization of B cells autoreactive for a cell surface antigen could be aided by Siglec-G, which will be recruited to the immunological synapse by a dense layer of sialic acid. The differential specificity of CD22 and Siglec-G is interesting in this regard. Outside the nervous system, there are two predominant linkages of sialic acid to underlying glyans: α2-3- and α2-6-sialosides. It is well known that the ratio of these two forms of sialic acid vary amongst different cell types, which is in large part due to differential expression of the appropriate 2-6- and 2-3-sialyltransferases (27–30). For instance, hepatocytes and B cells have high levels of α2-6-sialosides due to high expression of ST6Gal1. By extension, cell types that express ST6Gal1 at lower levels will have more α2-3-sialosides. As mentioned above, CD22 exhibits a strong preference for α2-6- over α2-3-sialosides, while Siglec-G clearly has the ability to bind α2-6-sialosides. Thus it will be of interest to determine if CD22 and Siglec-G act cooperatively to mediate peripheral B cell tolerance to autoreactive cell surface antigen through their combined ability to recognize a broader range of sialoside sequences than either siglec alone.
As a part of these studies, we used a recently developed platform for polyclonal stimulation of B cells, which are liposomes decorated with the Fab fragment of an anti-IgM reactive-antibody (18). These were initially used to allow us to investigate both B2 and B1a B cell subtypes, since peritoneal B1a cells from MD4 mice do not express the IgMHEL transgene (31). B1a cells constitute the main population of B1 cells in the peritoneal cavity and can be distinguished from B1b cells by their surface expression of CD5. Based on evidence that Siglec-G deficient mice have an increased number of B1a B cells, Siglec-G is believed to play a significant role in the development of this B cell subset. In particular, the expansion in Siglec-G deficient mice is believed to be the result of hyper-reactivity of B1a cells to BCR stimulation (4, 7). In contrast, CD22 deficient mice show enhanced BCR-simulated B cell activation in B2 cells (32–35). Using the anti-IgM liposomes, we find that Siglec-G is capable of inhibiting B cell activation to a roughly equivalent extent in B1a cells from the peritoneal cavity and B2 cells from the spleen. This suggests that, beside its important function in B1a cells, Siglec-G is equally capable of inhibiting BCR signaling in splenic B2 cells upon encounter of its cognate antigen in the context of Siglec-G ligands. While B1 and marginal zone B cells are thought to be the primary B cell subtypes that are responsible for immune responses toward T-independent type II antigens (36), follicular B2 cells are more adapted for T-dependent immune responses (37). Taken together with our finding that tolerance is induced toward both T-independent type-2 and T-dependent antigens, this suggests an important function of Siglec-G in maintaining tolerance toward sialylated antigens in various B cell compartments.
Consistent with the hypothesis of Siglec-G playing a role in various B cell compartments, we find equivalent cell surface expression of Siglec-G in B1a cells from the peritoneal cavity and B2 cells from the spleen. Furthermore, Siglec-G is developmentally expressed at constant levels throughout B cell development in the bone marrow, even before expression of IgM in immature B cells (Figure 1). This observation is consistent with the suggestion that Siglec-G influences the fate of B cells during early development, as evidenced by the enhanced number of B1a cells in the peritoneal cavity of Siglec-G deficient mice (38). In contrast to Siglec-G, CD22 is only weakly expressed in immature B cells before release into the periphery, and both CD22 expression and the Lyn/CD22/Shp-1 regulatory access are upregulated in mature peripheral B cells (9, 39–41). Thus, CD22 is not believed to play a role in central tolerance (39, 40, 42, 43). Based on the constant expression of Siglec-G in the earliest stages of B cell development, we believe that studies on its potential contribution to central B cell tolerance are warranted.
In summary, we describe antigenic liposomes decorated with a high affinity Siglec-G ligand as a novel tool to investigate the tolerogenic effect of Siglec-G towards antigens presented with sialosides on the same membrane. We find that Siglec-G is expressed at a constant level throughout B cell subsets and may contribute to maintaining peripheral B cell tolerance independently of, but in conjunction with, CD22.
Supplementary Material
Acknowledgments
We thank Anna Tran-Crie for help with the preparation of the manuscript, and Jessica Lu, Jeffel Medina, and Britni Arlian-Cruz for technical help.
This work was supported by the National Institutes of Health Grants R01AI050143 and R01AI099141. F.P. was supported by a fellowship of the German Academic Exchange Service (DAAD) and M.S.M. by a fellowship of the Human Frontier Science Program (HSFP).
Abbreviations
- Siglec
sialic acid-binding immunoglobulin-like lectin
- 6’-BPANeuGc
9-Biphenylacetyl-N-glycolylneuraminic acid-α2-6-galactose-β1-4-N-acetylglucosamine-β-ethylamine
- 3’-BPANeuGc
9-Biphenylacetyl-N-glycolylneuraminic acid-α2-3-galactose-β1-4-N-acetylglucosamine-β-ethylamine
- 3’-NeuGc
N-glycolylneuraminic acid-α2-3-galactose-β1-4-N-acetylglucosamine-β-ethylamine
- HEL
hen egg lysosyme
- NP
nitrophenol
- CTV
cell track violet.
References
- 1.Reth M. Antigen Receptors on Lymphocytes-B. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 2.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 3.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 4.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 × Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 5.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 6.Ding C, Liu Y, Wang Y, Park BK, Wang CY, Zheng P. Siglecg limits the size of B1a B cell lineage by down-regulating NFkappaB activation. PLoS One. 2007;2:e997. doi: 10.1371/journal.pone.0000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, Winkler TH, Kneitz B, Crocker PR, Nitschke L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jellusova J, Nitschke L. Regulation of B Cell Functions by the Sialic Acid-Binding Receptors Siglec-G and CD22. Front Immunol. 2011;2:96. doi: 10.3389/fimmu.2011.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 11.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitney G, Wang S, Chang H, Cheng KY, Lu P, Zhou XD, Yang WP, McKinnon M, Longphre M. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen GY, Chen X, King S, Cavassani KA, Cheng J, Zheng X, Cao H, Yu H, Qu J, Fang D, Wu W, Bai XF, Liu JQ, Woodiga SA, Chen C, Sun L, Hogaboam CM, Kunkel SL, Zheng P, Liu Y. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29:428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 16.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 17.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC, Nemazee D. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, Paulson JC. J Clin Invest. doi: 10.1172/JCI69187. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen Delivery to Macrophages Using Liposomal Nanoparticles Targeting Sialoadhesin/CD169. PLoS One. 2012;7:e39039. doi: 10.1371/journal.pone.0039039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nycholat CM, Rademacher C, Kawasaki N, Paulson JC. In Silica-Aided Design of a Glycan Ligand of Sialoadhesin for in Vivo Targeting of Macrophages. Journal of the American Chemical Society. 2012;134:15696–15699. doi: 10.1021/ja307501e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115:4778–4786. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins BE, Blixt O, Han S, Duong B, Li H, Nathan JK, Bovin N, Paulson JC. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, killing of B cells. J Immunol. 2006;177:2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Chokhawala H, Karpel R, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J Am Chem Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 24.Haniuda K, Nojima T, Ohyama K, Kitamura D. Tolerance Induction of IgG(+) Memory B Cells by T Cell-Independent Type II Antigens. Journal of Immunology. 2011;186:5620–5628. doi: 10.4049/jimmunol.1100213. [DOI] [PubMed] [Google Scholar]
- 25.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci U S A. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, Trent RJ, Basten A. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. J Immunol. 2009;183:5442–5448. [PubMed] [Google Scholar]
- 27.Kitagawa H, Paulson JC. Differential expression of five sialyltransferase genes in human tissues. J Biol Chem. 1994;269:17872–17878. [PubMed] [Google Scholar]
- 28.Paulson JC, Weinstein J, Schauer A. Tissue-specific expression of sialyltransferases. J Biol Chem. 1989;264:10931–10934. [PubMed] [Google Scholar]
- 29.Kono M, Ohyama Y, Lee YC, Hamamoto T, Kojima N, Tsuji S. Mouse beta-galactoside alpha 2,3-sialyltransferases: comparison of in vitro substrate specificities and tissue specific expression. Glycobiology. 1997;7:469–479. doi: 10.1093/glycob/7.4.469. [DOI] [PubMed] [Google Scholar]
- 30.Ulloa F, Real FX. Differential distribution of sialic acid in alpha2,3 and alpha2,6 linkages in the apical membrane of cultured epithelial cells and tissues. J Histochem Cytochem. 2001;49:501–510. doi: 10.1177/002215540104900410. [DOI] [PubMed] [Google Scholar]
- 31.Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol Rev. 2004;197:206–218. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 32.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 33.O'Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 34.Otipoby KL, Andersson KB, Draves KE, Klaus SJ, Farr AG, Kerner JD, Perlmutter RM, Law CL, Clark EA. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 35.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, Tedder TF. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 36.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 37.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 38.Jellusova J, Duber S, Guckel E, Binder CJ, Weiss S, Voll R, Nitschke L. Siglec-G regulates B1 cell survival and selection. J Immunol. 2010;185:3277–3284. doi: 10.4049/jimmunol.1001792. [DOI] [PubMed] [Google Scholar]
- 39.Tedder TF, Poe JC, Haas KM. CD22: a multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv Immunol. 2005;88:1–50. doi: 10.1016/S0065-2776(05)88001-0. [DOI] [PubMed] [Google Scholar]
- 40.Poe JC, Tedder TF. CD22 and Siglec-G in B cell function and tolerance. Trends Immunol. 2012;33:413–420. doi: 10.1016/j.it.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferry H, Crockford TL, Silver K, Rust N, Goodnow CC, Cornall RJ. Analysis of Lyn/CD22 double-deficient B cells in vivo demonstrates Lyn- and CD22-independent pathways affecting BCR regulation and B cell survival. European journal of immunology. 2005;35:3655–3663. doi: 10.1002/eji.200535247. [DOI] [PubMed] [Google Scholar]
- 43.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.