Abstract
We investigated the influence of allograft primary vascularization on alloimmunity, rejection and tolerance in mice. First, we showed that fully allogeneic primarily vascularized and conventional skin transplants were rejected at the same pace. Remarkably, however, short-term treatment of mice with anti-CD40L antibodies achieved long-term survival of vascularized skin and cardiac transplants but not conventional skin grafts. Non-vascularized skin transplants triggered vigorous direct and indirect pro-inflammatory type 1 T cell responses (IL-2 and γIFN) while primarily-vascularized skin allografts failed to trigger a significant indirect alloresponse. Similar lack of indirect alloreactivity was also observed after placement of different vascularized organ transplants including hearts and kidneys while hearts placed under the skin (non-vascularized) triggers potent indirect alloresponses. Altogether, these results suggest that primary vascularization of allografts is associated with lack of indirect T cell alloreactivity. Finally, we show that long-term survival of vascularized skin allografts induced by anti-CD40L antibodies was associated with a combined lack of indirect alloresponse and a shift of the direct alloresponse towards a type 2 cytokine (IL-4, IL-10) secretion pattern but no activation/expansion of regulatory T cells. Therefore, primary vascularization of allografts governs their immunogenicity and tolerogenicity.
Keywords: Transplantation, allorecognition, tolerance, T cells, skin graft, costimulation blockade
Introduction
Transplantation of allogeneic organs and tissues induces a potent inflammatory immune response that invariably results in early acute allograft rejection. The anti-donor immune response is initiated by recipient T cells activated in the recipient’s secondary lymphoid organs via two distinct pathways: the direct pathway in which T cells recognize intact donor MHC molecules on transplanted cells (1) and the indirect pathway which involves the recognition of donor peptides processed and presented by host APCs (2). Fully allogeneic skin grafts trigger potent pro-inflammatory T cell responses via both pathways (3). Either direct or indirect alloresponse is sufficient to mediate acute rejection of skin allografts (4). In contrast, the relative contribution of these pathways to acute rejection of vascularized solid organ transplants, including hearts and kidneys, is less clear. Currently, direct alloreactivity is thought to be the driving force behind early acute rejection of these transplants while the indirect pathway is rather involved in chronic rejection (5), a late process characterized by perivascular inflammation, fibrosis and arteriosclerosis involving intimal thickening and luminal occlusion of graft vessels (6). This conclusion was drawn based on the assumption that the direct alloresponse is short-lived due to the rapid elimination of donor passenger leukocytes while the indirect alloresponse is perpetuated via continuous presentation of alloantigens by host APCs. In addition, indirect alloimmunity drives alloantibody production which is essential to the chronic rejection process (7). Finally, de novo induction of indirect alloresponses via allopeptide immunization has been shown to trigger chronic rejection of allografts in various animal models (5, 8). Therefore, while indirect alloreactivity is presumably an essential element of the chronic rejection process, its contribution to acute rejection of primarily vascularized solid organ allografts remains to be demonstrated.
Advances in surgical techniques and the development of immunosuppressive agents have rendered possible large-scale transplantation of some allogeneic organs in patients with minimal risks for early acute rejection. However, continuous widespread immunosuppression treatments are associated with susceptibility to infection and neoplasia in transplanted patients. Additionally, these drugs are nephrotoxic and ineffective in preventing chronic rejection. Altogether, this underscores the need for the development of more efficient and selective immune-based strategies in transplantation. Some protocols involving T cell costimulation blockade and/or donor hematopoietic chimerism have achieved immunological tolerance (indefinite graft survival without immunosuppression and chronic rejection) to some vascularized solid organ transplants in rodents and primates (9-12). However, tolerance to skin allografts has proven to be more arduous. The high immunogenicity of skin allografts is traditionally attributed to the presentation of highly immunogenic skin-specific antigens (13) by a large population of resident DCs (14-16). Until now, this has not been demonstrated.
In the present study, we show that initial vascularization of skin allografts renders these transplants susceptible to tolerance via protocols effective with vascularized solid organ transplants. The mechanisms by which vascularization governs the immunogenicity and susceptibility to tolerogenesis of allografts are investigated.
Materials and Methods
Mice and transplantation
Mice were bred and maintained at MGH animal facilities under specific pathogen-free conditions. All animal care and handling were performed according to institutional guidelines. Non-vascularized “conventional” full-thickness trunk skin allografts were placed using standard techniques (17). Skin was harvested from euthanized donor mice, the s.c. fat was removed, and the skin was cut into 2-cm pieces and placed in sterile PBS until used for transplantation (<30 min). Recipient mice were anesthetized and shaved around the chest and groin. The skin allograft was placed in a slightly larger graft bed prepared over the groin or chest of the recipient and secured using Vaseline gauze and a bandage. For vascularized skin grafts, a 2×3 cm full thickness flap was outlined in the groin and raised. The epigastric vessels were dissected, the distal superficial and deep femoral vessels were ligated and the femoral artery and vein were separated. The femoral artery and vein were then divided. For the recipient, same size defect was created in groin area. The femoral artery and vein, right below the inguinal ligament, were separated and prepared for anastomosis. End-to-end anastomosis was performed for arteries and end to side for the veins (Supplemental Figure 2). After the patency of the vessels confirmed the flap was sutured to the defect with interrupted sutures. Bandages were removed on day 7, and the grafts were then visually scored daily for evidence of rejection. The allograft was considered fully rejected when it was >90% necrotic. In selected animals, allograft rejection was confirmed histologically.
Preparation of sonicates
Stimulator spleen cells were suspended at 3 × 107 cells/ml in AIM-V containing 0.5 % FCS, and sonicated with 10 pulses of 1 sec each. The resulting suspension was frozen in a dry ice/ethanol bath, thawed at room temperature and centrifuged at 300g for 10 min to remove intact cells as described elsewhere (3).
ELISPOT assays
Direct and indirect alloresponses by T cells were measured as previously described (3). Briefly, 96-well ELISPOT plates (Polyfiltronics, Rockland, MA) were coated with an anti-cytokine capture mAb in sterile PBS overnight. On the day of the experiment, the plates were washed twice with sterile PBS, blocked for 1.5 h with PBS containing 1 % BSA, then washed 3 times with sterile PBS. Responder cells or purified T cells were added to wells previously filled with either intact donor cells (direct response) or syngeneic APCs together with donor sonicate (indirect response) and cultured for 24 hours at 37°C, 5% CO2. After washing, biotinylated anti-lymphokine detection antibodies were added overnight and the plates were washed and developed using 800 ul AEC (Pierce, Rockford, IL, 10 mg dissolved in 1 ml dimethyl formamide) mixed in 24 ml 0.1M sodium acetate, pH 5.0, plus 12 ul H202. The resulting spots were counted and analyzed on a computer-assisted ELISA spot image analyzer (C.T.L., Cleveland, OH).
Anti-CD40L antibody treatment
Recipient mice were injected intraperitoneally with 0.5 mg of anti-CD40L monoclonal antibodies (MR1) at the time of transplantation and day 4 and 6 post-transplantation, as previously reported (18).
Regulatory T cell (Tregs) assays
Cell enrichment
CD4+ cells were enriched using either CD4 (L3T4) MACs MicroBeads or the CD4+ T Cell Isolation Kit II, mouse (Miltenyi Biotec, Auburn, CA). CD4+CD25neg and CD4+CD25+ T cells were enriched using the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Antigen presenting cells (APCs) were mouse splenocytes depleted of T cells using CD90.2 MicroBeads, mouse (Miltenyi Biotec). Flow cytometry data were acquired either on a FACsCalibur using CellQuest software (BD Biosciences, San Diego, CA) or a LSRII using FACSDiva software (BD Biosciences). Data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR). Live cells were gated using propidium iodide and singlet cells were gated for analysis as FSC-Wlo and SSC-Alo. The suppression assays were performed as previously described (19). Briefly, 2 × 105 cells/well MACs-enriched CD4+CD25− T cells were plated in triplicate in 96-well, round-bottom plates along with 1×105 irradiated (3,000 cGy) CD90-depleted splenocytes per well. MACs enriched CD4+CD25+ T cells were added at T effector/Treg ratios of 2:1, 4:1, 8:1 or 16:1. Medium consisted of RPMI 1640 supplemented with 15% Hybrid-MAX (Sigma-Aldrich, Corp., St. Louis, MO), 1 mM of glutamine, 1 mM of sodium pyruvate, 0.1 mM of NEAA, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 50 μg/ml of gentamycin, and 25 μM of 2-mercaptoethanol with a total volume in each well of 200 μl. Cultures were incubated for 4 days at 37°C in 5-7% CO2. 3H-thymidine (2 μCi/well) was added for the last 18 hr of culture. Percent inhibition was calculated as 100 – (avg cpm sample cultures with CD4+CD25+ T cells / avg cpm sample cultures without CD4+CD25+ T cells × 100).
Statistical analyses
Statistical analyses were performed using STATView software (Abacus Concepts, Inc., Berkeley, CA). P-values were calculated using Log-rank and paired τ-test. A P-value < 0.05 was considered statistically significant.
Results
Anti-CD40L antibody treatment achieves long-term survival of primary vascularized skin allografts
First, we compared the rejection of skin allografts that were either vascularized at the time of their placement or not. Fully allogeneic skin grafts from C57BL/6J (B6, H-2b) mice were transplanted on C3H/HeJ (C3H, H-2k) or BALB/cJ (BALB/c, H-2d) recipients. All of the non-vascularized grafts were rejected in an acute fashion (MST: BALB/c: 9 ± 2 days, C3H: 10 ± 1 days). Vascularized skin grafts were also acutely rejected although in a slight delayed manner MST: (BALB/c 11 ± 2 days, C3H: 12 ± 2 days). Similar data were obtained when B6 were used as recipients of BALB/c grafts (see survival curves in Supplemental Fig. 1).
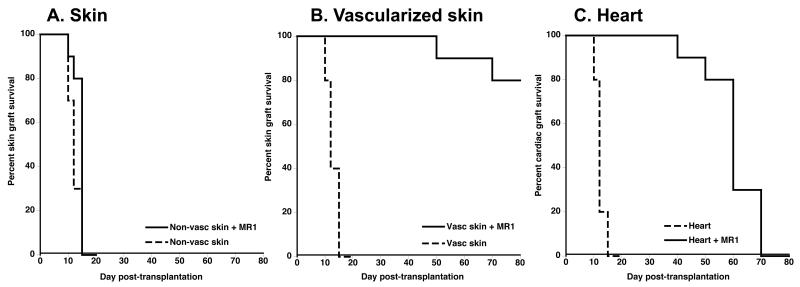
It is well known that prolonged survival or tolerance of skin allografts is difficult to accomplish. In contrast, this is regularly achieved is heart-transplanted mice via multiple treatments including costimulation blockade (9, 18). Here, we hypothesized that this difference relies on graft vascularization at the time of transplantation. To test this, we compared the survivals of conventional skin allografts with those of vascularized skin and heart allografts in recipients treated with anti-CD40L mAbs (MR1, 0.5 mg injected I.P. at day 0, 2 and 4) using the B6 to C3H donor-recipient combination. This model was chosen because short-term treatment of recipients with anti-CD40L monoclonal antibodies (MR1) has been shown to markedly prolong the survival of heart but not conventional skin allografts (18). Delayed rejection of cardiac allografts in this model is essentially due to inhibition of alloreactive CD4+ T cells (20). Our experiments showed that, as anticipated, anti-CD40L-treatment had no substantial impact on the survival of conventional skin allografts (MST: 10d) while it delayed the rejection of cardiac allografts (MST: 55d) (Fig. 1). However, remarkably, MR1 treatment achieved long-term survival of vascularized skin transplants (MST:82d) (Figure 1). Actually, these skin allografts survived significantly longer than their cardiac counterparts (P = 0.0012). In addition, it is noteworthy that late rejection of vascularized skin allografts did not follow a common course in that they shrunk gradually starting at day 60-70 post-transplantation and never exhibited apparent signs of inflammation or necrosis. As they reduced in size, the allografts became replaced by the recipient’s own skin, a process which were frequently completed by d100 post-transplantation. Therefore, primary vascularization is sufficient to accomplish long-term survival of skin allografts via anti-CD40L mAb costimulation blockade.
Figure 1. Anti-CD40L mAb treatment prolongs the survival of vascularized skin allografts.
C3H mice were transplanted with a conventional B6 skin allograft (panel A), a vascularized B6 skin allograft (B) or a B6 heart (C) and injected intraperitoneally with PBS (dotted lines) or with anti-CD40L mAbs (MR1 given i.p., 0.5 mg at d0, and 2 and 4 post-transplantation) (solid lines). The results are shown as percent graft survival over time after transplantation. Four to eight mice were tested in each group. Graft survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test.
Primarily vascularized allografts induce direct but no indirect T cell-mediated alloresponses
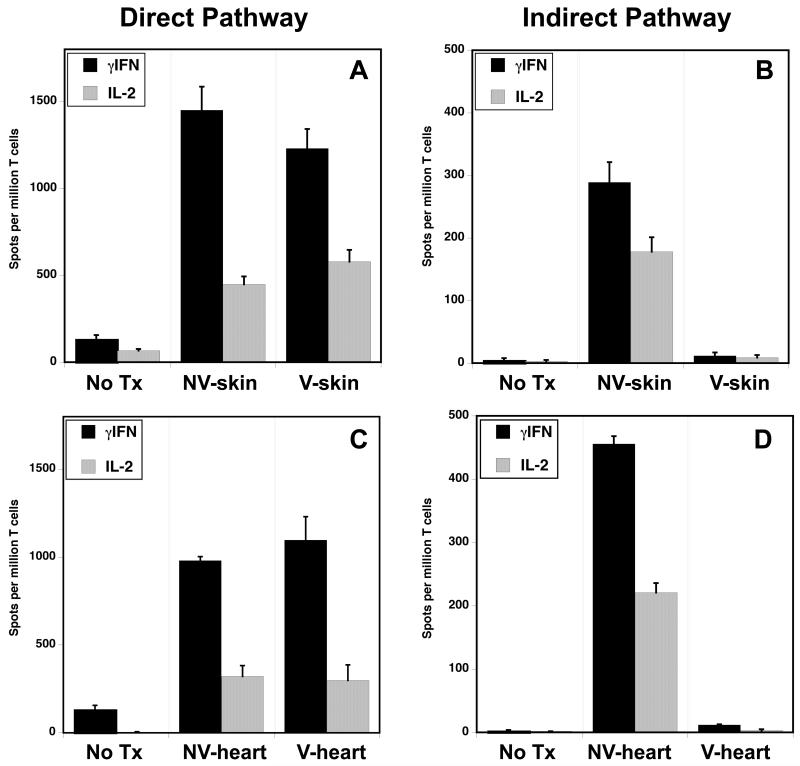
These observations suggested that graft vascularization could influence the nature and/or magnitude of the T cell alloresponse. Typical non-vascularized skin allografts are known to induce potent direct and indirect pro-inflammatory alloresponses by recipient T cells (3). Here, we investigated the effect of primary skin graft vascularization on the recipient’s anti-donor T cell response. T cells were isolated from the lymph nodes and spleens of C3H mice transplanted with a conventional or primarily vascularized B6 skin allograft (9-11 days POD). The frequencies of T cells secreting pro-inflammatory IL-2 and γIFN cytokines following a 24-48 hours culture in the presence of intact donor APCs (direct pathway) or recipient APCs + donor sonicates (indirect pathway) were measured by ELISPOT as previously described (3). As anticipated, recipients of standard skin allografts mounted potent direct and indirect T cell alloresponses characterized by the expansion of activated T cells secreting pro-inflammatory (type 1) cytokines, IL-2 and γIFN (Figure 2A and B). In contrast, recipients of vascularized skin grafts mounted a direct but not indirect alloresponse (Figure 2A and B). No significant production of type 2 cytokines (IL-4 and IL-10) was recorded with either type of skin graft (Supplemental Fig. 3 A and B). Third-party allogeneic APCs from an irrelevant donor induced a low direct response corresponding to a primary MLR response (< 100 γIFN spots/million T cells) (Supplemental Fig. 3C). Therefore, vascularization of skin allografts is associated with lack of a pro-inflammatory indirect alloresponse by recipient T cells.
Figure 2. Primary vascularization of allografts is associated with lack of inflammatory T cell indirect alloreactivity.
The frequencies of T cells activated either via direct (panel A and C) or indirect allorecognition (panel B and D) and secreting pro-inflammatory cytokines type 1 cytokines were measured by ELISPOT. Alloresponses were measured using spleen T cells collected from naïve C3H mice (no Tx) and from C3H mice transplanted (d10 POD) with either conventional (non-vascularized, NV-skin) or primarily vascularized (V-skin) fully allogeneic B6 skin allografts (panel A and B). Panel C and D show direct and indirect alloresponses recorded in C3H mice transplanted with a typical vascularized B6 heart (V-heart) or a non-vascularized B6 heart (NV-heart). The results are expressed as spots per million T cells for each cytokine ± SD. 5-8 mice were tested in each group.
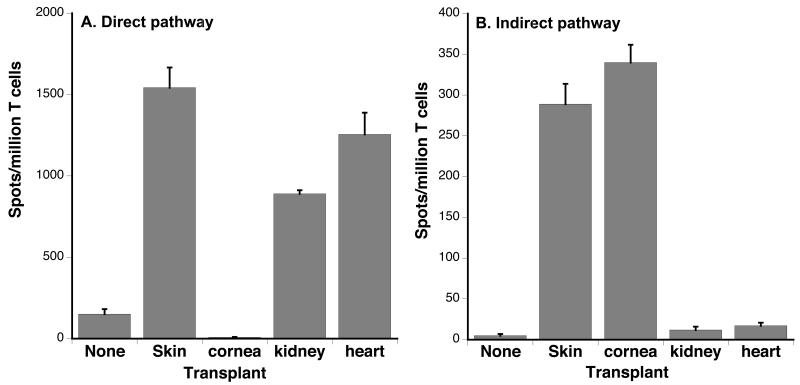
To further investigate the influence of graft vascularization on the nature of T cell alloreactivity, we measured direct and indirect responses of T cells in C3H recipients of either vascularized or non-vascularized (placed under the skin) B6 cardiac allografts. Both types of transplants induced a vigorous direct alloresponse (Fig. 2C). In contrast, only recipients of non-vascularized cardiac transplants mounted an indirect alloresponse (Figure 2D). Together with our observations in the skin graft model, these results suggested that the ability to trigger an indirect alloresponse is not an intrinsic property of the transplant but it depends upon its vascularization at the time of placement. In further support of this view was the observation that vascularized kidney allografts also failed to induce an indirect alloresponse, while it was triggered by corneal allografts that are non-vascularized grafts (Figure 3). Therefore, primary vascularization controls the immunogenicity of allografts in that it governs their ability to initiate a proinflammatory indirect T cell alloresponse.
Figure 3. Direct and indirect alloresponses induced in different transplant models.
The frequencies of alloreactive T cells secreting γIFN though the direct (A) or indirect (B) pathway were measured by ELISPOT using spleen T cells collected from C3H mice transplanted with either non-vascularized (skin or cornea) or vascularized (heart or kidney) fully allogeneic B6 transplants. The results are expressed as numbers of γIFN-forming spots per million T cells ± SD and are representative of 4-18 mice tested individually in each group.
Anti-CD40L mAb treatment suppresses direct but not indirect alloresponses by T cells
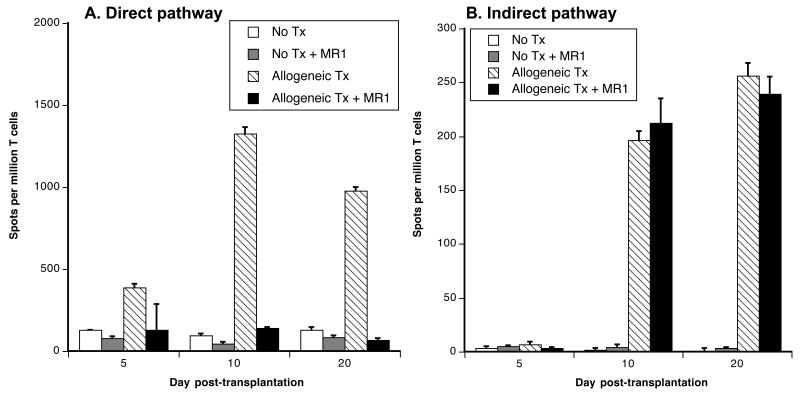
It is firmly established that anti-CD40L mAb (MR1) treatment inhibits T cell direct alloreactivity and prevent the acute rejection of heart but not conventional skin allografts (21). At the same time, we showed that MR1 delays the rejection of skin allografts when they are vascularized at the time of transplantation (Fig. 1). Since only conventional skin allografts induce an indirect alloresponse, this suggested that MR1 might not prolong the survival of non-vascularized skin grafts because it fails to suppress the indirect alloresponse. To address this possibility, direct and indirect pro-inflammatory alloresponses (γIFN) were measured in mice transplanted with a conventional skin allograft and treated with anti-CD40L mAbs. We observed that the direct alloresponse was thwarted by day 5 post-transplantation (Fig. 4A) whereas the anti-CD40L mAb treatment reduced but did not abolish the indirect alloresponse by γIFN-secreting T cells (Fig. 4B). Similar results were obtained following stimulation of T cells with donor-derived peptides thus excluding that persistence of the indirect alloresponse was due to a non-specific effect of the sonicates (Supplemental Figure 4). It is plausible that this “MR1-resistant” residual pro-inflammatory indirect alloresponse causes the rejection of conventional skin allografts in MR1-treated mice.
Figure 4. Anti-CD40L mAb treatment abrogates direct but not indirect T cell alloresponses.
C3H mice were transplanted with a conventional B6 skin allograft and treated with anti-CD40L mAbs (MR1, 0.25 mg given i.p. at d2, 4 and 6 post-transplantation) (solid bars). The frequencies of T cells secreting γIFN following activation through the direct (panel A) or indirect (panel B) allorecognition pathway were tested at d5, d10 and d20 post-transplantation. Control non-transplanted mice (white bars) and non-transplanted mice treated with MR1 (striated bars) as well as mice transplanted but not treated (grey bars) were also tested. The results are expressed as numbers of γIFN-forming spots per million T cells ± SD and are representative of 8-12 mice tested individually in each group.
Tolerance to vascularized skin allografts is donor-specific
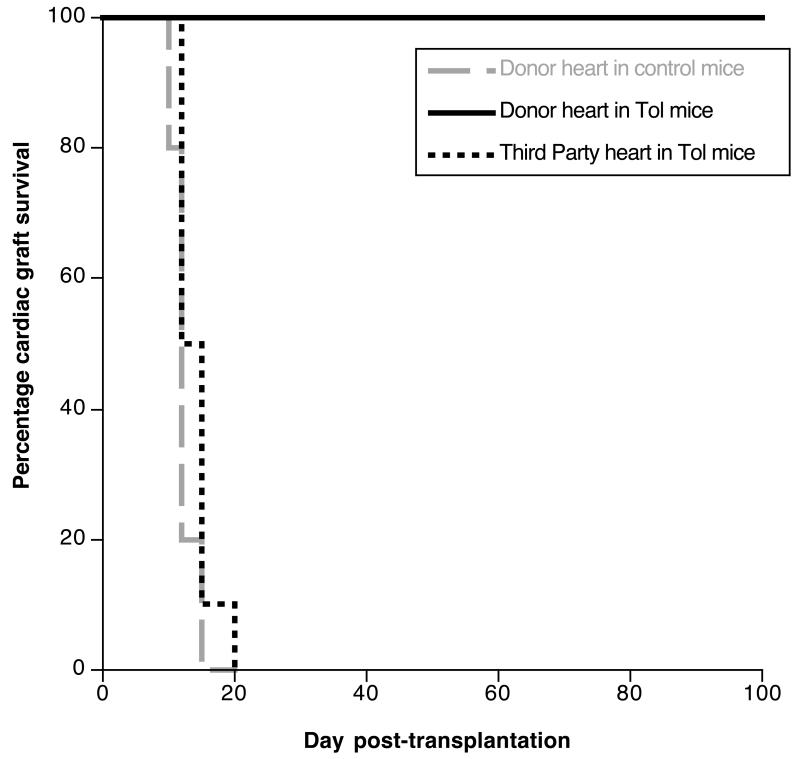
Next, we investigated the mechanisms by which MR1 treatment achieved long-term survival of vascularized skin transplants. It was possible that MR1 mediated its effect simply via suppressing the direct alloresponse. At the same time, combined costimulation blockade (MR1) along with alloantigen presentation (allograft) could have triggered some regulatory mechanisms inducing transplant tolerance. To address this question, MR1-treated BALB/c mice, which had accepted a B6 vascularized skin allograft for 50 days, were transplanted with a donor-matched (B6) or third-party (C3H) heart allotransplant. B6 cardiac transplants survived indefinitely and showed no signs of chronic rejection while third-party C3H hearts were rejected acutely (Fig. 5). This result shows that anti-CD40L mAb-treatment had induced donor-specific tolerance in recipients of vascularized skin allografts. The observation that secondary heart transplants (Fig. 5) survived much longer than their primary counterparts (Fig. 1) (p < 0.005) presumably reflects the tolerogenic effects exerted by vascularized skin grafts placed in MR1-treated recipients.
Figure 5. Long-term survival of vascularized skin allografts in MR1-treated mice is associated with donor-specific tolerance.
MR1-treated C3H mice, which had accepted a vascularized B6 skin allograft for 50 days, were transplanted with a heart from the same B6 donor (black solid line) or a third-party BALB/c heart (black dotted line). Control non-treated C3H mice were transplanted with a B6 heart (grey dotted line). The results are shown as percent graft survival over time after transplantation. Four to eight mice were tested in each group. Graft survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test.
Long-term survival of vascularized skin allografts is associated with directly activated T cells producing type 2 cytokines
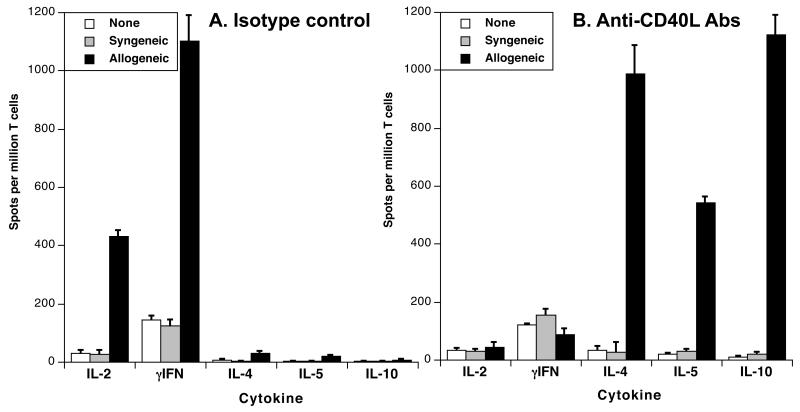
Some evidence has been provided suggesting that short-term blockade of signals given to T cells via CD28/B7 and CD40L/CD40 suppresses TH1 responses while sparing TH2 immunity (22, 23). This incited us to compare the frequencies of activated T cells producing either type 1 (IL-2, γIFN) or type 2 (IL-4, IL-10) cytokines in mice treated with MR1 anti-CD40L Abs or a control Ab displaying the same isotype, 10 days after placement of a vascularized skin allograft. In MR1-treated mice, the frequencies of type 1 cytokine-secreting cells were markedly reduced as compared to untreated recipients (Fig. 6A). Concurrently, these mice mounted a potent direct alloresponse mediated by T cells producing type 2 cytokines, IL-4, IL-5 and IL10 (Fig. 6B). No cytokine secretion was observed with unstimulated T cells or T cells exposed to syngeneic stimulators. Therefore, anti-CD40L mAb treatment of mice transplanted with a vascularized skin allograft was associated with an immune deviation of the alloresponse towards the TH2 phenotype.
Figure 6. MR1 treatment promotes the activation of T cells secreting type 2 cytokines.
C3H mice were transplanted with a B6 conventional skin allograft and injected with medium (panel A) or MR1 anti-CD40L mAbs as described earlier. Ten days later, spleen T cells were collected and restimulated in vitro with PBS (white bars), syngeneic APCs (grey bars) or irradiated donor APCs (solid bars) (direct allorecognition). The frequencies of activated T cells secreting type 1 (IL-2 and γIFN) and type 2 (IL-4, IL-5 and IL-10) cytokines were measured by ELISPOT. The results are expressed as numbers of cytokine-forming spots per million T cells ± SD and are representative of 3-5 mice tested individually in each group.
Tolerance of skin allografts is not associated with activation or expansion of FoxP3+ regulatory T cells
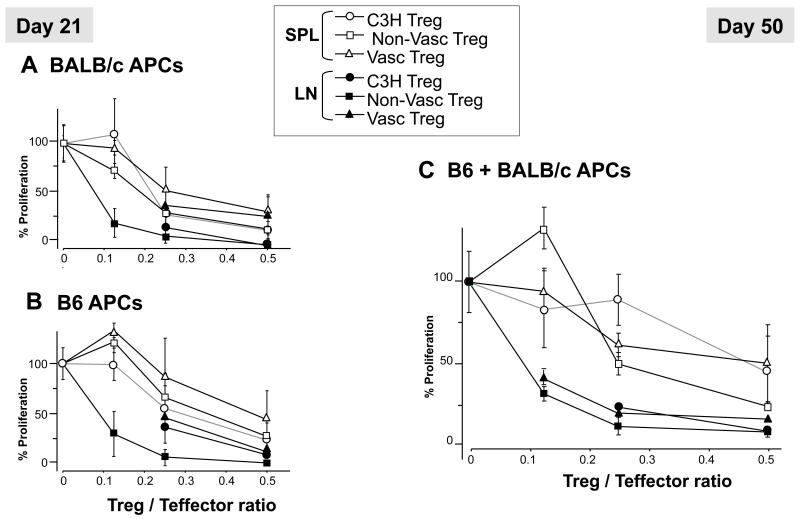
Tolerance of vascularized solid organ transplants has been associated with the emergence of CD4+CD25+Foxp3+ suppressive Treg cells in murine, primate and human transplantation models (24-26). Thus, we next evaluated the impact of graft vascularization on Tregs. Tregs numbers and suppressive functions were evaluated in naïve C3H mice and recipients of vascularized or non-vascularized skin grafts treated with MR1. The overall frequencies of CD4+CD25+Foxp3+ and their percentages among CD4+ T cells, measured in the spleen at d7, d21 and d50 post transplantation, were similar in both transplanted groups (12.5 +/− 2% and 12.7 +/− 1% for the non-vascularized and vascularized transplants, respectively). We observed a modest but not significant increase in the percentages of Tregs from the lymph nodes of mice grafted with a conventional skin as compared to naïve mice and recipients of a vascularized graft (10.8 +/− 1% versus 8.8 +/− 1% and 9.3 +/− 2%, respectively; p = 0.07). Finally, we compared the ability of Tregs collected from either spleen or lymph nodes of each group of mice at d21 (Fig. 7A and B) and d50 (Figure 7C) after skin grafting. As shown in Figure 7, lymph node Tregs (LN:solid symbols) were more potent suppressors than their splenic counterparts (SPL:open symbols) but there was no significant differences in the suppressive ability of Tregs isolated from recipients of vascularized and non-vascularized skin grafts. The alloresponses of C3H effector CD4+ T cells to donor BALB/c (Figure 7) and third-party B6 APCs (Figure 7) were inhibited in a similar fashion by C3H Tregs, suggesting a lack of donor antigen-specificity of Treg suppression. Finally, treatment of recipients with PC61, an anti-CD25 mAb (PC61) known to deplete CD25highFoxP3+ Tregs (27), given at the time of transplantation did not affect the survival of vascularized skin allografts in MR1-treated mice (data not shown). Collectively, these results support the view that long-term survival of primarily vascularized skin allografts induced by MR1 Abs was not associated with an increase in the frequency and function of peripheral FoxP3+ Treg cells.
Figure 7. Treg suppression assays.
Naïve CD4+CD25− T effector cells from graft recipient-type (C3H) were incubated with CD90-depleted irradiated splenocytes from donor B6, third party (BALB/c), or a 1:1 mixture of BALB/c and B6 cells. CD4+CD25+ Treg cells were isolated from naïve C3H mice (circles) and C3H mice transplanted with either a conventional (squares) or a vascularized (triangles) B6 skin allograft. Tregs were added to the coculture at the indicated Treg/T effector ratios (X-axis), and suppression of alloreactive cell proliferation was measured by thymidine incorporation. (A and B): suppression of alloresponses to third-party (A) or donor (B) by spleen (SPL, opened symbols) and lymph node (LN, closed symbols) Tregs collected 21 days after transplantation. (C): Similar studies were performed using Tregs collected at d50 post-transplantation from spleen and lymph nodes and CD4+ T effector cells stimulated by an equal number of B6 and BALB/c APCs.
Discussion
Primary vascularization of fully allogeneic skin grafts did not improve their survival. This finding confirms previous results obtained in rodents transplanted with skin flaps, composite tissue allografts and skin grafts parked under kidney capsules (28, 29). Indeed, our study shows that these allografts induce potent direct pro-inflammatory alloresponses, which are sufficient to provoke their early acute rejection.
In contrast to conventional skin allografts that activate T cells via both direct and indirect pathways, primarily vascularized skin transplants did not trigger a substantial indirect alloresponse. Similar results were obtained with vascularized heart and kidney transplants, while non-vascularized cardiac allografts triggered a potent indirect alloresponse. Altogether, these observations indicate that primary graft vascularization is generally associated with poor indirect alloreactivity. This suggests that the direct alloresponse is the only driving force behind acute rejection of vascularized transplants. In support of this view, previous studies from H. Auchincloss’ and R. Gill’s groups show that donor rather than recipient MHC class II expression, which triggers the CD4+ T cell direct alloresponse, is required for the rejection of heart allografts (30, 31). At first glance, this conclusion might appear in disagreement with previous reports involving indirect alloresponses in the acute rejection of kidney and cardiac allografts. However, most of these studies were performed either with recipients presensitized with donor MHC peptides emulsified in adjuvant or adoptively transferred with large number of indirectly activated T cells (32, 33). Although important, these results demonstrated that T cells activated in an indirect fashion can acutely reject vascularized grafts, but they did not conclude on the relevance of this pathway in unmanipulated hosts. Finally, it is noteworthy that, in contradiction with our results, a recent report by Brennan, T et al. shows induction of vigorous indirect alloresponses in BALB/c mice transplanted with a B6 heart (34). However, evidence of the contribution of this response to the acute rejection of these allografts was not provided. While indirect alloreactivity is not involved in acute rejection of solid organ transplants, it is possible that the sustained presentation of alloantigens by recipient APCs may lead to perpetuation of some oligoclonal alloresponse associated with chronic graft rejection (8, 30, 35). Finally, it is important to keep in mind that the indirect allorecognition pathway could contribute to acute rejection of vascularized organ transplants in “sensitized” recipients displaying indirectly activated and expanded memory T cells at the time of graft placement (36). We surmise that exposure to allo-MHC molecules following pregnancy, blood transfusion or a previous transplantation are among the elements accounting for the differentiation of long-lived memory T cells recognizing alloantigens in an indirect fashion in humans.
It is still unclear why the lack of primary vascularization results in potent indirect allosensitization of T cells after transplantation of conventional skin allografts. Since these transplants become vascularized only 4-5 days after their placement, it is conceivable that initial blood deprivation results in cell death, tissue damage and increased local inflammation. These circumstances are expected to enhance shedding of donor proteins and subsequent presentation of processed allopeptides by recipient APCs to T cells residing in draining lymph nodes. In addition, in the absence of vascularization, donor passenger leukocytes are likely to leave the graft exclusively through the lymphatics and concentrate in the recipient’s draining lymph nodes where the indirect alloresponse is likely to take place (15, 37). Indeed, this process is critical to the rejection process as evidenced by the seminal “pedicle” experiments of Barker and Billingham and recent studies from F. Lakkis’s group using aly/aly mice showing that alteration of cell trafficking via lymphatics after skin transplantation leads to prolonged allograft survival (38, 39). Alternatively, primary vascularization of allografts is presumably associated with a rapid emigration of donor passenger leukocytes via blood vessels rather than lymphatics and spreading of these cells throughout the body, a process which may not favor indirect priming of pro-inflammatory alloreactive T cells (40).
Treatment of mice with anti-CD40L mAbs prolonged the survival of cardiac but not conventional skin allografts in the B6 to C3H donor/recipient combination, a result consistent with previous reports from C. Larsen’s group (18). Remarkably, however, the same treatment significantly extended the survival of vascularized skin allotransplants. This demonstrates that vascularization rendered these skin grafts susceptible to tolerogenesis via costimulation blockade. This shows that, unlike traditionally believed, skin allografts are not intrinsically resistant to tolerance induction as compared to solid organ transplants.
Our study provides new insights into the mechanisms by which conventional skin allografts are rejected acutely despite costimulation blockade using anti-CD40L mAbs. It is firmly established that MR1 administration of C3H mice blocks the direct activation of alloreactive CD4+ T helper cells and subsequent differentiation of CD8 cytotoxic T cells (41). Therefore, in the absence of an indirect T cell-mediated alloresponse, inhibition of CD4+ T cell direct alloreactivity via MR1 treatment was sufficient to prevent acute rejection of cardiac allografts. Our results show that the same reasoning applies to the rejection of primarily vascularized skin allografts. On the other hand, since MR1 Abs failed to thwart indirect activation of T cells, it is likely that the indirect alloresponse was responsible for the acute rejection of conventional skin allografts in MR1-treated mice. Work is in progress in an effort to determine why allospecific T cells activated through the indirect pathway are resistant to anti-CD40L mAb treatment.
The mechanisms by which short-term MR1 treatment ensures long-term survival of vascularized skin allografts are not entirely clear. On one hand, it is possible that anti-CD40L mAbs prevent acute rejection primarily by suppressing the direct alloresponse. Since these allografts trigger a suboptimal indirect alloresponse, inhibition of direct alloreactivity is apparently sufficient to prevent the acute rejection process. On the other hand, MR1 treatment promotes some donor-specific tolerance effect since mice transplanted with a vascularized skin allograft subsequently accept heart transplants from the same donor but not a third-party one. Transplant tolerance has been previously achieved via MR1 treatment combined with donor specific transfusion (DST) (42-44). In this model, tolerance was attributed to regulatory T cells (Tregs) activated in an indirect fashion (45). At the same time, Auchincloss’ group has reported that MR1-induced long-term survival of cardiac allografts cannot be achieved in K14 mice, which lack the ability to mount a CD4+ T cell indirect alloresponse (46). Collectively, these studies suggested that transplant tolerance relies on self-MHC class II-restricted activation (indirect pathway) of Tregs (19). Furthermore, a recent study by Horibe et al. shows that indefinite survival of vascularized skin allografts achieved via injection of rapamycin-conditioned alloantigen pulsed DCs relied on Treg expansion (47). However, we did not obtain any evidence indicating the contribution of FoxP3+ Tregs to tolerance of vascularized skin allografts in our model. In turn, we showed that MR1 treatment is associated with immune deviation toward anti-inflammatory type 2 immunity as evidenced the high frequencies of activated T cells producing IL-4, IL-5 and IL-10 cytokines found in MR1-treated recipients of a vascularized allograft. Likewise, several studies have reported the activation of TH2, TH3 and/or FoxP3− Tr1 cells secreting IL-10 or TGFβ following systemic antigen administration via blood or oral routes (48-51). However, it is noteworthy that former studies by Bishop’s group showed that, in the absence of a TH1 response, activation of alloreactive TH2 cells was associated with an aggressive form of cardiac transplant rejection (52, 53). This suggests that donor-specific FoxP3− Tr1 cells, which secrete IL-10 and are known to prevent allograft rejection (54, 55), rather than “classical” FoxP3+ Tregs or TH2 cells contribute to long-term survival of vascularized skin allografts in MR1-treated mice.
Successful transplantation of allogeneic skin patches is essential to the treatment of patients with major burn injuries and recipients of composite tissue allografts. However, allogeneic skin grafts are invariably rejected in an acute fashion. While current immunosuppressive treatments are effective in preventing the early rejection of organ transplants such as kidneys, they have little or no effect in skin transplantation. Additionally, until now, many attempts to engineer artificial skin or to grow autologous skin tissue in vitro have been poorly effective. As a result, current clinical skin transplantation is largely confined to auto-transplantation of relatively small skin pieces. Seminal “pedicle” studies performed in the 1960’s by Barker and Billingham have demonstrated the key role of afferent lymphatics rather than blood vessels in the early allosensitization to and rejection of skin allografts (38). However, it has been difficult to adapt this principle in order to achieve immune tolerance in skin transplantation. Indeed, some studies have demonstrated that tolerance to conventional skin allografts can be reliably achieved in some animal models upon accomplishment of high level and stable donor hematopoietic mixed chimerism (11, 56). However, this procedure involves recipient’s whole body irradiation, profound depletion of peripheral lymphocytes, donor bone marrow transplantation and treatment with immunosuppressive drugs. Our study shows that primarily vascularized skin allografts are susceptible to tolerance induction via short-term costimulation blockade, a protocol that has only been effective thus far with kidney and heart transplants. This finding may have important implications in clinical skin transplantation. Further dissection of the tolerogenic effects associated with transplant vascularization and systemic alloantigen delivery through blood vessels will help unveil the basic mechanisms underlying transplantation tolerance.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH NIAID R03AI094235 and R21AI100278 to Gilles Benichou.
Footnotes
Disclosure of potential conflicts of interest:
No potential conflicts of interest were disclosed
Literature cited
- 1.Lechler RI, Lombardi G, Batchelor JR, Reinsmoen N, Bach FH. The molecular basis of alloreactivity. Immunology today. 1990;11:83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- 2.Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175:305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 4.Auchincloss H, Jr., Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci U S A. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illigens BM, Yamada A, Anosova N, Dong VM, Sayegh MH, Benichou G. Dual effects of the alloresponse by Th1 and Th2 cells on acute and chronic rejection of allotransplants. Eur J Immunol. 2009;39:3000–3009. doi: 10.1002/eji.200838980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell PS, Chase CM, Colvin RB. Alloantibody- and T cell-mediated immunity in the pathogenesis of transplant arteriosclerosis: lack of progression to sclerotic lesions in B cell-deficient mice. Transplantation. 1997;64:1531–1536. doi: 10.1097/00007890-199712150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Suciu-Foca N, Liu Z, Harris PE, Reed EF, Cohen DJ, Benstein JA, Benvenisty AI, Mancini D, Michler RE, Rose EA, et al. Indirect recognition of native HLA alloantigens and B-cell help. Transplantation proceedings. 1995;27:455–456. [PubMed] [Google Scholar]
- 8.Lee RS, Yamada K, Houser SL, Womer KL, Maloney ME, Rose HS, Sayegh MH, Madsen JC. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci U S A. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakkis FG, Konieczny BT, Saleem S, Baddoura FK, Linsley PS, Alexander DZ, Lowry RP, Pearson TC, Larsen CP. Blocking the CD28-B7 T cell costimulation pathway induces long term cardiac allograft acceptance in the absence of IL-4. J Immunol. 1997;158:2443–2448. [PubMed] [Google Scholar]
- 10.Larsen CP, Pearson TC. The CD40 pathway in allograft rejection, acceptance, and tolerance. Current opinion in immunology. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 11.Sykes M, Szot GL, Swenson KA, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nature medicine. 1997;3:783–787. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr., Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinmuller D. Skin allograft rejection by stable hematopoietic chimeras that accept organ allografts sill is an enigma. Transplantation. 2001;72:8–9. doi: 10.1097/00007890-200107150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nature medicine. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billingham RE, Medawar PD. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385. [Google Scholar]
- 18.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 19.LeGuern C, Akiyama Y, Germana S, Tanaka K, Fernandez L, Iwamoto Y, Houser S, Benichou G. Intracellular MHC class II controls regulatory tolerance to allogeneic transplants. J Immunol. 184:2394–2400. doi: 10.4049/jimmunol.0803664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingaman AW, Ha J, Durham MM, Waitze SY, Tucker-Burden C, Cowan SR, Pearson TC, Larsen CP. Analysis of the CD40 and CD28 pathways on alloimmune responses by CD4+ T cells in vivo. Transplantation. 2001;72:1286–1292. doi: 10.1097/00007890-200110150-00018. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Alexander DZ, Hollenbaugh D, Elwood ET, Ritchie SC, Aruffo A, Hendrix R, Pearson TC. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61:4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kishimoto K,DVMISFEVWAMYASMBGAHJGMJ. The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest. 2000;106:63, 72. doi: 10.1172/JCI9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobbold SP, Adams E, Graca L, Daley S, Yates S, Paterson A, Robertson NJ, Nolan KF, Fairchild PJ, Waldmann H. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunological reviews. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Torrealba JR, Katayama M, Fechner JH, Jr., Jankowska-Gan E, Kusaka S, Xu Q, Schultz JM, Oberley TD, Hu H, Hamawy MM, Jonker M, Wubben J, Doxiadis G, Bontrop R, Burlingham WJ, Knechtle SJ. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. J Immunol. 2004;172:5753–5764. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 26.Salama AD, Najafian N, Clarkson MR, Harmon WE, Sayegh MH. Regulatory CD25+ T cells in human kidney transplant recipients. Journal of the American Society of Nephrology: JASN. 2003;14:1643–1651. doi: 10.1097/01.asn.0000057540.98231.c1. [DOI] [PubMed] [Google Scholar]
- 27.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perloff LJ, Barker CF. Studies of the immediately vascularized skin allograft. Br J Surg. 1980;67:289–293. doi: 10.1002/bjs.1800670420. [DOI] [PubMed] [Google Scholar]
- 29.Iwata T, Kamei Y, Esaki S, Takada T, Torii S, Yamashita A, Tomida S, Tamatani T, Miyasaka M, Yoshikai Y. Immunosuppression by anti-ICAM-1 and anti-LFA-1 monoclonal antibodies of free and vascularized skin allograft rejection. Immunobiology. 1996;195:160–171. doi: 10.1016/S0171-2985(96)80036-2. [DOI] [PubMed] [Google Scholar]
- 30.Yamada A, Laufer TM, Gerth AJ, Chase CM, Colvin RB, Russell PS, Sayegh MH, Auchincloss H., Jr. Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am J Transplant. 2003;3:23–27. doi: 10.1034/j.1600-6143.2003.30105.x. [DOI] [PubMed] [Google Scholar]
- 31.Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106:1003–1010. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fangmann J, Dalchau R, Fabre JW. Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides. J Exp Med. 1992;175:1521–1529. doi: 10.1084/jem.175.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss MJ, Guenther DA, Mezrich JD, Sahara H, Ng CY, Meltzer AJ, Sayre JK, Cochrane ME, Pujara AC, Houser SL, Sachs DH, Rosengard BR, Allan JS, Benichou G, Madsen JC. The indirect alloresponse impairs the induction but not maintenance of tolerance to MHC class I-disparate allografts. Am J Transplant. 2009;9:105–113. doi: 10.1111/j.1600-6143.2008.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan TV, Jaigirdar A, Hoang V, Hayden T, Liu FC, Zaid H, Chang CK, Bucy RP, Tang Q, Kang SM. Preferential priming of alloreactive T cells with indirect reactivity. Am J Transplant. 2009;9:709–718. doi: 10.1111/j.1600-6143.2009.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suciu-Foca N, Ciubotariu R, Colovai A, Foca-Rodi A, Ho E, Rose E, Cortesini R. Persistent allopeptide reactivity and epitope spreading in chronic rejection. Transplantation Proceedings. 1999;31:100–101. doi: 10.1016/s0041-1345(98)01458-4. [DOI] [PubMed] [Google Scholar]
- 36.Valujskikh A, Heeger PS. CD4+ T cells responsive through the indirect pathway can mediate skin graft rejection in the absence of interferon-gamma. Transplantation. 2000;69:1016–1019. doi: 10.1097/00007890-200003150-00063. [DOI] [PubMed] [Google Scholar]
- 37.Steinmuller D. Passenger leukocytes and the immunogenicity of skin allografts. The Journal of investigative dermatology. 1980;75:107–115. doi: 10.1111/1523-1747.ep12521331. [DOI] [PubMed] [Google Scholar]
- 38.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. The Journal of experimental medicine. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nature medicine. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 40.Austyn JM, Larsen CP. Migration patterns of dendritic leukocytes. Implications for transplantation. Transplantation. 1990;49:1–7. doi: 10.1097/00007890-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Williams MA, Trambley J, Ha J, Adams AB, Durham MM, Rees P, Cowan SR, Pearson TC, Larsen CP. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165:6849–6857. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 42.Bushell A, Karim M, Kingsley CI, Wood KJ. Pretransplant blood transfusion without additional immunotherapy generates CD25+CD4+ regulatory T cells: a potential explanation for the blood-transfusion effect. Transplantation. 2003;76:449–455. doi: 10.1097/01.TP.0000083043.84630.99. [DOI] [PubMed] [Google Scholar]
- 43.Pearl JP, Xu H, Leopardi F, Preston E, Kirk AD. CD154 blockade, sirolimus, and donor-specific transfusion prevents renal allograft rejection in cynomolgus monkeys despite homeostatic T-cell activation. Transplantation. 2007;83:1219–1225. doi: 10.1097/01.tp.0000259929.04596.d5. [DOI] [PubMed] [Google Scholar]
- 44.Chalermskulrat W, McKinnon KP, Brickey WJ, Neuringer IP, Park RC, Sterka DG, Long BR, McNeillie P, Noelle RJ, Ting JP, Aris RM. Combined donor specific transfusion and anti-CD154 therapy achieves airway allograft tolerance. Thorax. 2006;61:61–67. doi: 10.1136/thx.2005.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–5816. [PubMed] [Google Scholar]
- 46.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss H., Jr. Recipient MHC class II expression is required to achieve long-term survival of murine cardiac allografts after costimulatory blockade. Journal of Immunology. 2001;167:5522–5526. doi: 10.4049/jimmunol.167.10.5522. [DOI] [PubMed] [Google Scholar]
- 47.Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, Marsteller D, Ferreira LM, Thomson AW, Lee WP, Feili-Hariri M. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008;18:307–318. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Faria AM, Weiner HL. Oral tolerance. Immunological reviews. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, McDevitt HO. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci U S A. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 51.Valujskikh A, VanBuskirk AM, Orosz CG, Heeger PS. A role for TGFbeta and B cells in immunologic tolerance after intravenous injection of soluble antigen. Transplantation. 2001;72:685–693. doi: 10.1097/00007890-200108270-00022. [DOI] [PubMed] [Google Scholar]
- 52.Csencsits K, Wood SC, Lu G, Magee JC, Eichwald EJ, Chang CH, Bishop DK. Graft rejection mediated by CD4+ T cells via indirect recognition of alloantigen is associated with a dominant Th2 response. Eur J Immunol. 2005;35:843–851. doi: 10.1002/eji.200425685. [DOI] [PubMed] [Google Scholar]
- 53.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 54.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. The Journal of experimental medicine. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roncarolo MG, Gregori S, Lucarelli B, Ciceri F, Bacchetta R. Clinical tolerance in allogeneic hematopoietic stem cell transplantation. Immunol Rev. 2011;241:145–163. doi: 10.1111/j.1600-065X.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 56.Horner BM, Randolph MA, Duran-Struuck R, Hirsh EL, Ferguson KK, Teague AG, Butler PE, Huang CA. Induction of tolerance to an allogeneic skin flap transplant in a preclinical large animal model. Transplantation proceedings. 2009;41:539–541. doi: 10.1016/j.transproceed.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.