Abstract
The osteoblast-specific secreted molecule osteocalcin behaves as a hormone-regulating glucose and lipid metabolism, but the role of osteocalcin in cardiovascular disease (CVD) is not fully understood. In the present study, we investigated the effect of osteocalcin on autophagy and endoplasmic reticulum (ER) stress secondary to diet-induced obesity in the vascular tissue of mice and in vascular cell models and clarified the intracellular events responsible for osteocalcin-mediated effects. The evidences showed that intermittent injections of osteocalcin in mice fed the high-fat diet were associated with a reduced body weight gain, decreased blood glucose and improved insulin sensitivity compared with mice fed the high-fat diet receiving vehicle. Simultaneously, the administration of osteocalcin not only attenuated autophagy and ER stress but also rescued impaired insulin signaling in vascular tissues of mice fed a high-fat diet. Consistent with these results in vivo, the addition of osteocalcin reversed autophagy and ER stress and restored defective insulin sensitivity in vascular endothelial cells (VECs) and vascular smooth muscle cells (VSMCs) in the presence of tunicamycin or in knockout XBP-1 (a transcription factor which mediates ER stress response) cells or in Atg7−/− cells. The protective effects of osteocalcin were nullified by suppression of Akt, mammalian target of rapamycin (mTOR) or nuclear factor kappa B (NFκB), suggesting that osteocalcin inhibits autophagy, ER stress and improves insulin signaling in the vascular tissue and cells under insulin resistance in a NFκB-dependent manner, which may be a promising therapeutic strategies of cardiovascular dysfunction secondary to obesity.
Keywords: Akt, ER stress, NFκB, autophagy, mTOR, obesity, osteocalcin
Introduction
Obesity, especially the visceral type, is a fast growing problem that is reaching epidemic proportions worldwide, which increases the risk of cardiovascular disease (CVD).1,2 Obesity often clusters with various cardiovascular risk factors such as lipid abnormalities, glucose intolerance and high blood pressure, thus leading to endothelial dysfunction and atherosclerosis. Adipose tissue is also recognized to release a variety of large number of bioactive mediators such as adiponectin, resistin and tumor necrosis factor (TNF)-α to mediate insulin resistance.3-5 Although considerable progress has been made in understanding the mechanisms underlying these individual disorders, satisfactory treatment modalities remain limited.6,7
Osteocalcin, one of the most mature secretory products from osteoblasts,8 has recently emerged as an important regulator of glucose and lipid metabolism.9,10 For instance, genetic osteocalcin deficiency is associated with decreased β cell proliferation, glucose intolerance and insulin insensitivity,11 and no matter intermittent or continuous delivery, osteocalcin both promotes insulin secretion, improves insulin sensitivity and weakens the severity of insulin resistance in obese mice.12 Several clinical investigations have also reported serum osteocalcin concentrations were negatively related with carotid atherosclerosis and peripheral vascular disease, and its reduced levels were involved in all-cause mortality and CVD-related deaths in older men.13,14 In addition, serum osteocalcin is inversely associated with the severity of coronary artery disease and risk of coronary heart disease in Chinese adults,15 providing further evidence to the correlation between osteocalcin and CVD.
Previous studies also suggest a crucial role for osteocalcin in autophagy and endoplasmic reticulum (ER) stress in several physiological and pathological processes.16,17 Abnormal autophagy is implicated in multiple tissues of obesity;18 for instance, autophagosome formation is enhanced in pancreatic β cells and adipose tissue in obese mice, and enhanced autophagy was observed in advanced glycation end products (AGEs)-induced early injury of human umbilical vein endothelial cells and the process of AGEs-induced proliferation of VSMCs.19,20 On the other hand, ER stress could trigger autophagic imbalance and defective insulin signaling in mammalian cells.21-24 In recent studies, osteocalcin expression was suppressed by tunicamycin, an ER stress inducer, in mouse calvarial-derived osteoblasts, and inhibition of the cAMP response element-binding protein H (CREBH) expression reverses tunicamycin-suppressed osteocalcin expression,25 suggesting that osteocalcin might be a promising new link among ER stress, autophagy and obesity in vascular tissue in obesity.
Based on above-mentioned observations gathered in several laboratories, osteocalcin, as a bone-derived hormone, is inversely associated with the severity of coronary artery disease, which is involved in the regulation of endothelial dysfunction and atherosclerosis. In the present study, we provided the protective evidence and potential mechanisms for daily injection of osteocalcin involved in vascular tissues against diet-induced obesity. These results support the emerging notion that osteocalcin could be a treatment for the cardiovascular imbalance secondary to obesity and diabetes.
Results
Daily injections of osteocalcin improve glucose tolerance and insulin sensitivity in mice with diet-induced obesity
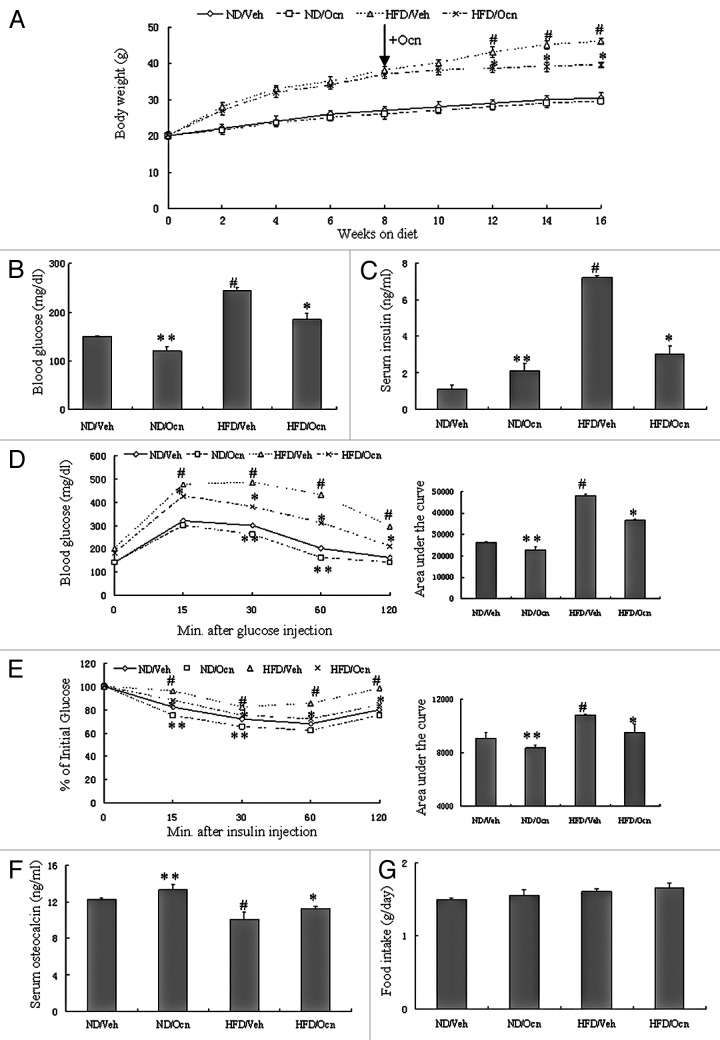
To evaluate the potential therapeutic relevance of osteocalcin in vivo, we investigated the effect of osteocalcin on glucose metabolism and insulin sensitivity in a classical model of diet-induced obesity.26 Daily injections of osteocalcin or vehicle were initiated after mice were fed a high-fat diet or a normal diet for 8 wk. As expected, after a 16-wk study period, mice fed the high-fat diet and not receiving osteocalcin displayed obesity, elevated blood glucose, higher insulin level, decreased osteocalcin level and developed glucose intolerance and insulin insensitivity as measured by glucose tolerance testing (GTT) and insulin tolerance testing (ITT) (Fig. 1A–F). However, mice fed the high-fat diet and injected with osteocalcin gained significantly less body weight and higher osteocalcin level, and their blood glucose and serum insulin levels were lower than those of mice fed the high-fat diet and injected with vehicle (Fig. 1A–C and F). Daily injections of osteocalcin in mice fed the high-fat diet were also associated with improved glucose tolerance and insulin sensitivity compared with mice fed the high-fat diet receiving vehicle by GTT and ITT (Fig. 1D and 1E), even though there is no significant difference in food intake among the groups (Fig. 1G), suggesting that the administration of osteocalcin improves glucose tolerance mainly by increasing insulin sensitivity in this model of obesity.
Figure 1. Daily injections of osteocalcin improve glucose tolerance and insulin sensitivity in mice with diet-induced obesity. All analyses were performed in mice fed a normal diet (ND) or a high-fat diet (HFD) for 8 wk and then injected daily with 30 ng/g/day of osteocalcin (Ocn) or with vehicle (Veh) for 8 wk. (A) Body weight curves. The arrow indicates the beginning of the daily injection treatment. (B) Blood glucose. (C) Serum insulin. (D) Glucose tolerance testing (GTT). (E) Insulin tolerance testing (ITT). (F) Serum osteocalcin. (G) Food intake. Twelve to fifteen mice per group were analyzed. The data expressed as mean ± SD in each bar graph represent the average of three independent experiments. *p < 0.05 (HFD/Ocn vs. HFD/Veh).#p < 0.05 (HFD/Veh vs. ND/Veh). **p < 0.05 (ND/Ocn vs. ND/Veh).
Daily injections of osteocalcin suppress high-fat diet-induced autophagy and ER stress in the vascular tissue of mice
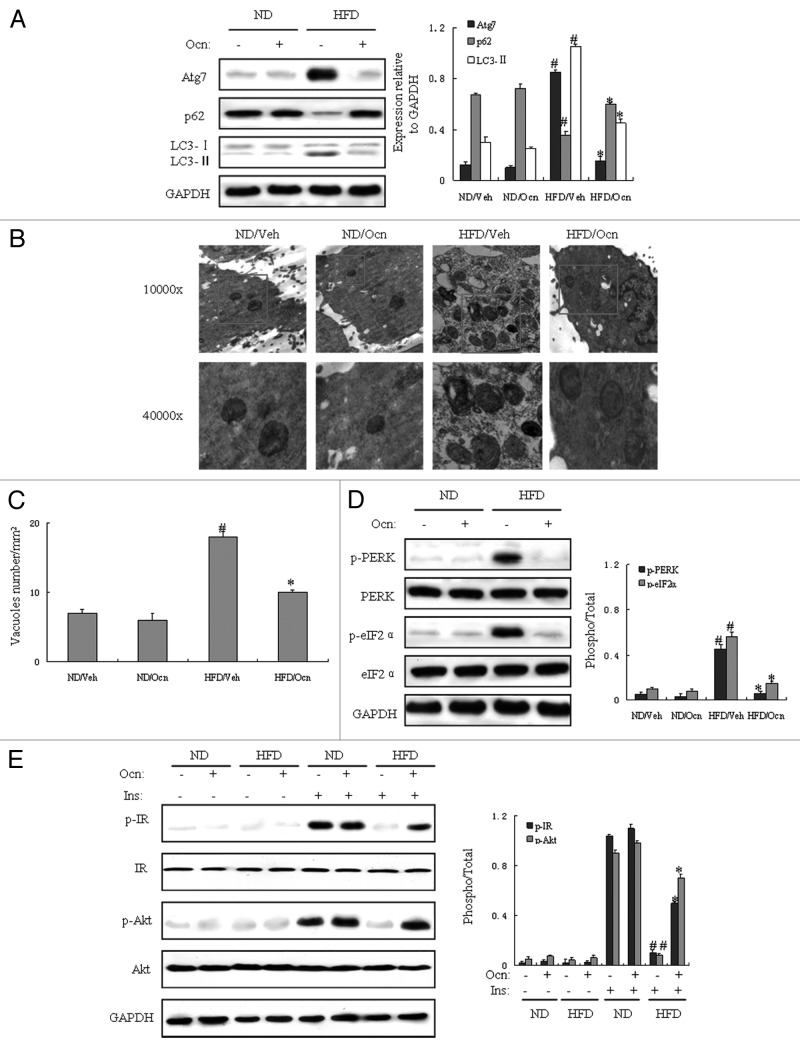
To determinate the role of autophagy and ER stress in vascular tissue of obese mice, we investigated whether autophagy and ER stress were abnormal in the vascular tissue of mice with diet-induced obesity, and whether osteocalcin could inhibit autophagy and ER stress and improve insulin receptor signaling in the vascular tissue of obese mice. We first measured protein expression of autophagy and ER stress indicators by western blot in aorta samples. Autophagic indicators such as Atg7 and light chain 3 II (LC3-II) are markedly increased in vascular tissue of obese mice. In contrast, p62 (also called SQSTM1, which is involved in aggresome formation and degraded through autophagy)27 was decreased in the vascular tissue of mice fed the high-fat diet and injected with vehicle compared with mice fed the normal diet (Fig. 2A). However, autophagy indicators such as Atg7 and LC3-II were significantly decreased, and p62 was increased in the aorta of mice fed the high-fat diet and injected with osteocalcin compared with mice fed the high-fat diet without osteocalcin (Fig. 2A). Electron microscopy (EM) examination of the aorta demonstrated significant increase in autophagosome/autolysosome formation in obese mice treated with vehicle as compared with mice fed the normal diet, whereas autophagosome/autolysosome formation was remarkably reduced in mice fed the high-fat diet and treated with osteocalcin compared with obese mice not receiving osteocalcin (Fig. 2B and C), supporting the biochemical alterations in key autophagy molecules. Meanwhile, the vascular tissue of mice fed the high-fat diet and injected with vehicle displayed activated ER stress, as evidenced by upregulation of phosphorylated protein kinase-like endoplasmic reticulum kinase (PERK) and eukaryotic initiation factor 2α (eIF2α) proteins compared with mice fed the normal diet (Fig. 2D). However, the vascular tissue of mice fed the high-fat diet with osteocalcin exhibited reduced ER stress on the grounds of downregulation of phosphorylated PERK and eIF2α proteins as compared with mice fed the high-fat diet and injected with vehicle (Fig. 2D).
Figure 2. Daily injections of osteocalcin suppress high-fat diet-induced autophagy and ER stress in the vascular tissue of mice with diet-induced obesity. All analyses were performed in mice fed a normal diet (ND) or a high-fat diet (HFD) for 8 wk and then injected daily with 30 ng/g/day of osteocalcin (Ocn) or with vehicle (Veh) for 8 wk. For insulin signaling, they were injected with 2 IU/kg of insulin. All indicators were measured in aorta samples at protein level, and the samples were observed by electron microscopy. The relative quantity of proteins was analyzed using Quantity One software. (A) Protein expression of Atg7, p62 and LC3. (B) Representative electron micrographs (10,000× and 40,000×) of aorta samples. Autophagolysosomes are marked with a red rectangle. (C) Quantification of autophagolysosome-like vacuoles per field in the EM images. (D) Phosphorylation of PERK and eIF2α in aorta samples. (E) IRβ subunit tyrosine 1162/1163 phosphorylation and Akt serine 473 phosphorylation in aorta samples. Twelve to fifteen mice per group were analyzed. A representative blot from three independent experiments is shown and the data expressed as mean ± SD in each bar graph represent the average of three independent experiments. *p < 0.05 (HFD/Ocn vs. HFD/Veh).#p < 0.05 (HFD/Veh vs. ND/Veh).
Next, we analyzed protein expression of indicators of insulin receptor signaling by western blot. Compared with controls, we found that there was a significant reduction in insulin-stimulated tyrosine 1162/1163 phosphorylation of IRβ subunit and serine 473 phosphorylation of Akt in the vascular tissue of mice fed the high-fat diet, demonstrating inhibited insulin receptor signaling and severe insulin resistance in obese mice. However, along with the acts of osteocalcin on autophagy and ER stress, our study also underlined that the administration of osteocalcin could reverse insulin resistance, which was proved by the increase of insulin-stimulated phosphorylation of insulin receptor β (IRβ) subunit and Akt Ser-473 in the vascular tissue of mice fed the high-fat diet (Fig. 2E), indicating that daily injections of osteocalcin enhance insulin sensitivity in the vascular tissues possibly by the recovery of abnormal autophagy and ER stress in mice with diet-induced obesity.
Tunicamycin-induced autophagy and ER stress are inhibited by osteocalcin in mouse VECs and VSMCs
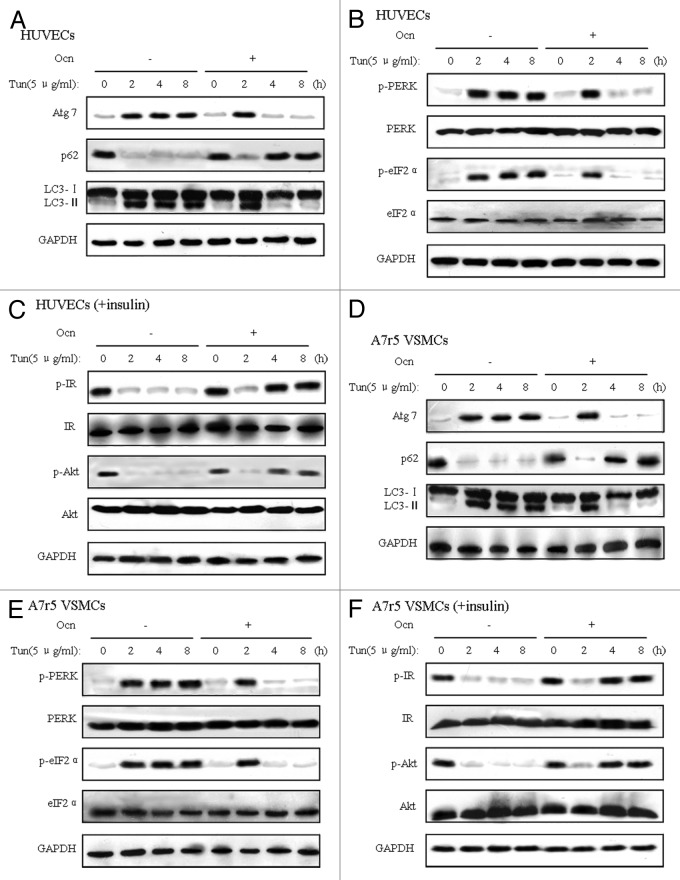
To determine whether ER stress interferes with autophagy and insulin action and whether osteocalcin suppresses tunicamycin-induced autophagy and ER stress and improves insulin receptor signaling in vitro, we first treated mouse VECs with osteocalcin and tunicamycin (an agent commonly used to induce ER stress) at several time points (2, 4 and 8 h) and then measured protein expression of indicators of autophagy, ER stress and insulin receptor signaling by western blot. Tunicamycin not only significantly elevated autophagy, but also increased the phosphorylation of PERK and eIF2α and decreased insulin-stimulated phosphorylation of IRβ subunit and Akt Ser-473 in VECs (Fig. 3A–C). Consistent with what we found in the animal models, 5 ng/ml osteocalcin treatment for 4 h and 8 h in the presence of tunicamycin both decreased dramatically the levels of Atg7 and LC3-II and increased the low level of p62 compared with VECs only exposed to tunicamycin (Fig. 3A); similarly, ER stress indicators were reduced (Fig. 3B), and the phosphorylation of IRβ subunit and Akt Ser-473 was significantly elevated (Fig. 3C). Thus, we found the optimum action time of 5ng/ml osteocalcin is 4 h, and all subsequent experiments were performed for 4 h. In addition, the same indication as VECs was evident in VSMCs (Fig. 3D–F).
Figure 3. Tunicamycin-induced autophagy and ER stress are inhibited by osteocalcin in mouse VECs and VSMCs. Tunicamycin (Tun) 5 μg/ml for 4 h was used to induce ER stress. To determine the effects of osteocalcin, mouse VECs and VSMCs were treated with 5 ng/ml of osteocalcin for 0, 2, 4 and 8 h. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 min. All indicators were measured at protein level. The relative quantity of proteins was analyzed using Quantity One software. (A) Protein expression of Atg7, p62 and LC3 in VECs. (B) Phosphorylation of PERK and eIF2α in VECs. (C) IRβ subunit tyrosine 1162/1163 phosphorylation and Akt serine 473 phosphorylation in VECs. (D) Protein expression of Atg7, p62 and LC3 in VSMCs. (E) Phosphorylation of PERK and eIF2α in VSMCs. (F) IRβ subunit tyrosine 1162/1163 phosphorylation and Akt serine 473 phosphorylation in VSMCs. A representative blot from three independent experiments is shown.
Low-dose tunicamycin-induced autophagy and ER stress are suppressed by osteocalcin in mouse VECs and VSMCs transfected with XBP-1 siRNA
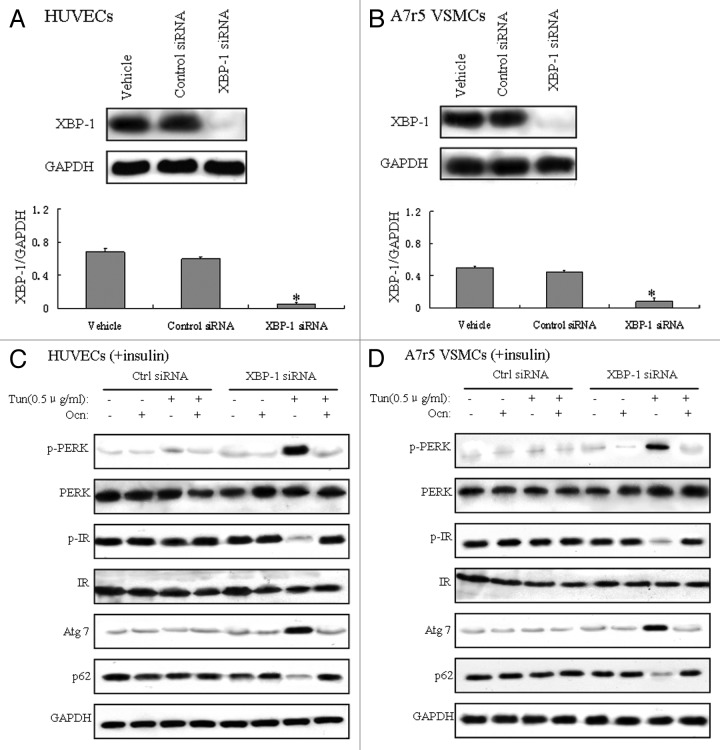
The spliced or processed form of XBP-1 (XBP-1s) is a key factor in ER stress.28,29 XBP-1−/− mouse embryonic fibroblasts (MEFs) are hypersensitive to ER stress because of the decreased ER folding capacity.30 To investigate whether osteocalcin could regulate ER stress and insulin signaling in XBP-1−/− cells, we first knocked down XBP-1 via XBP-1 siRNA in mouse VECs and VSMCs. XBP-1 siRNA was validated by measurement of reduced XBP-1 protein expression by western blot in XBP-1 siRNA-transfected VECs and VSMCs (Fig. 4A and B). ER stress and autophagy were activated, and insulin signaling was impaired in XBP-1−/− VECs and VSMCs treated with low-dose (0.5 μg/ml) tunicamycin (Fig. 4C and D), indicating the hypersensitivity of XBP-1−/− VECs and VSMCs to tunicamycin to induce ER stress and autophagy, but when this XBP-1−/− cells were predisposed with osteocalcin and low-dose (0.5 μg/ml) tunicamycin, the expression of Atg7 and PERK phosphorylation were reduced;in contrast, the protein level of p62 and IR phosphorylation were increased (Fig. 4C and D), from which we can conclude that osteocalcin reverses autophagy and ER stress in an XBP-1-independent manner.
Figure 4. Low-dose tunicamycin-induced autophagy and ER stress are suppressed by osteocalcin in mouse VECs and VSMCs transfected with XBP-1 siRNA. Tunicamycin (Tun) 0.5 μg/ml for 4h was used to induce ER stress. Cells were cultured in the presence or absence of osteocalcin (Ocn) with or without 100 nM XBP-1 siRNA. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 min. All indicators were measured at protein level. The relative quantity of proteins was analyzed using Quantity One software. (A) Protein expression of XBP-1 in VECs. (B) Protein expression of XBP-1 in VSMCs. (C) Protein expression of Atg7 and p62, and the phosphorylation of PERK and IR in VECs. (D) Protein expression of Atg7 and p62, and the phosphorylation of PERK and IR in VSMCs. A representative blot from three independent experiments is shown and the data expressed as mean ± SD in each bar graph represent the average of three independent experiments. *p < 0.05 (XBP-1 siRNA vs. control siRNA).
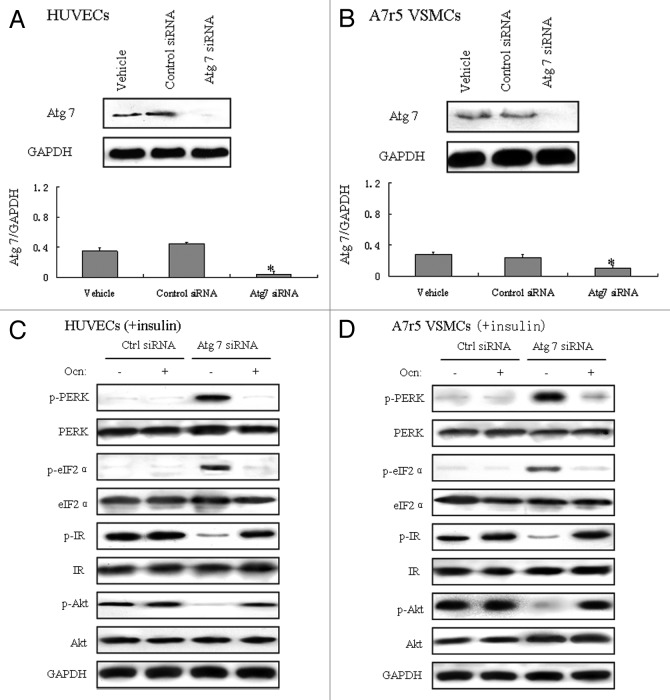
Suppression of autophagy results in ER stress and insulin resistance, but osteocalcin alleviated ER stress and improves insulin signaling in mouse VECs and VSMCs transfected with Atg7 siRNA
Experimental ER stress can induce autophagy in mammalian cells with several canonical unfolded protein response (UPR) pathways implicated in this interaction.31 To investigate the impact of autophagy on metabolic regulation, we interfered with autophagy via Atg7 siRNA in mouse VECs and VSMCs, and its validation was measured by reduction of Atg7 protein expression by western blot. ER stress was induced in Atg7-deficient VECs and VSMCs. Meanwhile, there was a significant reduction in insulin-stimulated tyrosine phosphorylation of IRβ subunit, demonstrating severe insulin resistance. Of interest, osteocalcin treatment in Atg7-deficient cells decreased the phosphorylation of PERK and eIF2α, and elevated the phosphorylation of IR and Akt compared with Atg7-deficient cells treated without osteocalcin (Fig. 5C and D), which demonstrated that the amelioration of ER stress and insulin action by osteocalcin is not a result of attenuated autophagy, even though in Atg7-deficient vascular cells, osteocalcin still inhibits ER stress and improves insulin signaling.
Figure 5. Suppression of autophagy results in ER stress and insulin resistance, but osteocalcin alleviated ER stress and improves insulin signaling in mouse VECs and VSMCs transfected with Atg7 siRNA. Mouse VECs and VSMCs were cultured in the presence or absence of osteocalcin (Ocn) with or without 100 nM Atg7 siRNA. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 min. All indicators were measured at protein level. The relative quantity of proteins was analyzed using Quantity One software. (A) Protein expression of Atg7 in VECs. (B) Protein expression of Atg7 in VSMCs. (C) Phosphorylation of PERK, eIF2α, IR and Akt in VECs. (D) Phosphorylation of PERK, eIF2α, IR and Akt in VSMCs. A representative blot from three independent experiments is shown and the data expressed as mean ± SD in each bar graph represent the average of three independent experiments. *p < 0.05 (Atg7 siRNA vs. control siRNA).
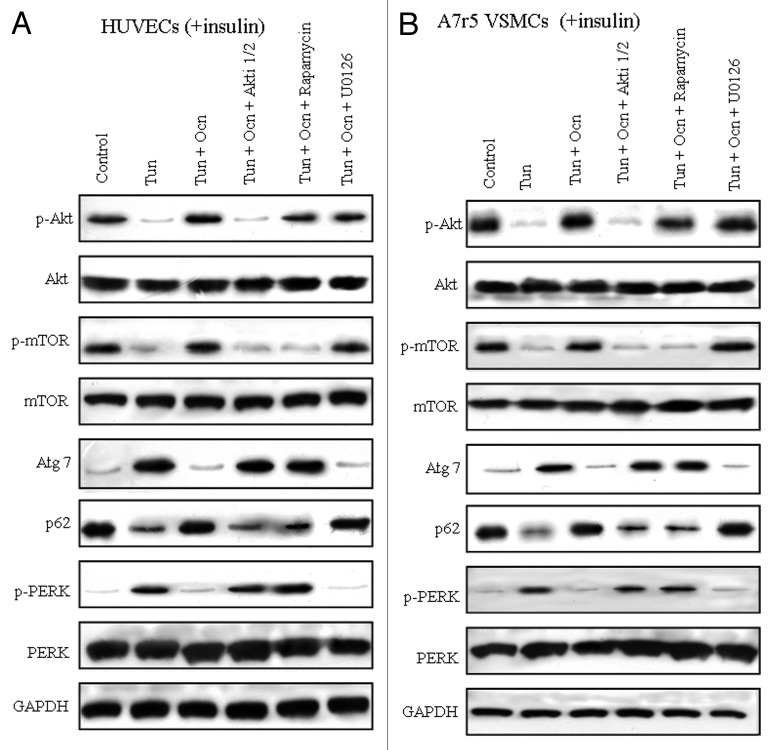
Osteocalcin activates Akt/mTOR signaling pathways in mouse VECs and VSMCs under ER stress
Akt/mTOR signaling pathways antagonize both autophagy and ER stress.32 Based on our findings that osteocalcin improved insulin sensitivity by elevating insulin-stimulated Akt Ser-473 phosphorylation in our experimental models, we postulated that intracellular Akt/mTOR signaling pathway was implicated in the action of osteocalcin. To understand the molecular mechanism, we cultured mouse VECs in the presence of insulin, tunicamycin and/or osteocalcin, with or without specific inhibitors of each signaling pathway such as Akti 1/2 (an Akt inhibitor), rapamycin (an mTOR inhibitor) and U0126 (a MAPK inhibitor). Osteocalcin significantly elevated the phosphorylation of Akt Ser-473, an indicator of Akt activity, and the phosphorylation of mTOR, an indicator of mTOR activity in VECs under ER stress without specific inhibitors (Fig. 4A). The addition of Akti 1/2 or rapamycin in the culture medium of osteocalcin- and tunicamycin-treated VECs significantly reversed the effects of osteocalcin on autophagy and ER stress, whereas the addition of U0126 did not (Fig. 6A). Similar results were also observed in VSMCs (Fig. 6B).
Figure 6. Osteocalcin activates Akt/mTOR signaling pathways in mouse VECs and VSMCs under ER stress. Tunicamycin (Tun) 5 μg/ml for 4 h was used to induce insulin resistance. Mouse VECs and VSMCs were cultured in the presence or absence of osteocalcin (Ocn) with or without specific signaling pathway inhibitors such as 10 μM Akti-1/2 (an AKT inhibitor), 10 nMRapamycin (an mTOR inhibitor) or 10 μM U0126 (a MAPK inhibitor) for 4 h. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 min. All indicators were measured at protein level. The relative quantity of proteins was analyzed using Quantity One software. (A) Protein expression of Atg7 and p62, and the phosphorylation of Akt, PERK and m-TOR in VECs. (B) Protein expression of Atg7 and p62, and the phosphorylation of Akt, PERK and m-TOR in VSMCs. A representative blot from three independent experiments is shown.
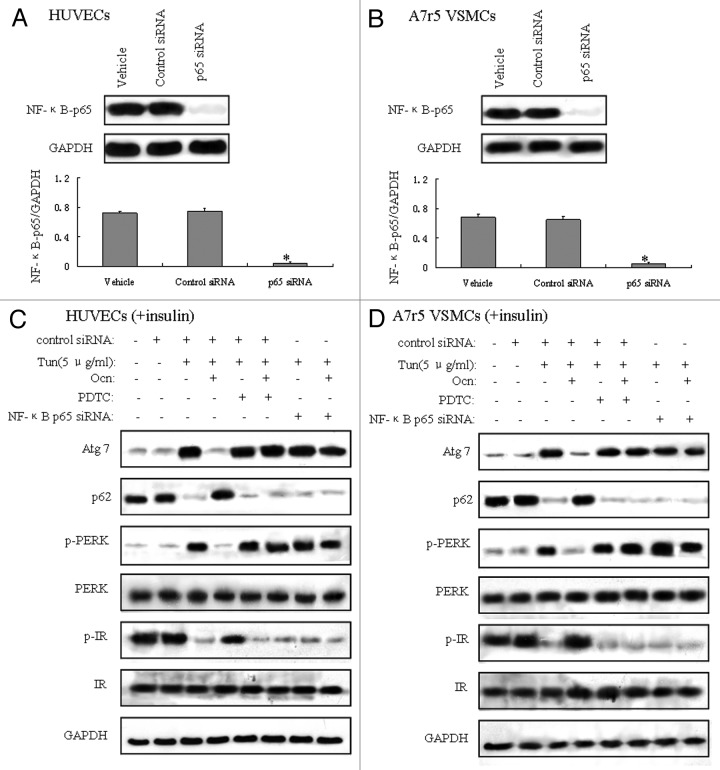
Osteocalcin regulates autophagy and ER stress via NFκB-p65-dependent mechanisms
NFκB pathway is involved in the control of inflammation, stress response and many other physiological processes in cellular signaling. The activation of NFκB followed by Akt phosphorylation plays an important role in the development of insulin resistance.33 To confirm that NFκB pathway was implicated in the regulation of osteocalcin on autophagy and ER stress, we blocked NFκB pathway via pyrrolidinedithiocarbamate (PDTC), a known NFκB inhibitor and NFκB p65 siRNA, respectively, in mouse VECs and VSMCs in the presence of tunicamycin and osteocalcin. NFκB p65 siRNA-transfected cells exhibited reduced p65 protein expression compared with control siRNA-transfected cells (Fig. 7A and B). Osteocalcin treatment under ER stress suppressed autophagy and ER stress and activated insulin signaling, but blockade of NFκB by the addition of PDTC in the culture medium or by transfection with NFκB p65 siRNA in cells nullified the protective effect of osteocalcin (Fig. 7C and D), which suggests that functions of osteocalcin upon autophagy and ER stress occur in a NFκB-p65-dependent manner in vitro.
Figure 7. Osteocalcin regulates autophagy and ER stress via NFκB-p65-dependent mechanisms. Tunicamycin (Tun) 5 μg/ml for 4 h was used to induce insulin resistance. Mouse VECs and VSMCs were cultured in the presence or absence of osteocalcin (Ocn) with or without 1 μM PDTC (an NFκB inhibitor) and 100 nM NFκB-p65 siRNA. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 min. All indicators were measured at protein level. The relative quantity of proteins was analyzed using Quantity One software. (A) Protein expression of NFκB-p65 in VECs. (B) Protein expression of NFκB-p65 in VSMCs. (C) Protein expression of Atg7 and p62, and the phosphorylation of PERK and IR in VECs. (D) Protein expression of Atg7 and p62, and the phosphorylation of PERK and IR in VSMCs. A representative blot from three independent experiments is shown, and the data expressed as mean ± SD in each bar graph represent the average of three independent experiments. *p < 0.05 (NFκB-p65 siRNA vs. control siRNA).
Discussion
Understanding the mechanisms of osteocalcin in the progress of insulin resistance is of both scientific and clinical significance. In the present study, our results displayed that increased autophagy, activated ER stress and impaired insulin signaling induced by obesity were effectively corrected by daily injections of osteocalcin. Consistent with these findings, osteocalcin effectively inhibited autophagy and ER stress, and restored insulin sensitivity via NFκB signaling pathway in VECs and VSMCs.
Osteocalcin, a classical marker of bone formation, has recently received attention as a circulating hormone implicated in the regulation of glucose and lipid metabolism. The OCN knockout mice not only had both increased bone mass and accumulation of visceral fat but are associated with insulin resistance and glucose intolerance.8 Furthermore, mice lacking Esp, an osteoblast-specific tyrosine phosphatase gene involved in the posttranscriptional gamma-carboxylation of glutamate residues of OCN, exhibited enhanced osteocalcin bioactivity on insulin and adiponectin regulation, severe hypoglycemia and protection against diet-induced obesity and type 2 diabetes.34 Our results demonstrated that daily injections of osteocalcin improve glucose tolerance and insulin sensitivity in mice with diet-induced obesity in line with previous studies that intermittent and continuous deliveries of osteocalcin partially restored insulin signaling, glucose tolerance and energy expenditure.11,12 However, the definite effect of exogenous osteocalcin on human remains to be determined, even though several clinical investigations support the notion that an association between glucose metabolism and osteocalcin exists in humans.35,36
Others have reported that patients with osteoporosis are known to have an increased incidence of CVD, and, for another, it was shown that low bone density and low bone formation markers, BAP and osteocalcin, were found in patients with severe atherosclerosis,37 which all revealed an important relationship between serum osteocalcin concentrations and CVD. Moreover, our experiments demonstrated that daily injections of osteocalcin improved the insulin sensitivity, autophagy and ER stress of vascular tissue in obese mice, and in vitro, osteocalcin played a protective role on mouse VECs and VSMCs from the detrimental effect of autophagy and ER stress. In addition, insulin has a specific and physiological action to vasodilate skeletal muscle vasculature in humans, and this hemodynamic action appears to be important both for the maintenance of vascular tone and the modulation of substrate uptake,38 from which the question of whether our daily injections of osteocalcin act on vascular tissue directly or indirectly by improving overall insulin sensitivity emerges and remains to be addressed in further studies.
It has been proved that ER stress and autophagy play a key role as a chronic stimulus in the development of obesity and type 2 diabetes in terms of the integrated deterioration of systemic glucose homeostasis.39 In our study, osteocalcin suppressed autophagy and ER stress and restored insulin action in vascular tissues in experimental models of insulin resistance. These results demonstrate that osteocalcin enhanced the adaptive capacity of the ER and might act as a potential application in the treatment of obesity and coronary artery disease. The exact mechanisms triggering autophagy and ER stress in obesity are still unclear, but likely involve multiple signals, including chronically increased demand on synthetic machinery along with profound alterations in energy fluxes in metabolically active tissues. The enhancement of ER function with osteocalcin during these alterations provides a unique approach to manage metabolic abnormalities associated with obesity in vascular tissues.
The intracellular events responsible for osteocalcin-mediated effects in insulin resistance remain largely unknown. An unbiased approach based on the ability of osteocalcin to increase cAMP production in Leydig cells and on its dichotomy of function between male and female gonads led to the identification of Gprc6a as the osteocalcin receptor.40 However, the possibility that Gprc6a could be a specific receptor for osteocalcin has never been tested through biochemical or genetic means, and it is still hard to clearly define the early stages of osteocalcin-mediated signaling from the plasma membrane. Blockade of Akt pathway effectively prevented increased XBP-1 and lipid droplet formation in human renal proximal tubular cells under stimulation of high glucose,41 indicating that Akt pathway mediated high glucose-induced XBP-1 expression. In our study, osteocalcin could reduce ER stress and improve insulin signaling in multiple cell types in an XBP-1-independent manner. It remains to be addressed whether osteocalcin promotes nuclear localization of XBP-1 via Akt signaling, resulting in enhanced ER folding capacity.
Our data demonstrated that the activation of Akt/mTOR signaling but not of the MAPK pathway is required for osteocalcin-mediated autophagy and ER stress alleviation as well as restoration of insulin signaling. Our study enhances understanding of the mechanisms by which Akt/mTOR contributes to the regulation of osteocalcin-induced modulation in vascular tissues. mTOR is a major intracellular hub for integrating autophagy-related signals; in deprivation, stress convergence and low insulin/IGF1 signaling, numerous signaling pathways inactivate mTOR kinase activity, which both suppresses cell growth to reduce energy demand and induces autophagy to enable stress adaptation and survival,42 in accordance with our results that osteocalcin attenuated autophagy by activating mTOR via AKT signaling.Previous studies in other cell types have shown direct effects of insulin on osteocalcin synthesis primarily through the activation of the Akt and MAPK pathways in osteoblasts,43 whereas osteocalcin enhancement during osteogenic differentiation is promoted through ERK and MAPK pathways in human periodontal ligament stem cells and human osteoblastic cells.44 Although this experimental evidence has shed light on the signaling pathways, the transcription factor(s) ultimately mediating the effects of osteocalcin on glucose and lipid metabolism remain to be identified.
Previous evidence indicated that the activation of NFκB p65 by the Akt pathway involves p65 Ser-536 phosphorylation by IκB kinase, suggesting an interaction between Akt and NFκB.45-48 We hypothesized that osteocalcin inhibits autophagy and ER stress and restores insulin signaling by inducing the activity of NFκB in vascular tissues. Our experiments in cultured cells confirmed the role of NFκB in osteocalcin-mediated actions. More significantly, the inhibition of NFκB activity by PDTC (a specific NFκB inhibitor) nullified the protective effect of osteocalcin on autophagy, ER stress and insulin sensitivity. The neutralization of the p65 siRNA further supports a functional interaction between NFκB-p65 and osteocalcin in the regulation of autophagy, ER stress and insulin signaling. Depletion of endogenous osteocalcin had a dramatic effect in a condition that is close to the cellular endocrine environment in vivo.10 It is therefore reasonable to speculate that osteocalcin functions effectively in an endocrine manner. In the current study, osteocalcin stimulated nuclear translocation of NFκB and initiated a cascade of target genes involved in cell proliferation and anti-apoptosis, clearly demonstrating the relationship between NFκB activation and osteocalcin function. Further studies are required to determine the relative roles of the upstream activators and downstream effectors for NFκB during osteocalcin-mediated processes.
All these findings suggest a reciprocal function interaction between ER stress, autophagy and insulin signaling via the NFκB-dependent mechanism. As shown in Figure 8, in tunicamycin-induced cells, osteocalcin may induce NFκB nuclear translocation, which modulates abnormal autophagy and ER stress. As a result, blockade of NFκB in the cells nullified the protective effect of osteocalcin. Meanwhile, inhibited ER stress can also regulate autophagy by triggering mTOR activity via the increase of insulin-stimulated phosphorylation of IRβ and Akt Ser-473. Thus, osteocalcin treatment may reverse abnormal autophagy and ER stress via the Akt/mTOR signaling pathway in a NFκB-p65-dependent manner.
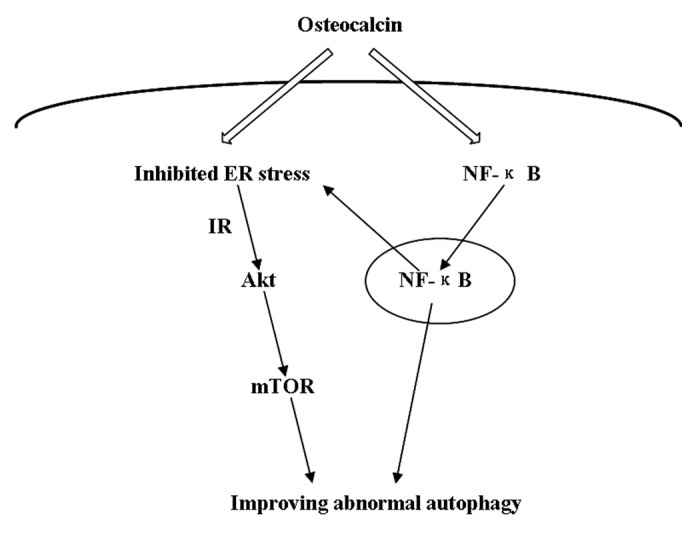
Figure 8. Schematic representation of the putative mechanisms characterizing the functional interaction between autophagy, ER stress and insulin signaling. In tunicamycin-induced cells, osteocalcin may activate NFκB-dependent intracellular signaling pathway via inducing NFκB nuclear translocation, leading to decreased ER stress and autophagy. Simultaneously, inhibited ER stress can activate Akt/mTOR signaling pathway, thereby autophagic dysfunction is reversed by triggering mTOR activity. As a result, osteocalcin treatment may suppress autophagy and ER stress via the NFκB-p65-dependent mechanism.
In conclusion, our findings suggest that osteocalcin attenuated autophagy, reversed ER stress and restored insulin signaling in the vascular tissue of mice with diet-induced obesity via NFκB signaling pathway, whereas its functional role in metabolic dysfunction warrants further investigation.
Materials and Methods
Materials
All chemicals used were of analytical grade and were purchased from Sigma unless stated otherwise. The following antibodies were used: anti-Atg7, anti-p62, anti-LC3, anti-p-PERK, anti-PERK, anti-p-eIF2α and anti-eIF2α (Cell Signaling Technology Inc); anti-p-IRβ, anti-IRβ, anti-p-Akt, anti-Akt, anti-p-mTOR, anti-mTOR, anti-Xbp-1, anti-NFκB-p65, anti-GAPDH and peroxidase goat anti-rabbit IgG and peroxidase goat anti-mouse IgG (Santa Cruz Biotechnology Inc).
Purification of recombinant uncarboxylatedosteocalcin
Purification of bacterially produced mouse recombinant uncarboxylatedosteocalcin was performed as described.49 Briefly glutathione S-transferase (GST)-osteocalcin fusion protein was produced and purified on glutathione-sepharose according to the standard procedure. Then osteocalcin was cleaved out from the GST moiety by using thrombin. Purity (> 95%) of the osteocalcin preparation was assessed by Tris-tricine SDS/PAGE staining. Concentration of the recombinant osteocalcin protein was determined using an osteocalcin RIA kit (Immunotope).
Cell culture and treatment
Mouse VECs were isolated from the aorta by enzymatic detachment using collagenase. VECs were cultured in DMEM/F-12 (HyClone, Thermo Fisher Scientific Inc) containing 10% fetal bovine serum (FBS) (HyClone, Thermo Fisher Scientific Inc). Mouse VSMCs were purchased from American Type Culture Collection (ATCC) and cultured in DMEM containing 10% FBS. Prior to each experiment, the medium was replaced by a fresh medium. ER stress was induced by 4 h of pretreatment with 5 μg/ml of tunicamycin. For effect of osteocalcin, cells were treated with 5 ng/ml of osteocalcin for 2, 4 or 8 h. For insulin signaling, cells were stimulated with 10 nM of insulin for 10 min; media were replaced by a fresh medium (10% FBS/DMEM).
Animal care
The experimental procedures performed in this study were in accordance with the guidelines of the Institutional Animal Ethics Committee for the Care and Use of Laboratory Animals. Recombinant osteocalcin was freshly diluted in saline solution (0.9% NaCl) at a concentration of 3ng/μl. For the in vivo study, C57BL/6J mice (4-wk-old) were housed under standard conditions with a 12 h light/dark cycle (darkness from 7:30 p.m. to 7:30 a.m.). Mice were distributed in four groups (n = 12–15per group): (1) vehicle (normal chow diet, 4% of energy as fat, 3.85 kcal/g); (2) osteocalcin (normal chow plus 30ng/g of osteocalcin implant); (3) high fat + vehicle (diet containing 60% of energy as fat, 5.24 kcal/g);(4) high fat + osteocalcin (high-fat diet + 30ng/g of osteocalcin implant). Daily injections of osteocalcin or vehicle were initiated after mice were fed a high-fat diet or a normal chow diet for 8 wk. Mice were injected once a day (6 p.m.) intraperitoneally (i.p.) with 10 μl/g of osteocalcin solution or with saline solution (vehicle) for 8 wk. At the end of the 16 wk study period, all mice received an intraperitoneal injection of insulin at a dosage of 2 IU/kg; 15 min after the injection, the animals were euthanized, and their blood and aorta samples were obtained and stored at −80°C for subsequent analysis.
Measurement of blood parameters
After collection, serum was stored at −80°C before analysis. Morning blood glucose was measured using an Accu-check glucometer. Insulin levels were determined by an ultrasensitive mouse-specific ELISA (Crystal Chem) with intra- and inter-assay C.V.s of 3.9–6.6% and 5.6–5.8%, respectively. Concentrations of serum osteocalcin were quantified using electrochemiluminescence immunoassay (Roche Diagnostics GmbH) with intra- and inter-assay coefficients of variation of 1.2–4.0% and 1.7–6.5%, respectively.
Metabolic tests
Glucose tolerance testing (GTT) was performed after the mice were fasted overnight. A total of 2 g/kg of glucose was administrated through an i.p. injection, and blood glucose was measured at the indicated time points. Insulin tolerance testing (ITT) was performed after the animals had fasted for 4 h. Then, 0.75 U/kg insulin was administered via i.p. injection, and blood glucose was measured at the indicated time points.
Physiological measurements
To measure food intake, mice were individually housed in metabolic cages and fed ad libitum. Food consumption was determined by weighing the powdered diet before and after a 24 h period.
Electron microscopy (EM) analysis
Aorta samples were fixed in 4% paraformaldehyde/2% glutaraldehyde/0.1 M sodium cacodylate pH 7.3, post-fixed in 1% osmium tetraoxide and embedded in epoxy resin (Epon). Ultrathin sections (80 nm) were stained with aqueous uranyl acetate and lead citrate and examined with a JEOL 2000FX transmission electron microscope (JEOL). For quantification of autophagolysosome-like vacuoles, the numbers of autophagolysosomal-like vacuoles were counted in each field and normalized by the surface area.
Gene silencing
Cells were transfected with a siRNA targeted for mouse XBP-1 (the sequence GGGATTCATGAATGGCCCTTA), mouse Atg7 (Cell Signaling, catalog number 6604), mouse NFκB-p65 (Santa Cruz, catalog number sc-29411) usingLipofectamine 2000 (Invitrogen). A siRNA consisting of a scrambled sequence of similar length was similarly transfected as control siRNA. One day before transfection, cells were plated in 500 μl of growth medium without antibiotics, such that they were 30–50% confluent at the time of transfection. The transfected cells were cultured in DMEM containing 10% fetal calf serum for 72 h after transfection. To determine knockdown efficiency, we evaluated protein expression of Xbp-1, Atg7 and NFκB-p65 in transfected cells by western blot.
Western blot
Tissues and cells under various treatments were lysed in lysis buffer containing 25 mM TRIS-HCl (pH 6.8), 2% SDS, 6% glycerol, 1% 2-mercaptoethanol, 2 mMphenylmethylsulfonyl fluoride, 0.2% bromophenol blue, and a protease inhibitor cocktail for 20 min. Nuclear extracts were obtained using a nuclear extraction kit (Sigma). Western blotting was performed by utilizing a standard protocol as described.21
Statistics
Statistical analyses were performed using SPSS 17.0 software. Statistical analysis between the two groups was performed using unpaired, two-tailed Student’s t-test or ANOVA followed by post hoc tests. Differences were considered significant when the p value was less than 0.05.
Acknowledgments
We appreciate the technical support and materials from the electron microscope center of Xi’an Jiaotong University. This work was funded by the programs from the National Natural Science Foundation of China (Key Program no. 30930105 and General Program no. 30971392, no. 81170741 and no. 81071440) and the New Century Excellent Talents in University from the Ministry of Education, China (NCET-08-0435).
Glossary
Abbreviations:
- AGEs
advanced glycation end products
- CVD
cardiovascular disease
- eIF2α
eukaryotic initiation factor 2α
- EM
electron microscopy
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GTT
glucose tolerance testing
- IRβ
insulin receptor β
- IR
insulin receptor
- ITT
insulin tolerance testing
- LC3
light chain 3
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NEFA
nonesterified fatty acid
- NFκB
nuclear factor kappa B
- T2DM
type 2 diabetes mellitus
- TNF
tumor necrosis factor
- VEC
vascular endothelial cell
- VSMC
vascular smooth muscle cell
- PERK
protein kinase-like endoplasmic reticulum kinase
- UPR
unfolded protein response
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24929
References
- 1.Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/S0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Smith SR, Wilson PW. Free fatty acids and atherosclerosis--guilty or innocent? J Clin Endocrinol Metab. 2006;91:2506–8. doi: 10.1210/jc.2006-1018. [DOI] [PubMed] [Google Scholar]
- 4.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab. 2006;91:2542–7. doi: 10.1210/jc.2006-0195. [DOI] [PubMed] [Google Scholar]
- 5.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 6.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blais J, Bell JC. Novel therapeutic target: the PERKs of inhibiting the integrated stress response. Cell Cycle. 2006;5:2874–7. doi: 10.4161/cc.5.24.3597. [DOI] [PubMed] [Google Scholar]
- 8.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 9.Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22:187–94. doi: 10.1007/s00198-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–75. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45–9. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- 14.Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol. 2010;163:265–72. doi: 10.1530/EJE-10-0414. [DOI] [PubMed] [Google Scholar]
- 15.Zhou M, Ma X, Li H, Pan X, Tang J, Gao YC, et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161:723–9. doi: 10.1530/EJE-09-0585. [DOI] [PubMed] [Google Scholar]
- 16.Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle. 2012;11:2092–9. doi: 10.4161/cc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik SA, Mariño G, BenYounès A, Shen S, Harper F, Maiuri MC, et al. Neuroendocrine regulation of autophagy by leptin. Cell Cycle. 2011;10:2917–23. doi: 10.4161/cc.10.17.17067. [DOI] [PubMed] [Google Scholar]
- 18.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y, You SJ, Zhang YL, Han Q, Cao YJ, Xu XS, et al. Protective role of autophagy in AGE-induced early injury of human vascular endothelial cells. Mol Med Rep. 2011;4:459–64. doi: 10.3892/mmr.2011.460. [DOI] [PubMed] [Google Scholar]
- 20.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012;29:613–8. doi: 10.3892/ijmm.2012.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Zhou B, Xu L, Sun H. IRS-2, but not IRS-1, can sustain proliferation and rescue UBF stabilization in InR or InR defective signaling of 32D myeloid cells. Cell Cycle. 2009;8:3218–26. doi: 10.4161/cc.8.19.9759. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Zang W, Zhou B, Xu L, Wu S. DHEA suppresses longitudinal bone growth by acting directly at growth plate through estrogen receptors. Endocrinology. 2011;152:1423–33. doi: 10.1210/en.2010-0920. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 24.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang WG, Kim EJ, Koh JT. Tunicamycin negatively regulates BMP2-induced osteoblast differentiation through CREBH expression in MC3T3E1 cells. BMB Rep. 2011;44:735–40. doi: 10.5483/BMBRep.2011.44.11.735. [DOI] [PubMed] [Google Scholar]
- 26.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958–66. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 27.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calfon M, Zeng HQ, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z, Fan Q, Zhang Z, Zou Y, Cai R, Wang Q, et al. SENP1 deficiency promotes ER stress-induced apoptosis by increasing XBP1 SUMOylation. Cell Cycle. 2012;11:1118–22. doi: 10.4161/cc.11.6.19529. [DOI] [PubMed] [Google Scholar]
- 30.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–9. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 32.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Morrison A, Sun H, De Luca F. Nuclear factor-kappaB (NF-kappaB) p65 interacts with Stat5b in growth plate chondrocytes and mediates the effects of growth hormone on chondrogenesis and on the expression of insulin-like growth factor-1 and bone morphogenetic protein-2. J Biol Chem. 2011;286:24726–34. doi: 10.1074/jbc.M110.175364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulló M, Moreno-Navarrete JM, Fernández-Real JM, Salas-Salvadó J. Total and undercarboxylated osteocalcin predict changes in insulin sensitivity and β cell function in elderly men at high cardiovascular risk. Am J Clin Nutr. 2012;95:249–55. doi: 10.3945/ajcn.111.016642. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 2010;29:761–5. doi: 10.1016/j.clnu.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 37.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 38.Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol. 1994;266:E248–53. doi: 10.1152/ajpendo.1994.266.2.E248. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–63. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 40.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao S, Zhu L, Duan H, Liu S, Liu Q, Liu W, et al. PI3K/Akt pathway mediates high glucose-induced lipid accumulation in human renal proximal tubular cells via spliced XBP-1. J Cell Biochem. 2012;113:3288–98. doi: 10.1002/jcb.24207. [DOI] [PubMed] [Google Scholar]
- 42.Narasimhan SD, Mukhopadhyay A, Tissenbaum HA. InAKTivation of insulin/IGF-1 signaling by dephosphorylation. Cell Cycle. 2009;8:3878–84. doi: 10.4161/cc.8.23.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, et al. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J Biol Chem. 2011;286:19149–58. doi: 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–76. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Zhang X, Zheng J, Yang J. High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem. 2010;338:115–22. doi: 10.1007/s11010-009-0344-6. [DOI] [PubMed] [Google Scholar]
- 46.Wu S, Zang W, Li X, Sun H. Proepithelin stimulates growth plate chondrogenesis via nuclear factor-kappaB-p65-dependent mechanisms. J Biol Chem. 2011;286:24057–67. doi: 10.1074/jbc.M110.201368. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–3. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 48.Sun HZ, Yang TW, Zang WJ, Wu SF. Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. J Endocrinol. 2010;204:311–8. doi: 10.1677/JOE-09-0270. [DOI] [PubMed] [Google Scholar]
- 49.Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-κB signaling pathway. Endocrinology. 2013;154:1055–68. doi: 10.1210/en.2012-2144. [DOI] [PubMed] [Google Scholar]