Abstract
Dietary modification such as caloric restriction (CR) has been shown to decrease tumor initiation and progression. We sought to determine if nutrient restriction could be used as a novel therapeutic intervention to enhance cytotoxic therapies such as radiation (IR) and alter the molecular profile of triple-negative breast cancer (TNBC), which displays a poor prognosis. In two murine models of TNBC, significant tumor regression is noted with IR or diet modification, and a greater regression is observed combining diet modification with IR. Two methods of diet modification were compared, and it was found that a daily 30% reduction in total calories provided more significant tumor regression than alternate day feeding. At the molecular level, tumors treated with CR and IR showed less proliferation and more apoptosis. cDNA array analysis demonstrated the IGF-1R pathway plays a key role in achieving this physiologic response, and multiple members of the IGF-1R pathway including IGF-1R, IRS, PIK3ca and mTOR were found to be downregulated. The innovative use of CR as a novel therapeutic option has the potential to change the biology of tumors and enhance the opportunity for clinical benefit in the treatment of patients with TNBC.
Keywords: caloric restriction, breast cancer, radiation, IGF, tumor regression, cytotoxic therapy
Introduction
Caloric restriction (CR) is an intervention that has been shown to extend life and reduce age-related and chronic diseases, such as cardiovascular disease and cancer.1-3 Although the positive benefits of CR were first described nearly a century ago,4 there now is renewed interest in CR with an, increasing body of literature demonstrating a direct connection between dietary practices, cancer outcomes and obesity. One such cancer that has been shown in vivo to respond to CR is breast cancer5. Caloric restriction has been shown to not only decrease the incidence, but also the progression of breast cancers6,7 Most recently CR has been shown in vivo to decrease metastases from breast cancer.7 Human epidemiologic studies indicate the relevance of dietary intake on breast cancer incidence and treatment. Population studies on obese patients have shown increase rates of breast cancer mortality, while those assessing anorexia nervosa have found a ~50% decreased risk of breast cancer compared with the normal population.8
At the molecular level, CR has optimal characteristics to combat cancer, as it has been shown to increase apoptosis and decrease the rate of proliferation, angiogenesis, hormones levels and growth factors.9 In fact, many novel therapeutic agents for cancer treatment are targeted against individual molecules known to also be targets of CR, such as IGF1-R/Akt pathway, mTOR and AMP-K10. These observations have led to investigations into CR as a potential treatment for cancer. To date, CR has proven to be very important, because it can inhibit mTOR11,12 and can increase the effectiveness of chemotherapy13,14 while protecting normal tissue.12,15,16 Interestingly, many of those same molecular targets, including mTOR, are also altered with radiation therapy.17-20 Therefore, in this study we sought to determine if CR could be used in conjunction with radiation therapy to render cancer cells more susceptible to treatment.
The use of caloric restriction to augment radiation treatment has not been previously investigated. In this study, an in vivo model of breast cancer was used to determine if CR could increase the efficacy of radiation on established tumors. This is the first study to report the use of CR as a novel therapy in combination with radiation for breast cancer.
Results
Physiologic response of dietary intervention
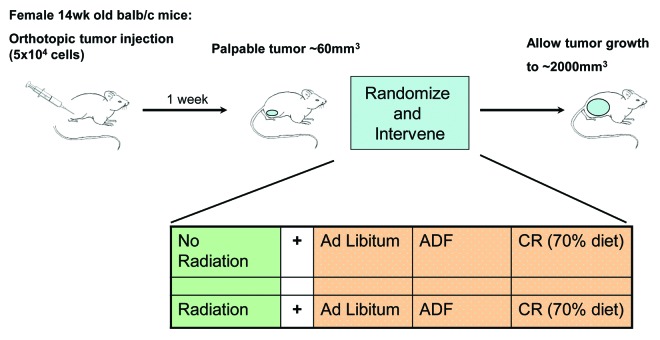
Dietary caloric intake has a strong positive correlation for the incidence of many human cancers,8,21 and spontaneous tumor development in mouse models.22,23 Studies have also shown that large reductions (40–60% reduction) in caloric intake can slow the growth of implanted tumors in mouse models.7,24,25 Using an in vivo model of breast cancer that mimics the clinical presentation in patients, we sought to determine if diet modification could slow established tumor growth with and without radiation (Fig. 1).
Figure 1. Experimental design. Tumors were generated by injecting either 67NR or 4T1 cells into the mammary fat pad of balb/c mice. Once tumors were palpable, mice were treated with IR to the primary tumor alone, treated with a dietary intervention of either ADF or CR or treated with a combination of the two and compared with control mice given no IR and an ad libitum diet.
Mice were implanted orthotopically with one of two triple-negative breast cancer (TNBC) cell lines: 4T1, which are highly metastatic, or 67NR, which are locally aggressive. When palpable tumors formed, feeding changes were instituted; when tumors reached 100 mm3, radiation was administered, and tumor growth was recorded.
Mice with both types of TNBC tumors were treated with one of four conditions: control, which was ad libitum feeding (AL), alternate day feeing (ADF), IR or ADF + IR. Alternate day feeding was used, since it has previously been reported to cause a 25–30% reduction in caloric intake over time.17 Measurement of food intake over the course of the experiment showed an average intake of 3.71 g per day per mouse for ad libitum fed animals, whereas ADF mice consumed an average of 6.78 g per fed day or 3.39 g per day per mouse, about a 9% decrease in caloric intake. The difference in our observed decrease in intake from published reports for ADF may be due to the relatively short timeline of the experiment (38 d).
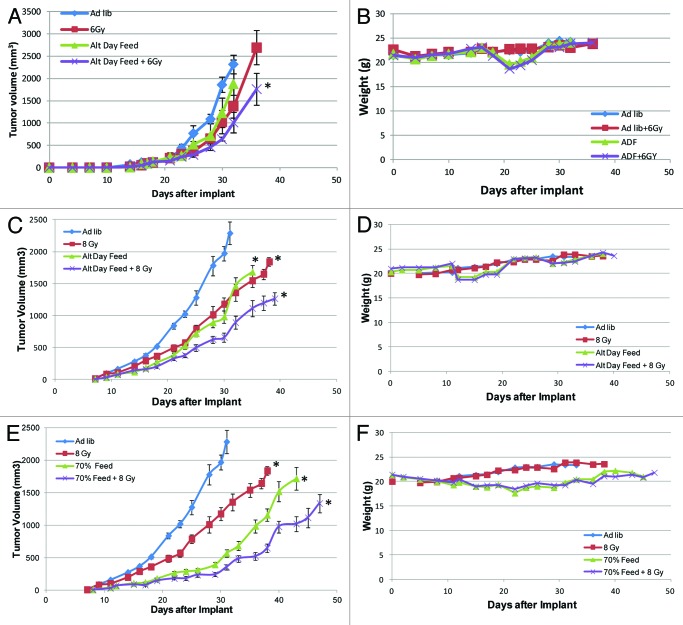
ADF did cause a decrease in tumor growth rate when compared with AL controls (Fig. 2A and B) for both 4t1 and 67NR implants. At 1,000 mm3, ADF caused an average growth delay of 16% (NS) for 67NR tumors and 30% (p value 1.37E-06) for 4t1 tumors. We also sought to determine how dietary restriction interacts with ionizing radiation (IR). To determine the proper dose of radiation to use for each cell line-derived tumor in vitro, clonogenic expansion assays were performed, and the radiation dose that achieved 1 log cell kill was determined to be 6 Gy for 67NR cells and 8 Gy for 4t1 cells (Fig. S1). IR alone caused a delay of 14% (NS) for 67NR tumors and 11% (p value 9.64E-07) for 4T1 tumors, and ADF combined with IR caused an average growth delay of 25% for 67NR tumors (p-value 0.0069) and 45% for 4T1 tumors (p value 1.32E-07).
Figure 2. Tumor re-growth-delay curves for tumors created from two TNBC cell lines: 4T1 and 67NR cells using two different methods of caloric restriction. Mice were treated with each of the following conditions for 67NR (A) cell lines: ad libitum (AL) diet, radiation (RT), alternate day feeding (ADF) or ADF + RT, and their weights were recorded (B). From the reference of the AL cohort for 67NR tumors at 1,000 mm3, RT gave a 16% growth delay, ADF 14%, ADF + RT 25%. From the reference of the AL cohort for 4T1 and weights were tumors at 1,000 mm3 (C), RT gave a 23% growth delay, ADF 30%, ADF + RT 45% also recorded for this cohort (D). Using the 4T1 tumors, the ADF method was then compared with a 30% reduction in calories (CR) (E) using: AL, RT, CR and CR + RT. From the reference of the AL cohort for 4T1 tumors at 1,000 mm3, RT gave a 23% growth delay, CR 56%, CR+RT 82% with an average weight reduction (F) of 12%. Based on this, the optimal method for performing future studies would be a constant reduction of 30% of the caloric intake. *Denotes p < 0.05 by Student’s t-test at 1,000 mm3.
Since these results seemed encouraging, but the 20–30% reduction in calories was not achieved, another cohort of mice were then treated after palpable 4T1 tumors were generated with caloric restriction (CR) that constituted controlled daily feeding of 70% of normal intake, and an additional arm which received both CR and IR. Compared with AL fed mice at 1,000 mm3, CR provided a growth delay of 56% (p value 2.29E-06), while the CR + IR group yielded a more than additive growth delay of 82% (p value 1.18E-05) (Fig. 2C). All growth delays were considered significant if p < 0.05 by Student’s t-test.
The weight of each mouse was measured three times a week for the duration of the experiment and recorded (Figs. 2D–F). Minor and temporary weight loss was noted with ADF treated mice that was not observed in AL cohorts (Fig. 2D and E). Weight loss was observed in CR-treated mice, with an average weight loss of 12% (Fig. 2F).
Molecular response of dietary interevention
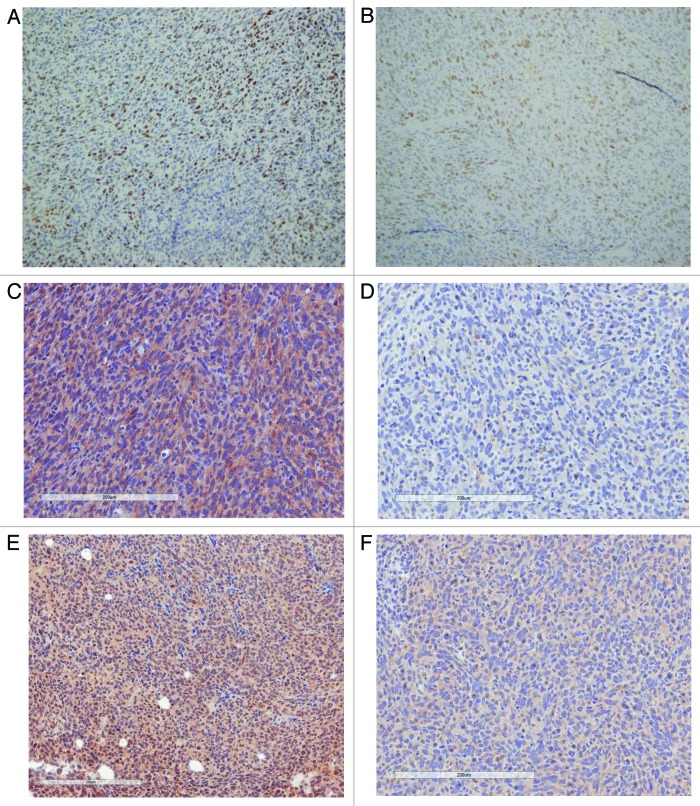
The molecular changes responsible for the physiologic tumor regression noted were explored. Histologic evaluation on hematoxylin and eosin for all treatment conditions revealed high-grade tumors with central necrosis. Tumors were noted to have decreased proliferation with CR alone, IR alone and an even further reduction with CR + IR when Ki-67 was assessed (Fig. 3A). Apoptosis was increased with each treatment alone, and when CR was combined with IR, even more apoptosis was noted, as measured with bcl-2 (Fig. 3B).
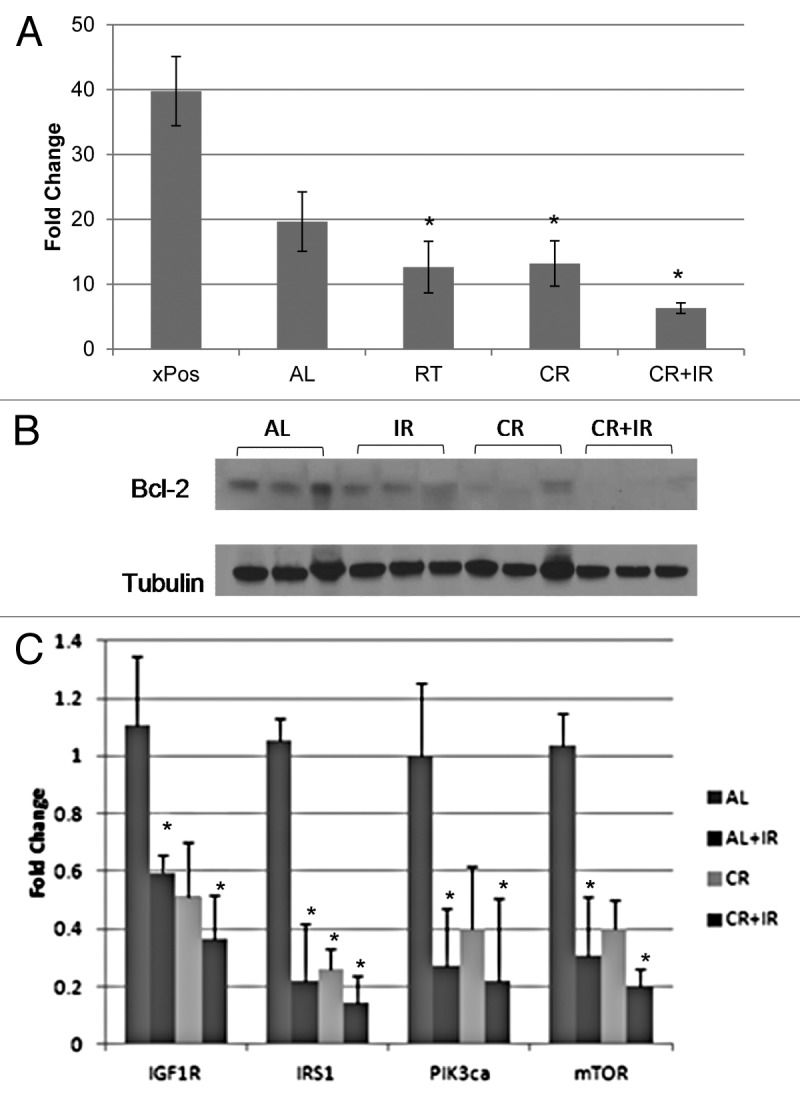
Figure 3. Molecular effects of treatment. Proliferation was evaluated by Ki-67 (A), and bcl-2 was used to evaluate apoptosis (B) for all conditions: Ad lib, CR, IR and CR + IR. Combination therapy was noted to decrease proliferation and increase apoptosis in tumors. qRT-PCR of IGF-1R pathway molecules shows downregulation (C) with either CR, IR or both. *Notes statistical significance with a p < 0.05.
Evidence demonstrates that caloric restriction provides a benefit in breast cancer by acting on multiple molecular targets, such as the insulin-like growth factor-1 receptor (IGF-1R) pathway, inflammatory pathways and estrogen and leptin signaling. To determine the pathway most important in our triple-negative breast cancer models, cDNA arrays were done for the 4T1 tumors exposed to AL, IR, CR and CR + IR. After analyzing by GO-Elite version 1.2.5 (www.genmapp.org/go_elite), we used the GOID system to compare the CR + IR condition with controls; one of the five most significant pathways included the IGF pathway (Table 1). Since the IGF-1R pathway has been implicated as a possible therapeutic target for TNBCs, because its downregulation leads to apoptosis, this seemed like an appropriate pathway to query. In addition, CR26,27 and IR28,29 are both independently noted in the literature to decrease members of the pathway.
Table 1. Top 5 GO categories significantly altered with CR and RT combined.
| GO name | Z score | AVG-log fold change | STDEV-log fold change | p value |
|---|---|---|---|---|
| Gluconeogenesis |
8.385544 |
1.21299 |
0.188822 |
< 0.001 |
| Insulin-like growth factor binding |
8.385544 |
1.400017 |
0.182701 |
< 0.001 |
| Peptide cross-linking |
7.797834 |
1.585857 |
0.574637 |
< 0.001 |
| Extracellular region part |
6.449937 |
1.208581 |
0.759949 |
< 0.001 |
| Extracellular region | 5.283875 | 1.287505 | 0.774612 | < 0.001 |
Expression of members of the IGF-1R pathway, including IGF-1R, IRS, PIk3ca and mTOR, were evaluated. qRT-PCR reactions showed a reduction in all members assessed (Fig. 3C), which implicates this pathway in mediating the tumor regression noted. Immunohistochemical evaluation confirmed these results with representative images shown for IGF-1R (Fig. 4B) and GSK-3β (Fig. 4C), demonstrating that both of these proteins stained the ad libitum samples with 3+ intensity and stained the CR + IR with 1+ intensity.
Figure 4. Immunohistochemical investigation of proliferation and IGF-1R pathway. Proliferation was also evaluated by immunohistochemistry (IHC) with Ki-67 staining, which was noted to have higher proliferation in the ad libitum tumors (A) compared with the tumors treated with CR and IR. IGF-1R was noted to have positive staining (3+) in AL tumors and negative (1+) in CR + IR samples while the downstream target of GSK-3B revealed positive staining (3+) in AL tumors and 2+ staining in CR + IR tumors.
Mice were euthanized for 2,000 mm3 tumors or for humane endpoints such as shortness of breath due to the metastatic tumor burden in the lung. The tumor regression curves demonstrate that breathing difficulties were reached significantly later in ADF or CR mice than in AL fed controls, indicating that metastatic growth is delayed with dietary restriction.
Discussion
Triple-negative breast cancers (TNBC) account for nearly 20% of breast malignancies and are more aggressive, have poor prognosis and a higher recurrence rate. Caloric restriction (CR) has been shown to decrease the incidence and progression of spontaneous breast cancers in murine models and protect normal tissue during chemotherapy. Our results show that a nutritional intervention represses tumor growth in an additive manner when combined with radiation in two different aggressive murine models (4T1 and 67NR) of breast cancer. The average growth delay noted was 15–30% when treated with ADF alone and greater than 50% when treated with CR alone. In combination with IR, a more than additive growth delay was noted at greater than 80%. The significant increase in growth delay observed with combination therapy is a promising result, as it points to CR as a viable adjuvant therapy for breast cancer patients receiving radiation. Local control of disease is clearly affected, but it is also intriguing that the survival of the mice is increased with combination therapy.
Concern has been raised regarding the use of a dietary intervention with standard cancer therapy. Cancer patients, particularly those with advanced disease may be at risk of weight loss or cachexia due to the molecular properties of advanced disease. In addition, cancer patients may be on weight-altering chemotherapeutic drugs. In our study, two different regimens of dietary intervention were evaluated including ADF and CR. This demonstrated that mice placed on a regimen of alternate day feeding do not lose weight, while the mice on a CR diet, lost an average of 12% (4 g), which was a temporary loss. Similarly, other published data has not shown irreversible weight loss or cachexia caused by caloric restriction in cancer models.30 While one human trial examining the effects of caloric restriction in combination with radiation has just begun, and effects are therefore unknown, a series by Longo et al. shows that ten patients were able to undergo short-term fasting during chemotherapy, and this treatment actually decreased the typical side effects of the drugs used.15 This effect has also been shown in vitro.12,16 Several other clinical trials testing the effects of ketogenic and low-carbohydrate diets on brain, lung and even cancers known to be associated with cachexia, including pancreatic cancer and metastatic cancers, are currently enrolling patients.31,32 Carefully selecting proper eligibility criteria to enroll patients onto trials with a dietary intervention will be crucial to their success.
At the molecular level, CR has been noted to increase apoptosis while augmenting anti-proliferative effects and decreasing DNA synthesis.33-36 Our results are congruent with that, and show that combination therapy with CR and IR cause even more apoptosis and less proliferation. The ability of CR to slow tumor growth is likely attributable to the induction of a number of molecular changes on targets such as inflammation, adipokines, mTOR11,12 and in the insulin-like growth factor (IGF-1R) pathway.30,37-39 Longo et al. have implied that short-term starvation, or 48 h fasting (comparable to ADF), reduces IGF-1 levels by 70%, reduces glucose by 60% and increases IGFBP-1 11-fold.2 Previous studies have suggested that fasting induces several metabolic changes; the major change being a shift to alternative nutrient sources and the reduction of growth factors such as IGF-1.33,40,41
Our cDNA array analysis confirmed the importance of the IGF-1R pathway in our model. Components of this pathway play an important role in carcinogenesis, and elevation of pathway components are prognostic for poor outcomes.37,42-44 Consistent with that notion, downregulation of the IGF-1R signaling cascade leads to widespread apoptosis of cancer cells through the PI3K/AKT pathway.45-48 In addition, it is also known that breast tumors often express higher IGF-1 levels than normal breast tissues counterparts.34,49,50 Genotoxic therapies for breast cancer, including radiation, are reported to decrease components of the IGF-1R signaling pathway.35,36,51,52 Our results at the molecular level demonstrate that various components of the IGF-1R pathway are downregulated with CR and RT used separately and are further decreased by the combination of treatments. This suggests that this pathway might be a driving force in the anti-proliferative effect noted.
Our data suggests that nutrient restriction could be used in combination with radiation therapy to confer a clinical benefit. In fact, many novel therapeutic agents for cancer treatment target molecules known to also be targets of CR, such as IGF1-R, the PI3K/Akt pathway, mTOR and AMP-K.2,9,10,39,45,46,53 However, while clinical studies to test these new drugs seem promising, further studies may be limited by overlapping toxicity profiles of the agents targeted at members of those pathways being used.54 Although CR has never been used as a treatment in oncologic clinical trials,15 CR is being successfully implemented with diligent dietary counseling and behavior modification for other diseases in trials throughout the country.44,47,55,56 CR may be an ideal complementary treatment modality for cancer, because it might alter many molecular targets concurrently, and it is free of adverse effects associated with multiple targeted agents.
Materials and Methods
Mice and tumor cell injections
Eighty female Balb/c mice were obtained from NCI Frederick at 6–8 wk of age and treated on a NCI Animal Care and Use Committee approved protocol. At 14-wk-old, 20 mice were implanted with 67NR tumor cells (triple-negative locally advanced cells) to determine efficacy of the treatment intervention (gift from Dr Fred Miller, Prentis Cancer Research Center, Karmanos Cancer Institute). Sixty mice were then implanted with 4T1 tumor cells, which are highly metastatic and randomized to a treatment arm (gift from Dr Patricia Steeg, NCI). All cells were cultured in DMEM supplemented with 10% FBS and pen/strep.
For implantation, mice were anesthetized, shaved, and a small incision (~0.5 cm) was made through the skin just anterior to the rear leg in order to visualize the #4 mammary fat pad, so that tumors could be created in situ. Five × 104 4T1 cells in 50 µL of sterile PBS were injected into the fat pad and the site closed with a staple. Tumors were measured three times per week using manual calipers, and their volumes were determined by the formula: 1/2 length × width2. Mice were euthanized when the tumor reached 2,000 mm3 or earlier if required by humane endpoints.
67NR pilot intervention
A pilot trial was first performed to determine if tumor regression in a locally aggressive triple-negative cell line could be noted with a dietary modification. To do this, after implanting 67NR cells and waiting for palpable primary tumors with an average size of 60 mm3 to develop, mice were placed in four treatment cohorts of five mice each. The conditions for the cohorts included (1) mice given ad libitum (AL) feeding; (2) mice treated with radiation (IR) to the primary tumor; (3) mice given alternate day feeding (ADF) and (4) mice treated with IR to the primary tumor and given ADF.
Average daily food intake per animal was determined for all groups by weighing food 2–3 times per week. Intake baseline was established at least 2 wk prior to implantation and/or altering feeding protocol. All animals had unrestricted access to water regardless of feeding protocol. Ad libitum fed (AL): AL animals were given unrestricted access to standard NIH31 mouse diet for the duration of the experiment. Alternate day feeding (ADF): The dietary intervention of ADF is noted in the literature to be equivalent to a global reduction of 30% calories. Therefore, for this pilot intervention, animals were fasted and fed in alternating 24 h cycles starting when tumors became palpable around 1 wk after implant. During the fed state, mice were given ad libitum access to NIH31 mouse diet. Ionizing radiation (IR): Mammary fat pad tumors in the IR groups were irradiated when the tumors were palpable with an average size of 100 mm3. Mice were sedated, placed into a restraint jig with shielding to isolate the tumor, and the tumor with margin was treated with a 6 Gy dose for tumors generated from the 67NR cells using a Pantak H-320 (320 kV) Precision X-Ray.
4T1 tumor intervention
Since benefit was noted with a pilot trial of a locally aggressive cancer, a larger study was undertaken to look at the efficacy of dietary intervention in the 4T1 model, which is also a triple-negative cell line, but is highly metastatic. We chose to evaluate radiation in conjunction with two different dietary interventions including ADF and caloric restriction, instituting a 30% reduction in calories to determine if both regimens would have equivalent efficacy as previously reported. Therefore, 6 cohorts of 10 mice were evaluated in this portion of the study: (1) AL feeding; (2) mice treated with (IR) to the primary tumor; (3) mice given ADF; (4) caloric restriction (CR) or a 30% reduction in their daily intake; (5) mice treated with IR to the primary tumor and given ADF and (6) mice treated with IR to the primary tumor while on a CR diet. Caloric restriction (CR): At 10-wk-old, each animal was singly housed, and baseline average daily food intake of NIH31 mouse diet was calculated per individual over 2 wk. Starting 2 d after tumor implant, each animal was fed once per day a weighed portion equivalent to 90% of its individual average daily intake. Over an 18 d period, animals were stepped down in 5% increments every 4–5 d, ensuring weight loss was stabilized before each step until the animals were calorically restricted to 70% of baseline. IR: As described above 12 d after implant, however, tumors generated from the 4T1 cells were treated to 8Gy using a Pantak H-320 (320 kV) Precision X-Ray.
Clonogenic survival curves
To determine an equivalent dose of radiation to treat mice implanted with two different cell types, 4T1 or 67NR cells were trypsinized and plated in 6-well plates at the appropriate densities and then incubated for 6–8 h at 37°C. Cells were then irradiated with a dose of 0, 2, 4, 6 or 8 Gy. The plates were then incubated for 10 d and colonies were stained with crystal violet. Colonies of 50 or more cells were counted, and the fraction of surviving cells at each dose was calculated. A dose of radiation was chosen to achieve a 90% cell kill in vitro. This tuned out to be 6Gy for 67NR cells and 8Gy for 4T1 cells as denoted in Figure S1.
RNA isolation and RT-PCR
Tumors were grossly dissected and immediately stabilized by placement in RNAlater (Ambion) per manufacturer’s instructions. Tissue was placed in TRIzol reagent (Invitrogen) with lysing matrix D (MP Biomedicals) and homogenized using manufacturer’s suggested settings for a FastPrep-24 machine (MP Biomedicals). Following lysis, extraction was performed using a standard phenol-chloroform method. Following extraction, 1 μl of 20 mg/ml glycogen (Fermentas) and 1.5 volumes of 100% ethanol was added to each sample. Total RNA was then purified using RNeasy Mini columns (Qiagen) using standard manufacturer’s protocol. RNA concentration was determined and integrity was confirmed by viewing 18S and 28S by denaturing agarose gel electrophoresis. mRNA expression for IGF-1R, IRS-1, PIk3ca, mTOR, were assessed using the high capacity cDNA reverse transcription kit (ABI) as per manufacturer’s instructions. RT-PCR reactions were performed using 20 ng of cDNA.
cDNA microarrays
RNA from primary tumors treated with AL, CR, IR and CR + IR were labeled with Cy3-dUTP and compared with the reference RNA (Stratagene Universal Reference), which was labeled with Cy5-dUTP. The method was previously described by Khan et al.57 Microarray slides were obtained from the Radiation Oncology Sciences Program Microarray Lab at the National Institutes of Health.58 Slides were 8 k human slides printed on site using a Named Genes clone set from Research Genetics, spotted onto poly-Llysine coated slides using an OmniGrid arrayer (GeneMachines). Hybridization and washing were done as previously described and slides were then scanned at 10 microns intervals using a Genepix® 4000 scanner (Axon Instruments), and images and data were stored in a database (mAdb) maintained by the Center for Information Technology, National Institutes of Health.59 The microarray data have been deposited in National Center for Biotechnology Information Gene Expression Omnibus.60
Data analysis
Data was extracted into two categories using the mAdb system: “all genes” and “outliers.” “All genes” were extracted excluding spots flagged as Bad/Not found, and spots with target diameters less than 50 μm or greater than 300 μm. Only spots with signal-to-background ratios of > 2, a minimum background corrected signal of 250 counts and 80% of pixels in the spots with intensity greater than a SD plus background were used excluding spots flagged as Bad/Not found. Local Mean Across Element Signal Intensity (LOESS) was applied to dye-normalization. Ratio data were analyzed by LIMMA statistics to obtain significant genes with selection criteria p ≤ 0.01, Bonferroni correction by using R version 2.11.1 and BioConductor package limma version 3.4.3. To identify biological pathways, including miRNA which altered by IR or/and 70% calorie restriction, pathway analysis was conducted using GO-Elite and GenMAPP-CS.
Tissue staining and scoring
Formalin-fixed, paraffin-embedded tissue sections were cut at 6 µm and deparaffinized by standard techniques. Antigen retrieval was performed by heating the sections in 10 mmol/L citrate buffer pH 6.0 for 50 min with the use of a pressure cooker. A rabbit monoclonal antibody for Ki-67 at 1:600 dilution (AbCam; catalog no. ab16667) for 60 min at room temperature. The immune complexes were visualized with Mouse ABC (Vector Laboratories, Inc) and the chromogenic substrate Dako Liquid DAB_Substrate-Chromogen Solution (Dako North America, Inc; catalog no. K3468; diaminobenzidine tetrahydrochloride) for 3 min. Ki-67 labeling index was determined by counting ≥ 500 nuclei in areas of the section with the highest labeling rates and was considered high when ≥ 10% of tumor cells were stained.61 The scoring was performed by an experienced staff pathologist and was also interpreted with the Aperio Image analysis system in order to obtain the number and percentage of positive cells in each tumor.
Statistical analysis
Statisitical significance between tumor growth curves was calculated as previously described.62 Briefly, tumor growth data were fit using an exponential growth equation; the tumor growth time (days) for control animals was calculated and then subtracted from all treated groups. SDs were derived and used to calculate the Student’s t-test and p values for the differences between the various groups. Comparisons comparing statisitical significance for RT-PCR anaylsis was made by the two-tailed Student’s t-test. A difference between groups of p < 0.05 was considered significant.
Supplementary Material
Acknowledgements
This research was supported in part by the American Society for Radiation Oncology, the Intramural Research Program of the NIH and the Kimmel Cancer Center Radiation Oncology Facility, which is supported partly by NCI Cancer Center Grant P30 CA56036. During the course of this work, Drs Lisanti and Sotgia were funded by the resources of Thomas Jefferson University. Also, Dr Lisanti’s and Dr Sotgia’s current affiliation is the University of Manchester, where they receive funding from the Manchester Cancer Research Centre (MCRC), Breakthrough Breast Cancer (BBC) and The European Research Council (ERC).
Glossary
Abbreviations:
- TNBC
triple-negative breast cancer
- CR
caloric restriction
- IGF
insulin-like growth factor
- IR
ionizing radiation
- AL
ad libitum
- ADF
alternate day feeding
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/25016
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25016
References
- 1.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoro EJ. Dietary restriction: current status. Aging (Milano) 2001;13:261–2. doi: 10.1007/BF03353421. [DOI] [PubMed] [Google Scholar]
- 4.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5:155–71, discussion 172. [PubMed] [Google Scholar]
- 5.Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. JAMA. 2004;291:1226–30. doi: 10.1001/jama.291.10.1226. [DOI] [PubMed] [Google Scholar]
- 6.Phoenix KN, Vumbaca F, Fox MM, Evans R, Claffey KP. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res Treat. 2010;123:333–44. doi: 10.1007/s10549-009-0647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzúa P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis. 2011;32:1381–7. doi: 10.1093/carcin/bgr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 9.Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989;49:4130–4. [PubMed] [Google Scholar]
- 10.Yuen JS, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as a treatment for cancer. Expert Opin Ther Targets. 2008;12:589–603. doi: 10.1517/14728222.12.5.589. [DOI] [PubMed] [Google Scholar]
- 11.Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–8. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 12.Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011;3:1039–40. doi: 10.18632/aging.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:24ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068–111. doi: 10.18632/oncotarget.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9:4474–6. doi: 10.4161/cc.9.22.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–33. doi: 10.18632/oncotarget.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varady KA, Roohk DJ, Hellerstein MK. Dose effects of modified alternate-day fasting regimens on in vivo cell proliferation and plasma insulin-like growth factor-1 in mice. J Appl Physiol. 2007;103:547–51. doi: 10.1152/japplphysiol.00209.2007. [DOI] [PubMed] [Google Scholar]
- 18.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–7. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, DeGraff W, Kaufman D, Krishna MC, Samuni A, Finkelstein E, et al. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch Biochem Biophys. 1991;289:62–70. doi: 10.1016/0003-9861(91)90442-L. [DOI] [PubMed] [Google Scholar]
- 20.Samuni Y, Cook JA, Choudhuri R, Degraff W, Sowers AL, Krishna MC, et al. Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic Biol Med. 2010;49:667–73. doi: 10.1016/j.freeradbiomed.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–13. doi: 10.1177/0300985809357753. [DOI] [PubMed] [Google Scholar]
- 22.Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, Maihle NJ. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol Biomarkers Prev. 2002;11:836–43. [PubMed] [Google Scholar]
- 23.Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grambsch PL, Grande JP, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer. 2009;61:265–75. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 24.Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71:4484–93. doi: 10.1158/0008-5472.CAN-10-3973. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res. 2004;10:5622–9. doi: 10.1158/1078-0432.CCR-04-0308. [DOI] [PubMed] [Google Scholar]
- 26.Rogozina OP, Bonorden MJ, Grande JP, Cleary MP. Serum insulin-like growth factor-I and mammary tumor development in ad libitum-fed, chronic calorie-restricted, and intermittent calorie-restricted MMTV-TGF-alpha mice. Cancer Prev Res (Phila) 2009;2:712–9. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- 27.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–72. [PubMed] [Google Scholar]
- 28.Turney BW, Kerr M, Chitnis MM, Lodhia K, Wang Y, Riedemann J, et al. Depletion of the type 1 IGF receptor delays repair of radiation-induced DNA double strand breaks. Radiother Oncol. 2012;103:402–9. doi: 10.1016/j.radonc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Suman S, Johnson MD, Fornace AJ, Jr., Datta K. Exposure to ionizing radiation causes long-term increase in serum estradiol and activation of PI3K-Akt signaling pathway in mouse mammary gland. Int J Radiat Oncol Biol Phys. 2012;84:500–7. doi: 10.1016/j.ijrobp.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzúa P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis. 2011;32:1381–7. doi: 10.1093/carcin/bgr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28:1028–35. doi: 10.1016/j.nut.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7:e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 34.Peyrat JP, Bonneterre J, Hecquet B, Vennin P, Louchez MM, Fournier C, et al. Plasma insulin-like growth factor-1 (IGF-1) concentrations in human breast cancer. Eur J Cancer. 1993;29A:492–7. doi: 10.1016/S0959-8049(05)80137-6. [DOI] [PubMed] [Google Scholar]
- 35.Wu JD, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–60. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 36.Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, et al. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332–9. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Ji QS, Mulvihill M, Pachter JA. Inhibition of the IGF-I receptor for treatment of cancer. Kinase inhibitors and monoclonal antibodies as alternative approaches. Recent Results Cancer Res. 2007;172:59–76. doi: 10.1007/978-3-540-31209-3_5. [DOI] [PubMed] [Google Scholar]
- 38.Holly J. Insulin-like growth factor-I and new opportunities for cancer prevention. Lancet. 1998;351:1373–5. doi: 10.1016/S0140-6736(05)79438-1. [DOI] [PubMed] [Google Scholar]
- 39.Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 40.Ligibel J. Obesity and breast cancer. Oncology (Williston Park) 2011;25:994–1000. [PubMed] [Google Scholar]
- 41.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 42.Tannenbaum A. The dependence of tumor formation on the composition of the calorie-restricted diet as well as on the degree of restriction. 1945. Nutrition. 1996;12:653–4. [PubMed] [Google Scholar]
- 43.Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 2010;176:2997–3006. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Pennington CALERIE Team Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, et al. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–41. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–9. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. Washington University School of Medicine CALERIE Group One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–50. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–26. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 49.Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L, et al. Elevated insulin receptor content in human breast cancer. J Clin Invest. 1990;86:1503–10. doi: 10.1172/JCI114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surmacz E. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:95–105. doi: 10.1023/A:1009523501499. [DOI] [PubMed] [Google Scholar]
- 51.Anguiano A, Tuchman SA, Acharya C, Salter K, Gasparetto C, Zhan F, et al. Gene expression profiles of tumor biology provide a novel approach to prognosis and may guide the selection of therapeutic targets in multiple myeloma. J Clin Oncol. 2009;27:4197–203. doi: 10.1200/JCO.2008.19.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beauchamp MC, Knafo A, Yasmeen A, Carboni JM, Gottardis MM, Pollak MN, et al. BMS-536924 sensitizes human epithelial ovarian cancer cells to the PARP inhibitor, 3-aminobenzamide. Gynecol Oncol. 2009;115:193–8. doi: 10.1016/j.ygyno.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Lashinger LM, Malone LM, Brown GW, Daniels EA, Goldberg JA, Otto G, et al. Rapamycin partially mimics the anticancer effects of calorie restriction in a murine model of pancreatic cancer. Cancer Prev Res (Phila) 2011;4:1041–51. doi: 10.1158/1940-6207.CAPR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. NeoALTTO Study Team Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin CK, Anton SD, Han H, York-Crowe E, Redman LM, Ravussin E, et al. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–90. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, et al. Development of adherence metrics for caloric restriction interventions. Clin Trials. 2011;8:155–64. doi: 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, et al. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res. 1998;58:5009–13. [PubMed] [Google Scholar]
- 58.Chuang YY, Chen Y, Gadisetti, Chandramouli VR, Cook JA, Coffin D, et al. Gene expression after treatment with hydrogen peroxide, menadione, or t-butyl hydroperoxide in breast cancer cells. Cancer Res. 2002;62:6246–54. [PubMed] [Google Scholar]
- 59.Szkanderová S, Port M, Stulík J, Hernychová L, Kasalová I, Van Beuningen D, et al. Comparison of the abundance of 10 radiation-induced proteins with their differential gene expression in L929 cells. Int J Radiat Biol. 2003;79:623–33. doi: 10.1080/09553000310001606821. [DOI] [PubMed] [Google Scholar]
- 60.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–30. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–37. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell JB, Choudhuri R, Fabre K, Sowers AL, Citrin D, Zabludoff SD, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16:2076–84. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.