Abstract
Objective
We measured the spatial, temporal and developmental patterns of gamma activity augmented by picture- and auditory-naming tasks and determined the clinical significance of naming-related gamma-augmentation.
Methods
We studied 56 epileptic patients (age: 4-56 years) who underwent extraoperative electrocorticography. The picture-naming task consisted of naming of a visually-presented object; the auditory-naming task consisted of answering an auditorily-presented sentence question.
Results
Naming-related gamma-augmentation at 50-120 Hz involved the modality-specific sensory cortices during stimulus presentation and inferior-Rolandic regions during responses. Gamma-augmentation in the bilateral occipital and inferior/medial-temporal regions was more intense in the picture-naming than auditory-naming task, whereas that in the bilateral superior-temporal, left middle-temporal, left inferior-parietal, and left frontal regions was more intense in the auditory-naming task. Patients above 10 years old, compared to those younger, showed more extensive gamma-augmentation in the left dorsolateral-premotor region. Resection of sites showing naming-related gamma-augmentation in the left hemisphere assumed to contain essential language function was associated with increased risk of post-operative language deficits requiring speech therapy (p < 0.05).
Conclusions
Measurement of gamma-augmentation elicited by either naming task was useful to predict postoperative language deficits.
Significance
A smaller degree of frontal engagement in the picture-naming task can be explained by no requirement of syntactic processing or less working memory load. More extensive gamma-augmentation in the left dorsolateral-premotor region in older individuals may suggest more proficient processing by the mature brain.
Keywords: Epilepsy surgery, Intracranial ECoG recording, Ripples, High-frequency oscillations (HFOs), Language, Speech, Outcome
1. INTRODUCTION
Important cognitive functions in humans include overt naming to visually- and auditorily-presented stimuli. Regardless of the modalities of stimuli initially perceived by sensory cortices, naming behaviors commonly consist of understanding of the meaning of stimuli, followed by formation and articulation of a relevant answer. To localize the brain regions responsible for overt naming, investigators have measured naming-related augmentation of gamma activity at 50-120 Hz recorded on intracranial electrocorticography (ECoG) (Sinai et al., 2005; Tanji et al., 2005; Towle et al., 2008; Wu et al., 2010; Miller et al., 2011; Kojima et al., 2012a; 2012b). Thereby, cortical sites showing naming-related gamma-augmentation were co-localized within language-related sites defined by electrical stimulation with statistically-significant accuracy. Our previous ECoG study of 13 epileptic patients with left-hemispheric language dominance on Wada test demonstrated that resection of the left superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic sites showing auditory-naming-related gamma-augmentation predicted postoperative language deficits requiring speech therapy (Kojima et al., 2012a). Thus, we defined the summation of these four regions of interest as the ‘canonical language region’. Our recent ECoG study of 77 epileptic patients demonstrated that the risk of postoperative language deficits requiring speech therapy was predicted by resection of the sites showing auditory-naming-related gamma-augmentation in the canonical language region of the left hemisphere assumed to contain essential language function (Kojima et al., 2012b); thereby, left-handed patients with left-sided seizure foci and early-onset left-sided neocortical lesions were assumed to have right-hemispheric language dominance, while the remaining patients were assumed to have essential language function still existing in the left hemisphere (Rasmussen and Milner, 1977; Akanuma et al., 2003; Möddel et al., 2009). Importantly, the prediction performance of gamma-augmentation measures remained significant, even after controlling for the effects of electrical stimulation or the extent of resection involving the canonical language region (Kojima et al., 2012b). Thus, measurement of auditory-naming-related gamma-augmentation is warranted in presurgical evaluation of epilepsy.
As it has been previously suggested that auditory and visual naming tasks engage different cortices, a visual naming task may also improve the presurgical prediction of essential language sites. In this study, we addressed the following issues: (i) We determined the spatial-temporal patterns of picture- and auditory-naming-related gamma-augmentation on ECoG, and determined the common and differential gamma-augmentation elicited by two different naming tasks. It remains uncertain how extensively regions outside the modality-specific sensory cortices will show common or differential gamma-augmentation. If differential gamma-augmentation is observed extensively outside the sensory cortices, assignment of two naming tasks will be encouraged in presurgical evaluation to increase the sensitivity for localizing the cortical regions involved in naming. (ii) We determined whether patients above 10 years, compared to those younger, showed more extensive engagement in the left dorsolateralpremotor and right inferior-frontal regions during the picture-naming task, as demonstrated in our previous study of auditory-naming-related gamma-augmentation (Kojima et al., 2012b) as well as studies using functional MRI (fMRI) (Adleman et al., 2002; Gaillard et al., 2003; Brown et al., 2005; Szaflarski et al., 2006). The replication of such developmental ECoG changes in a naming task involving a separate sensory modality would further support the concept of age-dependent utilization of the aforementioned sites. (iii) We finally determined whether a post-operative language deficit requiring speech therapy would be predicted by a logistic regression model incorporating picture-naming-related gamma-augmentation measures.
2. METHODS
2.1. Patients
This study has been approved by the Institutional Review Board at Wayne State University. The inclusion criteria consisted of: (i) patients with focal epilepsy who underwent extraoperative subdural ECoG recording as a part of presurgical evaluation in Children's Hospital of Michigan or Harper University Hospital in Detroit between January 2007 and May 2012; (ii) language mapping using measurement of gamma-augmentation elicited by the picture-naming task (Wu et al., 2011) and that elicited by the auditory-naming task (Brown et al., 2012; Kojima et al., 2012a); and (iii) written informed consent obtained by patients or their guardians.
2.2. Definition of anatomical regions of interest
The anatomical regions of interest included: the superior-temporal region (BA 22/41/42), inferior-frontal region (inferior frontal gyrus involving BA 44/45), dorsolateral-premotor region (dorsolateral portion of BA 6), and inferior-Rolandic region (BA 4/3/1/2 not more than 4 cm superior from the sylvian fissure; Haseeb et al., 2007; Fukuda et al., 2008) (Figure 1). The summation of these four regions was referred to here as the ‘canonical language region’ (Kojima et al., 2012b).
Figure 1. Definition of anatomical regions of interest.
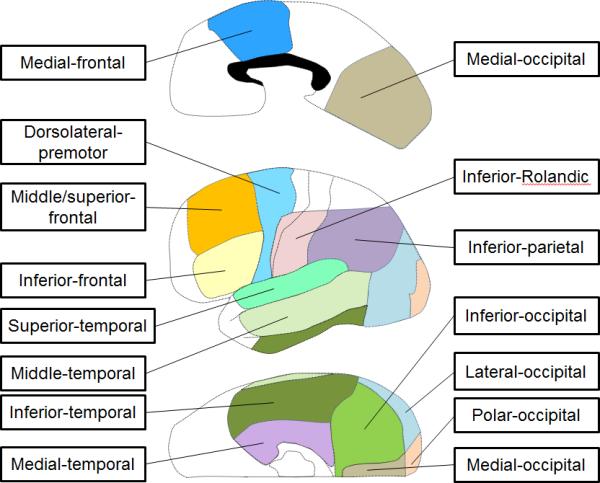
Regions outside of the aforementioned 14 regions were collectively defined as ‘other’, in each hemisphere.
In addition, the present study described: the middle-temporal region (middle temporal gyrus involving BA 21/37), inferior-temporal region (inferior temporal gyrus involving BA 20/37), medial-temporal region (BA 27/28/34/35/36), medial-occipital region (medial portion of BA 17/18), polar-occipital region (polar portion of BA 17/18), inferior-occipital region (inferior portion of BA 19/37), lateral-occipital region (lateral portion of BA 19/37), inferior-parietal region (BA 39/40), middle/superior-frontal region (lateral portion of BA 46/9/8) and medial-frontal region (medial portion of BA 6/8 and posterior portion of BA 24/32/33), bilaterally (Brodmann, 1909; Mitelman et al., 2003; Matsuzaki et al., 2012; Kojima et al., 2012b; Figure 1).
2.3. Preoperative estimation of reorganization of essential language function to the right hemisphere
The language-dominant hemisphere was clinically assumed for each patient, based on handedness and neuroimaging data obtained during Phase-I presurgical evaluation (Rasmussen and Milner, 1977; Akanuma et al., 2003; Möddel et al., 2009; Kojima et al., 2012b). Namely, left-handed patients with left-sided seizure focus and early-onset left-sided neocortical lesions (defined as either congenital or perinatal lesions such as dysplasia, encephalomalacia and porencephaly) were assumed to have right-hemispheric language dominance. All other patients with left-sided seizure focus were assumed to have either left-sided language dominance or bilateral language representation regardless of handedness; thus, the surgical hemisphere was assumed to still contain essential language cortex. Those with a right-sided seizure focus were assumed to have left-sided language dominance, regardless of handedness, since reorganization of essential language function to the epileptic right hemisphere is exceptionally rare (Drane et al., 2012). We discussed the advantage and limitation of this assumption in our recent ECoG study of 77 epileptic patients (Kojima et al., 2012b).
2.4. Subdural electrode placement
Platinum macro-electrodes were surgically placed in the subdural space over left, right, or bilateral cortical regions (intercontact distance: 10 mm; diameter: 4 mm; median: 112 electrodes per patient [standard deviation: 23]). Placement of intracranial electrodes was clinically guided by the results of Phase-I presurgical evaluation including: scalp video-EEG recording, MRI, and 2-deoxy-2-[18F] fluoro-D-glucose (FDG) positron emission tomography (PET) (Asano et al., 2009a). All electrode plates were stitched to adjacent plates or the edge of dura mater, to avoid movement of subdural electrodes after intracranial implantation. In all patients, intraoperative photographs were taken with a digital camera before dural closure as well as after re-opening during the second stage of surgery. All electrodes were displayed on the three-dimensional brain surface reconstructed from high-resolution MRI, as previously described in detail (Alkonyi et al., 2009; Wu et al, 2011). We confirmed the spatial accuracy of electrode display on the three-dimensional brain surface by using intraoperative digital photographs (Wellmer et al., 2002; Dalal et al., 2008).
2.5. Extraoperative video-ECoG recording
ECoG signals were obtained for 3-5 days with a sampling rate of 1,000 Hz, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc, Foothill Ranch, CA, USA). The averaged voltage of ECoG signals derived from the fifth and sixth intracranial electrodes on the amplifier was used as the original reference; ECoG signals were then re-montaged to a common average reference. Channels contaminated with large interictal epileptiform discharges or artifacts were visually identified and excluded from the average (Laufs et al., 2006), in order to minimize their contamination on ECoG traces. Usage of a common average reference is a widely-accepted practice in assessment of event-related gamma-augmentation recorded on subdural grid electrodes; its advantages and limitations were previously discussed (Crone et al., 2001; Asano et al., 2009b; Nagasawa et al., 2011; Kojima et al., 2012a). Electrooculography electrodes were placed 2.5 cm below and 2.5 cm lateral to the left and right outer canthi. ECoG traces were visually inspected with a band-pass filter low-frequency cut-off at 53 Hz and a sensitivity of 20 μV/mm; thereby, irregular broadband signals synchronized with facial and ocular muscle activities seen on electrooculography electrodes were treated as artifacts (Otsubo et al., 2008; Jerbi et al., 2009; Kovach et al., 2011; Kojima et al., 2012a). Seizure onset was defined as a sustained rhythmic change on ECoG accompanied by subsequent clinically typical seizure activity, not explained by state changes, and clearly distinguished from background ECoG and interictal activity (Asano et al., 2009a).
2.6. Picture naming task
The task employed to measure picture-naming-related gamma-augmentation on ECoG was previously described (Wu et al., 2011). Patients were comfortably seated on the bed in a dimly lit room. Patients were instructed to overtly name objects presented sequentially in the picture naming task. Stimuli were presented sequentially on a 19-inch LCD monitor placed 60 cm in front of patients. Picture stimuli consisted of 60 common line-drawn objects (such as ‘dog’ and ‘banana’; Rossion and Pourtois, 2004), of which size ranged from 11 to 16 cm in height and width. Picture stimuli were presented at the center of the monitor, in grayscale, on the black background, for 5,000 msec with an interstimulus interval randomly ranging 2,000 - 2,500 msec. TTL trigger signals synchronized with the onset and offset of each stimulus presentation were delivered to the ECoG recording system (Reale et al., 2007). These audible visual-language sessions were recorded using a Digital Voice Recorder (WS-300M, Olympus America Inc, Hauppauge, NY, USA) concurrently with ECoG recording, and the amplified audio waveform was integrated into the Digital ECoG Recording System (Brown et al., 2008). ECoG traces were aligned to: (i) stimulus (picture) onset; and (ii) response (answer) onset. The response time was defined as the period between the onset of stimulus presentation and the onset of overt responses.
2.7. Auditory naming task
The task employed to measure auditory-naming-related gamma-augmentation on ECoG was previously described (Brown et al, 2012; Kojima et al., 2012a). Patients were instructed to overtly verbalize a one- or two-word answer (e.g.: ‘Ears’) to a given auditory question (e.g.: ‘What do you hear with?). Each patient was presented up to 90 questions. ECoG traces were aligned to: (i) stimulus (question) onset; (ii) stimulus offset; and (iii) response (answer) onset.
2.8. Time-frequency analysis
Each ECoG trial was transformed into the time-frequency domain using complex demodulation (Papp and Ktonas, 1977) via BESA® software (BESA GmbH, Gräfelfing, Germany; Hoechstetter et al., 2004; Kojima et al., 2012b). A given ECoG signal was assigned an amplitude (a measure proportional to the square root of power) as a function of time and frequency (in steps of 10 ms and 5 Hz). The time-frequency transform was obtained by multiplication of the time-domain signal with a complex exponential, followed by a band-pass filter. The band-pass filter used here was a finite impulse response filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform. The filter had a full width at half maximum of 2 × 15.8 ms in the temporal domain and 2 × 7.1 Hz in the frequency domain. The corresponding time-frequency resolution was ±15.8 ms and ±7.1 Hz (defined as the 50% power drop of the finite impulse response filter). We determined ‘when,’ ‘where,’ and ‘how much’ gamma activity at 50-120 Hz averaged across trials were augmented compared to the resting periods. Further methodological details (such as the reference periods) are described in the Supplementary Figure S1. We also determined whether the degree of such gamma-augmentation in each time-frequency bin reached significance using a studentized bootstrap statistic followed by Simes’ correction (Brown et al., 2008; Koga et al., 2011). Finally, sites surviving correction showing significant amplitude augmentation spanning (i) at least 20-Hz in width and (ii) at least 20-ms in duration (Kojima et al., 2012b) were defined as significant gamma-augmentation elicited by a given task. We previously discussed the advantage and limitation of this analytic approach (Wu et al., 2011; Brown et al., 2012; Kojima et al., 2012b).
2.9. Delineation of the spatial-temporal characteristics of naming-related gamma-augmentations
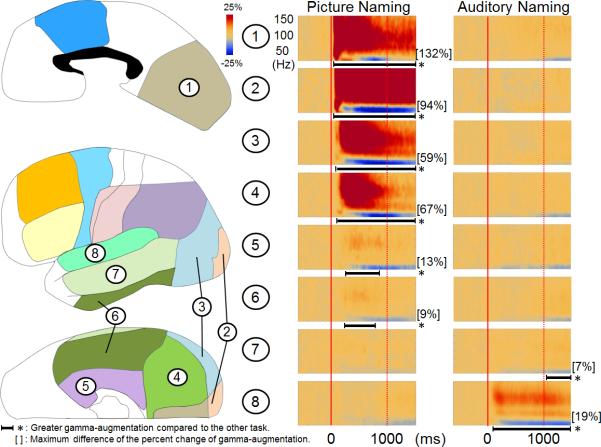
The grand-average of ECoG amplitudes in each time-frequency bin across all electrodes in each region of interest in each hemisphere was calculated for each naming task (Figure 2). We determined whether ECoG amplitudes in each region of interest differed between picture- and auditory-naming tasks, using a studentized bootstrap statistic (McIntosh et al., 1998, Efron and Tibshirani, 1986; Zhou et al., 1997) followed by Simes’ correction (Simes, 1986). A p-value < 0.05 after the correction was considered significant in all analyses. The statistical power to detect significant difference in amplitudes between tasks was dependent on the number of analyzed electrodes in each region of interest and not on the number of trials in each naming task. Each region of interest contained at least 36 analyzed electrodes (Supplementary Table S1). Analysis was conducted by excluding seizure onset sites in order to control for the effects of the seizure onset zone on naming-related gamma-augmentation (Kojima et al., 2012b). Patients assumed to have essential language function reorganized to the right hemisphere were also excluded from this analysis.
Figure 2. Grand-average of ECoG amplitudes across each region of interest.
(A) The results of time-frequency analyses relative to the stimulus onset are shown. Picture-naming, compared to auditory-naming, elicited greater gamma-augmentation in the left medial-occipital, polar-occipital, lateral-occipital, inferior-occipital, medial-temporal, and inferior-temporal regions. Conversely, auditory-naming elicited greater gamma-augmentation in the left superior-temporal and middle-temporal regions. *: duration of gamma-augmentation significantly different across two naming tasks (corrected p < 0.05). The magnitude of the difference in gamma-augmentation across naming tasks was large in the occipital and superior-temporal regions but small in the medial-, inferior- and middle-temporal regions. (B) The results of time-frequency analyses relative to the response onset are shown. Gamma-amplitudes in the left middle/superior-frontal, inferior-frontal, dorsolateral-premotor, inferior-parietal, and superior-temporal regions were greater during the auditory naming task. The results of time-frequency analyses in the right hemisphere are shown in the Supplementary Figure S2.
2.10. Assessment of the effect of age on the spatial pattern of picture-naming-related gamma-augmentation
Likewise, we determined whether ECoG amplitudes in each region of interest differed between patients above 10 years and those younger, using a studentized bootstrap statistic followed by Simes’ correction. Seizure onset sites were excluded from this analysis, and patients assumed to have essential language function reorganized to the right hemisphere were also excluded.
2.11. Prediction of a new language deficit requiring postoperative speech therapy
Electrical stimulation was conducted prior to resective surgery as a part of clinical procedures (Fukuda et al., 2008; Kojima et al., 2012b). The extent of cortical resection was determined after the team had extensive discussion with the patient or the legal guardian(s), regarding the risks and benefits of surgical resection of eloquent areas defined by electrical stimulation. The results of language mapping using naming-related gamma-augmentations on ECoG were not used for surgical decision-making in this study period; each cortical site showing naming-related gamma-augmentation was considered to participate in but not necessarily essential for language function unless electrical stimulation proved it to be essential.
We determined whether the language outcome would be predicted by a logistic regression model incorporating the extent of resection of sites showing picture-naming-related gamma-augmentation in the left hemisphere assumed to contain essential language function or that in the left canonical language region. The outcome measure of interest was a categorical variable for the occurrence of a new language deficit requiring postoperative speech therapy (Kojima et al., 2012b).
3. RESULTS
3.1. Patients
This cohort study included a consecutive series of 56 English-speaking patients who satisfied these criteria (age range: 4-56 years; median age: 14 years) (Supplementary Table S1). All 56 patients were included in our recent study of 77 patients who were assigned an auditory-naming task (Kojima et al., 2012b); 21 of the 77 patients were not included in the present study since they were not assigned the picture-naming task.
3.2. Preoperative assumption of language dominant hemisphere
Two left-handed children had early-onset neocortical lesions (cortical dysplasia with and without encephalomalacia) in the left-hemisphere preoperatively suggested on neuroimaging. According to the definition described earlier, these two children were assumed to have essential language function reorganized to the right hemisphere. The remaining 54 patients were assumed to have essential language function still represented in the left hemisphere or in both hemispheres.
3.3. The characteristics of naming-related gamma-augmentations
Figure 2 shows the grand-average time-frequency map in each region of interest across the 54 patients with essential language function assumed to remain in the left hemisphere. Both naming tasks elicited gamma-augmentation at 50-120 Hz initially involving the modality-specific sensory cortices during stimulus presentation. Both tasks commonly elicited gamma-augmentation in bilateral medial-frontal regions prior to responses, bilateral inferior-Rolandic regions prior to and during responses and bilateral superior-temporal regions during responses. Statistical analysis revealed that picture naming, compared to auditory naming, elicited greater gamma-augmentation in the entire occipital regions bilaterally as well as inferior- and medial-temporal regions bilaterally following the onset of stimulus presentation (corrected p < 0.05). Auditory naming, compared to picture naming, elicited greater gamma-augmentation in bilateral superior-temporal and left middle-temporal regions following the onset of stimulus presentation as well as in the left middle/superior-frontal, inferior-frontal, dorsolateral-premotor, and inferior-parietal regions prior to the onset of responses. The spatial and spectral profiles of naming-related gamma-augmentations at each region of interest in each hemisphere are summarized in the Supplementary Figures S3 and S4 as well as the Supplementary Tables S2 and S3.
3.4. Effect of age on the spatial pattern of picture-naming-related gamma-augmentation
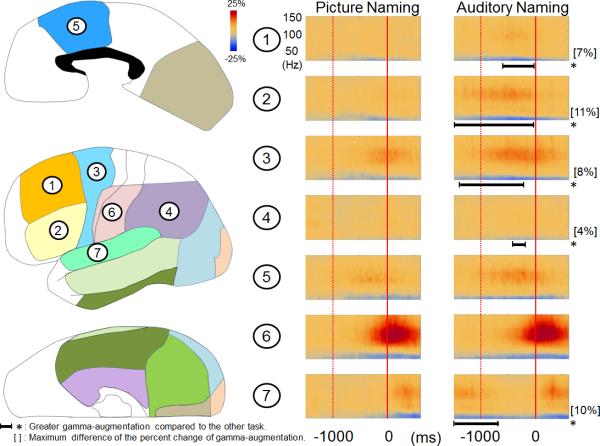
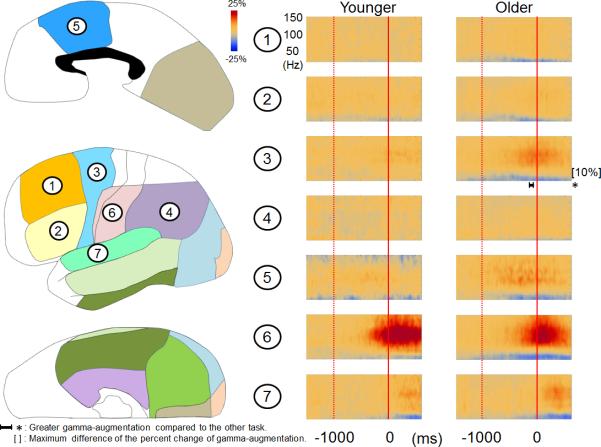
Out of the 54 patients with essential language function assumed to remain in the left hemisphere, 13 patients were 10 years old or younger while the remaining 41 were older than 10 years. There was no difference in verbal comprehension index between these two age groups (mean: 83.0 in the younger group and 84.9 in the older group [p = 0.8]). The median response time of 2.21 s in the younger patients was somewhat longer than 1.71 s in the older patients, but not significantly (p = 0.07 on Mann-Whitney U test). The older patients showed larger picture-naming-related gamma-augmentation in bilateral medial-occipital and left polar-occipital regions immediately following the onset of stimulus presentation as well as in the left dorsolateral-premotor region prior to responses (corrected p < 0.05; Figure 3).
Figure 3. Picture-naming-related ECoG amplitudes in younger and older patients.
(A) The results of time-frequency analyses relative to the stimulus onset are shown. Patients older than 10 years showed larger picture-naming-related gamma-augmentation in the left medial- and polar-occipital regions. *: duration of gamma-augmentation significantly different across two age groups (corrected p < 0.05). The older patients also showed larger gamma-augmentation in the left superior-temporal region; this finding could be partly attributed to a shorter response time compared to that in the younger patients. (B) The results of time-frequency analyses relative to the response onset are shown. The older patients showed larger gamma-augmentation in the left dorsolateralpremotor region prior to the onset of vocalization. The results of time-frequency analyses in the right hemisphere are shown in the Supplementary Figure S5.
3.5. Prediction of language outcome
A total of 11 out of all 56 patients developed a new language deficit requiring speech therapy following resection of the presumed epileptogenic zone. The incidence of such a deficit, the primary outcome measure of this study, was not associated with patient age at surgery (p = 0.2 on the Mann-Whitney U test).
Both picture- and auditory-naming-related gamma-augmentations are predictive of the language outcome. The univariate logistic regression model suggested that the postoperative language deficit was predicted by the number of resected sites showing picture-naming-related gamma-augmentation in the left hemisphere assumed to contain essential language function (r2 = 0.28; p = 0.049; odds ratio = 2.2 [95% CI: 1.0-5.0]). This finding indicates that a resection of each site showing picture-naming-related gamma-augmentation increased the odds of language deficit by 2.2 and that 28% of the outcome measures can be explained by this univariate model. Likewise, the postoperative language deficit was predicted by the number of resected sites showing auditory-naming-related gamma-augmentation in the left hemisphere estimated to contain essential language function (r2 = 0.48; p = 0.002; odds ratio = 1.9 [95% CI: 1.3 to 2.8]).
Subsequently, we determined the significance of naming-related gamma-augmentation in the canonical language region. The postoperative language deficit failed to be predicted by the number of resected sites showing picture-naming-related gamma-augmentation in the canonical language region in the left hemisphere assumed to contain essential language function (p > 0.05). In contrast, the language deficit was predicted by the number of resected sites showing auditory-naming-related gamma-augmentation in the canonical language region in the left hemisphere assumed to contain essential language function (r2 = 0.52; p = 0.018; odds ratio = 5.8 [95% CI: 1.4 to 24.9]).
4. DISCUSSION
4.1. Significance of picture-naming-related gamma-augmentation
Picture-naming-related gamma-augmentation involved bilateral occipital, and medial/inferior-temporal regions (Figure 2), which are known as the ventral visual pathways playing a role in visual object recognition (Mishkin and Ungerleider, 1982; Goodale and Milner, 1992). Our observations of relatively less extensive auditory-naming-related gamma-augmentation in these regions (the Supplementary Figures S3-S4) support the notion that the ventral visual pathways are involved in visual more than auditory language function. The postoperative/acute language deficits were predicted by the extent of resection of sites showing picture-naming-related gamma-augmentation in the left hemisphere assumed to contain essential language function. Since there is no added risk, measurement of picture-naming-related gamma-augmentation is warranted in presurgical evaluation.
Compared to auditory-naming, picture-naming-related gamma-augmentation less extensively involved the left inferior/middle/superior-frontal and dorsolateral-premotor regions. This finding can be explained by no requirement of syntactic processing and less working memory load in the picture-naming task used in this present study. For localization of the left frontal region involved in language function, measurement of gamma-augmentation using an auditory-naming task may be preferred to that using a picture-naming one.
4.2. Developmental changes in naming-related gamma-augmentation
Patients older than 10 years showed more extensive picture-naming-related gamma-augmentation in the left dorsolateral-premotor region (Figure 3B). A similar observation was reported in our recent study of auditory-naming-related gamma-augmentation (Kojima et al., 2012b). These findings suggest that more proficient utilization of this region for naming is independent of modalities of external language stimuli. Since gamma-augmentation in the left dorsolateral-premotor region occurs immediately prior to the onset of responses (Figure 3B), age-dependent utilization of this region may be related to preparation of speedy/appropriate naming. A behavioral study of healthy 2- to 5-year-old children reported that phonological sensitivity increases as a function of age (Lonigan et al., 1998). A study of healthy 5- to 12-year-old children reported age-related linear increase in performance of phonological processing and speeded naming between 5 and 10 years of age (Korkman et al., 2001). The present study found that the response time was somewhat shorter in the older compared to younger patients, though the difference did not reach significance.
In addition, the older patients showed larger picture-naming-related gamma-augmentation in a portion of bilateral occipital and superior temporal regions (Figure 3 and the Supplementary Figure S5). A potential explanation for these findings is that the older patients may have been more attendant to presented stimuli and self-vocalized responses.
4.3. Methodological issues
The limitations of our ECoG study include sampling limitations. Although the seizure onset zones were excluded from the assessment of the age effects on gamma-augmentation, we cannot exclude the effects of other confounding factors that are difficult to control for, such as different type and number of oral antiepileptic drugs across subjects. The present study was not designed to correlate the predictor measures with the postoperative neuropsychological measures, since neuropsychological data were not systematically collected before and after surgery. Further studies incorporating systematically obtained pre- and post-operative neuropsychological measures are warranted to determine whether picture-naming-related gamma-augmentation can predict long-term language outcome.
Supplementary Material
Highlights.
Picture- and auditory-naming tasks differentially increased the amplitude of gamma activity in human cerebral cortex.
Patients above 10 years old, compared to those younger, showed more extensive naming-related gamma-augmentation in the left dorsolateral-premotor region.
Measurement of picture- and auditory-naming-related gamma-augmentation contributed to prediction of post-operative language deficits requiring speech therapy.
Table 1.
Demographic and clinical characteristics of the patients.
| Age - year | |
| Median | 14 |
| Range | 4 - 56 |
| Gender - number (%) | |
| Male - number (%) | 29 (51.8) |
| Female - number (%) | 27 (48.2) |
| Antiepileptic drugs | |
| 1 - number (%) | 16 (28.6) |
| 2 - number (%) | 29 (51.8) |
| 3 - number (%) | 10 (17.9) |
| 4 - number (%) | 1 (1.8) |
| VCI score (41 patients) | |
| Median | 80 |
| Standard deviation | |
| VIQ score (6 patients) | |
| Median | 73 |
| Standard deviation | 10 |
| PPVT score (41 patients) | |
| Median | 85 |
| Standard deviation | 19 |
| CELF score (21 patients) | |
| Median | 75 |
| Standard deviation | 23 |
| Handedness | |
| Right - number (%) | 47 (83.9) |
| Left - number (%) | 8 (14.3) |
| Ambidextrous - number (%) | 1 (1.8) |
| Subdural electrodes | |
| Median | 112 |
| Standard deviation | 23 |
Oxcarbazepine: 29 patients. Levetiracetam: 21. Lacosamide: 13. Lamotrigine: 12. Valproic acid: 8. Topiramate: 8. Phenytoin: 7. Carbamazepine: 6. Zonisamide: 2. Clobazam: 2.
Acknowledgement
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano) as well as Japan Foundation for Neuroscience & Mental Health, Japan Epilepsy Research Foundation, and Japan-North America Medical Exchange Foundation (to K. Kojima). We are grateful to Harry T. Chugani, MD, Csaba Juhász, MD, PhD, Sarah Minarik, RN, BSN, Carol Pawlak, REEG/EPT, and Farah Huq at Children's Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Akanuma N, Alarcón G, Lum F, Kissani N, Koutroumanidis M, Adachi N, et al. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44:408–18. doi: 10.1046/j.1528-1157.2003.24502.x. [DOI] [PubMed] [Google Scholar]
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009a;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009b;45:477–89. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalizationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth; Leipzig: 1909. [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, et al. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–31. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Muzik O, Rothermel R, Matsuzaki N, Juhász C, Shah AK, et al. Evaluating reverse speech as a control task with language-related gamma activity on electrocorticography. Neuroimage. 2012;60:2335–45. doi: 10.1016/j.neuroimage.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–82. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS. Localization of neurosurgically implanted electrodes via photograph-MRI-radiograph coregistration. J Neurosci Methods. 2008;174:106–15. doi: 10.1016/j.jneumeth.2008.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Roraback-Carson J, Hebb AO, Hersonskey T, Lucas T, Ojemann GA, et al. Cortical stimulation mapping and Wada results demonstrate a normal variant of right hemisphere language organization. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2012.03573.x. doi: 10.1111/j.1528-1167.2012.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75. [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, et al. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–85. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–5. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Haseeb A, Asano E, Juhász C, Shah A, Sood S, Chugani HT. Young patients with focal seizures may have the primary motor area for the hand in the postcentral gyrus. Epilepsy Res. 2007;76:131–9. doi: 10.1016/j.eplepsyres.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–8. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, et al. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topogr. 2009;22:18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Koga S, Rothermel R, Juhasz C, Nagasawa T, Sood S, Asano E. Electrocorticographic correlates of cognitive control in a stroop task-intracranial recording in epileptic patients. Hum Brain Mapp. 2011;32:1580–91. doi: 10.1002/hbm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, et al. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012a;123:1917–24. doi: 10.1016/j.clinph.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Fuerst D, Matsuzaki N, et al. Clinical significance and developmental changes of auditory-language-related gamma activity. Clin Neurophysiol. 2012b doi: 10.1016/j.clinph.2012.09.031. doi: 10.1016/j.clinph.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: a cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20:331–54. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA, 3rd, Adolphs R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage. 2011;54:213–33. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, et al. Where the BOLD signal goes when alpha EEG leaves. Neuroimage. 2006;31:1408–18. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Burgess SR, Anthony JL, Barker TA. Development of phonological sensitivity in 2 to 5-year-old children. J Educ Psychol. 1998;90:294–311. [Google Scholar]
- Matsuzaki N, Nagasawa T, Juhász C, Sood S, Asano E. Independent predictors of neuronal adaptation in human primary visual cortex measured with high-gamma activity. Neuroimage. 2012;59:1639–46. doi: 10.1016/j.neuroimage.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Cabeza RE, Lobaugh NJ. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. J Neurophysiol. 1998;80:2790–6. doi: 10.1152/jn.1998.80.5.2790. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr. 2011;7:482–90. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann's areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–68. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50:1505–16. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Matsuzaki N, Juhász C, Hanazawa A, Shah A, Mittal S, et al. Occipital gamma-oscillations modulated during eye movement tasks: simultaneous eye tracking and electrocorticography recording in epileptic patients. Neuroimage. 2011;58:1101–9. doi: 10.1016/j.neuroimage.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, et al. High-frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clin Neurophysiol. 2008;119:862–8. doi: 10.1016/j.clinph.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–45. [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Reale RA, Calvert GA, Thesen T, Jenison RL, Kawasaki H, Oya H, et al. Auditory-visual processing represented in the human superior temporal gyrus. Neuroscience. 2007;145:162–84. doi: 10.1016/j.neuroscience.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–36. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–4. [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–70. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–93. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, et al. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, et al. Digital photography and 3D MRI-based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia. 2002;43:1543–50. doi: 10.1046/j.1528-1157.2002.30002.x. [DOI] [PubMed] [Google Scholar]
- Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, et al. Gamma-oscillations modulated by picture naming and word reading: intracranial recording in epileptic patients. Clin Neurophysiol. 2011;122:1929–42. doi: 10.1016/j.clinph.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, et al. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66:E407–9. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- Zhou XH, Gao S, Hui SL. Methods for comparing the means of two independent log-normal samples. Biometrics. 1997;53:1129–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.