Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia. Patients with AF have up to seven-fold higher risk of suffering from ischemic stroke. Better understanding of etiologies of AF and its thromboembolic complications are required for improved patient care, as current anti-arrhythmic therapies have limited efficacy and off target effects. Accumulating evidence has implicated a potential role of oxidative stress in the pathogenesis of AF. Excessive production of reactive oxygen species (ROS) is likely involved in the structural and electrical remodeling of the heart, contributing to fibrosis and thrombosis. In particular, NADPH oxidase (NOX) has emerged as a potential enzymatic source for ROS production in AF based on growing evidence from clinical and animal studies. Indeed, NOX can be activated by known upstream triggers of AF such as angiotensin II and atrial stretch. In addition, treatments such as Statins, antioxidants, ACEI or AT1RB have been shown to prevent post-operative AF; among which ACEI/AT1RB and Statins can attenuate NOX activity. On the other hand, detailed molecular mechanisms by which specific NOX isoform(s) are involved in the pathogenesis of AF and the extent to which activation of NOX plays a causal role in AF development remains to be determined. The current review discusses causes and consequences of oxidative stress in AF with a special focus on the emerging role of NOX pathways.
Keywords: oxidative stress, atrial fibrillation, NADPH oxidase, NOX2, NOX4, inflammation, fibrosis, structural and electrical remodeling
1. Atrial fibrillation and oxidative stress
Cardiac arrhythmias refer to abnormal rate or rhythm of the heartbeat caused by perturbed electrophysiology of the myocardium. Among many types of clinically significant arrhythmias, atrial fibrillation (AF) is most common; affecting 2.7 to 6.1 million adults in 2010 in the United States [1], among which 14–16% die of ischemic stroke [1]. AF incidence increases with aging. In particular, the percentage of stroke associated with AF rises steeply from 1.5% at age 50–59 years to 23.5 % at age 80–89 years [2]. AF also develops in 25–45% of patients with previous heart attack or cardiac surgery [3]. Several risk factors for AF have been identified including cardiopulmonary diseases (congenital heart disease [4], heart failure [5], valvular heart disease [6], hypertropic cardiomyopathy [1, 7]), hypertension [8], metabolic diseases (diabetes [3] or obesity [9]), hyperthyroidism [10], and heavy alcohol consumption [11]). Detailed molecular mechanisms underlying development of AF however, have remained elusive. Anti-arrhythmic drugs including β-blockers, Amiodarone, Dronedarone, Dofetilide, and Sotalol have been widely used for treatment of AF by blocking β-adrenergic receptors or ion channels [12]. Although therapy with these drugs is beneficial, many have been found to have limited long-term efficacy, off target side effects, or drug induced pro-arrhythmic effects [13].
Therefore, better understanding of molecular mechanisms underlying AF is essential for development of novel therapeutic strategies. Increasing evidence has demonstrated that oxidative stress likely plays a role in the pathogenesis of AF [14]. In myocardial tissues, increased levels of ROS such as superoxide and H2O2 have been found to be associated with AF [15–18], corresponding to a decrease in nitric oxide bioavailability [19]. The ratio of oxidized GSSG to reduced glutathione, and the ratio of oxidized cysteine to reduced cysteine, both of which as markers of oxidative stress, have been found increased in the blood samples of patients with AF [20]. Increased ROS levels result in damage to proteins, lipids, and DNA, and potentiate inflammation by augmenting cytokine production from activated inflammatory cells, which in turn further induces tissue damage. ROS are not only implicated in inflammation, but also involved in cardiac structural and electrical remodeling, all of which increase susceptibility to AF. For example, it has been shown that hydroxyl radical (OH−) and peroxynitrate (ONOO−) mediate oxidative damage of myofibrils in AF [21, 22], which in turn contributes to structural remodeling of atria. Atrial electrical remodeling has been known to be associated with intracellular calcium overload [23–25]. Oxidative stress induction of mitochondrial DNA damage in AF causes calcium overload by modulating calcium handling proteins or channels [26], which can promote atrial remodeling. On the other hand, treatment with antioxidants such as Vitamin C [27] and N-acetylcyctine (NAC) [28] has been shown to prevent post-operative AF [29]. Oral Vitamin C treatment was found beneficial in reducing early recurrence and inflammation after electrical cardioversion of persistent AF [30]. What remains to be defined, however, are the molecular mechanisms responsible for increased ROS production in AF, i.e. the enzymatic sources and their regulation; as well as better definition of the ROS-mediated downstream events, i.e. detailed pathways as to how calcium handling is modulated. Evidences accumulated in the past twenty years have shown that ROS generated in the cardiovascular system are primarily derived from NADPH oxidase (NOX), mitochondria, xanthine oxidase, and uncoupled eNOS [31, 32]. Among these enzymatic systems/complexes, NOX has emerged as a major initiating source for increased ROS production in cardiovascular diseases. In particular, latest studies have implicated a correlative and likely important causal role of NOX with AF, which will be discussed in depth in the current review.
2. NOX in the heart: NOX2 vs. NOX4
The NAD(P)H oxidases (NOXs) are a family of multi-subunit enzymatic complexes. NOX isoforms [NOX1–5 or dual oxidase 1–2 (Duox)] recruit separate regulatory subunits for enzymatic assembly and activation [33]. NOX1–4 require p22phox (“phox” stands for phagocyte oxidase) as the membrane binding partner for their activation. NOX1 is composed of p47phox and/or its homologue NOXO1, as well as NOXA1, p40phox, and Rac1. Similarly, NOX2 (gp91phox in leukocytes) requires p47phox, p67phox, p40phox, and Rac1/2 (Rac2 in leukocytes) for enzymatic assembly and activation. NOX3 is activated when NOX organizers (p47phox or NOXO1), activators (p67phox or NOXA1), and Rac1 are assembled and bound to NOX3. NOX4 activation however only requires p22phox binding but recently found regulated by Poldip2 and Tks5, which are cytosolic regulators [34]. Unlike other isoforms, NOX5 does not require any other subunits including the only membrane component p22phox for its activation [35, 36]. Of note, NOX5 does not exist in rodents but in humans, in which it is activated in a calcium-dependent manner [37–39]. Duox has a NOX2-like catalytic subunit and dual functions of acting as an oxidase, and as a peroxidase in the presence of H2O2. The pathophysiological regulation and function of each NOX isoform remains to be fully elucidated; but it is evident that some isoforms of NOX enzymes are pivotal in normal biological responses such as cell growth and gene regulation [34, 35, 40]. Excessive activations of these enzymes however contribute to cardiovascular pathogenesis.
Of note, tissue specific distribution of NOX isoforms has been documented [36, 41]. The heart expresses primarily NOX2 and NOX4 isoforms [42]. Recent studies have shown that NOX1 is also expressed in LAA [18] and left ventricle [43] at mRNA level and protein levels respectively, even though its expression appears to be much lower than NOX2 and NOX4 [44–47]. NOX2 is expressed in endothelial cells, cardiomyocytes, and fibroblasts, whereas NOX4 is expressed in all these cells and vascular smooth muscle cells. NOX2 and NOX4 have distinct cellular localization and consequences of activation. NOX2 is localized in plasma membrane and produces superoxide. Although it is still controversial, NOX4 has been found in intracellular compartments such as endoplasmic reticulum (in endothelium [48]) and mitochondrion (in myocardium [45]), and it has been suggested to produce H2O2 rather than superoxide due to its subcellular localization (i.e. only H2O2 that diffuses out of these compartments gets detected) [49, 50]. More importantly, each isoform has cell specific roles and often determines the specific reactive oxygen species (ROS) produced in the context. For instance, NOX4 promotes apoptosis in cardiomyocytes by generating H2O2 [45], while it also induces H2O2-dependent proliferation in fibroblasts [51], both of which contribute to pathogenesis of heart failure [52]. The role of NOX2 in angiotensin II (Ang II)-induced cardiac hypertrophy and fibrosis has been well established based on data from NOX2 knockout mice [47]. Recent data from NOX4 knockout [52] or transgenic mice [45] however demonstrated that NOX4 contributes to cardiac LV dysfunction, hypertrophy, and fibrosis [45, 52]. Consistently, NOX4 overproduction has been reported in heart failure patients with AF [18]. On the other hand, it should be noted that inducible deletion of endothelial NOX4 led to impaired angiogenesis and endothelial dysfunction [53]. Endothelium specific NOX4 transgenic mice demonstrated H2O2-mediated hyperpolarization and non-NO mediated vasodilation, resulting in lower blood pressure [54]. In the heart NOX4 overexpression has also been found to contribute to angiogenesis via H2O2 dependent mechanisms [55]. These observations seem consistent with previous notions that H2O2 can activate eNOS dependent or independent mechanisms to mediate physiological or compensatory vasodilatations [56–59]. Therefore, whereas cardiac activation of NOX4 has been reported to be pathogenic or protective, endothelial activation of NOX4 maybe protective.
3. Role of NOX in AF
AF is associated with oxidative stress [60]. Table 1 summarizes systemic or myocardial oxidative stress observed in AF patients and experimental models. It should be noted that each study used different atrial tissues and/or at different stages of AF.
Table 1.
Summary of studies on oxidative stress in AF
| Experimental Model |
Analyzed Tissues |
Oxidative Stress Markers |
Analyses | Ref |
|---|---|---|---|---|
| Animal Studies | ||||
| Porcine RAP (1 week) |
LA, LAA | ↓Bioavailable NO | NO specific microeletrode | [19] |
| LA | ↓eNOS | Western blot | ||
| ↑PAI −1 | ||||
| Swine RAP (1 week) |
LAA | ↑superoxide dependent on NOX and XO (NOX>XO) |
ESR, cytochrome C reduction |
[61] |
| LA | ↑Rac1 expression | Western blot | ||
| Goat RAP (2 weeks/6 months) |
LA | ↑NOX dependent superoxide (2 weeks) |
Lucigenin-enhanced chemiluminescence |
[64] |
| ↑Mitochondria derived (6 months) |
||||
| RA | ↑superoxide in 6 months- AF |
|||
| ↑Mitochondria and uncoupled eNOS derived |
||||
| Clinical Studies | ||||
| AF Patients (permanent AF, n=6; permanent from paroxymal n=4, paroxymal AF, n=5) |
RAA | ↑NOX activation | Lucigenin-enhanced chemiluminescence |
[16] |
| ↑eNOS uncoupling | DHI staining | |||
| AF Patients with mitral regurgitation (persistent AF, n=8; SR, n=8) |
RAA | ↑superoxide | Lucigenin-enhanced chemiluminescence |
[15] |
| ↑NOX activation -NOX 2 |
RT-PCR (no change) | IHC | ||
| ↑NOX 2 | ||||
| AF patients (SR=99, AF =71) CABG surgery |
RAA | ↑NOX activation | Lucigenin-enhanced chemiluminescence |
[17] |
| Persistent AF patients (n=8) |
RAA | ↓peroxiredoxin | Mass spectrometry | [105] |
| ↑NADH dehydrogenase flavoprotein |
Microarray | |||
| ↑Ubiquinol cytochrome C reductase core protein 1 |
||||
| Persistent AF patients (n=15) |
RA | ↑NOX in RA (↓NOX in serum) |
Nitrite assay (Griess reaction) |
[106] |
| ↑iNOS/eNOS | western blotting | |||
| ↑3-nitrotyrosine | immunostaining | |||
| Persistent AF (n=26) |
RA | ↑Rotenone/L-NAME inhibited superoxide - NOX2, NOX4, NOX5 |
Lucigenin-enhanced Chemiluminescence Western & real timePCR |
[64] |
| Post-operative AF (n=32) |
RA | ↑ NOX dependent superoxide |
Lucigenin-enhanced chemiluminescence |
[64] |
| ↑ p22phox, NOX2 | Western blot | |||
| ↓ H4B level | HPLC | |||
| AF patients (n=18) non-AF (n=17) |
LAA | ↑H2O2 | Amplex Red | [18] |
| ↑NOX4 - NOX1, NOX2 |
Real time PCR | |||
Animal studies
In a porcine model of pacing-induced AF, nitric oxide (NO) bioavailability was reduced, implicating a potential oxidative stress-mediated degradation mechanism in atrial tissue [19]. The study by Dudley et al. further demonstrated NOX-dependent ROS production, and highly upregulated Rac1 expression, in a similar model [61]. The expression of the other NOX isoforms/subunit, NOX1, NOX2, NOX4, and p22phox, was however unchanged. Nonetheless, Adam et al. also found increased Rac1 GTPase activity in AF [62]. Cardiac specific Rac1 overexpression in mice (RACET mice) resulted in AF in 44% and 75% of the mice at 10 month and 16 month old respectively, characterized by ECG analysis, and this response was reversed by Statin treatment. The animals also exhibited obvious cardiac hypertrophy and increased atrial collagen content [62]. A recent study by Yagi et al. demonstrated that Pitavastatin reduces not only incidence of Ang II-induced AF and left atrial enlargement, but also fibrosis and cardiac hypertrophy, via downregulation of Rac1 activity in eNOS null mice [63]. In addition, Reilly S et al. suggested that initial NOX activation likely accounts for early development of AF, whereas mitochondrion and uncoupled eNOS are involved in permanent AF [64]. In this study of pacing-induced AF in goats, NOX2 and p22phox expression were increased 2 weeks after AF induction but returned to baseline 6 months later. Taken together, the studies described above clearly demonstrate NOX activation in AF, which is highly dependent on Rac1 activity. However, it should be noted that mice with transgenic overexpression or knockout on different NOX isoform(s), have yet been employed to confirm whether particular NOX isoform(s) contribute to pathogenesis of AF. Therefore, usage of transgenic animal models may provide additional evidences for a potential causal role of specific isoform(s) of NOX in the development of AF.
Clinical studies
In agreement with animal studies, analysis of human LA myocardium in patients with AF showed significant upregulation of Rac1 GTPase and NOX activity compared to those in sinus rhythm (SR) (n=8) [62]. Likewise, Kim et al. also investigated sources of superoxide production in right atrial appendage (RAA) homogenates or isolated myocytes from 15 patients with AF (6 patients with persistent AF, 4 patients with persistent AF developed from paroxysmal AF, 5 patients with paroxymal AF) [16]. Superoxide production was inhibited in the presence of Diphenyleneiodonium (DPI), an inhibitor of flavin-containing oxidases, or Apocynin, an inhibitor of NOX by blocking p47phox translocation (not specific for NOX isoforms), implicating NOX-derived superoxide production in AF [16]. To a smaller degree, L-NAME-sensitive superoxide production was also increased, implicating a potential downstream role of uncoupled eNOS [16]. In a later study by Kim et al., the authors also showed that NADPH-driven superoxide production, reflective of NOX activity, was significantly increased in RAA from patients with postoperative AF [17]. Similarly, NADPH-driven superoxide in RAA was found attenuated by short term treatment of Atorvastatin (40 mg/day), indicating Rac1-dependent NOX activation in postoperative AF [65]. Chang and colleagues recently demonstrated borderline but significant correlation between NOX2-driven superoxide in LAA and left atrial enlargement in AF patients. They also observed NOX2 upregulation in RAA of AF patients and correlations between NOX2 upregulation, with myolysis and hypertrophy, implicating a possible role of NOX2-derived oxidative stress in atrial remodeling in AF [15].
In other studies, Reilly and colleagues demonstrated that NOX activation was increased along with increased expression of p22phox and NOX2, while eNOS was still coupled, in postoperative AF patients [64]. But in patients with permanent AF, expressions of NOX2, p22phox, NOX4 and NOX5 were not altered; and levels of superoxide production inhibitable by Rotenone and L-NAME were increased compared to SR, indicating increases in mitochondrion and uncoupled eNOS-derived superoxide in permanent AF [64]. In another recent study, it was found that mRNA expression of neither NOX2 nor NOX1 was increased in LAA of 18 AF patients [18]. Instead, NOX4 expression was significantly increased, and correlated well with a marked elevation in H2O2 production and higher blood pressure, implicating a potential role of NOX4 and NOX4-derived H2O2 in AF, particularly in those with hypertension [18]. NOX4 expression was also increased by Ang II stimulation in HL-1 atrial cells [18]. However, it cannot be excluded that NOX4 increase in this study might be in part associated with the severe pathological remodeling of the heart in these end stage heart transplant patients. Interestingly, recent data have shown that tachypacing of HL-1 atrial cells upregulates NOX2 and NOX4, leading to oxidative stress and myofibril degradation [66] (more see below for cellular studies). Taken together, it is evident that NOX derived ROS are involved in the pathogenesis of AF, although it remains unclear which NOX isoform(s) is(are) responsible in different types of AF. It is also possible that different NOX isoforms and other downstream sources of ROS including mitochondrion and uncoupled eNOS, are involved at different stages of the disease. It is known that regulation of one NOX isoform can affect other isoforms and their regulatory partners [52, 67, 68]. Therefore, larger trials with application of more selective, NOX-specific and NOX isoform-specific inhibitors are required to resolve these issues in future studies. Cell-type specific analyses of the NOX isoforms in heart tissues may also provide additional mechanistic information.
Cellular studies
In vitro studies using HL-1 atrial myocytes have implicated a role of NOX4 as well as NOX2 in increased TGFp1 expression, intracellular oxidative stress, and calpain activation for myofibril degradation [66]. In particular, tachypacing increased NOX2 and NOX4 protein expression. Tachypacing (at 4Hz, 24hrs) induced ROS production was also suppressed in both NOX2 and NOX4 siRNA transfected cell, indicating a role of NOX2 and NOX4 containing NOX as a source of oxidative stress in AF at cellular model. Additionally, neutralization of TGFp1 with specific antibody prevented NOX induced ROS production following tachypacing. These data seem to suggest that TGFp1 mediated NOX2/NOX4 activation induces subsequent atrial myocyte remodeling via myofibril degradation aside from TGFp1-smad3-myolysis pathway. Zhang and colleagues recently demonstrated that Ang II increases H2O2 production and NOX4 expression in HL-1 atrial cells, which is similar to findings observed in AF patients [18]. Given the well known effect of Ang II in structural remodeling in AF and the previously established role of H2O2 in inflammation and thrombosis, these data indicate that NOX4-derived H2O2 is possibly involved in electrophysiological changes promoting AF, as well as development of its thromboembolic complications.
4. Role of ROS in mediating initiation of AF and thromboembolic complication: AF begets AF as NOX begets NOX
Despite the clear link between oxidative stress and AF, it remains unclear whether oxidative stress is a causal factor for AF, or, conversely, a consequence of AF.
Pulmonary veins (PV) have been recognized as the sites of origin of premature atrial extrasystoles that can initiate AF [69, 70]. Indeed, ablation therapy has been known to be effective in patients with paroxysmal AF by disconnecting electrical conduction from PV to LA. Of note, H2O2 has been demonstrated to trigger irregular electrical firing of PV cardiomyocytes [71]. Moreover, NOX might be the source for increased H2O2 production. It is worth mentioning that H2O2 is cell permeable and known to diffuse to adjacent cells and tissues. On the other hand, in post-operative AF, oxidative stress induced by ischemia/reperfusion and mechanical stretch during cardiac surgery has been suggested to be a factor promoting electrical remodeling. For instance, mechanical stretch induces Ang II, which acts on multiple ion channels by stimulation of AT1R. This response is known to specifically destabilize cardiac myocyte Kv4.3 channel mRNA by activating NOX [72, 73]. Moreover, Ang II is a potent stimulator of NOX, which promotes ROS generation and oxidative modifications of protein targets, such as oxidative activation of CaMKII [31, 32, 74]. It has been reported that Ang II infusion leads to oxidation of Met 281/282 residues in the regulatory domain of CaMKII, which transforms CaMKII into a constitutively active form, leading to secondary electrical remodeling by inducing calcium overload via activation of L-type calcium channel and ryanodine receptor 2 (RyR2) hyperphosporylation [74, 75]. In the same study, expression of ox-CaMKII was found increased in atria of AF patients, indicating a potential role of ROS induced CaMKII activation in AF. Higher levels of ox-CaMKII were associated with sinoatrial node dysfunction in patients with heart failure [74]. Primary electrical disturbance induced by increased calcium loading can trigger electrical remodeling accompanied by structural remodeling of myocardium, which can subsequently lead to irreversible fibrosis. Thus oxidative stress is not only an early event in primary electrical remodeling, but also involved in secondary structural remodeling and consequent changes in electrophysiology. A similar relationship exists between inflammation and AF [76]. Calcium overload or Ang II receptor 1 activates NFkB, which is an inflammatory transcription factor and a known contributor of AF initiation and maintenance. Recently, NFκB has been shown to downregulate transcription of the cardiac sodium channel in response to oxidative stress [76], consistent with an Ang II-NOX-NFkB axis. Indeed, H2O2 can activate NF-kB. In addition, NF-kB is a transcription factor for TNF-α, iNOS, IL-1β, and MMPs, all of which are involved in structural remodeling and inflammation. Taken together, these data seem to indicate ROS could well be upstream of AF initiation, by contributing to electrical and structural remodeling, and activation of inflammatory pathways. As discussed earlier, activation of NOX in AF could lead to ROS production and possible downstream activations of uncoupled eNOS and mitochondrion [16, 45, 77–79]. It is proposed therefore, NOX might have an important role in “AF begets AF” [80].
5. Interventions potentially targeting NOX in AF
The spectrum of antioxidant treatments in AF has been reviewed extensively [29]. As summarized in Table 2, treatment with Vitamin C, Vitamin C in combination with Vitamin E or N-acetylcysteine (NAC), each of which has antioxidant actions, reduced post-operative AF. However, evidences as to whether general antioxidant treatments are effective to prevent AF are conflicting. Therefore, approaches targeting more specific ROS-generating pathways have received increasing attention.
Table 2.
Summary of studies on antioxidant treatments in AF
| Antioxidant | Effects | Action mechanism | Experimental Model |
Ref |
|---|---|---|---|---|
| Clinical Studies | ||||
| Vitamin C | ↓incidence of postoperative AF |
Downregulation of NADPH oxides (transcriptional or post translational level) |
coronary artery bypass grafting patients |
[107] |
| - incidence of postoperative AF |
[108] | |||
| Vitamin E with Vitamin C |
↓ncidence of AF | Scavenges ROS | Patients undergoing coronary artery bypass |
[109] |
| Statins | ↓incidence of postoperative AF, |
Counteracts the arrhythmogenic effects of angiotensin, reduces cholesterol, antioxidant, anti- inflammatory, anti- apoptosis, |
coronary artery bypass grafting patients |
[110, 111] |
| ↓incidence of postoperative AF |
reduces myocardial O2 and ONOO |
[65] | ||
| ↓incidence of postoperative AF |
reduces C-reactive protein levels |
[112, 113] | ||
| n-3 Polyunsaturated fatty acids (PUFAs) |
↓incidence of postoperative AF, |
increases electrical stability |
cardiac surgery patients |
[114, 115] |
| - incidence of postoperative AF |
[116–118] | |||
| Carvedilol | ↓incidence of postoperative AF | increases myocardial levels of the antioxidant enzymes superoxide dismutase and glutathione peroxidase, |
heart failure patients |
[119] |
| N-acetylcysteine | ↓incidence of postoperative AF |
ROS scavenger | Patients undergoing coronary artery bypass and/or valve surgery |
[28] |
Inhibition of RAS to inhibit initial activation of NOX
The RAS system is known to activate NOX to result in marked oxidative stress, and therefore a possible trigger of AF. It has been established that Ang II induces NOX activation [32] and consequent eNOS uncoupling in endothelial cells [81], hypertensive mice [82], and diabetic animals [83]. Similar to the findings that Ang II upregulates NOX4/AT1 expression and H2O2 production in HL-1 atrial cells [18], it was reported that Ang II induction of superoxide was attenuated by AT1RB Losartan in HL-1 cells [84]. AT1 receptor expression was found markedly increased in left atrium of patients with AF compared to those in SR [85]. Activation of the AT1 receptor leads to activation of plasminogen activator inhibitor (PAI-1) and tissue factor (TF), which in turn mediates thrombosis and fibrinolysis [86, 87]. In addition, stimulation of AT1 receptor activates monocyte chemoattractant protein (MCP-1), vascular adhesion molecule (VCAM-1), intracellular adhesion molecule (ICAM-1) and tumor necrosis factor α (TNF-α) [88]. Of note, elevated ICAM-1 expression occurs in patients with post-operative AF [89]. Endothelial VCAM-1 expression was also found increased in RAA of AF patients [90]. Pre-treatment with Olmesartan attenuated RAP (rapid atrial pacing) induced VCAM-1 expression in human atrial tissue slices [90]. Olmesartan significantly prevented RAP-induced downregulation of endocardial tissue factor platelet inhibitor (TFPI), thrombomodulin, and eNOS at protein levels without affecting mRNA expression of these proteins [91]. Upregulation of PAI-1 by RAP was also inhibited by AT1 receptor blockade. A recent study by Bodiga S. et al. reported accelerated adverse remodeling of the extracellular matrix and worsening of systolic function, along with increased NOX activity and increased superoxide production in response to pressure overload in ACE2 null mice, which was attenuated by p47phox depletion [92]. This finding suggests that Ang II-mediated NOX2 activation may serve as an early regulator of cardiac remodeling and that targeting ACE2 might be an effective therapeutic strategy. Furthermore, a recent meta-analysis based on 23 randomized trials showed that RAS inhibition with either ACEI or ARB is effective in preventing AF, despite considerable variation among different trials [93]. Taken together, targeting Ang II-mediated oxidative stress may be effective in attenuating several processes including atrial fibrosis, inflammation, electrical and structural remodeling of AF, all of which are involved in the pathogenesis of AF. What underlies the effectiveness on AF prevention of Ang II signaling attenuation could be inhibition of the NOX pathway.
Statins as NOX inhibitor
Statin therapy has been shown to decrease NOX activation by downregulation of Rac1 subunit. Statins and Probucol, which act as antioxidants in addition to their lipid lowering effects, reduced AF incidence [94]. Prubucol reduces cholesterol and inhibits oxidation of LDL cholesterol. It has been shown to cross membranes easily and act as a scavenger of oxygen radicals. In a dog AF model, levels of malondialdehyde and calpain 1, which contribute to structural remodeling, were both abrogated by Prubucol treatment [95]. Simvastatin attenuated Ang II-stimulated superoxide production in HL-1 atrial myocytes [84]. Likewise, it was shown that Probucol inhibits NOX activation [94]. A recent clinical study suggested that levels of myocardial superoxide and peroxynitrate are associated with length of hospitalization and duration of ionic support, whereas short term treatment with Atorvastatin (40 mg/day) reduces myocardial superoxide and peroxynitrate (ONOO−) due to decreased NOX activation in RAA from SR patients, suggesting that Statin usage for a longer period perhaps would have some benefits in preventing post-operative AF through inhibition of NOX [96]. Reilly S et al. also showed that Atovastatin treatment is effective to inhibit Rac1-dependent NOX activation in postoperative AF but not in permanent AF [64]. Taken together, Statins seem effective in preventing post-operative AF at least to some extent, potentially via modulation of NOX activity. Nonetheless, the efficacy of Statins in the prevention of AF is not yet conclusive [97].
6. Conclusions
AF has become more and more prevalent and now a major public health problem [98]. Although AF is clearly associated with aging, and cardiovascular conditions such as hypertension, mitral valve disease and heart failure, the underlying molecular mechanisms have remained elusive [98]. Recent developments suggest that AF is promoted by atrial structural and electrical remodeling, and that AF itself further augments these responses to perpetuate AF [99, 100]. The known risk factors for AF have been linked to oxidative stress, although it is still unclear whether oxidative stress is the initiating factor for AF. Antioxidant treatment and interventions targeting Ang II signaling appear to have some preventive effects on post-operative AF [101, 102], and sometimes on recurrence and new onset of AF [103, 104]. Latest advances from experimental and clinical approaches seem to suggest that NOX isoforms such as NOX2 and NOX4 are major sources of ROS production in AF. NOX-derived ROS, superoxide and H2O2, activate several processes including atrial inflammation, fibrosis, and structural and electrical remodeling as shown in Figure 1. These processes confer upstream activators of NOX such as Ang II and atrial stretch, forming a vicious cycle of NOX activation promoting AF, and AF promoting NOX activation. Therefore, inhibition of specific isoforms of NOX may serve as a novel therapeutic strategy for breaking the NOX-AF vicious cycle. So far, NOX2 and NOX4 have been identified as the specific NOX isoforms involved in AF. Nontheless, further investigations using specific inhibitors or siRNAs targeting different NOX isoforms, and knockout and transgenic animals targeting different NOX isoforms, are necessary to further elucidate detailed, NOX isoform(s)-dependent mechanisms involved in AF pathogenesis, and subsequently novel therapeutic strategies for the prevention and treatment of AF.
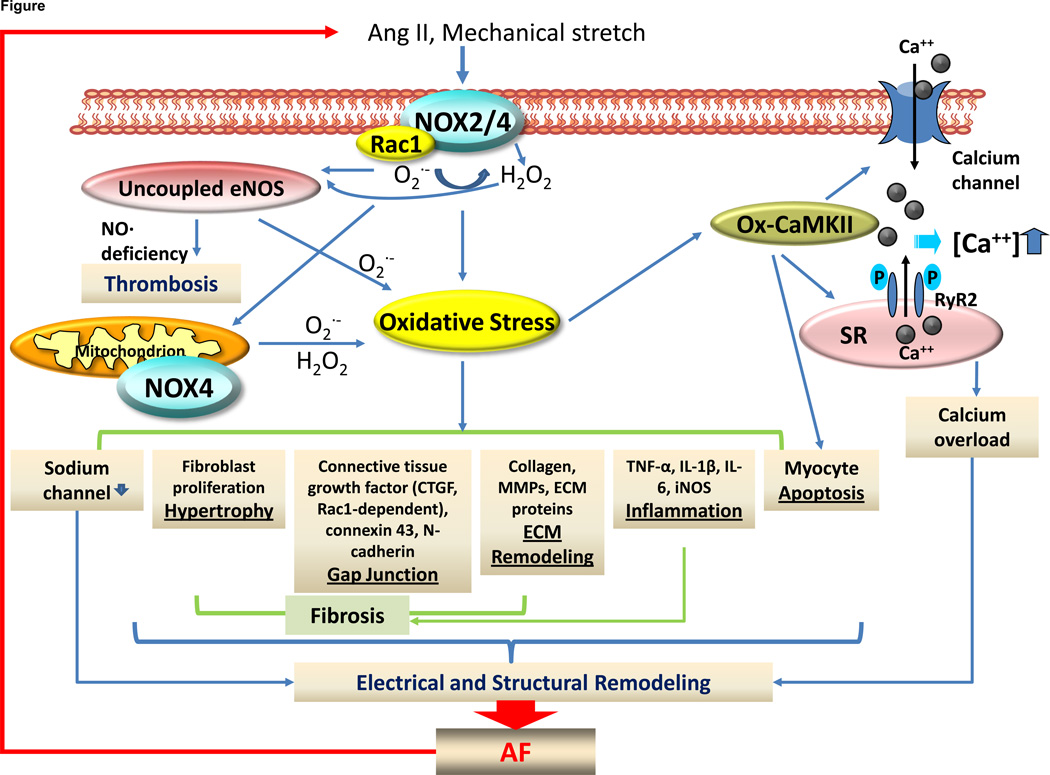
Figure 1.
Role of NADPH oxidase (NOX)-derived oxidative stress in atrial fibrillation (AF). Production of superoxide and H2O2 from activated NADPH oxidasae (NOX) isoforms 2 and 4 (NOX2, NOX4 respectively) leads to activation of downstream reactive oxygen species (ROS)-generating systems including mitochondrion and uncoupled eNOS, resulting in sustained oxidative stress which in turn stimulates myocyte apoptosis, atrial inflammation, fibrosis, and structural and electrical remodeling. Examples of key mediators downstream of increased ROS production include oxidized CaMKII (Ox-CaMKII) and activated nuclear factor kB (NF-kB). ox-CaMKII activates ryanodine receptor 2 (RyR2) hyperphospohrylation, which in turn causes secondary electrical remodeling and calcium overload-induced cardiac injury. NF-kB is a well known redox-sensitive transcriptional factor for inflammation and structural remodeling by activating TNF-alpha, iNOS, IL-1β, and MMPs. All these processes confer to NOX activators such as Ang II and atrial stretch, thus forming vicious cycle of NOX activation promoting AF, and AF promoting NOX activation. Endocardial nitric oxide deficiency due to oxidative stress and eNOS uncoupling may also contribute to thromboembolic complications of AF.
Highlights.
Oxidative stress has been implicated in the pathogenesis of AF;
Activation of NADPH oxidase (NOX), particularly isoforms 2 and 4, occurs in humans with AF and experimental models of AF;
NOX can be activated by upstream substrates of AF such as Ang II and atrial stretch;
Inhibition of NOX by ACEI/AT1RB or Statins may have beneficial effects in preventing post-operative AF
ACKNOWLEDGEMENT
The authors work has been supported by National Heart, Lung and Blood Institute (NHLBI) Grants HL077440 (HC), HL081571 (HC), HL088975 (HC), HL101228 (PPP, JNW, HC), HL108701 (HC, DGH), and an American Heart Association Established Investigator Award 12EIA8990025 (HC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Kalus JS, White CM, Caron MF, Coleman CI, Takata H, Kluger J. Indicators of atrial fibrillation risk in cardiac surgery patients on prophylactic amiodarone. Ann Thorac Surg. 2004;77:1288–1292. doi: 10.1016/j.athoracsur.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 4.Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol. 1998;32:197–204. doi: 10.1016/s0735-1097(98)00221-6. [DOI] [PubMed] [Google Scholar]
- 5.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. Jama. 1994;271:840–844. [PubMed] [Google Scholar]
- 7.Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. 2000;140:878–885. doi: 10.1067/mhj.2000.111108. [DOI] [PubMed] [Google Scholar]
- 8.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr., et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coromilas J. Obesity and atrial fibrillation: is one epidemic feeding the other? Jama. 2519–20;292 doi: 10.1001/jama.292.20.2519. [DOI] [PubMed] [Google Scholar]
- 10.Bielecka-Dabrowa A, Mikhailidis DP, Rysz J, Banach M. The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Res. 2009;2:4. doi: 10.1186/1756-6614-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427–436. doi: 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- 12.Patel C, Salahuddin M, Jones A, Patel A, Yan GX, Kowey PR. Atrial fibrillation: pharmacological therapy. Curr Probl Cardiol. 2011;36:87–120. doi: 10.1016/j.cpcardiol.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sanguinetti MC, Bennett PB. Antiarrhythmic drug target choices and screening. Circ Res. 2003;93:491–499. doi: 10.1161/01.RES.0000091829.63501.A8. [DOI] [PubMed] [Google Scholar]
- 14.Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil. 2008;15:735–741. doi: 10.1097/HJR.0b013e328317f38a. [DOI] [PubMed] [Google Scholar]
- 15.Chang JP, Chen MC, Liu WH, Yang CH, Chen CJ, Chen YL, et al. Atrial myocardial nox2 containing NADPH oxidase activity contribution to oxidative stress in mitral regurgitation: potential mechanism for atrial remodeling. Cardiovasc Pathol. 2011;20:99–106. doi: 10.1016/j.carpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Youn JY, Kim AY, Ramirez RJ, Gao L, Ngo D, et al. NOX4-Dependent Hydrogen Peroxide Overproduction in Human Atrial Fibrillation and HL-1 Atrial Cells: Relationship to Hypertension. Front Physiol. 2012;3:140. doi: 10.3389/fphys.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 20.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babusikova E, Kaplan P, Lehotsky J, Jesenak M, Dobrota D. Oxidative modification of rat cardiac mitochondrial membranes and myofibrils by hydroxyl radicals. Gen Physiol Biophys. 2004;23:327–335. [PubMed] [Google Scholar]
- 22.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 23.Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS, et al. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33:1231–1237. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 24.Li GR, Nattel S. Properties of human atrial ICa at physiological temperatures and relevance to action potential. Am J Physiol. 1997;272:H227–H235. doi: 10.1152/ajpheart.1997.272.1.H227. [DOI] [PubMed] [Google Scholar]
- 25.Daoud EG, Knight BP, Weiss R, Bahu M, Paladino W, Goyal R, et al. Effect of verapamil and procainamide on atrial fibrillation-induced electrical remodeling in humans. Circulation. 1997;96:1542–1550. doi: 10.1161/01.cir.96.5.1542. [DOI] [PubMed] [Google Scholar]
- 26.Bukowska A, Schild L, Keilhoff G, Hirte D, Neumann M, Gardemann A, et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp Biol Med (Maywood) 2008;233:558–574. doi: 10.3181/0706-RM-155. [DOI] [PubMed] [Google Scholar]
- 27.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 28.Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, et al. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J. 2008;29:625–631. doi: 10.1093/eurheartj/ehn011. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigo R, Vinay J, Castillo R, Cereceda M, Asenjo R, Zamorano J, et al. Use of vitamins C and E as a prophylactic therapy to prevent postoperative atrial fibrillation. Int J Cardiol. 2010;138:221–228. doi: 10.1016/j.ijcard.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, et al. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102:321–326. doi: 10.1016/j.ijcard.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 31.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 33.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 38.Pandey D, Gratton JP, Rafikov R, Black SM, Fulton DJ. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol Pharmacol. 2011;80:407–415. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, et al. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 41.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol. 2011;50:408–416. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol. 2012;302:C597–C604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cave A, Grieve D, Johar S, Zhang M, Shah AM. NADPH oxidase-derived reactive oxygen species in cardiac pathophysiology. Philos Trans R Soc Lond B Biol Sci. 2005;360:2327–2334. doi: 10.1098/rstb.2005.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 47.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 48.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 52.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 54.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 57.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 58.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012;18:286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 61.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 62.Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, et al. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 63.Yagi S, Akaike M, Aihara K, Ishikawa K, Iwase T, Ikeda Y, et al. Endothelial nitric oxide synthase-independent protective action of statin against angiotensin II-induced atrial remodeling via reduced oxidant injury. Hypertension. 2010;55:918–923. doi: 10.1161/HYPERTENSIONAHA.109.146076. [DOI] [PubMed] [Google Scholar]
- 64.Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, et al. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 65.Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59:60–70. doi: 10.1016/j.jacc.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 66.Yeh YH, Kuo CT, Chan TH, Chang GJ, Qi XY, Tsai F, et al. Transforming growth factor-beta and oxidative stress mediate tachycardia-induced cellular remodelling in cultured atrial-derived myocytes. Cardiovasc Res. 2011;91:62–70. doi: 10.1093/cvr/cvr041. [DOI] [PubMed] [Google Scholar]
- 67.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, et al. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol. 2005;288:H7–H12. doi: 10.1152/ajpheart.00637.2004. [DOI] [PubMed] [Google Scholar]
- 68.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 69.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 70.Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 71.Lin YK, Lu YY, Chen YC, Chen YJ, Chen SA. Nitroprusside modulates pulmonary vein arrhythmogenic activity. J Biomed Sci. 2010;17:20. doi: 10.1186/1423-0127-17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou C, Ziegler C, Birder LA, Stewart AF, Levitan ES. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res. 2006;98:1040–1047. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sridhar A, Nishijima Y, Terentyev D, Khan M, Terentyeva R, Hamlin RL, et al. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc Res. 2009;84:227–236. doi: 10.1093/cvr/cvp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao G, Dudley SC., Jr Redox regulation, NF-kappaB, and atrial fibrillation. Antioxid Redox Signal. 2009;11:2265–2277. doi: 10.1089/ars.2009.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin YK, Lin FZ, Chen YC, Cheng CC, Lin CI, Chen YJ, et al. Oxidative stress on pulmonary vein and left atrium arrhythmogenesis. Circ J. 2010;74:1547–1556. doi: 10.1253/circj.cj-09-0999. [DOI] [PubMed] [Google Scholar]
- 78.Nishijima Y, Sridhar A, Bonilla I, Velayutham M, Khan M, Terentyeva R, et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res. 2011;91:71–79. doi: 10.1093/cvr/cvr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–H415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 80.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 81.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol. 2009;47:752–760. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56:118–126. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 84.Tsai CT, Wang DL, Chen WP, Hwang JJ, Hsieh CS, Hsu KL, et al. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 85.Boldt A, Wetzel U, Weigl J, Garbade J, Lauschke J, Hindricks G, et al. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. J Am Coll Cardiol. 2003;42:1785–1792. doi: 10.1016/j.jacc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 86.Ridker PM, Vaughan DE. Potential Antithrombotic and Fibrinolytic Properties of the Angiotensin Converting Enzyme Inhibitors. J Thromb Thrombolysis. 1995;1:251–257. doi: 10.1007/BF01060734. [DOI] [PubMed] [Google Scholar]
- 87.Sironi L, Calvio AM, Arnaboldi L, Corsini A, Parolari A, de Gasparo M, et al. Effect of valsartan on angiotensin II-induced plasminogen activator inhibitor-1 biosynthesis in arterial smooth muscle cells. Hypertension. 2001;37:961–966. doi: 10.1161/01.hyp.37.3.961. [DOI] [PubMed] [Google Scholar]
- 88.Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, et al. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 89.Verdejo H, Roldan J, Garcia L, Del Campo A, Becerra E, Chiong M, et al. Systemic vascular cell adhesion molecule-1 predicts the occurrence of post-operative atrial fibrillation. Int J Cardiol. 2011;150:270–276. doi: 10.1016/j.ijcard.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 90.Goette A, Bukowska A, Lendeckel U, Erxleben M, Hammwohner M, Strugala D, et al. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation. 2008;117:732–742. doi: 10.1161/CIRCULATIONAHA.107.730101. [DOI] [PubMed] [Google Scholar]
- 91.Yamashita T, Sekiguchi A, Kato T, Tsuneda T, Iwasaki YK, Sagara K, et al. Angiotensin type 1 receptor blockade prevents endocardial dysfunction of rapidly paced atria in rats. J Renin Angiotensin Aldosterone Syst. 2007;8:127–132. doi: 10.3317/jraas.2007.021. [DOI] [PubMed] [Google Scholar]
- 92.Bodiga S, Zhong JC, Wang W, Basu R, Lo J, Liu GC, et al. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47(phox) NADPH oxidase subunit. Cardiovasc Res. 2011;91:151–161. doi: 10.1093/cvr/cvr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 94.Umeji K, Umemoto S, Itoh S, Tanaka M, Kawahara S, Fukai T, et al. Comparative effects of pitavastatin and probucol on oxidative stress, Cu/Zn superoxide dismutase, PPAR-gamma, and aortic stiffness in hypercholesterolemia. Am J Physiol Heart Circ Physiol. 2006;291:H2522–H2532. doi: 10.1152/ajpheart.01198.2005. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Sheng L, Li W, Liu W, Gong Y, Xue H, et al. Probucol attenuates atrial structural remodeling in prolonged pacing-induced atrial fibrillation in dogs. Biochem Biophys Res Commun. 2009;381:198–203. doi: 10.1016/j.bbrc.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, et al. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation. 2010;122:S66–S73. doi: 10.1161/CIRCULATIONAHA.109.927376. [DOI] [PubMed] [Google Scholar]
- 97.Rahimi K, Emberson J, McGale P, Majoni W, Merhi A, Asselbergs FW, et al. Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ. 2011;342 doi: 10.1136/bmj.d1250. d1250. [DOI] [PubMed] [Google Scholar]
- 98.Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace. 2008;10:647–665. doi: 10.1093/europace/eun130. [DOI] [PubMed] [Google Scholar]
- 99.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 100.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 101.Harling L, Rasoli S, Vecht JA, Ashrafian H, Kourliouros A, Athanasiou T. Do antioxidant vitamins have an anti-arrhythmic effect following cardiac surgery? A meta-analysis of randomised controlled trials. Heart. 2011;97:1636–1642. doi: 10.1136/heartjnl-2011-300245. [DOI] [PubMed] [Google Scholar]
- 102.McCarty MF. Practical prevention of cardiac remodeling and atrial fibrillation with full-spectrum antioxidant therapy and ancillary strategies. Med Hypotheses. 2010;75:141–147. doi: 10.1016/j.mehy.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 103.Bhuriya R, Singh M, Sethi A, Molnar J, Bahekar A, Singh PP, et al. Prevention of recurrent atrial fibrillation with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: a systematic review and meta-analysis of randomized trials. J Cardiovasc Pharmacol Ther. 2011;16:178–184. doi: 10.1177/1074248410389045. [DOI] [PubMed] [Google Scholar]
- 104.Huang G, Xu JB, Liu JX, He Y, Nie XL, Li Q, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers decrease the incidence of atrial fibrillation: a meta-analysis. Eur J Clin Invest. 2011;41:719–733. doi: 10.1111/j.1365-2362.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- 105.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–594. doi: 10.1016/j.jacc.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 106.Han W, Fu S, Wei N, Xie B, Li W, Yang S, et al. Nitric oxide overproduction derived from inducible nitric oxide synthase increases cardiomyocyte apoptosis in human atrial fibrillation. Int J Cardiol. 2008;130:165–173. doi: 10.1016/j.ijcard.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 107.Papoulidis P, Ananiadou O, Chalvatzoulis E, Ampatzidou F, Koutsogiannidis C, Karaiskos T, et al. The role of ascorbic acid in the prevention of atrial fibrillation after elective on-pump myocardial revascularization surgery: a single-center experience--a pilot study. Interact Cardiovasc Thorac Surg. 2011;12:121–124. doi: 10.1510/icvts.2010.240473. [DOI] [PubMed] [Google Scholar]
- 108.Bjordahl PM, Helmer SD, Gosnell DJ, Wemmer GE, O'Hara WW, Milfeld DJ. Perioperative supplementation with ascorbic acid does not prevent atrial fibrillation in coronary artery bypass graft patients. Am J Surg. 2012;204:862–867. doi: 10.1016/j.amjsurg.2012.03.012. discussion 7. [DOI] [PubMed] [Google Scholar]
- 109.Sisto T, Paajanen H, Metsa-Ketela T, Harmoinen A, Nordback I, Tarkka M. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann Thorac Surg. 1995;59:1519–1523. doi: 10.1016/0003-4975(95)00197-s. [DOI] [PubMed] [Google Scholar]
- 110.Marin F, Pascual DA, Roldan V, Arribas JM, Ahumada M, Tornel PL, et al. Statins and postoperative risk of atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2006;97:55–60. doi: 10.1016/j.amjcard.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 111.Goette A, Lendeckel U, Klein HU. Signal transduction systems and atrial fibrillation. Cardiovasc Res. 2002;54:247–258. doi: 10.1016/s0008-6363(01)00521-1. [DOI] [PubMed] [Google Scholar]
- 112.Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 113.Sun Y, Ji Q, Mei Y, Wang X, Feng J, Cai J, et al. Role of preoperative atorvastatin administration in protection against postoperative atrial fibrillation following conventional coronary artery bypass grafting. Int Heart J. 2011;52:7–11. doi: 10.1536/ihj.52.7. [DOI] [PubMed] [Google Scholar]
- 114.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 115.Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, et al. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg. 2009;57:276–280. doi: 10.1055/s-0029-1185301. [DOI] [PubMed] [Google Scholar]
- 116.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, et al. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. Jama. 2012;308:2001–2011. doi: 10.1001/jama.2012.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heidarsdottir R, Arnar DO, Skuladottir GV, Torfason B, Edvardsson V, Gottskalksson G, et al. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010;12:356–363. doi: 10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 118.Saravanan P, Bridgewater B, West AL, O'Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010;3:46–53. doi: 10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 119.Arumanayagam M, Chan S, Tong S, Sanderson JE. Antioxidant properties of carvedilol and metoprolol in heart failure: a double-blind randomized controlled trial. J Cardiovasc Pharmacol. 2001;37:48–54. doi: 10.1097/00005344-200101000-00006. [DOI] [PubMed] [Google Scholar]