Abstract
Objective
Sleep complaints are associated with adverse health consequences. We hypothesized that non-disabled older persons with more sleep complaints have an increased risk of developing disability.
Methods
Subjects included 908 older clergy participating in the Religious Order Study without clinical dementia, history of stroke or Parkinson’s disease. At baseline, participants rated their difficulty falling asleep, frequency of nocturnal awakenings, sleep efficacy, and napping frequency, from which a summary dyssomnia measure was derived. Self-report assessment of disability included instrumental activities of daily living (IADLs), basic activities of daily living (ADLs), and Rosow-Breslau mobility disability at baseline and at annual evaluations.
Results
Mean follow-up was 9.6 (SD=4.2) years. At baseline, more than 60% had 1 or more sleep complaints. In a series of Cox proportional hazards models controlling for age, sex, and education, a 1-point higher dyssomnia score at baseline was associated with about 20% increased risk of IADL disability (hazard ratio=1.20; 95% CI=1.04-1.39, x21=7.62, p<0.05), about 27% increased risk of ADL disability (hazard ratio=1.27; 95% CI=1.10-1.47, x21=12.15, p<0.01), and about 27% increased risk of mobility disability (hazard ratio=1.27, 95% CI=1.09-1.48, x21=11.04, p<0.01). These associations did not vary by age, sex, or education and remained significant after controlling for potential confounders including body mass index, chronic medical conditions, and several common medications. Controlling for depressive symptoms attenuated the association between sleep complaints and incident IADL and ADL disabilities but the association between sleep complaints and incident mobility disability remained significant.
Conclusion
Non-disabled older adults with more sleep complaints have an increased risk of developing disability.
Keywords: Aging, Dyssomnia, Sleep, Disability
BACKGROUND
Disability is common in older adults. Of those aged 65 years and older, approximately 35-50% are disabled or identify some limitation in activities of daily living. (1, 2) Since persons aged 65 and over represent the fastest growing segment of the U.S. population, their loss of independence and the financial costs of their growing disability is a critical public health challenge. (3, 4) Thus, there is an urgent need to identify factors which can be used to distinguish older individuals who are at increased risk of developing disability, so as to facilitate early interventions.
Sleep complaints are common in older adults (5) and are associated with various adverse health outcomes. (5-15) While cross-sectional studies have demonstrated the association between sleep complaints and functional impairment (16-18), including disability (19), there are a paucity of studies which have examined whether sleep complaints predict the subsequent development of disability.
In the current study, we tested the hypothesis that more sleep complaints in older individuals without disability are associated with incident disability. We used data from more than 900 older persons without dementia participating in the Religious Orders Study, a community-based cohort study of chronic conditions of aging. (20, 21) Participants underwent sleep assessment at study entry as well as assessments of disability at baseline and at annual follow-up exams. In secondary analyses, we also examined whether baseline chronic medical conditions, depressive symptoms, or medications might affect the association of baseline sleep complaints and incident disability.
METHODS
Subjects
Subjects were from the Religious Orders Study, which is a longitudinal clinical-pathologic investigation of aging and cognition in older Catholic nuns, priests, and brothers recruited from approximately 40 groups in the United States. (20, 21) Subjects signed an informed consent agreeing to annual clinical evaluations and organ donation at time of death. The study was in accordance with the latest version of the Declaration of Helsinki and was approved by the Institutional Review Board of Rush University Medical Center.
Eligibility for current analyses required completion of baseline sleep and disability assessments and 1 or more valid follow-up assessments of disability. We excluded participants with clinical evidence of dementia (see below) or a history of stroke or Parkinson’s disease (PD). At the time of these analyses, 1139 persons had enrolled in the study and completed the baseline evaluation. Of these, 185 participants were excluded from these analyses (80 had evidence of clinical dementia and 105 participants had a history of stroke or PD). 28 were missing follow-up disability assessments and 18 had not been in the study long enough or died before follow-up assessment. This left 908 persons included in these analyses. Their average age at study entry was 75.0 (SD=6.9) years, 69.3% were women, average years of education was 18.2 (SD=3.4) years, and average mini-mental state examination score was 28.5 (SD=1.6). Additional clinical details about these participants at baseline are included in Table 1.
Table 1. Characteristics of Participants at Baseline (n=908).
| Baseline characteristics | Mean (SD) |
|
| |
| Age (years) | 75.0 (6.9) |
| Sex (No. of females) | 629 (69.3%) |
| Education (years) | 18.2 (3.4) |
| Body mass index (kg/m2) | 27.4 (5.4) |
| Mini-Mental State Examination score | 28.5 (1.6) |
| Depressive symptoms (CES-D score) | 1.0 (1.5) |
|
| |
| Medical risk factors | No. of subjects (%) |
|
| |
| Diabetes | 68 (7.5%) |
| Hypertension | 316 (34.8%) |
| Thyroid disease | 136 (15.0%) |
| Head injury with loss of consciousness | 67 (7.4%) |
| Cancer | 252 (27.8%) |
|
| |
| Medications reported | No. of subjects (%) |
|
| |
| Psychoactive substances | 105 (11.6%) |
| Psychoactive hypnotics | 234 (25.8%) |
| Antidepressants | 279 (30.7%) |
Clinical Diagnoses
Each subject had a uniform structured evaluation which included a medical history, neurological and medical examination, and cognitive testing. The diagnosis of dementia was made in a multi-step process. Participants had detailed cognitive testing that included 21 cognitive performance tests. The cognitive test data were reviewed by an experienced neuropsychologist who determined if cognitive impairment was present. Based on review of all available data, a clinician experienced in the evaluation of older persons determined whether the participant met the clinical criteria for dementia and Alzheimer’s disease, stroke, or Parkinson’s disease, using criteria previously described. (20, 22-25)
Assessment of Sleep Complaints
To assess sleep complaints, we used specific questions which have been used by other investigators for assessment of community-dwelling older persons. (26-28) Participants rated the frequency of abnormalities of 4 aspects of sleep including: (1) “how often do you have trouble falling asleep?”, a proxy measure for sleep latency; (2) “how often are you troubled by waking up during the night?”, a proxy measure for sleep consolidation; (3) “how often do you get so sleepy during the day or evening that you have to take a nap?”, a proxy measure for daytime sleepiness; (4) “how often do you feel really rested when you wake up in the morning?”, a proxy measure for sleep quality. For the first 3 questions, answers to the questions were coded on a 0-4 scale, with higher scores indicative of worse sleep (i.e. 0=never; 1=rarely; 2=sometimes; 3=often; 4=very often). For question 4, the answers were reverse coded in accordance to the other questions so that higher scores also reflected worse sleep (i.e. 4=never; 3=rarely; 2=sometimes; 1=often; 0=very often). Responses to the four sleep items were averaged to yield a dyssomnia score which was used for these analyses. To assess number of sleep complaints per participant, responses to the four sleep items were dichotomized such that a participant was considered to have a sleep complaint if the response to questions 1, 2, or 3 was “often” or “very often”, or if the response to question 4 was “never” or “rarely”. A fifth question regarding total hours of nighttime sleep (“How many hours do you usually sleep at night?”), a proxy measure for total sleep time) was also asked.
Self-Report of Assessment of Disability
Disability was assessed using 3 scales. Instrumental activities of daily living (IADLs) were assessed using items adapted from the Duke Older Americans Resources and Services project. This instrument assesses the following 8 activities: telephone use, meal preparation, money management, medication management, light and heavy housekeeping, shopping, and local travel. (29) Basic activities of daily living (ADLs) were assessed using a modified version of the Katz Basic ADL scale. This scale assesses the following 6 activities: feeding, bathing, dressing, toileting, transferring, and walking across a small room. (30) Mobility disability was assessed using the Rosow-Breslau Functional Health scale. This instrument assesses 3 activities: walking up and down a flight of stairs, walking one-half mile, and doing heavy housework (e.g. washing windows, walls, and floors). (31) Participants reported the amount of help needed as: no help, help, unable to do. For the purposes of these analyses, subjects were classified as disabled for each of the 3 scales if they reported either needing help or were unable to perform 1 or more tasks.
Other Covariates
Date of birth, sex, and years of education were collected through a participant interview. Body mass index (BMI) was calculated by dividing measured weight represented in kilograms with the square of measured height represented in meters.
Chronic medical conditions that could influence sleep or disability were assessed at baseline. Stroke was diagnosed based on a uniform structured neurological examination and medical history, as previously reported. (24) Classification of diabetes, hypertension, and thyroid disease was made by self-reported medical history and medication inspection, as previously reported. (20) Head injury and cancer were based on participant report at the time of interview. The presence of each of these chronic conditions at baseline was used in analyses.
Depressive symptoms were assessed at baseline with a 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D). (32) Persons indicated whether they had experienced any of 10 symptoms much of the time during the previous week.
Medications were inspected at the baseline exam. For these analyses, we included dummy variables to indicate the use of several common classes of medications which can affect sleep: psychoactive substances, psychoactive hypnotics, and antidepressants.
Statistical Analyses
We first examined the crude associations of sleep complaints with age, sex, and education and other covariates at baseline. To examine whether sleep complaints predicted incident disability, we used a discrete-time Cox proportional hazards model which adjusted for age, sex, and education and included a term for sleep complaints. In subsequent analyses, we added interaction terms to the previous model to determine if the association between sleep complaints and incident disability varied with demographic variables. Finally, we added terms for several covariates to determine if they affected the association of sleep complaints and incident disability. A priori level of statistical significance was 0.05. Model validation was performed graphically and analytically and did not show evidence of nonlinearity or nonproportionality. Models were programmed using SAS Version 9.2.
RESULTS
Descriptive Properties of Sleep Complaints
There were a total of 908 participants in these analyses. Baseline characteristics of these participants are summarized in Table 1. On average, participants reported sleeping for 7.0 (SD=1.1) hours. The dichotomized responses to the four sleep items demonstrated that more than 60% of participants had one or more of the 4 sleep complaints. (Figure 1) The summary dyssomnia measure scores ranged from 0 to 3.75, with higher scores reflecting more sleep complaints [mean (SD)=1.56 (0.74)]. Dyssomnia was associated with age (rs=0.14, p<0.001) but not associated with sex (rs=0.04, p=0.26) or education (rs= −0.05, p=0.16). Dyssomnia was associated with depressive symptoms (rs=0.36, p<0.001), hypertension (rs=0.09, p<0.05), diabetes (rs=0.07, p<0.05), and history of head injury (rs=0.07, p<0.05). Dyssomnia was not associated with body mass index (rs=0.03, p=0.44), thyroid disease (rs=0.03, p=0.30), or cancer (rs=0.04, p=0.27).
Figure 1.
Number of Sleep Complaints at Baseline.
Sleep Complaints and Instrumental Activities of Daily Living (IADL) Disability
This analysis was restricted to the 510 participants without IADL disability at baseline. Over a mean follow-up of 9.9 (SD=4.1) years, 400 (78.4%) developed IADL disability. In a proportional hazards model adjusting for age, sex, and education, a higher level of dyssomnia (a 1-point higher dyssomnia score at baseline) was associated with about a 20% greater risk of developing IADL disability. (Table 3, Model 1)
Table 3. Dyssomnia and Incident Disability.
| Terms | IADL | ADL | Mobility |
|---|---|---|---|
| 1. Core model | 1.20 (1.04-1.39, x21=7.62) * | 1.27 (1.10-1.47, x21=12.15) ** | 1.27 (1.09-1.48, x21=11.04) ** |
| 2. Core + body mass index | 1.21 (1.05-1.40, x21=8.20) * | 1.28 (1.10-1.48, x21=12.41) * | 1.27 (1.08-1.48, x21=11.03) ** |
| 3. Core + chronic medical conditions | 1.17 (1.02-1.36, x21=5.90) * | 1.24 (1.07-1.44, x21=9.94) * | 1.25 (1.07-1.46, x21=9.76) *** |
| 4. Core + hypnotic medications | 1.19 (1.03-1.38, x21=6.84) * | 1.26 (1.09-1.46, x21=11.16) ** | 1.26 (1.08-1.47, x21=10.44) ** |
| 5. Core + psychoactive medications | 1.21 (1.05-1.40, x21=8.42) ** | 1.29 (1.12-1.50, x21=14.49) *** | 1.26 (1.09-1.48, x21=11.13) ** |
| 6. Core + antidepressant medications | 1.17 (1.01-1.35, x21=5.68) * | 1.18 (1.02-1.37, x21=6.14) * | 1.23 (1.05-1.43, x21=7.90) ** |
| 7. Core + depressive symptoms | 1.10 (0.94-1.29, x21=2.12) | 1.11 (0.95-1.30, x21=2.23) | 1.23 (1.03-1.41, x21=6.58) * |
Core model includes terms for dyssomnia, age, sex, and education. Models 2-7 included the terms from the core model as well as the covariate listed. Values are the hazard ratio (95% confidence interval) for the dyssomnia term in a Cox proportional hazards model for incident disability.
IADL: instrumental activity of daily living; ADL: activity of daily living.
p<0.05
p<0.01
p<0.001
To interpret the association between dyssomnia and incident IADL disability, we compared this increased risk to a more familiar metric: the increased risk of IADL disability associated with higher age at baseline. In this model, baseline age was also associated with incident IADL disability (HR=1.08, 95% CI=1.06-1.10, x21=99.3, p<0.001). In comparing the estimates of dyssomnia and age in this model (0.20/0.08), the increased risk of disability associated with a 1-point higher dyssomnia score is similar to a participant being 2.5 years older at baseline. (Figure 2)
Figure 2.
Cumulative Hazard of Disability in Instrumental Activities of Daily Living (IADL).
The association of dyssomnia and incident IADL disability did not vary by age, sex, or education (data not shown).
Sleep Complaints and Activities of Daily Living (ADL) Disability
This analysis was restricted to the 826 participants without ADL disability at study entry. Over a mean follow-up of 9.6 (SD=4.2) years, 349 (42.3%) subjects reported impairment in ADL. Using a Cox proportional hazards model adjusting for age, sex, and education, a higher level of dyssomnia (a 1-point higher dyssomnia score at baseline) was associated with about a 27% greater risk of developing ADL disability. (Table 3, Model 1) This risk is equivalent to the risk of developing ADL disability associated with a subject being 2.4 years older at baseline. (Figure 3)
Figure 3.
Cumulative Hazard of Disability in Activities of Daily Living (ADL).
The association of dyssomnia with incident ADL disability did not vary by age, sex, or education (data not shown).
Sleep Complaints and Mobility Disability
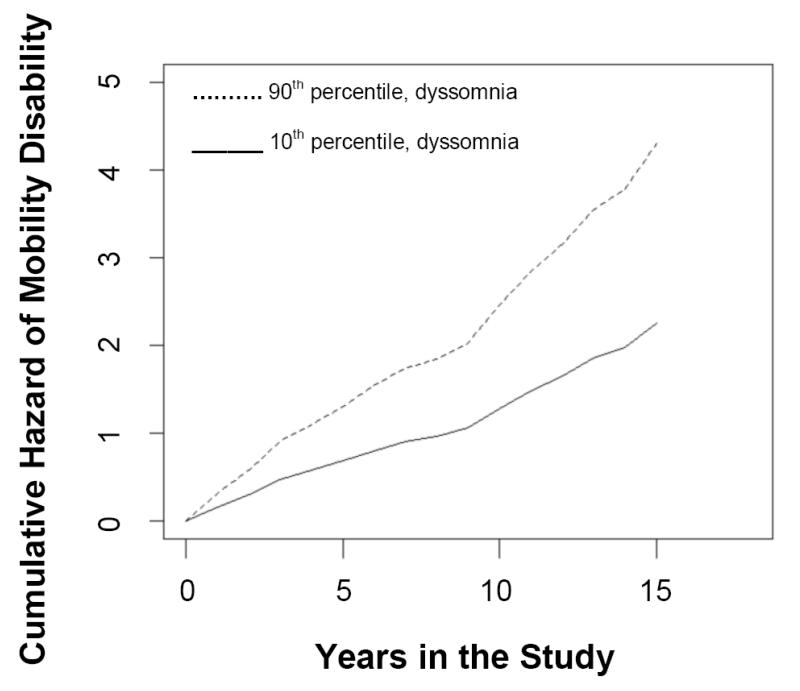
This analysis was restricted to the 519 participants without mobility disability at baseline. Over a mean follow-up of 9.9 (SD=4.2) years, 370 (71.3%) subjects developed mobility disability. Using the same Cox proportional hazards model adjusting for age, sex, and education, dyssomnia (about a 1-point higher dyssomnia score at baseline) was associated with about a 27% greater risk of developing mobility disability. (Table 3, Model 1) This risk is the equivalent to the risk of developing mobility disability associated with a subject being 2.7 years older at baseline. (Figure 4)
Figure 4.
Cumulative Hazard of Disability in Mobility Disability.
The association of dyssomnia and incident mobility disability did not vary by age, sex, or education (data not shown).
Sleep Complaints, Other Covariates, and Disability
Body composition and chronic medical conditions can affect either sleep or disability or both and may attenuate the link between sleep and incident disability. Adding terms to adjust for baseline reports of BMI and chronic medical conditions, the association of dyssomnia and all 3 disabilities remained significant. (Table 3, Models 2 and 3)
Several common medications can also affect sleep. When we added terms adjusting for use of these medications at baseline, the association between dyssomnia and disability remained significant. (Table 3, Models 4, 5, and 6)
Depression is commonly associated with sleep complaints. When we included terms for depressive symptoms, the association between dyssomnia and incident disability was attenuated for IADL and ADL disabilities. However, the association with incident mobility disability remained significant. (Table 3, Model 7)
Individual sleep complaints and disability
In secondary analyses, we examined the association of individual sleep complaints and incident disability. Participants reporting trouble falling asleep (question 1) had an increased risk of IADL disability. Reports of waking up at night (question 2) and not feeling rested in the morning (question 4) were associated with incident ADL disability. Participants reporting daytime napping (question 3) or not feeling rested in the morning (question 4) had an increased risk of developing mobility disability. (see Supplemental Table)
DISCUSSION
In this large community-based study of more than 900 older individuals, sleep complaints were common, with more than 60% reporting some degree of sleep difficulty. Furthermore, the number of sleep complaints at baseline predicted the subsequent development of a broad range of disabilities, including IADL, Katz, and mobility disabilities. These associations did not vary by age, sex, or education and remained significant even after adjusting for a wide variety of potential confounders including BMI, chronic health conditions, and common medications. Controlling for depressive symptoms at baseline attenuated the associations between sleep complaints and incident IADL and ADL disabilities, but did not affect the association between sleep complaints and incident mobility disability. These findings suggest sleep assessment in non-disabled older adults may identify individuals at higher risk for developing mobility disability, providing a potential means of decreasing disability in old age.
Prior studies have reported that sleep complaints are more common in older adults. (5) Similarly, sleep complaints were very common in our participants, with more than 60% reporting at least one or more sleep complaints. There is increasing recognition of the importance of assessing sleep complaints because they are associated with a wide variety of adverse health consequences including cognitive decline and mortality. (5, 7) Previous cross-sectional studies have reported the association between sleep complaints and physical function. (33-35) The current longitudinal study extends these findings and provides evidence that more sleep complaints in older individuals without disability predicts the subsequent development of a wide range of common disabilities. These results lend further support for the accumulating evidence of the adverse consequences of sleep impairments in older adults. These findings may have important translational consequences, as sleep complaints may help identify a group of older persons at increased risk for the subsequent development of disability. Further research is needed to determine whether treatment of sleep complaints can reduce the growing burden of disability in our rapidly aging population.
The basis for the association between sleep complaints and disability is uncertain. Although subjective and objective sleep measures do not always correlate, subjective sleep complaints are recognized as independently correlating with a wide range of health indicators, including cardiovascular function and morbidity. (36) If sleep disturbances do lead to incident disability, potential mediators that may account for these associations include insulin resistance (37, 38), autonomic nervous system dysregulation (37, 39-42), metabolic derangement (43, 44), and inflammation (45, 45, 46). In the present study, we attempted to account for several of these factors at baseline including body composition, diabetes, vascular diseases, and vascular risk factors. However, the association between sleep complaints and incident disability remained robust. This suggests that there are likely additional pathways through which sleep can affect physical function. Prior studies have suggested that pain and depression may mediate the relationship between sleep disturbances and disability. (47, 48) This may account in part for the attenuation of the risk of IADL and ADL disabilities we observed when we adjusted our analyses for depressive symptoms. Since depression and sleep complaints are strongly linked, it is possible that sleep complaints may be a proxy for depression for these particular disability measures. It is also possible that sleep disturbance increases sensitivity to pain within the musculoskeletal system. (49, 50) This may partially explain why the association between sleep complaints and mobility disability remained robust even after controlling for depressive symptoms. Future studies are needed to further clarify the biologic substrate which links sleep complaints and the motor system as it relates to mobility disability.
The current study has several limitations. The observational nature of the study limits causal inferences. It is therefore possible that disability and sleep complaints share a common pathophysiology rather than a causal relationship. Self-report sleep complaints were assessed without physiological data on sleep so it is unknown whether nocturnal sleep disorders such as sleep apnea accounted for these results. Objective measures of sleep are needed to confirm the current results. Findings are also based on a selected cohort that differs in important ways from older persons in the general population with regards to education, socioeconomic status, and lifestyle. It will be important to investigate these findings in more diverse cohorts.
Nevertheless, the homogeneity of the cohort might also be considered a strength as it helps to control for factors that may otherwise be considered confounders. The current study has other several strengths. Data were obtained longitudinally from a large sample of community dwelling elderly, allowing control for important covariates. Use of a community based sample, rather than a participants from a clinic-based sample, reduces a certain type of selection bias. Finally, while objective sleep data may have yielded interesting results, specifically whether there is a correlation between subjective complaints of sleep, objective sleep parameters, and incident disability, the brief self-report measurements utilized in this study allowed for collection of data on a large sample within the community.
CONCLUSIONS
Older persons with more sleep complaints have a higher risk of developing disability. Sleep complaints may serve to identify individuals at risk for disability. Further research is needed to determine whether interventions to treat these sleep complaints help decrease the burden of disability in our aging population.
Supplementary Material
Table 2. Frequency of Sleep Complaints at Baseline (n=908).
| Sleep question | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| Trouble falling asleep | 241 (27%) | 380 (42%) | 186 (20%) | 52 (6%) | 49 (5%) |
| Waking up at night | 140 (15%) | 203 (22%) | 222 (24%) | 160 (18%) | 183 (20%) |
| Daytime napping | 192 (21%) | 201 (22%) | 190 (21%) | 159 (18%) | 166 (18%) |
| (Not) rested upon awakening | 311 (34%) | 356 (39%) | 138 (15%) | 75 (8%) | 28 (3%) |
Values rounded to the nearest %.
Question 1: How often do you have trouble falling asleep?
Question 2: How often are you troubled by waking up during the night?
Question 3: How often do you get so sleepy during the day or evening that you have to take a nap?
Question 4: How often do you feel really rested when you wake up in the morning?
ACKNOWLEDGEMENTS
We are indebted to the participants and the staff of the Religious Orders Study and the Rush Alzheimer’s Disease Center for this work, and Woojeong Bang, MS and Liping Gu, MS for help with statistical programming.
FUNDING SOURCE:
This material is based upon work supported by National Institute on Aging grants R01AG15819 and P30AG10161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENTS:
No Disclosures to Report.
REFERENCES
- 1.Nevitt MC, Cummings SR, Kidd S, et al. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 2.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46:M164–70. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association 2008 Alzheimer’s disease facts and figures. Alzheimers Dement. 2008;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association 2009 Alzheimer’s disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 7.Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 8.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliwise DL, Foley DJ, Vitiello MV, et al. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10:540–548. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensrud KE, Blackwell TL, Redline S, et al. Sleep disturbances and frailty status in older community-dwelling men. J Am Geriatr Soc. 2009;57:2085–2093. doi: 10.1111/j.1532-5415.2009.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi K, Hoshide S, Ishikawa S, et al. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J Am Soc Hypertens. 2010;4:255–262. doi: 10.1016/j.jash.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Bidulescu A, Din-Dzietham R, Coverson DL, et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES) BMC Public Health. 2010;10:581. doi: 10.1186/1471-2458-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzierzewski JM, Williams JM, Roditi D, et al. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: evidence of covariation over time. J Am Geriatr Soc. 2010;58:925–930. doi: 10.1111/j.1532-5415.2010.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–1423. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 17.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–235. [PubMed] [Google Scholar]
- 18.Roth T, Jaeger S, Jin R, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60:1364–1371. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein MB, Belik SL, Jacobi F, et al. Impairment associated with sleep problems in the community: relationship to physical and mental health comorbidity. Psychosom Med. 2008;70:913–919. doi: 10.1097/PSY.0b013e3181871405. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 23.Wilson RS, Schneider JA, Bienias JL, et al. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60:539–544. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 26.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 27.Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 28.Tractenberg RE, Singer CM, Cummings JL, et al. The Sleep Disorders Inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s disease. J Sleep Res. 2003;12:331–337. doi: 10.1046/j.0962-1105.2003.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 30.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 31.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 32.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 33.Foley KA, Sarsour K, Kalsekar A, et al. Subtypes of sleep disturbance: associations among symptoms, comorbidities, treatment, and medical costs. Behav Sleep Med. 2010;8:90–104. doi: 10.1080/15402001003622842. [DOI] [PubMed] [Google Scholar]
- 34.Gradinger F, Glassel A, Gugger M, et al. Identification of problems in functioning of people with sleep disorders in a clinical setting using the International Classification of Functioning Disability and Health (ICF) Checklist. J Sleep Res. 2010 doi: 10.1111/j.1365-2869.2010.00888.x. [DOI] [PubMed] [Google Scholar]
- 35.Mesas AE, Lopez-Garcia E, Rodriguez-Artalejo F. Self-reported sleep duration and falls in older adults. J Sleep Res. 2011;20:21–27. doi: 10.1111/j.1365-2869.2010.00867.x. [DOI] [PubMed] [Google Scholar]
- 36.Budhiraja R, Roth T, Hudgel DW, et al. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 38.Leproult R, Copinschi G, Buxton O, et al. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 39.Hall M, Vasko R, Buysse D, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 40.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–193. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 41.Novati A, Roman V, Cetin T, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–1585. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab. 2007;92:3044–3051. doi: 10.1210/jc.2006-2788. [DOI] [PubMed] [Google Scholar]
- 44.Friese RS. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Crit Care Med. 2008;36:697–705. doi: 10.1097/CCM.0B013E3181643F29. [DOI] [PubMed] [Google Scholar]
- 45.Okun ML, Reynolds CF, 3rd, Buysse DJ, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 47.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7:75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 48.Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain. 2007;127:243–252. doi: 10.1016/j.pain.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Salo P, Oksanen T, Sivertsen B, et al. Sleep disturbances as a predictor of cause-specific work disability and delayed return to work. Sleep. 2010;33:1323–1331. doi: 10.1093/sleep/33.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman AB, Enright PL, Manolio TA, et al. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.