Abstract
Enterohemorrhagic Escherichia coli serotype O157:H7 is a food- and waterborne pathogen that causes significant morbidity and mortality in both developing and industrialized nations. The present review focuses on the history, epidemiology and evolution of the pathogen; provides a mechanistic overview of major virulence factors (including Shiga toxins, locus of enterocyte effacement pathogenicity island and pO157 plasmid); discusses host immune responses to infection; considers available animal models; and provides an overview of current and potential future management considerations.
Keywords: Escherichia coli, Host response, Irritable bowel syndrome, Pathogenicity, Treatment
Abstract
L’Escherichia coli de sérotype O157:H7 entérohémorragique est un pathogène d’origine alimentaire et hydrique qui provoque une morbidité et une mortalité considérables, tant dans les pays en développement que dans les pays industrialisés. La présente analyse s’attarde sur l’histoire, l’épidémiologie et l’évolution du pathogène, présente un aperçu mécaniste des principaux facteurs de virulence (y compris les toxines Shiga, les îlots de pathogénicité du locus d’effacement des entérocytes et le plasmide pO157), aborde les réponses immunitaires de l’hôte à l’infection, traite des modèles animaux disponibles et contient un aperçu de la prise en charge actuelle et éventuelle.
Escherichia coli was first discovered in 1885 by Theodore Escherich as a Gram-negative, facultative anaerobe that typically inhabits the lower intestinal tract of many warm-blooded animals (1). In humans, the organism is acquired as a commensal bacterium in the colon shortly after weaning, and persists for life as part of the commensal gut microbiota within the lumen and mucus layer of the large intestine.
Pathogenic E coli evolved from commensal E coli through the acquisition of multiple virulence determinants such as toxins, adhesins and secreted effector proteins that modulate multiple host responses, the acquisition of mobile virulence plasmids and phages, and through lateral gene transfer of pathogenicity islands. The combined effects of different virulence factors determine the extent of E coli pathogenesis and the severity of human disease. Infectious strains resulting in common diseases are grouped as pathotypes, including enteropathogenic E coli (EPEC), atypical enteropathogenic, enterotoxigenic E coli, diffusely adherent E coli, enteroaggregative E coli, enterohemorrhagic E coli (EHEC), enteroinvasive E coli, adherent-invasive E coli, sepsis/meningitis-causing E coli and uropathogenic E coli(2). Within each pathotype, E coli strains are further characterized according to antigenic variants including O-antigen (lipopolysaccharide), H-antigen (flagellar) and K-antigen (capsular) types.
E coli serotype O157:H7 is the prototypical EHEC, and was first associated with hemorrhagic colitis in 1983 in an outbreak of food-borne-related illness associated with eating undercooked ground hamburger meat in a fast-food chain in the United States (3). This pathogen was subsequently shown have cytotoxic effects in Vero cells (African green monkey kidney cells) and, henceforth, was referred to as verocytotoxin-producing E coli (VTEC) (4). The parallel discovery that this pathogen produced Shiga toxins gave rise to the alternative name Shiga toxin-producing E coli (STEC).
While VTEC and STEC describe more than 200 serotypes of cytotoxin-producing E coli, ranging in human illness from severe disease to nonpathogens, Levine (5) first coined the term ‘EHEC’ to incorporate only E coli serotypes associated with hemorrhagic colitis and the hemolytic-uremic syndrome (HUS), classically reserved for use in describing E coli serotypes O157:H7 and O26:H11. However, a conflict in the naming schema for this pathotype of E coli remains: the term ‘VTEC’ remains widely used in Canada and Europe, while the term ‘STEC’ is commonly used in the United States and Asia. For purposes of consistency, the term ‘EHEC’ will be used throughout the present review, focusing on serotypes causing human enteric disease.
EPIDEMIOLOGY OF EHEC O157:H7
Although EHEC O157:H7 is a human pathogen responsible for numerous infectious outbreaks worldwide, it is also a resident commensal bacterium commonly found in the intestinal tract of ruminants such as cattle, sheep, goats and deer. Human exposure to this microbial pathogen is classically associated with the ingestion of undercooked ground beef (6); however, infections can also arise following the ingestion of ruminant feces-contaminated foodstuffs such as fruits, vegetables and drinking water, as well as person-to-person contact and hospital-acquired nosocomial infections (7).
EHEC outbreaks are attributed to its low infectious dose (<100 organisms) and high transmissibility, which can either remain isolated or develop into widespread international outbreaks. Examples of EHEC outbreaks in North America include a multistate outbreak resulting in approximately 200 cases of infection following ingestion of fecally contaminated spinach from a single commercial vendor in the fall of 2006 (8). In 2000, the largest EHEC outbreak in Canada occurred in Walkerton, Ontario, due to inadequately chlorinated drinking water, which resulted in approximately 2300 cases of infection and seven deaths (9). Another human outbreak occurred in Canada in 2012 due to EHEC O157:H7 infection arising from the fecal contamination of huge volumes of meat in a single processing plant situated in southern Alberta.
While individuals may be asymptomatic even though EHEC is detected in stools, others can develop severe cases of infection characterized by abdominal cramps and bloody diarrhea within three days of consuming contaminated foodstuffs (10). The most severe cases of infection typically occur in children <5 years of age, the elderly and in immune-compromised persons. In severe cases of infection, individuals present with hemorrhagic colitis, HUS (the leading cause of acute renal failure in children) characterized by microangiopathic hemolytic anemia (fragmented erythrocytes) and thrombocytopenia (low platelet count) in the setting of kidney dysfunction arising from toxin-induced endothelial cell damage in the capillary bed of the glomerulus. In sporadic cases particularly, E coli O157:H7 infection can mimic Crohn’s colitis in terms of clinical presentation as well as radiographic and endoscopic features (11). However, the short duration of the presenting symptoms, relative absence of fever and lack of thrombocytosis should alert the gastroenterologist to the potential of an intercurrent infection instead of the initial presence of an intercurrent infection. Initial reports of EHEC O157:H7 infection indicated the absence of fecal leukocytes, but this is incorrect. Pus cells are present in fecal smears and fecal calprotectin levels will be elevated, both indicative of an active neutrophilic colitis.
Long-term complications of EHEC infection include irritable bowel syndrome (IBS), as observed in a significant number of infected subjects experiencing postinfectious IBS eight years after the Walkerton outbreak, compared with those who did not experience acute enterocolitis (15.4% versus 5.4% in age-, community- and sex-matched uninfected controls [P<0.0001]) (12). The same group reported a higher prevalence of uninvestigated dyspepsia in the same cohort of patients (13). Postinfectious functional bowel disorders, including IBS and functional dyspepsia, have been confirmed by other research groups, including in Scandinavia (14).
In North America, approximately 75,000 cases of EHEC infections are reported annually. Of these, approximately 10% to 15% develop HUS, an additional 5% to 10% result in long-term complications and 3% to 5% of HUS cases are fatal. EHEC infections account for approximately 250 reported deaths in North America each year (15).
In addition to serotype O157:H7, multiple non-O157:H7 STEC also can cause human infections and disease outbreaks. In North America, E coli serotype O157:H7 accounts for approximately 75% of all STEC infections, while non-O157:H7 STEC account for the remaining 25% (16). However, the exact number of non-O157:H7 infections in North America is likely to be underestimated because serotyping techniques are rarely routinely used in the setting of a hospital clinical microbiology laboratory (17).
In certain parts of Europe and Australia, non-O157:H7 E coli is the predominant cause of infectious outbreaks. For example, a recent large non-O157 outbreak of infection occurred in Germany in May 2011, in which sprouts were contaminated with a Stx2-expressing enteroaggregative E coli of the serotype O104:H4. Over the course of three months, 3800 cases of infection were reported, with more than 800 individuals developing HUS and 54 fatalities (17).
EVOLUTION OF EHEC O157:H7
EHEC O157:H7 is considered to have evolved from enteropathogenic E coli O55:H7 approximately 4.5 million years ago (18). A current model proposes that EHEC O157:H7 evolved through a series of stepwise acquisitions of genes such as Shiga toxin-encoding stx genes; the loss of phenotypic markers including the ability to ferment sorbitol (SOR) and β-glucuronidase activity; and nucleotide changes in uidA (the gene that encodes β-glucuronidase), and fimA and fimH fimbrial encoding genes (19). In addition to these genetic changes, there was parallel evolution of both enteropathogenic and enterohemorrhagic E coli within related serogroups via multiple acquisitions of a locus of enterocyte effacement (LEE) pathogenicity island, the large virulence plasmids EAF and pO157, respectively, and stx-containing lambda-like bacteriophages in the case of EHEC (19). Specific O157:H7 genetic lineages appear to be hypervirulent and these are the strains that result in outbreaks of disease in humans (20).
VIRULENCE FACTORS OF EHEC O157:H7
The pathophysiology of EHEC O157:H7 infection is attributed to the effects of multiple virulence determinants including Shiga toxins, the LEE pathogenicity island and the pO157 plasmid (21).
Shiga toxins
One of the major virulence factors attributed to EHEC disease pathogenesis is phage-encoded Shiga toxins. The cytotoxic effects of these toxins were first documented in Vero cells (African green monkey kidney epithelial cells) in 1977 (22), and later confirmed in 1983 from a patient with an EHEC O157:H7 infection and HUS (23). Subsequent studies indicated that multiple E coli serotypes elicit the same cytotoxic effects (4), and further studies showed that EHEC contains two closely related, but genetically distinct, Shiga toxins encoded by bacteriophages (Stx1 and Stx2) (24).
EHEC Shiga toxins are quite similar to the toxin found in Shigella dysenteriae type 1; Stx1 displays 98% sequence homology, while Stx2 shares approximately 55% amino acid identity (24). Despite related primary amino acid sequences, Stx1 and Stx2 are immunologically distinct. Stx2 is many times more potent than Stx1 in mice, demonstrating a lethal dose in mice that is 400-fold lower than Stx1, resulting in greater mortality and increased renal damage and toxicity. Stx2 is the toxin most commonly associated with clinical isolates of STEC.
EHEC Shiga toxins are AB5 type multimeric toxins, comprised of a single 30 kDa A subunit and a pentamer of noncovalently attached identical 7 kDa B subunits. During infection, the toxins are secreted by EHEC into the extracellular milieu; the B subunits bind to the glycosphingolipid globotriosylceramide (Gb3) present on the surface of host cells triggering internalization of the toxin. It should be noted that while Stx classically enters the host cell through binding to the Gb3 receptor, there are reports that Stx also can enter Gb3 negative cells through retrograde trafficking (25) and bind to kidney and brain endothelial cells through globotetraosylceramide (Gb4) surface glycoplipid receptors (26). These data suggest that Gb3 likely is not the sole receptor involved in Stx-mediated disease pathogenesis.
Following internalization, the A subunit dissociates from the five B subunits and, via retrograde transport, traverses the Golgi stacks to the lumen of the endoplasmic reticulum where it depurinates 28S eukaryotic ribosomal RNA, thereby inhibiting protein synthesis and causing cell death. The toxins also induce programmed cell death (also referred to as apoptosis). During infection, the Shiga toxins target Gb3 receptors on glomerular endothelial cells, podocytes and various tubular epithelial cells in the kidney, thereby causing kidney damage. Toxin-induced activation of chemokine signalling pathways appears to be of particular importance in mediating endothelial cell injury and inducing nephrotoxicity (27).
LEE pathogenicity island
Following ingestion, EHEC travels to the distal ileum and large bowel, where it comes in contact with and binds intimately to surface epithelial cells, forming F-actin-enriched pedestals directly underneath sites of bacterial attachment to colonocytes (28). This phenotype is afforded to EHEC by genes encoded on a 42 kb pathogenicity island known as the LEE. LEE gene expression is highly regulated and encodes a type 3 secretion system (T3SS), which serves as a molecular syringe that translocates bacterial effector proteins from the prokaryote directly into the host eukaryotic cell cytoplasm. Once EHEC comes into intimate contact with host epithelial cells, the pathogen uses this T3SS to inject at least 50 bacterial effector proteins directly into the cell cytoplasm (29). One of these effectors is translocated intimin receptor (Tir). Following injection, Tir anchors itself onto the surface of the host cell plasma membrane and acts as a receptor for intimin, an outer membrane protein found on the surface of the EHEC bacterium.
Binding of Tir to intimin triggers the recruitment of another EHEC effector, EspFu, using the host proteins IRTKS and IRSp53 as adaptors. Once this complex is formed, EspFu binds to neural Wiskott-Aldrich syndrome protein (N-WASP), which activates the host Arp2/3 complex resulting in actin polymerization and pedestal formation. Interestingly, this process differs in EPEC, where the Tir molecule is phosphorylated and recruits the host cell protein Nck, which then binds and activates N-WASP and the Arp2/3 complex. In addition to experiments demonstrating that EHEC and EPEC Tir are not functionally interchangeable, these studies indicate that EHEC and EPEC use slightly different mechanisms to induce actin polymerization and pedestal formation.
In addition to Tir and EspFu, the T3SS injects several other EHEC proteins encoded by the LEE, most of which are known as E coli secreted proteins (Esp). EspA, EspB and EspD serve as structural proteins of the T3SS molecular syringe and are involved in delivery of other effectors into the cell. Additional LEE-encoded effectors include EspG, EspF, EspH and mitochondrial-associated protein, all of which interfere with host cell signalling.
The T3SS also allows for the delivery of bacterial protein effectors that are not encoded within the LEE and are largely categorized as non-LEE-encoded effectors. The findings highlight the multifunctional nature of the effectors and their ability to participate in redundant and overlapping roles in terms of subverting host cell processes (28).
pO157 plasmid
EHEC O157:H7 contains a highly conserved, nonconjugative F-like plasmid, referred to as pO157, which ranges in size between 92 kb and 104 kb (30). Sequence analysis shows a heterogeneous mixture of genetic elements, transposons and prophages, as well as parts of other plasmids which, collectively, are indicative of its mottled evolution. The complete sequence of pO157 reveals 100 open reading frames; among them, 43 show similarities to known proteins. However, the precise role of pO157 in disease pathogenesis still is not well defined because published studies have reported conflicting findings (30).
HOST IMMUNE RESPONSES TO EHEC O157:H7 INFECTION
One of the first lines of host defense against EHEC infection is through the activation of the innate immune system, using pattern recognition receptors to detect pathogen-associated molecular patterns expressed by microbes to elicit a protective antimicrobial immune response (31). Of the many host pattern recognition receptors, Toll-like receptors (TLRs) and Nod-like receptors (NLRs) are the principal pathogen recognition receptors involved in recognizing and responding to microbes, including bacterial antigens. TLRs are a family of membrane-bound receptors that recognize specific microbial components such as Gram-negative bacterial lipopolysaccharide (TLR 4) and flagellin (TLR 5) (32). In contrast, NLRs detect other microbial products, such as peptidoglycan by Nod-2, in the cytoplasm of the host cell (33). The activation of TLRs and NLRs leads to a general activation of the immune system, resulting in the release of immune mediators and immune cell activation that collectively serves to contain and clear the bacterial infection.
An important signalling cascade that is activated to clear a microbial pathogen is the interferon-gamma (IFNγ), Jak 1, 2, Stat1 signal transduction pathway (34). Proinflammatory cytokines, including IFNγ, are secreted into the extracellular environment by macrophages, natural killer T cells, and activated T cells following EHEC O157:H7 infection resulting in the activation of up to 2000 IFNγ-stimulated genes in recipient host cells that together mount the host defense against pathogenic microbes (35,36).
The IFNγ signal transduction pathway is critical for protective immunity against viruses, bacteria, fungi and parasites. In humans, defects in any part of the signalling cascade leads to recurrent, severe and often life-threatening microbial infections (37). Furthermore, the important antimicrobial aspects of the IFNγ pathway make it an attractive target for pathogen subversion (31). For instance, Listeria monocytogenes inhibits the IFNγ signalling pathway by upregulating suppressors of cytokine signalling activity, a downregulator of IFNγ activation (38). The parasite Leishmania donovani prevents phosphorylation of Stat-1 (39) while influenza A (40) and vaccinia viruses (41) prevent Stat-1 tyrosine phosphorylation and nuclear translocation. EHEC O157:H7 also suppresses the IFNγ signal transduction pathway, mediated, at least in part, by Shiga toxins (42).
ANIMAL MODELS OF EHEC INFECTION
Previous studies have successfully challenged rabbits, infant rabbits, and gnotobiotic piglets with EHEC O157:H7, and non-O157 STEC. Following orogastric challenge with viable organisms, attaching and effacing lesions are apparent on the apical plasma membrane of surface epithelia lining the terminal ileum, cecum and colon. However, such lesions are not observed following challenge of rodents with human STEC isolates, even though EHEC O157:H7 challenge of germ-free mice is lethal (43). With systemic injection of holotoxin, damage to vascular endothelial cells in the cecum and brain, and to renal tubular cells, is observed in rabbits and in mice (44).
To more closely approximate the human condition, a rabbit diarrheal pathogenic E coli (RDEC-1) expressing the LEE pathogenicity island was transduced with phages encoding either Stx1 or Stx2. A more severe disease phenotype ensues in rabbit ileal ligated loops challenged with the toxin-producing lapine-specific enteropathogen (45).
Rodents challenged with human STEC isolates do not develop either attaching-effacing lesions in the gut or renal vascular endothelial cell damage. A murine-specific enteric pathogen known as Citrobacter rodentium also encodes the LEE pathogenicity island and results in attaching-effacing lesions in the distal colon of mice some six days after orogastric challenge. C rodentium does not elaborate Shiga toxins, but when a Stx2-producing, mucus-activating strain was constructed, it resulted in lethality of mice and systemic injury characterized by damage to renal tubules (46). However, a major limitation to these models to date is that none develop thrombotic microangiopathy, anemia, thrombocytopenia or the glomerulopathy that is characteristic of HUS in humans.
MANAGEMENT OF EHEC INFECTIONS
Sporadic outbreaks of EHEC O157:H7 infection will continue until more reliable prevention strategies can be implemented. For instance, reducing gut colonization and fecal excretion in bovines is one potential option that would be served by the development of effective and safe animal vaccines. Under active investigation is active immunization of cattle with a vaccine against EHEC O157:H7 to prevent bacterial shedding in feces and animal-to-animal spread (47). Alternatively, general safety practices of regular hand washing with soap, chlorination of drinking water supplies and complete cooking of ground hamburger (ie, no pink meat) will reduce environmental exposures and person-to-person transmission in the setting of an outbreak.
Current treatment options for EHEC infection and HUS are largely supportive and consist of fluid resuscitation, peritoneal dialysis and plasma exchange (48). Careful attention to intravascular volume status and the liberal use of intravenous fluids, especially early during the course of the illness, may reduce the rates of developing acute renal failure in infected subjects (49). Antibiotic treatment against EHEC O157:H7 infection is not recommended because while effective in reducing pathogen elimination in stools, antibiotics may also induce toxin release from the pathogen and result in increased systemic exposure to the adverse effects of the potent nephrotoxin. In fact, antibiotic usage appears to increase the frequency of HUS occurrence among EHEC-infected children (24,50).
Alternative strategies to sequester and limit Shiga toxin-associated pathology have been proposed. For instance, Stx ligand mimics should sequester Stx from binding to host cells and thereby limit pathology. However, in the sole clinical trial of such a molecule, Synsorb Pk, the treatment failed to diminish disease severity in infected children (51). In clinical practice, it is likely that vascular endothelial damage may have already occurred before the ligand binding receptor analogues could prove to be of benefit. Alternatively, neutralizing Shiga toxin-specific monoclonal antibodies are highly protective when administered to animals challenged with lethal doses of the toxin (52). In addition, the divalent cation manganese (Mn2+) blocks endosome-to-Golgi trafficking of Stx in vitro, which could offer a novel therapeutic approach for managing human disease (53). In vitro studies indicate that beneficial microbes (probiotics) are another approach that should be tested further for potential use in preventing acquisition or spread of EHEC O157:H7 infection, particularly in the setting of an outbreak (54). Based on evidence that Shiga toxins activate complement in HUS, a few case reports of successful treatment by using the monoclonal antibody eculizumab (targeted against the complement protein C5) have been reported (55).
Vaccines for use in humans are another therapeutic avenue currently under active investigation. This approach could provide a long-term prevention for those at highest risk for contracting and spreading EHEC. Many EHEC-derived proteins are highly immunogenic and promising results in animal model studies have been described, including vaccines targeting antigenic epitopes on Shiga toxins (56).
CONCLUSIONS.
EHEC O157:H7 infection remains a significant cause of morbidity and mortality in both developing and industrialized nations. An overview of factors involved in EHEC O157:H7 disease pathogenesis is illustrated schematically in Figure 1. Studies of mechanisms of disease pathogenesis demonstrate a varied array virulence factors that work redundantly and cooperatively to subvert host cellular and immune processes. Antibiotic treatment is not recommended for patients infected with EHEC O157:H7 and care is supportive in nature, with a focus on maintaining intravascular volume homeostasis. Alternative preventive and therapeutic options to manage EHEC infection are currently under investigation. While it is clear that many of these therapeutic options are still some way from testing in the setting of clinical trials involving humans, initial results appear to be promising, and may well provide viable solutions for preventing and treating EHEC O157:H7 disease outbreaks in the not-too-distant future.
Recommendations for gastroenterologists
Continued sporadic cases and outbreaks of EHEC O157:H7 infection emphasize the importance to the gastroenterology community in Canada of recognizing acute infections that can mimic inflammatory bowel disease; maintaining universal precautions at endoscopic procedures to reduce potential nosocomial spread of infections; and considering the role of acute infections in chronic functional disorders involving the intestinal tract, including dyspepsia and IBS.
Figure 1).
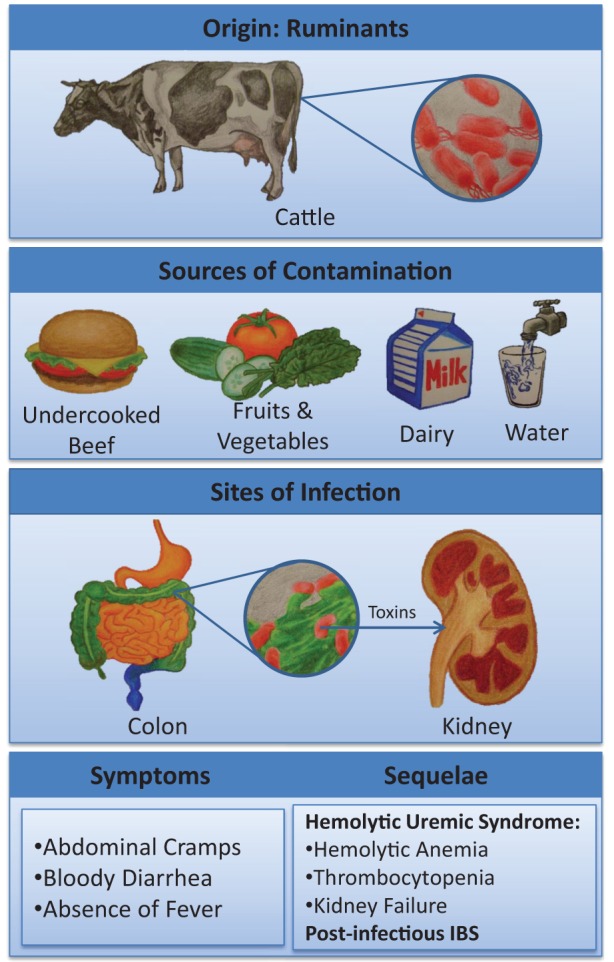
Summary of Escherichia coli O157:H7 infection including the source of infection, potential routes of human contamination, sites of infection and injury, major presenting clinical features and potential short-term complications. IBS Irritable bowel syndrome
REFERENCES
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–40. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 3.Riley LW, Remis RS, Helgerson SD, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–5. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 4.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–82. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 5.Levine MM. Escherichia coli that cause diarrhea: Enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–89. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 6.Bavaro MF. E. coli O157:H7 and other toxigenic strains: The curse of global food distribution. Curr Gastroenterol Rep. 2012;14:317–23. doi: 10.1007/s11894-012-0264-6. [DOI] [PubMed] [Google Scholar]
- 7.Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol. 2005;295:405–18. doi: 10.1016/j.ijmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Maki DG. Don’t eat the spinach – controlling foodborne infectious disease. N Engl J Med. 2006;355:1952–5. doi: 10.1056/NEJMp068225. [DOI] [PubMed] [Google Scholar]
- 9.Schuster CJ, Ellis AG, Robertson WJ, et al. Infectious disease outbreaks related to drinking water in Canada, 1974–2001. Can J Public Health. 2005;96:254–8. doi: 10.1007/BF03405157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton-Celsa A, Mohawk K, Teel L, O’Brien A. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol. 2012;357:67–103. doi: 10.1007/82_2011_176. [DOI] [PubMed] [Google Scholar]
- 11.Ilnyckyj A, Greenberg H, Bernstein CN. Escherichia coli O157:H7 infection mimicking Crohn’s disease. Gastroenterology. 1997;112:995–9. doi: 10.1053/gast.1997.v112.pm9041263. [DOI] [PubMed] [Google Scholar]
- 12.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–11. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Thabane M, Collins SM, et al. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: A cohort study. Gastroenterology. 2010;138:1727–36. doi: 10.1053/j.gastro.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Tornblom H, Holmvall P, Svenungsson B, Lindberg G. Gastrointestinal symptoms after infectious diarrhea: A five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461–4. doi: 10.1016/j.cgh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Serna At, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol. 2008;24:38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
- 16.Andreoli SP, Trachtman H, Acheson DW, Siegler RL, Obrig TG. Hemolytic uremic syndrome: Epidemiology, pathophysiology, and therapy. Pediatr Nephrol. 2002;17:293–8. doi: 10.1007/s00467-001-0783-0. [DOI] [PubMed] [Google Scholar]
- 17.Werber D, Krause G, Frank C, et al. Outbreaks of virulent diarrheagenic Escherichia coli – are we in control? BMC Med. 2012;10:11. doi: 10.1186/1741-7015-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng P, Lampel KA, Karch H, Whittam TS. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J Infect Dis. 1998;177:1750–3. doi: 10.1086/517438. [DOI] [PubMed] [Google Scholar]
- 19.Kyle JL, Cummings CA, Parker CT, et al. Escherichia coli serotype O55:H7 diversity supports parallel acquisition of bacteriophage at Shiga toxin phage insertion sites during evolution of the O157:H7 lineage. J Bacteriol. 2012;194:1885–96. doi: 10.1128/JB.00120-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laing CR, Buchanan C, Taboada EN, et al. In silico genomic analyses reveal three distinct lineages of Escherichia coli O157:H7, one of which is associated with hyper-virulence. BMC Genomics. 2009;10:287. doi: 10.1186/1471-2164-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton-Celsa A, Mohawk K, Teel L, O’Brien A. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol. 2012;357:67–103. doi: 10.1007/82_2011_176. [DOI] [PubMed] [Google Scholar]
- 22.Konowalchuk J, Speirs JI, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18:775–9. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;1:619–20. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 24.Obrig TG. Escherichia coli Shiga toxin mechanisms of action in renal disease. Toxins (Basel) 2010;2:2769–94. doi: 10.3390/toxins2122769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maluykova I, Gutsal O, Laiko M, Kane A, Donowitz M, Kovbasnjuk O. Latrunculin B facilitates Shiga toxin 1 transcellular transcytosis across T84 intestinal epithelial cells. Biochim Biophys Acta. 2008;1782:370–7. doi: 10.1016/j.bbadis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betz J, Bielaszewska M, Thies A, et al. Shiga toxin glycosphingolipid receptors in microvascular and macrovascular endothelial cells: Differential association with membrane lipid raft microdomains. J Lipid Res. 2011;52:618–34. doi: 10.1194/jlr.M010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petruzziello-Pellegrini TN, Yuen DA, Page AV, et al. The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice. J Clin Invest. 2012;122:759–76. doi: 10.1172/JCI57313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong AR, Pearson JS, Bright MD, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: Even more subversive elements. Mol Microbiol. 2011;80:1420–38. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- 29.Tobe T, Beatson SA, Taniguchi H, et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci U S A. 2006;103:14941–6. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim JY, Yoon J, Hovde CJ. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J Microbiol Biotechnol. 2010;20:5–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RM, Neish AS. Recognition of bacterial pathogens and mucosal immunity. Cell Microbiol. 2011;13:670–6. doi: 10.1111/j.1462-5822.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 33.Saleh M. The machinery of Nod-like receptors: Refining the paths to immunity and cell death. Immunol Rev. 2011;243:235–46. doi: 10.1111/j.1600-065X.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- 34.Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–48. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SY, Boisson-Dupuis S, Chapgier A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: Insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 36.Shea-Donohue T, Fasano A, Smith A, Zhao A. Enteric pathogens and gut function: Role of cytokines and STATs. Gut Microbes. 2010;1:316–24. doi: 10.4161/gmic.1.5.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–28. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoiber D, Stockinger S, Steinlein P, Kovarik J, Decker T. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J Immunol. 2001;166:466–72. doi: 10.4049/jimmunol.166.1.466. [DOI] [PubMed] [Google Scholar]
- 39.Nandan D, Reiner NE. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: Selective inhibition of signaling through Janus kinases and Stat1. Infect Immun. 1995;63:4495–500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uetani K, Hiroi M, Meguro T, et al. Influenza A virus abrogates IFN-gamma response in respiratory epithelial cells by disruption of the Jak/Stat pathway. Eur J Immunol. 2008;38:1559–73. doi: 10.1002/eji.200737045. [DOI] [PubMed] [Google Scholar]
- 41.Mann BA, Huang JH, Li P, et al. Vaccinia virus blocks Stat1-dependent and Stat1-independent gene expression induced by type I and type II interferons. J Interferon Cytokine Res. 2008;28:367–80. doi: 10.1089/jir.2007.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho NK, Ossa JC, Silphaduang U, Johnson R, Johnson-Henry KC, Sherman PM. Enterohemorrhagic Escherichia coli O157:H7 Shiga toxins inhibit interferon-gamma mediated cellular activation. Infect Immun. 2012;80:2307–15. doi: 10.1128/IAI.00255-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 44.Mohawk KL, O’Brien AD. Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J Biomed Biotechnol. 2011:258185. doi: 10.1155/2011/258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crane JK, Byrd IW, Boedeker EC. Virulence inhibition by zinc in shiga-toxigenic Escherichia coli. Infect Immun. 2011;79:1696–705. doi: 10.1128/IAI.01099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mallick EM, McBee ME, Vanguri VK, et al. A novel murine infection model for Shiga toxin-producing Escherichia coli. J Clin Invest. 2012;122:4012–24. doi: 10.1172/JCI62746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen KJ, Rogan D, Finlay BB, Potter AA, Asper DJ. Vaccination with type III secreted proteins leads to decreased shedding in calves after experimental infection with Escherichia coli O157. Can J Vet Res. 2011;75:98–105. [PMC free article] [PubMed] [Google Scholar]
- 48.Goldwater PN, Bettelheim KA. Treatment of enterohemorrhagic Escherichia coli (EHEC) infection and hemolytic uremic syndrome (HUS) BMC Med. 2012;10:12. doi: 10.1186/1741-7015-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hickey CA, Beattie TJ, Cowieson J, et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med. 2011;165:884–9. doi: 10.1001/archpediatrics.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong CS, Mooney JC, Brandt JR, et al. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: A multivariable analysis. Clin Infect Dis. 2012;55:33–41. doi: 10.1093/cid/cis299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trachtman H, Cnaan A, Christen E, et al. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: A randomized controlled trial. JAMA. 2003;290:1337–44. doi: 10.1001/jama.290.10.1337. [DOI] [PubMed] [Google Scholar]
- 52.Islam MS, Stimson WH. Production and characterization of monoclonal antibodies with therapeutic potential against Shiga toxin. J Clin Lab Immunol. 1990;33:11–6. [PubMed] [Google Scholar]
- 53.Mukhopadhyay S, Linstedt AD. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science. 2012;335:332–5. doi: 10.1126/science.1215930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 2005;73:5183–8. doi: 10.1128/IAI.73.8.5183-5188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–3. doi: 10.1056/NEJMc1100859. [DOI] [PubMed] [Google Scholar]
- 56.Cai K, Gao X, Li T, Wang Q, et al. Enhanced immunogenicity of a novel Stx2Am-Stx1B fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine. 2011;29:946–52. doi: 10.1016/j.vaccine.2010.11.035. [DOI] [PubMed] [Google Scholar]


