Abstract
It is known that heroin dependence and withdrawal are associated with changes in the hypothalamic-pituitary-adrenal (HPA) axis. The objective of these studies in rats was to systematically investigate the level of HPA activity and response to a heroin challenge at two time points during heroin withdrawal, and to characterize the expression of associated stress-related genes 30 minutes after each heroin challenge. Rats received chronic (10-day) intermittent escalating-dose heroin administration (3×2.5 mg/kg/day on day 1; 3×20 mg/kg/day by day 10). Hormonal and neurochemical assessments were performed in acute (12 hours after last heroin injection) and chronic (10 days after the last injection) withdrawal. Both plasma ACTH and corticosterone levels were elevated during acute withdrawal, and heroin challenge at 20 mg/kg (the last dose of chronic escalation) at this time point attenuated this HPA hyperactivity. During chronic withdrawal, HPA hormonal levels returned to baseline, but heroin challenge at 5 mg/kg decreased ACTH levels. In contrast, this dose of heroin challenge stimulated the HPA axis in heroin naïve rats. In the anterior pituitary, pro-opiomelanocortin (POMC) mRNA levels were increased during acute withdrawal and retuned to control levels after chronic withdrawal. In the medial hypothalamus, however, the POMC mRNA levels were decreased during acute withdrawal, and increased after chronic withdrawal. Our results suggest a long-lasting change in HPA abnormal responsivity during chronic heroin withdrawal.
Keywords: POMC, orexin, mu opioid receptor, gene expression, CRF, AVP
Introduction
Heroin, morphine and other short-acting opiates regulate the activity of endogenous opioid systems. In humans, long-term exposure to opiates leads to relative deficiency in beta-endorphin/mu opioid receptor (MOP-r) system [1]. Pro-opiomelanocortin (POMC) is a large peptide precursor that gives rise to several biologically active neuropeptides, including beta-endorphin and ACTH. In the hypothalamic-pituitary-adrenal (HPA) axis, stress increases both corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) release into the pituitary portal circulation from terminals of hypothalamic paraventricular nucleus. Both CRF type I receptor (CRF-R1) and AVP V1b receptor (V1b) are located on corticotropes in the anterior pituitary and drive the processing and release of the POMC peptides. POMC neurons are also identified in the rodent hypothalamus (arcuate nucleus) and some extra-hypothalamic brain regions [2,3]. During spontaneous or naloxone-precipitated opiate withdrawal, the presumed relative deficiency in endogenous beta-endorphin could lead to a hyperactive HPA axis and corresponding increases in hormone secretion. During acute opiate withdrawal, increases in HPA hormonal levels have been observed both in humans [4-7] and rats [8-10]. Furthermore, long-lasting alterations in HPA responses to various stressors may play an important role in continued vulnerability to relapse during chronic withdrawal [11,12].
Although early studies reported a lack of effect on beta-endorphin-immunoreactivity levels in many regions of the rodent brain [13,14], studies of POMC gene expression indicated that chronic morphine exposure causes significant decreases in mRNA levels in the hypothalamus [15,16]. In the arcuate nucleus, the MOP-r is a pre-synaptic autoreceptor in beta-endorphin neurosecretory neurons that negatively modulates the release of beta-endorphin from these cells [17]. Furthermore, in the lateral hypothalamus, about 50% of orexin/dynorphin (Dyn) neurons express MOP-r [18], and these are under tonic inhibition by endogenous opioids [19]. Recently, it has been demonstrated that early withdrawal from opiates is associated with increased orexin mRNA expression in this region [10,18]. Together, these several hypothalamic stress responsive systems may be important neurobiological substrates of intense cravings and heightened relapse vulnerability observed in heroin dependent individuals during acute withdrawal [20].
The present experiments were designed to further characterize the activity of the HPA axis during opiate withdrawal from chronic (10-day) escalating-dose heroin administration. This pattern of opiate exposure has been found to stimulate HPA activity during acute spontaneous withdrawal in rats [21]. The first aim was to investigate the effects on HPA activity of a heroin challenge at two phases of heroin withdrawal: (1) acute 12-hour withdrawal, when elevations of plasma ACTH and corticosterone (CORT) levels were found; and (2) chronic 10-day withdrawal, when plasma ACTH and CORT levels had returned to basal levels. We examined expression levels of several HPA-related genes at both hypothalamic and pituitary levels, including CRF, CRF type I receptor (CRF-R1), AVP, AVP V1b receptor (V1b), and POMC, as measured quantitatively by solution hybridization ribonuclease protection assays. The second aim of these experiments was to investigate alterations in another set of stress responsive genes in the hypothalamus and amygdala, including MOP-r, orexin and Dyn.
Methods and materials
1. Animals
Male Fischer rats (190-220 g, Charles River, Kingston, NY) were housed individually in a stress-minimized facility with free access to water and food. Animals were adapted to a standard 12-hr light/dark cycle (lights off from 7:00 h to 19:00 h) for seven days. All experiments followed the Principles of Laboratory Animal Care (NIH Publication No 86-23, 1996), and the specific protocols were approved by the Rockefeller University Animal Care and Use Committee.
2. Procedures
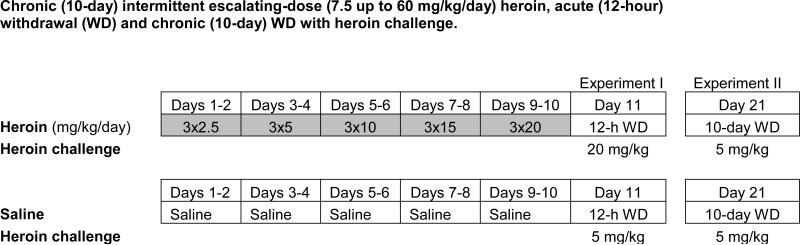
Animals were randomly assigned to two groups (chronic heroin and chronic saline control), and given either heroin or an equal volume of saline (1 ml/kg) three times daily with two 6-hour intervals and one 12-hour interval, beginning 4 hours after the start of their daily dark cycle (11:00 h, 17:00 h and 23:00 h) for 10 days. The regimen of chronic (10-day) escalating-dose heroin administration included a dose increase every second day (see Figure 1): 7.5 (3 × 2.5) mg/kg/day on days 1-2, 15 (3 × 5.0) mg/kg/day on days 3-4, 30 (3 × 10) mg/kg/day on days 5-6, 45 (3 × 15) mg/kg/day on days 7-8 and 60 (3 × 20) mg/kg/day on days 9-10. This pattern of opiate exposure using morphine or heroin has been found to induce physiological dependence in rats [10,22].
Figure 1.
Timelines for heroin administration regimens with acute heroin challenge.
In Experiment I, animals in each chronic heroin and saline group were randomly assigned to receive a heroin challenge (HC) or vehicle injection on experimental day 11, at 11:00 h (see Figure 1). This created four treatment groups (n = 8-9 each): chronic heroin and 20 mg/kg heroin challenge (Chronic heroin + HC); chronic saline and saline challenge (Chronic saline + Sal); chronic heroin and saline challenge (Chronic heroin + Sal); chronic saline and 5 mg/kg heroin challenge (Chronic saline + HC). The heroin challenge dose for this latter group had to be reduced because 20 mg/kg would be too high in opiate-naïve animals and result in overdose. All animals were sacrificed 30 min following the heroin (or saline) injection by decapitation after brief exposure to CO2 (within 15 seconds), and specific brain and pituitary regions, as well as plasma, were collected for subsequent mRNA and hormonal analyses.
Experiment II was performed exactly as described above, but the heroin and saline challenge injections were administered after 10 days of withdrawal (i.e., experimental day 21) (Figure 1). All animals were sacrificed 30 min following the challenge injection and specific brain regions, pituitary and plasma were collected for subsequent mRNA and hormonal analyses.
3. Preparation of RNA extracts
In each experiment, brains were removed from the skull and placed in a chilled rat brain matrix (ASI Instruments, Houston, Texas). A coronal slice containing the hypothalamus and/or amygdala was removed from the matrix and placed on a chilled petri dish. Dissection was carried out using razor blades and forceps under a dissecting microscope. The brain regions of interest were identified according to The rat brain in stereotaxic coordinates (Paxinos G & Watson C, 1989), as described in detail recently [21]. The lateral hypothalamus (LH), medial portion of the hypothalamus (MH, including the perifornical area, dorsomedial hypothalamus, PVN and arcuate nucleus), amygdala (Amy, including the central nucleus, medial nucleus and basolateral portion) and anterior pituitary (AP) were dissected on ice, homogenized in guanidinium thiocyanate buffer and extracted with acidic phenol and chloroform. After the final ethanol precipitation step, each extract was resuspended in diethylpyrocarbonate (DEPC)-treated H2O and stored at −80 °C.
4. Solution hybridization ribonuclease (RNase) protection-trichloroacetic acid (TCA) precipitation assay
The solution hybridization RNase protection-TCA precipitation protocol has been described in detail in earlier reports [21]. A 538 base pair (bp) fragment from the rat POMC cDNA, a 2100 bp fragment from the rat MOP-r cDNA, a 1700 bp fragment from the rat Dyn cDNA or a 760 bp fragment from the rat CRF cDNA was cloned into the polylinker region of either pSP64 or pSP65 plasmids (Promega, Madison, WI) in both the sense and antisense orientations. A 531 bp fragment from the rat orexin (or hypocretin) cDNA was cloned into the polylinker region of pBC SK+ (Stratagene, La Jolla, CA). A 2.5 k base fragment from the rat CRF-R1 cDNA was cloned into the polylinker region of pcDNA (Promega). A 502 bp fragment from the rat AVP cDNA and a 1201 bp fragment from the rat V1b cDNA were cloned into the polylinker region of pCR II (Invitrogen, Carlsbad CA). The plasmid pS/E (a pSP65 derivative) was used to synthesize riboprobe for the 18S rRNA to determine total RNA. 33P-labeled cRNA antisense probes and unlabeled cRNA sense standards were synthesized using a SP6, T3 or T7 transcription system. A denaturing agarose gel containing 1.0 M formaldehyde showed that a single full-length transcript had been synthesized from each plasmid.
RNA extracts were dried in 1.5 ml Eppendorf tubes and resuspended in 30 μl of 2 × TESS (10 mM N-Tris[hydroxy-methyl]methyl-2-aminoethane sulfonic acid, pH 7.4; 10 mM ethylenediaminetetraacetic acid [EDTA]; 0.3 M NaCl; 0.5% sodium dodecyl sulfate [SDS]) that contained 150 to 300 K cpm of a probe. Samples were covered with mineral oil and hybridized overnight at 75 °C. For RNase treatment, 250 μl of a buffer containing 0.3 M NaCl; 5 mM EDTA; 10 mM Tris-HCl (pH 7.5), 40 μg/ml RNase A (Worthington, Biochemicals, Freehold, NJ) and 2 μg/ml RNase T1 (Calbiochem, San Diego, CA) was added and each sample was incubated at 30 °C for 1 hour. TCA precipitation was effected by the addition of 1 ml of a solution that contained 5% TCA and 0.75% sodium pyrophosphate. Precipitates were collected onto a filter in sets of 24 using a cell harvester (Brandel, Gaithersburg, MD) and were measured in a scintillation counter with liquid scintillant (Beckman, Palo Alto, CA).
The procedure to measure mRNA levels involved a comparison of values obtained from experimental samples (brain extracts) to those obtained for a set of calibration standards. The calibration standards had known amounts of an in vitro sense transcript whose concentration was determined by optical absorbance at 260 nm. The set of calibration standards included those with no added sense transcript and those that contained between 1.25 and 80 pg of the sense transcript. To determine the total attomoles of each mRNA in each extract, the amounts calculated from the standard curves were multiplied by 2.73 for POMC, 0.25 for MOP-r, 4.3 for orexin, 1.25 for Dyn, 5.00 for AVP, 2.41 for AVP V1b, 2.15 for CRF or 1.11 for CRF-R1 to correct for the difference in length between the sense transcript and the full-length mRNA. A new standard curve was generated each time experimental samples were analyzed and all extracts of a particular tissue were assayed for each mRNA as a group in a single assay.
Total cellular RNA concentrations were measured by hybridization of diluted extracts to a 33P-labeled probe complementary to 18S rRNA at 75 °C. The calibration standards for this curve contained 10 μg of E. coli tRNA plus either 0.0, or from 2.5 to 40 ng of total RNA from rat brain whose concentration was determined by optical absorbance at 260 nm.
Selected samples were subjected to solution hybridization and RNase treatment followed by gel electrophoresis. The protected RNA:RNA hybrids were phenol-chloroform extracted, precipitated with 100% ethanol, and electrophoresed through nondenaturing 4% polyacrylamide gels, with 32P-labeled ΦX 174 RF DNA digested with Haelll as molecular weight markers. The gels were then dried and exposed to X-film. The protected species for each mRNA was approximately at the size, corresponding to an RNA:RNA hybrid formed by hybridization of the full-length cRNA probe with total cytoplasmic RNA samples extracted from the tissues (data not shown).
5. Radioimmunoassays
At the time of decapitation, blood from each rat was collected in tubes, placed on ice, and was spun in a refrigerated centrifuge. Plasma was separated and stored at −40 °C for hormonal measurements by radioimmunoassay. Corticosterone (CORT) levels were assayed using a rat corticosterone 125I kit from MP Biomedicals (Costa Mesa, CA). ACTH immunoreactivity levels were assayed from unextracted plasma by using a kit from DiaSorin Inc. (Stillwater, MN). All values were determined in duplicate in a single assay.
6. Data analysis
In Experiments I and II, group differences in mRNA levels of each gene in each brain region and in plasma hormonal levels were analyzed using a two-way analysis of variance (ANOVA) with Treatment (chronic heroin or saline in Experiment I and heroin or saline withdrawal in Experiment II) and Challenge (heroin or saline in both Experiments I and II), followed by Newman-Keuls post-hoc tests or planned comparisons or Student's t-tests when appropriate. The accepted level of significance for all tests was p < 0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc.).
Results
1. ACTH
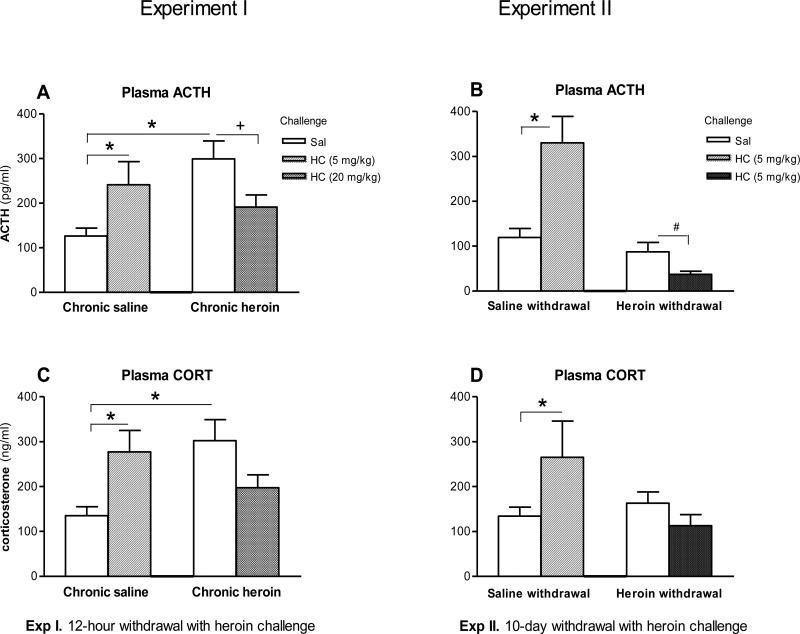
In Experiment I, the ANOVA revealed a significant Treatment × Challenge interaction (F (1,21) = 10.0, p < 0.01) (Figure 2A). Newman-Keuls post-hoc tests revealed that plasma ACTH levels were significantly higher in the Chronic heroin + Sal group in comparison to the Chronic saline + Sal group (p < 0.05). Also, plasma ACTH levels after 5 mg/kg heroin challenge in heroin naïve rats (Chronic saline + HC) were significantly higher than in the Chronic saline + Sal group (F (1,21) = 5.52, p < 0.05), and plasma ACTH levels in the Chronic heroin + HC group were significantly lower than levels in the Chronic heroin + Sal group (F (1,21) = 4.53, p < 0.05).
Figure 2.
Effects of chronic (10-day) intermittent escalating-dose heroin administration, 12-hour withdrawal, and 10-day withdrawal with heroin challenge on mean + SEM of plasma ACTH levels (pg/ml) (A, B) or corticosterone (CORT) levels (ng/ml) (C, D). A and C: In Experiment I (12-hour withdrawal with heroin challenge), rats received chronic heroin or saline for 10 days followed by heroin challenge (HC, 20 mg/kg) or saline (Sal) challenge on day 11, including four groups of rats: (1) Chronic saline + Sal, as saline control; (2) Chronic saline + HC, as acute heroin (heroin challenge at 5 mg/kg); (3) Chronic heroin + Sal: as 12-hour withdrawal; and (4) Chronic heroin + HC: as chronic heroin (heroin challenge at 20 mg/kg). B and D: In Experiment II (10-day withdrawal with heroin challenge), rats received chronic heroin or saline for 11 days, and then 10 days of withdrawal, followed by heroin challenge (HC, 5 mg/kg) or saline on day 21, including four groups of rats: (1) Saline withdrawal + Sal, as saline control; (2) Saline withdrawal + HC, as acute heroin (heroin challenge at 5 mg/kg); (3) Heroin withdrawal + Sal: 10-day heroin withdrawal + saline, as 10-day withdrawal; and (4) Heroin withdrawal + HC: as heroin challenge (5 mg/kg) after 10-day withdrawal. Significant differences are indicated: *, +, # p < 0.05 (n=6-9).
In Experiment II, the ANOVA revealed a significant main effect of Treatment (F (1,32) = 19.4, p < 0.001), a significant effect of Challenge (F (1,32) = 4.73, p < 0.05), and a significant Treatment × Challenge interaction (F (1,32) = 12.5, p < 0.005; Figure 2B). Newman-Keuls post-hoc tests revealed that plasma ACTH levels after 5 mg/kg heroin challenge in heroin naïve rats (Saline withdrawal + HC) were significantly higher than those in controls (Saline withdrawal + Sal; p < 0.001). Since other studies have reported suppression of HPA activity in the rat after 5 or more days of withdrawal from chronic administration of morphine [8], a planned comparison was employed to reveal that plasma ACTH levels in the Heroin withdrawal + HC group were significantly lower than in the Heroin withdrawal + Sal group (t (1,17) = 4.55, p < 0.05).
2. CORT
As shown in Figures 2C and 2D, plasma CORT levels showed a pattern of variation similar to plasma ACTH. In Experiment I, the ANOVA revealed a significant Treatment × Challenge interaction (F (1,25) = 10.2, p < 0.01; Figure 2C). Newman-Keuls post-hoc tests indicated that plasma CORT levels in the Chronic heroin + Sal group were significantly higher than those in the Chronic saline + Sal group (p < 0.05), and that plasma CORT levels in the Chronic saline + HC group was significantly higher than those in the Chronic saline + Sal group (p < 0.05). Further, levels of CORT were reduced in the Chronic heroin + HC, but this was not significantly different from the Chronic Heroin + Sal group (F (1,21) = 3.57, p = 0.07).
In Experiment II (Figure 2D), the ANOVA revealed a significant main effect of Treatment (F (1,32) = 4.28, p < 0.05) and a significant Treatment × Challenge interaction (F (1,32) = 8.10, p < 0.01). Newman-Keuls post-hoc tests indicated that plasma CORT levels in the Saline withdrawal + HC group were significantly higher than those in the Saline withdrawal + Sal group (p < 0.05). Plasma CORT levels in the Heroin withdrawal + HC group were not significantly different from that of the Heroin withdrawal + Sal group, although plasma CORT levels showed a reduction similar to plasma ACTH (Figure 2B).
3. POMC in MH
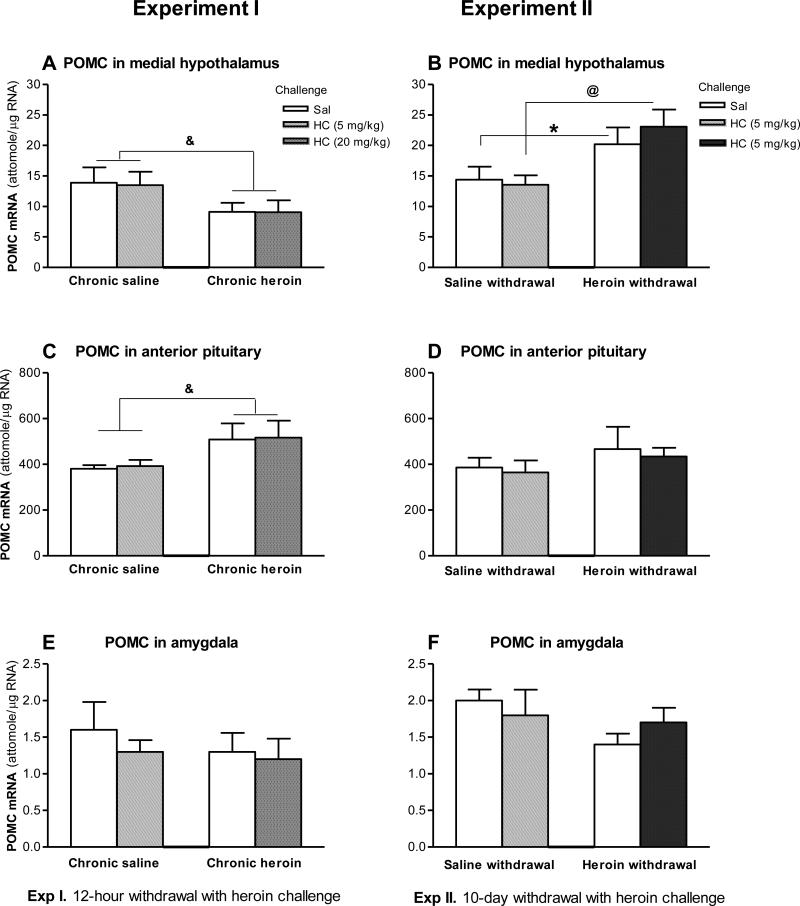
In Experiment 1, the ANOVA revealed a significant main effect of Treatment (F (1,24) = 4.35, p < 0.05), and Newman-Keuls post-hoc tests indicated that POMC mRNA levels were significantly lower in the Chronic heroin + HC and Chronic heroin + Sal groups in comparison to the Chronic saline + Sal and Chronic saline + HC groups (Figure 3A).
Figure 3.
Effects of chronic (10-day) intermittent escalating-dose heroin administration, 12-hour withdrawal, and 10-day withdrawal with heroin challenge on pro-opiomelanocortin (POMC) mRNA levels (attomole/μg total RNA) in the medial hypothalamus (A, B), anterior pituitary (C, D) and amygdala (E, F). A, C and E: In Experiment I (12-hour withdrawal with heroin challenge), rats received chronic heroin or saline for 10 days followed by heroin challenge (HC, 20 mg/kg) or saline (Sal) challenge on day 11. B, D and F: In Experiment II (10-day withdrawal with heroin challenge), rats received chronic heroin or saline for 11 days, and then 10 days of withdrawal, followed by heroin challenge (HC, 5 mg/kg) or saline on day 21. See details in Figure 2. There was a significant main effect for Chronic heroin, & p < 0.05. Newman-Keuls post-hoc tests showed significant effects: *, +, @ p < 0.05 (n=6-9).
In Experiment II (Figure 3B), the ANOVA revealed only a significant main effect of Treatment (F (1,32) = 13.5, p < 0.001). Newman-Keuls post-hoc tests indicated that POMC mRNA levels were significantly higher in the Heroin withdrawal + HC and Heroin withdrawal + Sal groups in comparison to Saline withdrawal + Sal and Saline withdrawal + HC groups.
4. POMC in AP
In Experiment 1 (Figure 3C), the ANOVA revealed a significant main effect of Treatment (F (1,26) = 5.50, p < 0.05). Newman-Keuls post-hoc tests suggested that POMC mRNA levels in the Chronic heroin + HC and the Chronic heroin + Sal groups were significantly higher than those in Chronic saline + Sal and Chronic saline + HC groups. In Experiment II (Figure 3D), there were no significant differences.
5. Orexin in LH
In Experiment I (Table 1), the ANOVA revealed a significant effect of Treatment (F (1,26) = 4.84, p < 0.05). Newman-Keuls post-hoc tests further revealed that levels of orexin mRNA in the Chronic heroin + Sal group were significantly higher than those in the Chronic saline + Sal group (p < 0.05). No group differences were found in Experiment II.
Table 1.
Effects of chronic (10-day) intermittent escalating-dose heroin administration, 12-hour withdrawal, and 10-day withdrawal with heroin challenge on mRNA levels (attomole/μg total RNA) of orexin and preprodynorphin (Dyn) in the lateral hypothalamus (LHyp) and amygdala (Amy).
| Experiment | Treatment | Orexin LHyp | Dyn LHyp | Dyn Amy |
|---|---|---|---|---|
| I. 12-hour withdrawal with heroin challenge | Chronic saline + Sal | 4.6 ± 0.48 | 4.9 ± 0.52 | 2.2 ± 0.48 |
| Chronic saline + HC | 4.8 ± 0.32 | 4.8 ± 0.49 | 1.9 ± 0.21 | |
| Chronic heroin + Sal | 6.9 ± 0.52* | 4.0 ±0.29 | 1.9 ± 0.21 | |
| Chronic heroin + HC | 5.9 ± 0.69 | 4.3 ± 0.25 | 2.0 ± 0.27 | |
| II. 10-day withdrawal with heroin challenge | Saline withdrawal + Sal | 5.0 ± 0.44 | 4.7 ± 0.27 | 2.1 ± 0.35 |
| Saline withdrawal + HC | 5.2 ± 0.58 | 4.6 ± 0.31 | 1.9 ± 0.16 | |
| Heroin withdrawal + Sal | 5.4 ± 0.48 | 4.7 ± 0.35 | 2.0 ± 0.21 | |
| Heroin withdrawal + HC | 5.1 ± 0.32 | 4.8 ± 0.33 | 2.2 ± 0.19 |
In Experiment I (12-hour withdrawal with heroin challenge), rats received chronic heroin or saline for 10 days followed by heroin challenge (HC, 20 mg/kg) or saline (Sal) on day 11, including four groups of rats: (1) Chronic saline + Sal, as saline control; (2) Chronic saline + HC, as acute heroin (heroin challenge at 5 mg/kg); (3) Chronic heroin + Sal: as 12-hour withdrawal; and (4) Chronic heroin + HC: as chronic heroin. In Experiment II (10-day withdrawal with heroin challenge), rats received chronic heroin or saline for 11 days, and then 10 days of withdrawal, followed by heroin challenge (HC, 5 mg/kg) or saline on day 21, including four groups of rats: (1) Saline withdrawal + Sal, as saline control; (2) Saline withdrawal + HC, as acute heroin (heroin challenge at 5 mg/kg); (3) Heroin withdrawal + Sal: as 10-day withdrawal; and (4) Heroin withdrawal + HC: as heroin challenge (5 mg/kg) after 10-day heroin withdrawal. Data shown in table are treatment group mean ± SEM. Significant differences are indicated:
p < 0.05 vs. Chronic saline + Sal (n=6-9).
6. MOP-r in LH
In Experiment I (Table 2), the ANOVA revealed a significant Treatment × Challenge interaction (F (1,21) = 4.59, p < 0.05). Newman-Keuls post-hoc tests indicated that MOP-r mRNA levels in the Chronic heroin + Sal were significantly higher than those in the Chronic saline + Sal group (p < 0.05). In Experiment II, no group differences were found (Table 2).
Table 2.
Effects of chronic (10-day) intermittent escalating-dose heroin administration, 12-hour withdrawal, and 10-day withdrawal with heroin challenge on mu-opioid receptor (MOP-r) mRNA levels (attomole/μg total RNA) in the lateral hypothalamus (LHyp) and amygdala (Amy).
| Experiment | Treatment | MOP-r LHyp | MOP-r Amy |
|---|---|---|---|
| I. 12-hour withdrawal with heroin challenge | Chronic saline + Sal | 0.38 ± 0.05 | 0.17 ± 0.02 |
| Chronic saline + HC | 0.37 ± 0.11 | 0.14 ± 0.01 | |
| Chronic heroin + Sal | 0.62 ± 0.07* | 0.17 ± 0.01 | |
| Chronic heroin + HC | 0.53 ± 0.11 | 0.15 ± 0.01 | |
| II. 10-day withdrawal with heroin challenge | Saline withdrawal + Sal | 0.44 ± 0.03 | 0.19 ± 0.01 |
| Saline withdrawal + HC | 0.43 ± 0.04 | 0.18 ± 0.02 | |
| Heroin withdrawal + Sal | 0.46 ± 0.03 | 0.17 ± 0.01 | |
| Heroin withdrawal + HC | 0.39 ± 0.04 | 0.19 ± 0.01 |
See details in Table 1. Data shown in table are treatment group mean ± SEM. Significant differences are indicated:
p < 0.05 vs. Chronic saline + Sal (n=6-9).
7. CRF and AVP in MH or CRF-R1 and V1b in AP
In either Experiment I or II, there was no significant group difference in mRNA levels of CRF and AVP in MH (Table 3A), or CRF-R1 and V1b in AP (Table 3B).
Table 3.
Effects of chronic (10-day) intermittent escalating-dose heroin administration, 12-hour withdrawal, and 10-day withdrawal with heroin challenge on mRNA levels (attomole/μg total RNA) of arginine vasopressin (AVP) and corticotropin-releasing factor (CRF) in the medial hypothalamus (A), and AVP V1b type receptor (V1b) and CRF type I receptor (CRF-R1) in the anterior pituitary (B).
| A. | |||
|---|---|---|---|
| Experiment | Treatment | AVP | CRF |
| I. 12-hour withdrawal with heroin challenge | Chronic saline + Sal | 103 ± 11 | 1.13 ± 0.09 |
| Chronic saline + HC | 104 ± 9 | 1.10 ± 0.15 | |
| Chronic heroin + Sal | 112 ± 9 | 1.30 ± 0.10 | |
| Chronic heroin + HC | 77 ± 8 | 0.94 ± 0.11 | |
| II. 10-day withdrawal with heroin challenge | Saline withdrawal + Sal | 93 ± 9 | 1.06 ± 0.18 |
| Saline withdrawal + HC | 90 ± 7 | 1.10 ± 0.09 | |
| Heroin withdrawal + Sal | 94 ± 15 | 1.13 ± 0.19 | |
| Heroin withdrawal + HC | 99 ± 9 | 1.11 ± 0.12 | |
| B. | |||
|---|---|---|---|
| Experiment | Treatment | V1b | CRF-R1 |
| I. 12-hour withdrawal with heroin challenge | Chronic saline + Sal | 1.7 ± 0.19 | 0.92 ± 0.10 |
| Chronic saline + HC | 1.8 ± 0.11 | 0.95 ± 0.18 | |
| Chronic heroin + Sal | 1.9 ± 0.34 | 1.20 ± 0.25 | |
| Chronic heroin + HC | 2.2 ± 0.41 | 1.11 ± 0.30 | |
| II. 10-day withdrawal with heroin challenge | Saline withdrawal + Sal | 1.9 ± 0.19 | 0.89 ± 0.16 |
| Saline withdrawal + HC | 2.0 ± 0.29 | 0.88 ± 0.27 | |
| Heroin withdrawal + Sal | 1.9 ± 0.10 | 0.98 ± 0.33 | |
| Heroin withdrawal + HC | 1.9 ± 0.26 | 0.92 ± 0.31 | |
See details in Table 1. Data shown in table are treatment group mean ± SEM (n=6-9).
8. Other measures
The mRNA levels of Dyn in LH, and Dyn, POMC or MOP-r in the amygdala were unaltered (Tables 1 and 2, Figure 3E and F).
Discussion
The present study confirmed that 12-hour acute heroin withdrawal, as stress, elevated HPA hormones. Under this stress condition, heroin inhibited the HPA activation. When the HPA hormonal levels returned to baseline following 10-day chronic withdrawal, the HPA activity was inhibited by heroin at a low dose, suggesting a long-lasting neuroadaptation during chronic heroin withdrawal. In acute withdrawal, a decreased POMC mRNA level in the medial hypothalamus was coupled with enhanced orexin and MOP-r gene expression in the lateral hypothalamus. After chronic withdrawal, conversely, an increased POMC mRNA level was found in the medial hypothalamus, with no lasting changes of orexin or MOP-r mRNA levels.
1. HPA axis
Consistent with earlier studies [8], we found that an acute heroin challenge (5 mg/kg) had a stimulatory effect on plasma ACTH and CORT release in heroin-naïve rats. After chronic (10-day) intermittent escalating-dose heroin administration, we have reported that the HPA axis did not respond to heroin, indicating tolerance of HPA activity, and in acute 12-h opiate withdrawal, activation of the HPA axis was observed [21]. The present experiment replicated the above results by showing that HPA hormonal levels were elevated after acute heroin withdrawal. Chronic intermittent exposure to opiates is a chronic stressor, and it may alter the responsivity of the HPA axis, as do many other stressors [23,24]. Under this particular stress condition (Experiment I), we examined the effect of heroin challenge on pituitary-adrenal function, and found that heroin challenge at 20 mg/kg decreased the elevation of plasma ACTH and CORT levels induced by acute opiate withdrawal stress.
In Experiment II, the present study examined basal levels of the stress hormones after chronic 10-day withdrawal as well as the alterations of HPA responses to heroin challenge, in order to compare with the HPA responsivity during acute withdrawal. We found that after 10-day heroin withdrawal, heroin challenge at a low dose, 5 mg/kg, did not stimulate the HPA axis as it did in heroin naïve rats. In contrast, it even had a slight, but significant, suppression on plasma ACTH levels. Our results indicate long-lasting neuroadaptation in the HPA system during chronic heroin withdrawal.
Although the mechanisms responsible for the interactions between heroin and withdrawal stress are not clear, the effects of opiates on HPA activity have been found to depend on the presence or absence of stress. In fact, an inhibitory effect of opiates on the HPA axis is found in heroin addicts [4]. It is consistently found that morphine suppresses cortisol release induced by surgical stress in humans [25,26]. In support of this concept, our animal study found that while either acute morphine or water restriction stress alone increased ACTH levels as an independent stimulus, morphine decreases plasma ACTH levels elevated by water restriction stress [27]. Both human and animal studies demonstrate that morphine or heroin effectively blunts the HPA activity caused by stress, indicating that opioids play a counter-regulatory role in modulating HPA stress responsivity under stress conditions. Although interesting, the HPA result of the current study should be interpreted with caution. No difference for the mRNA levels of CRF or AVP in the hypothalamus or of their receptors in the anterior pituitary was observed after acute or chronic heroin withdrawal with or without heroin challenge. In future experiments, information on the peptide levels of CRF and AVP in the hypothalamus, and the HPA axis responses at multiple time points or to other different stressors would be useful.
Although the mechanisms responsible for the interactions between heroin and withdrawal stress have not been elucidated, the inhibitory effects of opiates on HPA activity in rats have been reported to occur at the paraventricular nucleus [28] or median eminence [29], but not the anterior pituitary [30]. Moreover, although it has been reported that the stress responsive neuropeptides in the amygdala are involved in modulating HPA activity [3], we did not observe any change of POMC, MOP-r or Dyn in the amygdala during either acute (12 hours) or chronic (10 days) heroin withdrawal with or without heroin challenge.
2. POMC/MOP-r and orexin systems in the hypothalamus
In early studies using morphine pellets, it was found that POMC mRNA in the hypothalamus tended to decrease after chronic morphine in either normal rats [15,16], castrated male rats [31] or ovariectomized female guinea pigs [32]. In the current study, we examined POMC gene expression in two brain regions (medial hypothalamus and amygdala) after acute (12-h) withdrawal, and found a slight, but significant, decrease in the POMC mRNA levels only in the medial hypothalamus. Our data suggests a decrease in opioidergic activity in the hypothalamus caused by reduced POMC gene expression in acute heroin withdrawal, although there was a parallel increase in POMC mRNA expression in the anterior pituitary, which could result in an increased opioid activity at peripheral level.
Given that POMC neurons in the arcuate nucleus project to the lateral hypothalamus, and that beta-endorphin inhibits orexin neurons [19], we also measured orexin mRNA levels in the lateral hypothalamus, with MOP-r in the same region. We found that both orexin and MOP-r mRNA levels were increased at 12-hour withdrawal from chronic escalating-dose heroin administration. Orexin A peptide has been found to decrease the excitability of brain reward systems in the lateral hypothalamus [33]. Also, alteration of the orexin activity is likely involved in the negative affective state during drug withdrawal, which may be important in the modulation of drug-seeking behaviors [34]. Our results suggest that activation of orexin gene expression in the lateral hypothalamic neurons may be associated with a new set point of brain reward function established by chronic heroin exposure and withdrawal [3,35].
In an early study, blockade of beta-endorphin activity in the brain by icv infusion of naloxone was found to reduce the reinforcing effect of lateral hypothalamic stimulation, suggesting that endogenous beta-endorphins are involved in self-stimulation reward [36]. Behaviorally, rats self-administer this opioid peptide into the brain and this supports a role for central beta-endorphin in reinforcement [37]. It has been hypothesized that heroin dependent individuals self-administer heroin to escape the negative affective and emotional states associated with drug withdrawal in humans [20,24]. There is also evidence in rats and primates that the motivational properties of opiates are altered by spontaneous and naloxone-precipitated withdrawal and that opiate withdrawal can motivate opiate-seeking behavior [38-40]. It is reasonable, therefore, to postulate that decreased POMC gene expression with resultant decreases in beta-endorphin may initiate behaviors leading to opiate intake and thus normalization of endogenous opioid tone. This opioid-deficiency hypothesis is also supported by evidence in humans that heroin addicts have decreased opioid activity in regulating HPA activity [5,41,42], which is normalized by methadone (a potent MOP-r agonist) maintenance treatment [4].
Of interest, beta-endorphin acting on the MOP-r exerts tonic inhibition of CRF release from the paraventricular nucleus, and then of the HPA axis in both humans and rodents [20]. In acute heroin withdrawal, we found that acute opiate withdrawal dramatically activated HPA activity, which is associated with decreased POMC mRNA levels in the hypothalamus. The results of the present study support the concept that there is an inverse association between hypothalamic POMC activity and the HPA axis. To date, there is no evidence that orexin acting at its receptors has any direct relationship with or regulation of the HPA axis, although there is extensive evidence that orexin has actions in reward-related nuclei, such as the hypothalamus, nucleus accumbens and ventral tegmental area [3].
In sharp contrast, after 10-day chronic heroin withdrawal, we observed a rebound increase in the POMC mRNA levels in the medial hypothalamus. Although the exact timing and maximal magnitude of POMC gene expression remain to be further resolved, our results suggest that after chronic withdrawal, there may be an increase in the POMC biosynthesis and/or opioidergic activity from the hypothalamus, although there was no alteration of POMC mRNA expression in the anterior pituitary observed. In line with these animal studies, human studies have demonstrated that supersensitivity to naloxone-induced HPA stimulation is evident after administration of a single low dose of naloxone to an opiate dependent individual, indicating that enhanced opioid activity is present during withdrawal [7].
3. Summary
The present study confirmed that acute heroin withdrawal stimulated HPA activity. When the rats were under this stress condition, heroin suppressed the HPA activation, indicating that opioids play a counter-regulatory role in the stress response by inhibiting the stress response cascade. Our data further showed that when basal HPA hormonal levels returned to baseline following chronic withdrawal, the HPA activity was inhibited by heroin challenge at a low dose, suggesting a long-lasting neuroadaptation during chronic heroin withdrawal. Also, in the hypothalamus, a relative deficit in basal opioid activity in response to acute withdrawal (as reflected by decreased POMC mRNA levels) may lead to enhanced orexin and MOP-r gene expression in the lateral hypothalamus. Conversely, relative excess in basal opioid activity was found after chronic withdrawal, as reflected by increased POMC mRNA levels in the hypothalamus.
acknowledgments
This work was supported by NIH NIDA Research Center Grant DA-P50-05130 (MJK). Y.Z. and M.J.K. designed the research; Y.Z. performed the research; Y.Z. and A.H. analyzed the data. Y.Z., F.L., A.H. and M.J.K. wrote the paper. The authors would like to thank Dr. G. Aguilera for providing rat AVP and V1b receptor cDNAs; Dr. L. de Lecea for rat hypocretin (or orexin) cDNA; Dr. G. Uhl for rat MOP-r cDNA; Dr. J. Roberts for rat POMC cDNA; Drs. J. Douglass and O. Civelli for rat ppDyn cDNA; Dr. W. Vale for rat CRF-R1 cDNA; Dr. R. Thompson for rat CRF cDNA; and Drs. T. Nilsen and P. Maroney for 18S DNA. The authors would like to thank the NIDA Division of Drug Supply and Analytical Services for providing diacetylmorphine HCl (heroin) in this study.
Footnotes
Declarations of interest, funding All authors declare that they have no conflict of interest.
References
- 1.Kreek MJ. Opiates, opioids and addiction. Mol Psychiatry. 1996;1:232–254. [PubMed] [Google Scholar]
- 2.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. JAMA. 1973;223:665–668. [PubMed] [Google Scholar]
- 5.Kreek MJ, Ragunath J, Plevy S, Hamer D, Schneider B, Hartman N. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5:277–288. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- 6.Kosten TR, Kreek MJ, Raghunath J, Kleber HD. Cortisol levels during naltrexone maintenance treatment in ex-opiate addicts. Biol Psychiatry. 1986;21:217–220. doi: 10.1016/0006-3223(86)90150-2. [DOI] [PubMed] [Google Scholar]
- 7.Culpepper-Morgan JA, Kreek MJ. HPA axis hypersensitivity to naloxone in opioid dependence: A case of naloxone induced withdrawal. Metabolism. 1997;46:130–134. doi: 10.1016/s0026-0495(97)90289-4. [DOI] [PubMed] [Google Scholar]
- 8.Ignar DM, Kuhn CM. Effects of specific mu and kappa opiate tolerance and abstinence on hypothalamo-pituitary-adrenal axis secretion in the rat. J Pharmacol Exp Ther. 1990;255:1287–1295. [PubMed] [Google Scholar]
- 9.Martinez JA, Vargas ML, Fuente T, Garcia JDR, Milanes MV. Plasma beta-endorphin and cortisol levels in morphine-tolerant rats and in naloxone-induced withdrawal. Eur J Pharmacol. 1990;182:117–123. doi: 10.1016/0014-2999(90)90499-v. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- 11.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–231. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 12.Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudehithlu KP, Tejwani GA, Bhargave HN. b-Endorphin and methionine enkephalin levels in discrete brain regions, spinal cord, pituitary gland and plasma of morphine tolerant-dependent and abstinent rats. Brain Res. 1991;553:284–290. doi: 10.1016/0006-8993(91)90836-k. [DOI] [PubMed] [Google Scholar]
- 14.Le Merrer L, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocchetti I, Ritter A, Costa E. Down-regulation of proopiomelanocortin synthesis and b-endorphin utilization in hypothalamus of morphine-tolerant rats. J Mol Neurosci. 1989;1:33–38. doi: 10.1007/BF02896854. [DOI] [PubMed] [Google Scholar]
- 16.Bronstein DM, Przewlocki R, Akil H. Effects of morphine treatment on pro-opiomelanocortin systems in rat brain. Brain Res. 1990;519:102–111. doi: 10.1016/0006-8993(90)90066-k. [DOI] [PubMed] [Google Scholar]
- 17.Kelly MJ, Loose MD, Ronnekleiv OK. Opioids hyperpolarize beta-endorphin neurons via mu-opioid activiation of a potassium conductance. Neuroendocrinology. 1990;52:268–275. doi: 10.1159/000125597. [DOI] [PubMed] [Google Scholar]
- 18.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, Dileone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28:2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- 22.Seip KM, Reed B, Ho A, Kreek MJ. Measuring the incentive value of escalating doses of heroin in heroin-dependent Fischer rats during acute spontaneous withdrawal. Psychopharmacology. 2012;219:59–72. doi: 10.1007/s00213-011-2380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman M. Intermittent morphine administration induces dependence and is a chronic stressor in rats. Neuropsychopharmacology. 2003;28:1960–1971. doi: 10.1038/sj.npp.1300271. [DOI] [PubMed] [Google Scholar]
- 24.Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to bench. Cur Opin Pharmacol. 2009;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George JM, Reier CE, Lanese RR, Rower M. Morphine anesthesia blocks cortisol and growth hormone response to surgical stress in humans. J Clin Endocrinol Metab. 1974;38:736–741. doi: 10.1210/jcem-38-5-736. [DOI] [PubMed] [Google Scholar]
- 26.Cowen MJ, Bullingham RE, Paterson GM, McQuay HJ, Turner M, Allen MC, Moore A. A controlled comparison of the effects of extradural diamorphine and bupivacaine on plasma glucose and plasma cortisol in postoperative patients. Anesth Analg. 1982;61:15–18. [PubMed] [Google Scholar]
- 27.Zhou Y, Spangler R, Maggos CE, Wang XM, Han JS, Ho A, Kreek MJ. Hypothalamic–pituitary–adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. J Endocrinol. 1999;163:261–267. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]
- 28.Laorden ML, Milanes MV, Angel E, Tankosic, Burlet A. Quantitative analysis of corticotrophin-releasing factor and arginine vasopressin mRNA in the hypothalamus during chronic morphine treatment in rats: an in situ hybridization study. J Neuroendocrin. 2003;15:586–591. doi: 10.1046/j.1365-2826.2003.01037.x. [DOI] [PubMed] [Google Scholar]
- 29.Plotsky PM. Opioid inhibition of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation of rats. Reg Peptides. 1986;16:235–242. doi: 10.1016/0167-0115(86)90022-4. [DOI] [PubMed] [Google Scholar]
- 30.Buckingham JC. Secretion of corticotrophin and its hypothalamic releasing factor in response to morphine and opioid peptides. Neuroendocrinology. 1982;35:111–116. doi: 10.1159/000123364. [DOI] [PubMed] [Google Scholar]
- 31.Wardlaw SL, Kim J, Sobieszczyk S. Effect of morphine on pro-opiomelanocortin gene expression and peptide levels in the hypothalamus. Mol Brain Res. 1996;41:140–147. doi: 10.1016/0169-328x(96)00084-8. [DOI] [PubMed] [Google Scholar]
- 32.Fang Y, Kelly MJ, Rønnekleiv OK. Proopiomelanocortin (POMC) mRNA expression: distribution and region-specific down-regulation by chronic morphine in female guinea pig hypothalamus. Mol Brain Res. 1998;55:1–8. doi: 10.1016/s0169-328x(97)00348-3. [DOI] [PubMed] [Google Scholar]
- 33.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 35.Sharf R, Sarhan M, DiLeone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr KD. Effects of antibodies to dynorphin A and beta-endorphin on lateral hypothalamic self-stimulation in ad libitum fed and food-deprived rats. Brain Res. 1990;534:8–14. doi: 10.1016/0006-8993(90)90106-l. [DOI] [PubMed] [Google Scholar]
- 37.van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- 38.Negus S. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 39.Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 40.Cooper ZD, Truong YN, Shi YG, Woods JH. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326:920–929. doi: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakko J, von Wachenfeldt J, Svanborg KD, Lidström J, Barr CS, Heilig M. Mood and neuroendocrine response to a chemical stressor, metyrapone, in buprenorphine-maintained heroin dependence. Biol Psychiatry. 2008;63:172–177. doi: 10.1016/j.biopsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Schluger JH, Borg L, Ho A, Kreek MJ. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]