Abstract
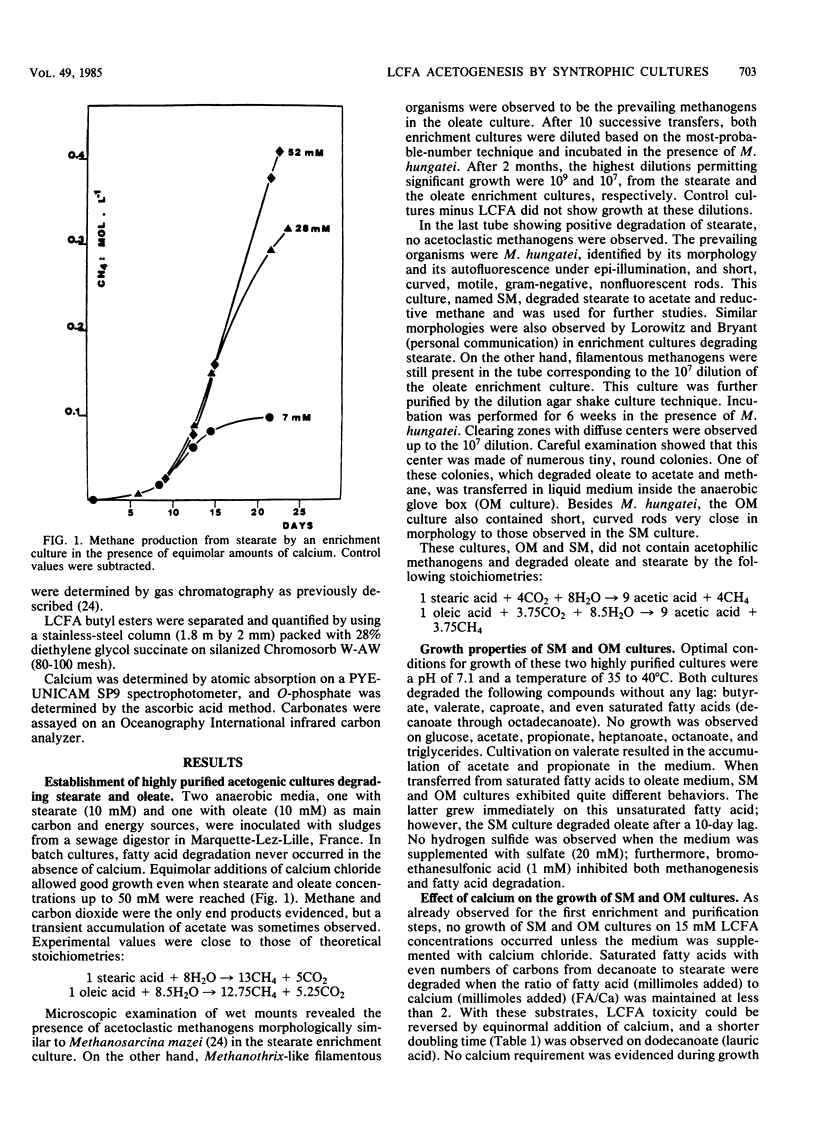
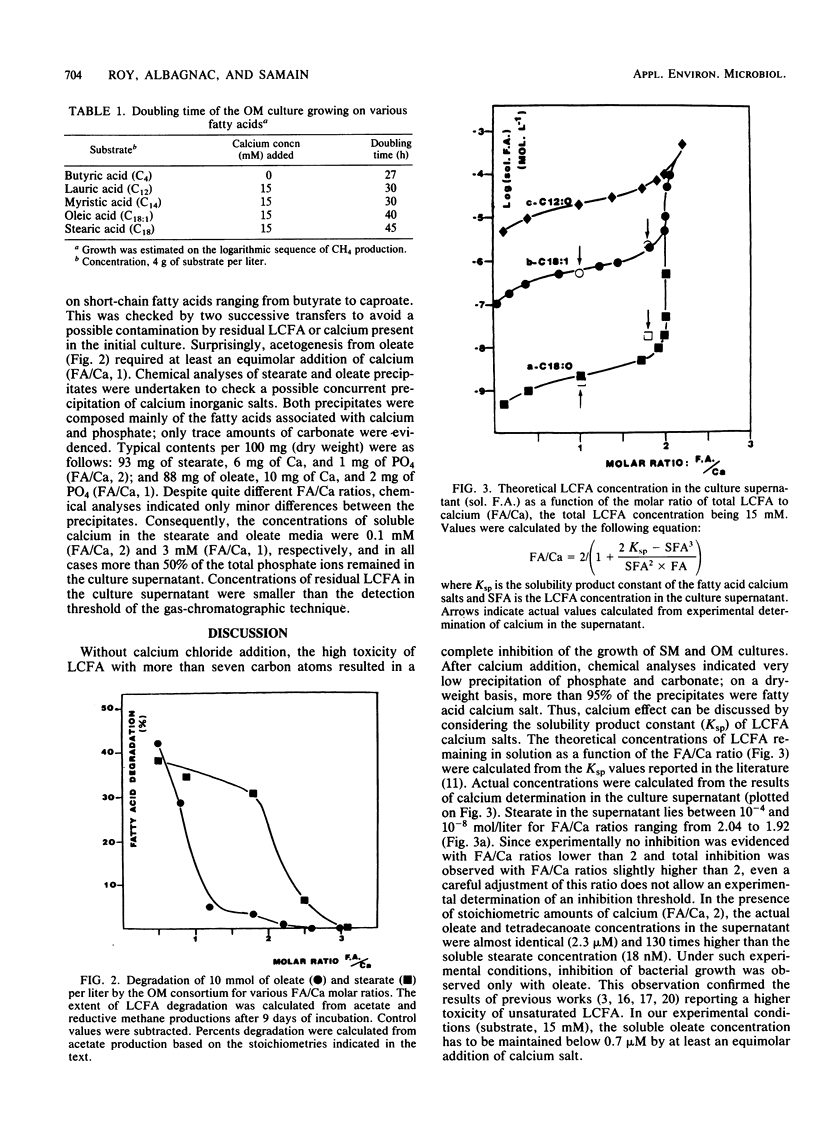
Two highly purified syntrophic associations resulting in acetogenesis from stearate (SM) and oleate (OM) were obtained from the sludges of a sewage digestor. In both cases, Methanospirillum hungatei together with short, motile, gram-negative, nonfluorescent rods morphologically similar to Syntrophomonas wolfei were identified by microscopic examination. Besides growing on volatile fatty acids (butyrate through caproate), both cultures grew on oleate (C18:1) and numerous even-numbered, saturated long-chain fatty acids (LCFA [decanoate through stearate]). In addition, during growth on LCFA, supplementation of the culture media with calcium chloride was an absolute requirement. The sole difference between the associations was observed when SM and OM cultures were transferred from a stearate to an oleate medium. The SM culture needed 10 days before starting to degrade oleate, whereas the OM culture grew immediately, but the OM culture also grew immediately when transferred to stearate medium. Saturated LCFA degradation occurred in the presence of equinormal amounts of calcium (fatty acid/Ca ratio, 2). On the other hand, OM degradation only took place in the presence of an equimolar amount of calcium (fatty acid/Ca ratio, 1). These observations are discussed by considering the solubility constants of LCFA as calcium salts and the toxicity of the free acids against microorganisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas-Rodriguez A., Smith H. W. The identification of the antimicrobial factors of the stomach contents of sucking rabbits. Biochem J. 1966 Jul;100(1):79–82. doi: 10.1042/bj1000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer D. I., Henderickx H. K. The effect of C18 unsaturated fatty acids of methane production in vitro by mixed rumen bacteria. Biochim Biophys Acta. 1967 Jun 6;137(3):484–497. doi: 10.1016/0005-2760(67)90130-0. [DOI] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B., Paton A. M., Thompson J. K. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J Appl Bacteriol. 1971 Dec;34(4):803–813. doi: 10.1111/j.1365-2672.1971.tb01019.x. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N., MANN S. O. The isolation of glycerol-fermenting and lipolytic bacteria from the rumen of the sheep. J Gen Microbiol. 1961 Jun;25:227–240. doi: 10.1099/00221287-25-2-227. [DOI] [PubMed] [Google Scholar]
- Henderson C. A study of the lipase produced by Anaerovibrio lipolytica, a rumen bacterium. J Gen Microbiol. 1971 Jan;65(1):81–89. doi: 10.1099/00221287-65-1-81. [DOI] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLAN C. E., MCNEILL J. J., TOVE S. B. BIOHYDROGENATION OF UNSATURATED FATTY ACIDS BY RUMEN BACTERIA. J Bacteriol. 1964 Oct;88:1056–1064. doi: 10.1128/jb.88.4.1056-1064.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins R. A., van Nevel C. J., Demeyer D. I. Pure culture studies of inhibitors for methanogenic bacteria. Antonie Van Leeuwenhoek. 1972;38(3):281–287. doi: 10.1007/BF02328099. [DOI] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):516–524. doi: 10.1128/jb.111.2.516-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Hag G. A., Miller T. B. Evaluation of whisky distillery by-products. VI. The reduction in digestibility of malt distiller's grains by fatty acids and the interaction with calcium and other reversal agents. J Sci Food Agric. 1972 Feb;23(2):247–258. doi: 10.1002/jsfa.2740230212. [DOI] [PubMed] [Google Scholar]



