Abstract
In the past decade, the growing field of telomere science has opened exciting new avenues for understanding the cellular and molecular substrates of stress and stress-related aging processes ver the lifespan. Shorter telomere length is associated with advancing chronological age and also increased disease morbidity and mortality. Emerging studies suggest that stress accelerates the erosion of telomeres from very early in life and possibly even influences the initial (newborn) setting of telomere length. In this review, we highlight recent empirical evidence linking stress and mental illnesses at various times across the lifespan with telomere erosion. We first present findings in the developmental programming of telomere biology linking prenatal stress to newborn and adult telomere length. We then present findings linking exposure to childhood trauma and to certain mental disorders with telomere shortening. Last, we review studies that characterize the relationship between related health-risk behaviors with telomere shortening over the lifespan, and how this process may further buffer the negative effects of stress on telomeres. A better understanding of the mechanisms that govern and regulate telomere biology throughout the lifespan may inform our understanding of etiology and the long-term consequences of stress and mental illnesses on aging processes in diverse populations and settings.
Keywords: Telomere length, telomerase, stress, lifespan, prenatal, fetal/developmental programming, childhood stress, mental health, depression, lifestyle
Introduction
Telomeres are the DNA-based caps and protein structures at the chromosome tips. Telomerase is the intracellular ribonucleoprotein that can help maintain and elongate telomeres. The telomere/telomerase maintenance system was originally studied in model systems, and now has been studied extensively in people. Telomeres are longest in germ cells, where they play an important role in cell replication throughout the lifespan, but are also important in any dividing tissue that must be replenished throughout life, from parts of the hippocampus, to blood, and bone. This complex cell aging system regulates the longevity of cells as well as senescence. In the last ten years, there has been a rapidly increasing epidemiological research body suggesting that telomere length (TL) serves as an early predictor of onset of disease and earlier mortality. It is interesting to note that although the word ‘aging’ is usually associated with old age, aging in the sense of telomeres is a life-time phenomenon that begins even before birth. Age-related diseases manifest mostly in old age but the aging process, at the cellular level, can be viewed as a lifelong progression. Indeed, abnormalities in telomere maintenance, resulting from mutations in telomere maintenance genes, are associated with premature aging in rare genetic diseases, collectively called ‘telomere syndromes’ (Armanios and Blackburn, 2012). Many clinical features of telomere syndromes are characteristic of geriatrics, and children with this disorder have a phenotype that resembles premature aging, signifying a causal link between telomere biology and aging.
Given the apparent centrality of this aging system in human health, it is important to identify the multitude of factors that shape TL early on in life, and promote TL maintenance throughout adulthood. While genetics play a role in regulating TL and telomerase activity, a wide range of environmental and behavioral factors also appear to affect TL. Stress has emerged as a major influence on telomere erosion. This brief review focuses on how life stress may impact telomere maintenance, starting from in utero (Figure 1). Stress shapes the biochemical milieu, in ways that may promote telomere damage, inflammation, and greater rate of leukocyte division in part through impairing telomerase mediated elongation, but also through other pathways, as explored elsewhere (Epel, 2012; Shalev, 2012). The shaping of stem cell health and turnover is influenced during development and early childhood. Novel research by Entringer and colleagues suggests that maternal stress during pregnancy may model offspring TL. Childhood adversity has been studied most, and appears to impact TL during the periods of exposure, as well as later in adulthood, although longitudinal studies are needed to establish how early adversity leads to longer-term effects. Depression, as well as other major mental disorders and physical disorders, have been linked to TL shortness, and it is likely that they are both influenced by cellular aging as well as contribute further to accelerate aging. Lastly, there are suggestions that healthy lifestyle factors may promote telomere maintenance or even lengthening; this may matter particularly in the face of adversity. Conversely, unhealthy lifestyle factors may significantly shorten telomeres. Together, a picture emerges that TL is an informative ‘clock’ that can be accelerated during critical periods or exposures, likely through different mechanisms. A better understanding of the mechanisms that mediate the effects of stress on telomere maintenance is an active avenue of investigation. Regardless of mechanism, shortened TL appears to index rate of biological aging and thus may provide insights into group and individual differences in early aging.
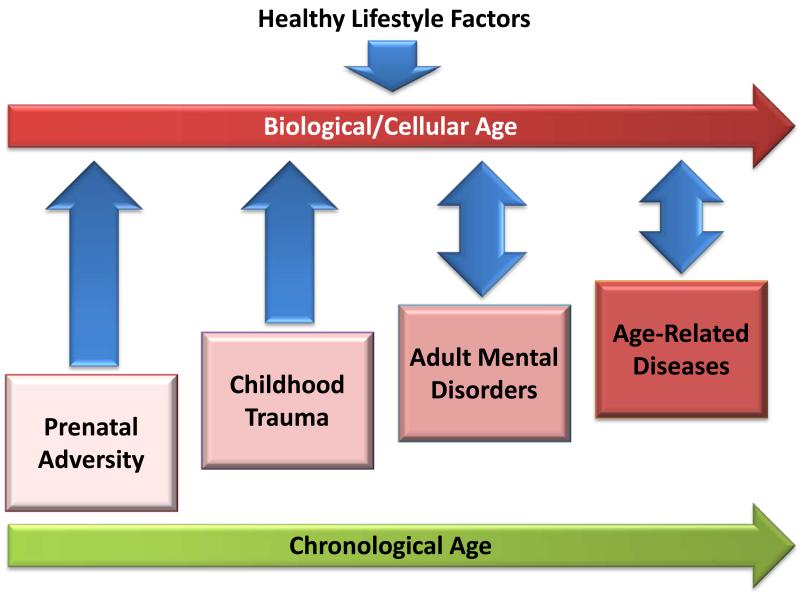
Figure 1. Schematic representation of lifespan influences on telomere length (biological/cellular age).
Horizontal arrows at the top and bottom of the figure illustrate the progression of biological aging in parallel to chronological aging. Boxes and arrows in the middle of the figure illustrate stress exposures at different points in the lifespan (pre-natal development, childhood, adulthood, and later life) that act to accelerate the pace of biological aging. Double-headed arrows for adult mental disorders and age-related diseases exposures indicate bidirectional influences between mental and physical health and cellular aging. The downward pointing arrow at the top of the figure indicates that healthy lifestyle factors may mitigate the deleterious effects of stress exposures on biological aging.
Fetal programming of telomere biology
Growing evidence from epidemiological, clinical, and molecular studies suggests that conditions during early development (i.e., embryonic, fetal and early postnatal periods of life) interact with the genome of an individual to exert a major impact on structural and functional integrity of the developing brain and other peripheral systems. This interaction, in turn, influence individual’s subsequent state of health and her or his propensity, or susceptibility, for developing one or more of the common physical or mental disorders that collectively represent the major burden of disease in society (i.e., the concept of fetal, or developmental, programming of health and disease risk). Consistent with this concept of fetal or developmental programming, we suggest that telomere biology (i.e., chromosomal TL and the activity of the enzyme telomerase) may be plastic during development and receptive to the influence of intrauterine and other early life conditions (Entringer et al., 2012a). The initial setting of TL in the newborn likely represents a critically-important aspect of an individual’s telomere biology system. For any given individual at any age TL depends on, first, the initial (newborn) setting of TL, and second, the magnitude of telomere erosion from birth onwards. Telomere erosion, in turn, depends on cell replication rate, cumulative exposure to agents that produce DNA damage (such as oxidative, inflammatory, endocrine and other forms of biological stress), and activity of the telomerase enzyme (Aviv, 2008). The consequences of shortened telomeres in adults are well-established. Thus, a reduction in the initial setting of TL likely confers greater susceptibility in later life for pathophysiological outcomes. Although at the present time there are no studies in humans linking early life telomere dynamics with later life health and disease risk, a recent study in zebra finches reported that TL measured in early life was a strong predictor of lifespan (Heidinger et al., 2012).
The determinants of newborn TL are poorly understood. Despite the relatively high heritability of TL, known genetic variants (from candidate gene as well as GWAS approaches) account for only a small proportion of the variance in TL (e.g.,(Mangino et al., 2012)). Indeed, it is likely that the initial setting of chromosomal TL and the activity of the enzyme telomerase may be plastic and receptive to the influence of intrauterine and other early life conditions (Entringer et al., 2012a). Extrinsic and intrinsic conditions representing energetic resources and challenges (threats) to survival and reproduction epitomize the key processes underlying natural selection and developmental plasticity, and thus intrauterine stress warrants particular consideration as a candidate mechanism implicated in the programming of the telomere biology system. Stress-related maternal-placental-fetal endocrine, immune and oxidative processes represent an attractive candidate mechanism. First, they are exquisitely sensitive to a diverse array of potentially adverse physiological (metabolic), social, environmental and clinical exposures (summarized in (Entringer et al., 2010)). Second, they serve as the key signaling molecules between the fetal and maternal compartments during intrauterine development (Wadhwa, 2005). And third, they may exert stable, long-term effects via epigenetic and other processes (e.g., actions on DNA methyltransferase) on key components of the developing telomere biology system that influence the initial setting of TL and the tissue- and stage-of-development-specific regulation of telomerase expression.
There is relatively little empirical literature to date that has addressed the issue of the link between exposure to prenatal adversity and telomere biology. Animal studies that have manipulated maternal nutrition during pregnancy (e.g., protein restriction) have reported effects on offspring TL in different tissues and organs. A recent study in chickens reported that prenatal administration of the stress hormone cortisol in the yolk resulted in a higher proportion of short telomeres (and increased levels of reactive oxygen metabolites as well as increased duration of the acute stress response) in the offspring compared to a non-treated control group (Haussmann et al., 2011). Human studies in this area have, for the most part, examined the effects of obstetric risk conditions during pregnancy, such as fetal growth restriction, diabetes and preeclampsia, on placental and newborn TL and telomerase activity (reviewed in (Entringer et al., 2012a)). Less is known about effects of stress exposure during the intrauterine life with telomere biology. We recently published the first human study of the association between maternal exposure during pregnancy to severe psychosocial stress and offspring TL in young adulthood (Entringer et al., 2011). The effect equated approximately to an additional 3.5 years of cellular aging in prenatally-stressed offspring, was more pronounced in women, and was unchanged after adjusting for potential confounders (subject characteristics, birth weight, and early-life and concurrent stress level). In a second, smaller prospective study we found that maternal pregnancy-specific stress (worries about the health of the unborn child) assessed in early pregnancy significantly predicted newborn leukocyte TL (Entringer et al., 2012b). After accounting for the effects of potential determinants of newborn leukocyte TL (gestational age at birth, weight, sex and exposure to antepartum obstetric complications), there was a significant, independent, linear effect of pregnancy-specific stress on newborn leukocyte TL that accounted for 25% of the variance in adjusted leukocyte TL, thereby replicating and extending our previously-published finding on prenatal stress exposure and adult offspring TL.
Thus, based on the theoretical considerations and empirical evidence outlined above, Entringer and colleagues (Entringer et al., 2012a) have advanced the hypothesis that context- and time-inappropriate levels of physiological stress exposure (maternal-placental-fetal endocrine, immune/inflammatory and oxidative stress) during the intrauterine period of development may alter or program the telomere biology system (i.e., the initial setting of TL and telomerase expression capacity) in a manner that accelerates cellular dysfunction, aging and disease susceptibility over the lifespan. It is likely that extreme levels of stress exposure in infants and children may also deeply impact telomere biology maintenance abilities, a new area of study.
Early life stress and telomere length
Childhood stress, a major public-health and social-welfare problem, is known to have a powerful direct effect on poor health in later life. But how can stress during early life lead to health problems that only emerge decades later? This direct effect requires one or more underlying mechanisms that can maintain it across the life-course. Now, new evidence suggests telomere erosion is a potential mechanism for the long-term cellular embedding of stress.
In the past few years, several studies of adult participants have provided support for an association between childhood history of stress and shorter TL (reviewed in (Price et al., 2013; Shalev, 2012)). In contrast to previous findings, one study failed to replicate the association between leukocytes TL and physical and sexual abuse in childhood in a large cohort of adult twins. In the first study of children, greater exposure to institutional care was significantly associated with shorter TL in buccal cells in middle childhood (Drury et al., 2011). These cross-sectional studies had documented a correlation between TL and stress. It remained unknown whether stress exposure, as opposed to its disease sequelae, caused telomere erosion. The hypothesis that childhood violence exposure would accelerate telomere erosion was recently tested in the first prospective-longitudinal study in children (Shalev et al., 2012). Based on evidence that the effects of stress are cumulative, the hypothesis was that cumulative exposure to violence would be associated with accelerated telomere erosion. Indeed, only children who experienced multiple forms of violence exposure (either exposure to maternal domestic violence, frequent bullying victimization or physical maltreatment by an adult) showed significantly more telomere erosion in buccal cells between age-5 baseline and age-10 follow-up measurements, even after adjusting for confounding factors (Shalev et al., 2012). This finding provided the first evidence that stress-related accelerated telomere erosion can be observed already at young age while children are experiencing stress. Importantly, the violence-exposed children who experienced more rapid telomere erosion had not yet developed chronic disease, suggesting that telomere erosion may be a link in the causal chain connecting early-life stress exposure to later life disease.
One of the most challenging questions concerns our understanding of the mechanisms linking early life stress, and stress in general, to telomere dynamics. With the case of childhood stress, the effect of stress on TL during sensitive developmental periods and age-dependent maturation of the brain and immune-system (Danese and McEwen, 2011) may play a critical role for precipitating this long-term damage. Currently, most of the insights about mechanisms associated with telomere erosion originate from research on inflammation and oxidative stress, indicating both as important influences on TL. Several studies have shown that childhood stress predicts elevated inflammation (Danese et al., 2007) and also that individuals with early life stress have heightened inflammatory response to psychosocial stress. Moreover, childhood adversity among older adults predicted both higher inflammatory markers and shorter TL in blood cells (Kiecolt-Glaser et al., 2011). Inflammation is also associated with increased proliferation of immune cells and, as a consequence, with more telomere erosion. These studies suggest a mediating role for inflammation linking early life stress to telomere erosion. The endocrine system is another plausible route for mediating the effects of early life stress. The connection between cortisol, oxidative stress and cell senescence is established (Behl et al., 1997). Cortisol has been associated with reduced telomerase activation of human T lymphocytes in culture, and higher levels of cortisol in response to a laboratory stressor were associated with shorter TL in buccal cells of 5-to-6-year old children (Kroenke et al., 2011). Overall, stress-induced secretion of cortisol may down-regulate the activity of telomerase and increase oxidative stress which in turn can lead to more rapid erosion of telomeres. More research is needed to test whether effects of stress on telomere erosion are mediated by immune- and endocrine-system changes, oxidative stress, mitochondria dysfunction, or other factors in children.
Mental health disorders and telomere maintenance
Common mental disorders like depression and anxiety may also be associated with changes in telomere maintenance. Major depressive disorder (MDD) and other serious mental illnesses are associated with high rates of comorbid medical illnesses, many of which are more common in the elderly, such as cardiovascular disease, stroke and dementia. One possible explanation for this comorbidity is that these mental illnesses are associated with accelerated rates of cellular/ biological aging. As reviewed above, shortening of leukocyte TL indexes increased risk of medical illness, and several studies have now characterized leukocyte TL in MDD and other psychiatric illnesses (reviewed in (Wolkowitz et al., 2011)). Fewer psychiatric studies have characterized the activity of telomerase, an enzyme that can elongate and preserve telomeric DNA, in psychiatric illness. Further, few studies have investigated the biochemical mediators of accelerated biological aging in psychiatric illness. Including an initial study by Simon et al. that demonstrated shortened leukocyte TL in MDD (Simon et al., 2006), 10 studies in MDD, two in bipolar disorder, three in schizophrenia or other non-affective psychoses and three in anxiety disorders have been reported. Although disparate findings have been published, certain characteristics may be associated with heightened risk of leukocyte TL shortening. Also, certain biochemical mediators that are associated with serious mental illnesses as well as with biological aging are being identified.
Of the 10 studies in MDD, six reported significant leukocyte TL shortening in depressed subjects, 3 failed to detect significant differences, and one was partially positive, finding significantly shortened leukocyte TL only in individuals with more chronic lifetime exposure to depression. The positive studies were often in individuals with more chronic depression or with greater severity of symptoms, perhaps suggesting a “dose-response” relationship with leukocyte TL shortening, whereas the negative studies tended to use non-standardized or only self-report diagnostic criteria for MDD over brief periods of time, included population-based samples rather than clinical psychiatric samples, or failed to have adequate control groups. Patients with bipolar disorder may also have shortened leukocyte TL, but one of the studies only reported leukocyte TL in a mixed group of mood disorder patients rather than in bipolar patients exclusively, and the other study found only a trend level of shortening of mean leukocyte TL, although it found a significantly higher percentage of “short” telomeres in the bipolar cohort. In the latter study, leukocyte TL shortening was proportional to the number of lifetime depressive episodes but not with length of time since first diagnosis. In three separate studies, psychotic individuals were also reported to have shortened leukocyte TL, but in one, only patients with poor response to antipsychotics showed this effect. Finally, some but not all reports on individuals with anxiety disorders have shown shortened leukocyte TL. In one study of individuals with various anxiety-type disorders, only older individuals (48-87 years old) showed shortened leukocyte TL compared to age-matched controls, perhaps suggesting more chronic exposure to the disorder was required for the leukocyte TL shortening to be seen. In another study (in phobic individuals), only those with more severe symptoms showed leukocyte TL shortening. In the final anxiety disorder study, individuals with post-traumatic stress disorder (PTSD) showed significantly shortened leukocyte TL compared to controls, but this effect was largely determined by the presence of substantial adverse childhood events (a risk factor itself for PTSD) in those subjects. In summary, findings remain inconclusive regarding leukocyte TL shortening in serious mental disorders. A preponderance of studies has found significant leukocyte TL shortening, especially when rigorous diagnostic criteria are applied and when individuals with longer lifetime duration of symptoms or with greater severity of symptoms are studied. The latter observations may suggest a ‘dose-response’ relationship. It should be emphasized that the degree of leukocyte TL shortening reported in the positive studies reviewed here is not trivial and ranges from approximately six to 25 years of accelerated aging compared to age-matched controls, even when sex, age, tobacco usage, body-mass index and medical illnesses are taken into account.
The possibility that shortened leukocyte TL is seen across a wide variety of serious mental disorders makes it extremely unlikely that this phenomenon is specific to any particular psychiatric diagnosis. One possibility is that histories of multiple adverse childhood experiences, which are substantially more common in individuals with serious mental disorders, explain the leukocyte TL shortening rather than the mental disorders themselves. Another possibility is that leukocyte TL shortening relates to certain pathophysiological processes that transcend traditional psychiatric diagnoses. For example, several of the psychiatric conditions reviewed here have been associated with increased oxidative stress and with chronic inflammation, and both of these factors have been associated with shortening of leukocyte TL. In studies of individuals with various medical illnesses or MDD, as well as in individuals undergoing chronic psychological stress, peripheral indices of oxidative stress and inflammation have both been related to shortening of leukocyte TL, suggesting these may at least partially mediate this effect.
Leukocyte TL is also a function of telomere reparative process such as telomerase activity. Mixed findings regarding peripheral blood mononuclear cell (PBMC) telomerase activity have been reported in individuals undergoing chronic psychological stress, as well as in individuals with serious mental disorders. One study in individuals with schizophrenia and one in caregivers noted decreased telomerase activity, but another study in caregivers and one in un-medicated individuals with MDD noted significant increases in telomerase activity. The authors of the reports that noted telomerase increases suggested that this might represent a compensatory attempt in the face of incipient cell damage or telomere shortening. In the MDD study, baseline (un-medicated) telomerase levels were inversely correlated with subsequent antidepressant response, as were treatment-associated increases in telomerase activity (Wolkowitz et al., 2012). These findings, along with recent preclinical data suggesting antidepressant effects of telomerase, suggest a novel mechanism regulating treatment response in MDD.
In summary, data regarding cellular/ biological aging in serious mental disorders remain inconclusive. However, tantalizing leads are emerging. These might provide insights into the high comorbidity of medical illnesses in individuals with mental disorders and might suggest new approaches to categorizing and treating these disorders (Wolkowitz et al., 2011).
Health behaviors and telomere biology
Early chronic disease onset and early mortality are accounted for in large part by chronic poor health behaviors, including physical inactivity, poor diet, poor sleep, smoking and other tobacco use, and excessive alcohol consumption (Murray et al., 2013). The importance of healthy behaviors to the prevention and treatment of disease cannot be understated (Fisher et al., 2011). Work over the past decade directs attention to the many protective cellular effects of healthy behaviors that are mechanistically implicated in disease pathogenesis and early mortality. These protective cellular effects include, but are not limited to, maintaining TL in immune and neural cells.
Many studies have evidenced that each behavior alone is associated with TL and/or telomerase levels (reviewed in (Lin et al., 2012)). A combination of these healthy behaviors is also associated with longer telomeres. Here we highlight the studies that indicate associations between behavior and telomere maintenance. We primarily emphasize the work on physical exercise, for two reasons. In part, there is a strong literature of animal model studies that have illuminated specific preceding and ensuing biological mechanisms through which voluntary exercise impedes immune and neural cell telomere erosion. Most other studies in other health behaviors, to date, have shown only associations between the behavior of interest and telomeres and/or telomerase. We also highlight the work on exercise and physical activity since there are now several studies demonstrating that activity can also protect individuals from the negative effects of stress on cell aging - relevant to the current review.
Endurance exercise and fitness
Endurance exercise that increases fitness delays cell aging processes in rodents. Endurance exercise in rodents increases telomerase activity and telomere-stabilizing proteins expression in myocytes, endothelial cells of the vascular wall, immune and neural cells, in turn preventing apoptosis and cellular senescence. In humans, self-reported physical activity (Cherkas et al., 2008) and objective markers of fitness are associated with longer telomeres. In one study, telomerase levels were higher in athletes compared to sedentary non-athletes, even in young adulthood (Werner et al., 2009).
Puterman and colleagues (Puterman et al., 2012; Puterman et al., 2010; Puterman et al., 2011), as well as others (Rethorst et al., 2011), have examined how maintaining an active lifestyle mitigates the relationship between stress and biomarkers of disease. In one study, engaging in activity levels at those recommended by the Center for Disease Control and Prevention moderated the association between perceived stress and TL. Specifically, the association between perceived stress and shorter telomeres was limited to the inactive women. For the active women, perceived stress was not significantly associated with shorter telomeres (Puterman et al., 2010). New data suggests that life stress over the course of one year may predict telomere shortening over the same period only in individuals with unhealthy lifestyles (Puterman et al., unpublished data). These studies suggest that unhealthy behaviors may compound the negative effects of stress on cell aging.
Dietary patterns
Food choices seem to also shape TL (reviewed in (Paul, 2011)). Eating foods high in fiber and vitamins (both dietary and supplemental) are related to longer telomeres, whereas eating processed meats and foods high in polyunsaturated fats is related to shorter telomeres. In one study, patients with heart disease who were low at baseline in dietary omega-3 fatty acids had the greatest decline in TL over 5 years. While no studies have examined how drive to overeat or calorically restrict is associated to TL, it is known that women who are preoccupied with restraining their food intake have both higher cortisol and shorter telomeres (Kiefer et al., 2008).
Sleep
The role of sleep in immune system health and function is well described by others. Liang and colleagues (Liang et al., 2011) recently demonstrated that women under 50 years old who sleep less than 6 hours a night on average have shorter telomeres compared to women who sleep the an average of 9 hours. Additionally, our work suggests that women who report poor sleep quality have shorter telomeres as well.
Substance use
Excessive alcohol consumption (Pavanello et al., 2011), and cigarette smoking and tobacco use (Valdes et al., 2005) have also been associated with shorter telomeres.
Discussion
The German-French philosopher Albert Schweitzer once said that “the tragedy of life is what dies inside a man while he lives”. Although he was not referring to telomeres, it echoes well with new evidence from the field of telomere science. What dies inside us, or at least becomes senescent, are our cells, and it seems that telomeres are key elements in the causal chain of normal and premature senescence from very early in life. Moreover, recent empirical studies suggest that the telomere dynamics are influenced by environmental stress exposure, mental disorders, and health behaviors, as well as resilience to stress and trauma.
The length of telomeres appears to be an important predictor of health and disease. Nonetheless, not all studies report significant associations between stress exposures, or mental health disorders, and TL, and it is still not known whether stress exposure (as opposed to its disease sequelae, for example) is causing the erosion of telomeres. It may be that those most vulnerable to adult stress exposures are those who also have some predisposition, whether genetic, or acquired, such as prenatal or childhood adversity. Caution should be taken as more research is needed to elucidate mechanisms that govern TL dynamics. Moreover, although recent findings support the hypothesis of stress-related acceleration of cellular aging, even at young ages, and more studies provide plausible mechanistic pathways, there are more questions that require further research (Shalev, 2012). Recent longitudinal findings indicate caution because the temporal process of telomere erosion is more complex than initially assumed. For example, telomere erosion is inversely correlated with baseline TL, and also, in some individuals, telomeres lengthen over time. In addition, there are controversies regarding the best ways to measure TL. Another methodological question concerns the measurement of TL in different types of tissue cells. Because of ethical difficulties obtaining blood from children in the community, most studies in children have used buccal cells, instead of the peripheral blood cells more commonly used in studies of adults, thus limiting the generalization of these findings to other tissues.
Meanwhile, emerging body of evidence in the new field of telomeres help to address a basic-science puzzle of how and when stress gets ‘under the skin’ at the cellular level. In this review, we provided evidence that stress-related telomere erosion can be observed from very early in life. Prenatal stress exposure was linked to shorter TL in young adulthood (Entringer et al., 2011). More studies have documented an association between childhood trauma and shorter TL in adulthood, and in fact, several reviews have been devoted to this topic (Price et al., 2013; Shalev, 2012). Studies in adult clinical populations have provided further support. Several, but not all, studies in mental health disorders, including depression, bipolar disorder, anxiety disorders, PTSD and schizophrenia, have reported shorter TL (Wolkowitz et al., 2011). Interestingly, higher telomerase activity was associated with MDD among un-medicated individuals, suggesting a potential compensatory mechanism to overcome the telomere erosion associated with MDD. More research is needed to explore the effects of TL and telomerase in clinical and non-clinical settings.
There is also hope, however, that stress effects can be mitigated. Lifestyle factors and a healthy environment can help to buffer the deleterious effects of stress on telomere erosion (Puterman and Epel, 2012). It is also tempting to speculate that some of those factors (e.g., diet, physical activity and stress-reduction methods) involve two of the main mechanistic pathways in telomere integrity: immune-system and oxidative stress. More research is needed to elucidate the complex cascade leading from stress exposure during early life to cellular aging via telomere biology. Given that individuals who are exposed to stress during their early years show a faster erosion rate of TL, early intervention and prevention strategies can potentially ameliorate the acceleration of physiological aging processes early in life.
In sum, increasing numbers of studies in humans have implicated age-related TL as an important predictor of morbidity and mortality. Stress exposure in early life is linked with the same patterns of increased morbidity and mortality as shorter telomeres. Thus, TL is a promising new target for research into the long-term effects of stress throughout the lifespan. Elucidating the molecular mechanisms that regulate telomere dynamics, identifying intervening biological substrates that could serve as potential treatment targets, and discovering coping resources that may protect individuals from the adverse effects of stress on telomere erosion are primary future directions in this field. This multidisciplinary research has the potential to identify novel targets for interventions to help young children and adults recover from exposure to chronic stress. Taken together, this body of evidence suggests the importance of integrating telomeres as stress markers in research to evaluate the effects of stress throughout the lifespan.
Acknowledgments
This article was based on the 2012 Annual ISPNE symposium entitled-Cellular aging: From physical to mental syndromes. I.S. is supported by NICHD grant HD061298 and by the Jacobs Foundation.
References
- Armanios M, Blackburn EH. The telomere syndromes. Nature reviews. Genetics. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63:979–983. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- Behl C, LezoualcH F, Trapp T, Widmann M, Skutella T, Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2011;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, Fox NA, Zeanah CH, Nelson CA. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular psychiatry. 2011;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sciene Signaling. 2012a;5(12) doi: 10.1126/scisignal.2003580. [DOI] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E513–518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, Simhan HN, Wadhwa PD. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. 2012b doi: 10.1016/j.ajog.2012.11.033. doi:10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. How “Reversible” Is Telomeric Aging? Cancer Prevention Research. 2012;5:1163–1168. doi: 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- Fisher EB, Fitzgibbon ML, Glasgow RE, Haire-Joshu D, Hayman LL, Kaplan RM, Nanney MS, Ockene JK. Behavior Matters. Am J Prev Med. 2011;40:e15–e30. doi: 10.1016/j.amepre.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc Biol Sci. 2011;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer A, Lin J, Blackburn EH, Epel ES. Dietary Restraint and Telomere Length in Pre- and Postmenopausal Women. Psychosom. Med. 2008;70:845–849. doi: 10.1097/PSY.0b013e318187d05e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, Lin J, Blackburn E, Stamperdahl JL, Boyce WT. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73:533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang GY, Schernhammer E, Qi L, Gao X, De Vivo I, Han JL. Associations between Rotating Night Shifts, Sleep Duration, and Telomere Length in Women. PLoS ONE. 2011;6:e23462. doi: 10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: Roles in cellular aging. Mutat Res-Fund Mol M. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mangino M, Hwang SJ, Spector TD, Hunt SC, Kimura M, Fitzpatrick AL, Christiansen L, Petersen I, Elbers CC, Harris T, Chen W, Srinivasan SR, Kark JD, Benetos A, El Shamieh S, Visvikis-Siest S, Christensen K, Berenson GS, Valdes AM, Vinuela A, Garcia M, Arnett DK, Broeckel U, Province MA, Pankow JS, Kammerer C, Liu YM, Nalls M, Tishkoff S, Thomas F, Ziv E, Psaty BM, Bis JC, Rotter JI, Taylor KD, Smith E, Schork NJ, Levy D, Aviv A. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet. 2012;21:5385–5394. doi: 10.1093/hmg/dds382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22:895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, Ferrara SD, Montisci M, Baccarelli A. Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer. 2011;129:983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biological psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Adler NE, Matthews KA, Epel ES. Financial Strain and Impaired Fasting Glucose: The Moderating Role of Physical Activity in the Coronary Artery Risk Development in Young Adults Study. Psychosom. Med. 2012;74:187–192. doi: 10.1097/PSY.0b013e3182448d74. [DOI] [PubMed] [Google Scholar]
- Puterman E, Epel E. An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Social and personality psychology compass. 2012;6:807–825. doi: 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Blackburn E, Epel E. A one-year prospective study on major events, lifestyle, and telomere biology. unpublished data. [Google Scholar]
- Puterman E, Lin J, Blackburn EH, O’Donovan A, Adler NE, Epel ES. The Power of Exercise: Buffering the Effect of Chronic Stress on Telomere Length. Plos One. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, O’Donovan A, Adler NE, Tomiyama AJ, Kemeny M, Wolkowitz OM, Epel ES. Physical Activity Moderates Effects of Stressor-Induced Rumination on Cortisol Reactivity. Psychosom. Med. 2011;73:604–611. doi: 10.1097/PSY.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Moynihan J, Lyness JM, Heffner KL, Chapman BP. Moderating Effects of Moderate-Intensity Physical Activity in the Relationship Between Depressive Symptoms and Interleukin-6 in Primary Care Patients. Psychosom. Med. 2011;73:265–269. doi: 10.1097/PSY.0b013e3182108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. BioEssays: news and reviews in molecular, cellular and developmental biology. 2012;34:943–952. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.32. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biological psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Scharhag J, Buchner N, Meyer T, Kindermann W, Haendeler J, Bohm M, Laufs U. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Eper ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson JC, Dhabhar FS, Blackburn EH. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Molecular psychiatry. 2012;17:164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues in clinical neuroscience. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]