Abstract
STAT6 plays a central role in IL-4-mediated allergic responses. Several studies indicate that regulatory T cells (Treg) can be modulated by IL-4 in vitro. We previously showed that STAT6−/− mice are highly resistant to allergic lung inflammation even when wild type Th2 effectors were provided and that they have increased numbers of Tregs. However, the role of STAT6 in modulating Tregs in vivo during allergic lung inflammation has not been thoroughly investigated. To investigate Treg and STAT6 interaction during allergic inflammation, STAT6−/−, STAT6×RAG2−/− and RAG2−/− mice were subjected to OVA sensitization and challenge following adoptive transfer of OVA-specific, wild type Th2 effectors with or without prior Treg depletion/ inactivation using anti-CD25 (PC61). As expected, STAT6−/− mice were highly resistant to airway inflammation and remodeling. In contrast, allergic lung inflammation was partially restored in STAT6−/− mice treated with PC61 to levels observed in STAT6×RAG2−/− mice. In some cases, STAT6×RAG2−/− mice were also given natural (n) Tregs along with Th2 effectors. Adoptive transfer of nTregs caused a substantial reduction in BAL eosinophil composition and suppressed airway remodeling and T cell migration into the lung in STAT6×RAG2−/− mice to levels comparable to those in STAT6−/− mice. These results demonstrate the STAT6-dependent suppression of Tregs in vivo in order to promote allergic airway inflammation.
INTRODUCTION
CD4+ Th2 cells are pivotal for the induction of allergic asthma (1-4). These cells secrete IL-4, 5, and 13 cytokines and cause changes in the airway including excessive mucus production, bronchial smooth muscle thickening, and eosinophilic inflammation (1, 5-9). IL-4 and IL-13 both bind to subunits of the IL-4 receptors to cause receptor activation and initiation of a complex signaling cascade that results in phosphorylation and induction of STAT (signal transducer and activator of transcription) 6, a transcription factor (TF), which has been shown to be important for Th2 differentiation and propagation of the allergic response (10-13). This allergic asthmatic response will not normally occur because of immune tolerance to allergens established by regulatory T cell lymphocytes (Tregs) (14-17). Tregs are CD4+ CD25+Foxp3+ T cells that regulate immune responses of effector lymphocytes (18-21). Tregs can be divided into 2 main groups. The first group, natural (n) Tregs, exit the thymus as CD4+ CD25+Foxp3+ T-cells (22-24). The second group, inducible (i) Tregs, leave the thymus as CD4+CD25−Foxp3− and convert to CD4+ CD25+Foxp3+ Tregs only after antigen exposure, in the presence of TGF-β (25, 26). Both types of regulatory T-cells have been shown to regulate allergic lung inflammation (16, 17, 27-29).
Various studies have indicated that immunosuppression and tolerance elicited by Foxp3+ Tregs are blocked by Th2 cytokines IL-4/13 (15, 30, 31). In fact, there may be direct interaction between the key Th2 TFs (Gata3 and STAT6) and Treg TF (Foxp3): Foxp3 can bind Gata3 to block expression of IL-5 and Th2 differentiation, while IL-4 and Gata3 hinder the in vitro differentiation of naïve CD4+ T cells into Foxp3+ Tregs, in the presence of TGF-β, and reduce their ability to suppress T cell proliferation (15, 32). Furthermore, in vitro experiments suggest an antagonistic interaction between STAT6 and Foxp3. IL-4 suppressed in vitro TGF-β-mediated induction of Foxp3 in a STAT6-dependent manner and a STAT6 binding site has been localized within the Foxp3 promoter (32, 33). However, the role of STAT6 in the control of Tregs during allergic airway inflammation in vivo has not been elucidated.
STAT6-deficient mice were previously shown to be highly resistant to allergic airway inflammation (34-36). This observation was not surprising, given that STAT6 is important for antigen-induced Th2 cell differentiation, Th2 migration, and other characteristics of IL-4 mediated allergic airway inflammation (10, 13, 34, 37, 38). However, we found that STAT6−/− mice were still resistant to allergic lung inflammation even when provided with wildtype bone marrow or WT Th2 effectors (34). Of additional interest, when STAT6−/− mice were crossed onto a lymphocyte-deficient genetic background (RAG2−/−), STAT6×RAG2−/− mice were able to develop moderate eosinophilic airway inflammation in the presence of WT Th2 cells (34). These results suggest that a Rag2-dependent cell type was able to efficiently suppress allergic inflammation. Furthermore, we showed that STAT6−/− mice had twice the number of CD4+CD25HiFoxp3+ Tregs in their lungs and spleen compared to WT mice under both steady state and inflammatory conditions while STAT6+/+ and STAT6−/− nTregs were equally efficient in suppressing in vitro T-cell proliferation responses (34).
These results suggested the hypothesis that STAT6−/− mice are highly resistant to allergic airway inflammation because they have increased numbers of Tregs. To test this hypothesis, we first depleted/ inactivated Tregs in STAT6−/− mice prior to transfer of Th2 effector cells and exposed the mice to allergen using a modified version of a classic murine OVA-induced allergic asthma model (39, 40). We demonstrate herein a significant restoration of previously absent eosinophilic airway inflammation and widespread allergic lung inflammation in STAT6−/− mice that undergo Treg depletion/ inactivation. Additionally, we found that adoptive transfer of nTregs reduced allergic airway inflammation, airway remodeling, and T-cell migration to the lung in STAT6×RAG−/− mice. These findings demonstrate that STAT6 suppresses Tregs during allergic lung inflammation and that STAT6−/− mice are resistant to airway inflammation due in part to their increased Treg cell population.
MATERIALS AND METHODS
Mice
BALB/c STAT6−/− mice were previously generated and described (10, 34) and were bred in the AALAC (Association for Assessment and Accreditation of Laboratory Animal Care) - approved animal care center at the University of Maryland, Baltimore (UMB). STAT6−/− mice were crossed to RAG2−/− mice to generate STAT6×RAG2−/− mice (34, 39). WT (BALB/c) RAG2−/− and D011.10×RAG2−/− mice were purchased from Taconic (Germantown, NY). The D011.10 Foxp3GFP(KI) mice were previously generated by crossing Foxp3GFP(KI) mice on a BALB/c background with D011.10 TCR transgenics and bred thereafter in the UMB animal care facility (19, 41). C57BL/6 STAT6−/− mice were obtained from Dr. Jonathan Bromberg (University of Maryland, Baltimore). All procedures described were performed in agreement with the animal protocol approved by the Institutional Animal Care and Use Committee at the University of Maryland, Baltimore School of Medicine.
FACS Analysis
Single cell suspensions made from spleens and lymph nodes were passed through 40 μM cell strainers (BD Falcon) (42). RBCs were lysed in Red Blood Cell Lysing Buffer (Sigma-Aldrich, St. Louis, MO). Cells prepared from individual mice or cells pooled from mice in the same experimental group were stained using conjugated antibodies to the following surface markers: CD4 (PE, PE-Cy7, or AlexaFluor647; BD Bioscience), mouse D011.10 TCR clone KJ126 (FITC, allophycocyanin (APC); eBioscience), CD25 (PE and PE-Cy7; BD Bioscience). In some cases, cells were stained with rat anti-mouse CD25 (clone PC61, BioXCell, West Lebanon, New Hampshire) followed by donkey anti-rat IgG (AlexaFluor 594, Molecular Probes, Eugene, Oregon). All cells were analyzed on a FACSCalibur Flow cytometer or a BD LSR Fortessa Cell Analyzer (Becton Dickinson, Franklin, NJ). Flow cytometric data were analyzed and forward and side scatter gating was performed using CELLQuest, BD FACSDiva, or FlowJo software.
Intracellular Staining
LN and spleen single cell suspensions were fixed using 4% paraformaldehyde for 15 minutes, washed, and stored in FACS Buffer for 18 hours as previously described (34). Cells were treated with Fixation/ Permeabilization solution for 30 minutes and permeabilized at 4°C. Anti-Foxp3-APC (Clone FJK-16s, eBioscience) was used to stain cells.
In vivo primed CD4+ T cell generation and adoptive transfer
D011.10×Rag2−/− mice were immunized i.p. with 100 μg/200 μL OVA (Sigma-Aldrich, St. Louis, MO) along with 2 mg of Aluminum hydroxide adjuvant (Alum, Sigma-Aldrich, St. Louis, MO or Pierce Biotechnology, Rockford, IL). Ten days later, lymph nodes and spleens were homogenized and the in vivo primed T cells were isolated from single cell suspensions. CD4+ T cells were purified using immunomagnetic separation and negative selection (Stem Cell Technologies, Vancouver, Canada) and adoptively transferred to recipient mice either i.v. or i.p. (2-4 ×106 cells/mouse), as described (39) .
Depletion/ inactivation of Tregs
The PC61.5.3 clone of rat-anti-mouse CD25 antibody (BioXCell, West Lebanon, New Hampshire) was administered to STAT6−/− mice i.p. (0.03 mg/kg) 48 hours prior to each sensitization to deplete/ inactivate CD4+CD25+Foxp3+ Tregs and Rat IGg1 isotype antibody (BioXCell, West Lebanon, New Hampshire) was used as a control (17, 43).
Adoptive Co-Transfer of CD4+CD25+ Tregs or control CD4+CD25− T cells and Th2 effector cells
To expand the CD4+CD25+Foxp3+Treg cell population, D011.10 Foxp3GFP(KI) mice were injected with a complex of IL-2 Ab (JES6-1 clone, eBioscience) and recombinant murine IL-2 cytokine (PeproTech, Rocky Hill, NJ) at a 1:2 molar ratio, respectively, i.p. once daily for three days as previously described (44, 45). Three days later, CD4+CD25+ Tregs or control CD4+CD25− T cells were isolated from harvested spleens and lymph nodes of D011.10 Foxp3GFP(KI) mice. Twenty four hours prior to OVA sensitization, 9×105 cells were co-transferred I.P. to STAT6×RAG2−/− mice along with in vivo primed Th2 effector cells (from D011.10×Rag2−/− mice) at a 1:2 ratio, respectively. FACS analysis (using the above mentioned Abs) indicates >90% of purified CD4+CD25+ T cells are Foxp3+ (16, 46).
Allergen Sensitization and Challenge
Mice were immunized i.p. on days 1 and 6 with chicken egg Ovalbumin (OVA, Sigma-Aldrich, St. Louis, MO) in Alum or with Alum alone. On days 12 and 14, all mice were challenged with aerosolized 1% OVA in PBS for 40 minutes using an Invacare Envoy Nebulizer (39).
Assessment of Allergic Airway Inflammation
Forty-eight hours following the last allergen challenge, bronchoalveolar lavage was performed on each mouse and BAL cells were cytospinned, stained, and enumerated as previously described (34, 39). Airway cytokine IL-5 was measured in BAL fluid by ELISA (R&D Systems, Minneapolis, MN) as per manufacturer's instructions.
Lung Histology
As previously described (11), 10 mL of PBS was used to flush lungs and to displace circulating blood. Lungs were removed and fixed in 10% Formalin (Fischer Scientific, Fairland, NJ) at 25°C for 120 min and subsequently stored in 70% ethanol. Lungs were paraffin-embedded and divided into tissue sections. De-paraffinized serial lung sections were stained with Hematoxylin and Eosin (H&E) or Periodic acid Schiff (PAS) at the Histology Core at University of Maryland School of Medicine (Baltimore, MD). Peroxidase was inactivated by washing slides with PBS followed by a 30 minute soak in a 0.3% H202 solution. A 1:100 dilution of rat anti-CD3 (Serotec, Raleigh, NC) and a subsequent 1:200 dilution of biotinylated anti-rat mouse antibodies (Vector Laboratories Inc. Burlingame, CA) were used to stain the slides.
Evaluation of Airway Remodeling
Serial lung sections were stained with Masson's Trichrome after paraffin embedding to illustrate collagen deposition (blue). Red color indicates muscle fibers and keratin. Images (40X magnification) of Trichrome-stained lung sections were analyzed with NIH Image J Software (National Institutes of Health, Bethesda, MD) to quantify the blue area stained for collagen relative to the entire area, as previously described (39, 47). Averages of forty airways per mouse group were analyzed. H&E stained lung sections were used to determine the average thickness of the airway smooth muscle. NIH Image J software was used to measure the cross-sectional diameter of the airway smooth muscle layer at three distinct regions surrounding the airway lumen (39, 48). Averages of forty airways were measured for each mouse group.
Statistical Analysis
Data averages are presented as mean ± SEM. To determine statistical significance and compare two groups, the two-tailed student t test (Microsoft Excel) and F-test for variance (Microsoft Excel) or Single Factor ANOVA (Microsoft Excel) were used. A p value of ≤0.05 or 0.001 was considered to be statistically significant.
RESULTS
Treg Depletion/ inactivation Restores Allergic Airway Inflammation in STAT6−/− mice in an OVA-induced Allergic Lung Inflammation Model
In previous studies we observed that BALB/c STAT6−/− mice bred and maintained in our animal facility had twice as many CD25+, Foxp3+ cells in the lungs and spleen as STAT6+/+ mice under basal and inflammatory conditions using cytoplasmic staining to detect Foxp3 (34). To confirm this finding in additional lines, we analyzed the lymph nodes and spleens from untreated D011.10 Foxp3GFP(KI) mice and D011.10×STAT6−/−Foxp3GFP(KI) mice for GFP expression (Fig. S1A). We found that compared to D011.10 Foxp3GFP(KI) mice , D011.10×STAT6−/−Foxp3GFP(KI) mice had 5-times the amount of CD4+Foxp3+ Tregs in their LN's and twice as many Tregs in their spleen. Additionally, C57BL/6 STAT6−/− mice had twice the number of CD4+Foxp3+ Tregs as WT mice (Fig. S1B). Therefore, the enhanced numbers of Tregs in STAT6−/− mice is not specific to a single mouse strain. These results suggest that STAT6 may influence induction of natural (n) Tregs in vivo in addition to the TGFβ-mediated induction of iTregs shown in vitro (32).
We hypothesized that if the observed increase in Treg numbers was responsible for the resistance of STAT6−/− mice to allergic airway inflammation, then Treg depletion/ inactivation would restore Th2-driven inflammation to levels observed in STAT6×RAG2−/− mice. To test this hypothesis, we provided OVA-specific Th2 cells to STAT6−/− and STAT6×RAG2−/− mice and immunized them as pictured in Fig.1A (39). Additionally, 2 days prior to each sensitization, STAT6−/− mice were treated with the PC61 clone of anti-mouse CD25 antibody or a control IgG antibody. The use of PC61 to deplete/ inactivate Tregs has been shown previously and is validated here in Fig.S2A (17, 43, 49-51).
Figure 1. Treg Depletion/ inactivation in STAT6−/− mice restores Th2-driven Airway Eosinophilia.
(A) This study utilized an Allergic Airway Inflammation protocol in which STAT6−/− and STAT6×RAG2−/− mice received in vivo primed D011.10 CD4+ T cells (described in Materials and Methods) and were immunized twice with Alum or 100 μg OVA in Alum and challenged 6 days later with 1% aerosolized OVA in PBS. Additional STAT6−/− groups received two i.p. treatments of PC61 to deplete/ inactivate Tregs or control IgG 48h prior to each immunization. The mice were analyzed 48 h following the last challenge. Allergic airway inflammation was induced in STAT6−/− and STAT6×RAG2−/− mice as described above. Bronchoalveolar lavage was performed and the eosinophils in the BAL were analyzed by differential cell counting. The average percentage of eosinophils (B) and absolute number of eosinophils (C) are depicted here in bar graphs ± SEM (n=2-3 Alum-treated mice group, n=3-5 OVA-treated mice/group). *p<0.05; n.s. indicates non-statistical significance (p > 0.05).
Control STAT6−/− mice treated with IgG did not demonstrate a significant increase in airway eosinophilia in response to OVA-priming (Fig.1B). Eosinophils represented only 6 ± 0.9% of the cell population recovered from their BAL and the cell composition was dominated by macrophages. However, when Tregs were depleted/ inactivated from STAT6−/− mice using PC61, the proportion of BAL eosinophils significantly increased to 16 ± 2.8% after OVA priming and challenge. STAT6×RAG2−/− mice had the greatest extent of eosinophilic expansion in their BAL (40 ± 1.5%). The depletion/ inactivation of Tregs from STAT6−/− mice also resulted in a 3-fold increase in total BAL eosinophils from IgG-treated STAT6−/− mice to STAT6−/− mice given PC61 (12,319 vs. 39,045, respectively) (Fig. 1C). The largest numbers of eosinophils were recovered from the BAL of STAT6×RAG2−/− mice (80,715 cells). Efficiency of Treg depletion/ inactivation was evaluated by FACS analyses of the lung-draining lymph nodes after completion of the full experimental protocol (Fig. S2B). Compared to the percentage of host CD4+Foxp3+ Tregs in control STAT6−/− mice administered IgG (1.29%), the proportion of host Tregs was reduced by ~ 50% of STAT6−/− mice given PC61 (0.78%). This consistent depression of Tregs was also observed when PBS was used as a control in place of IgG (data not shown).
Histological changes in the Lung following the Depletion/ inactivation of Tregs
For the purpose of examining lung tissue eosinophilia, serial lung sections from STAT6−/− mice (treated with either PC61 or control antibody) and STAT6×RAG2−/− mice were stained with H&E. (Fig.2). Enlarged images can be seen in Fig. S2C. IgG-treated STAT6−/− mice showed minor, insignificant increases in eosinophilic inflammation around airways or pulmonary vasculature after OVA-priming and challenge (Fig.2A,B). This result is consistent with previous observations of STAT6−/− mouse resistance to allergic lung inflammation (34). Conversely, the lungs of STAT6−/− mice with Treg depletion/ inactivation revealed a significantly increased proportion of eosinophils adjacent to their airways and vasculature (19±2.8% and 35±1.9%, respectively) (Fig.2A). The degree of eosinophilic inflammation in PC61-treated STAT6−/− mice was similar to that of STAT6×RAG2−/− mice, in which eosinophils composed 36% of the cells surrounding the airway lumen and almost half of the cells adjacent to the pulmonary vasculature (Fig.2A). STAT6×RAG2−/− mice consistently developed moderate levels of eosinophilic lung inflammation. These results demonstrate that resistance to eosinophilic lung infiltration in STAT6−/− mice in the presence of WT Th2 cells can be reversed after Treg depletion/ inactivation. PAS staining of lung sections revealed that in contrast to OVA-induced mucus production in WT mice, there was no increase in mucus production by epithelial cells in OVA-primed STAT6−/− or STAT6×RAG2−/− mice (Fig. S3A). This was not unexpected since epithelial cell STAT6 expression is required for mucus production and the experimental mice were all STAT6-deficient (35-37). While the epithelial expression of STAT6 is also required for eotaxin production (38), the production of IL-5 by Th2 cells is sufficient to support eosinophilic inflammation (36, 39).
Figure 2. Treg Depletion/ inactivation in STAT6−/− mice restores Th2-driven Allergic Lung Inflammation.
Allergic airway inflammation was induced as described in Figure 1. Lung sections were stained with H&E. (A) The percentage of eosinophils surrounding airways or blood vessels was quantified by differential counting in 9-15 HPF/group. Percentages are represented graphically ± SEM (n=3-5 mice/ group). *p<0.05; n.s. indicates non-statistical significance (p > 0.05). HPF: high power field. Data is representative of three independent experiments. (B) Representative H&E images of lung sections from OVA-primed mice adjacent to the airway lumen (left) or bordering the pulmonary vasculature (right) are shown at 10x, 40x, and 100x. Arrow heads identify eosinophils surrounding the airway or lung vasculature.
Effect of Treg Depletion/ inactivation on Remodeling of the Airway During Allergic Inflammation
Airway remodeling is a hallmark feature of allergic asthma that can be analyzed by measuring the extent of collagen deposition and the thickness of the smooth muscle layer surrounding the airway lumen (52-54). To determine the extent of pulmonary collagen deposition, lung sections from STAT6−/− and STAT6×RAG2−/− mice were stained with Masson's Trichrome (Fig.3A). Image J software was used to quantify the average area of collagen. A small increase in collagen deposition was detected in the lungs of STAT6−/− mice given IgG (9.48±0.26%) (Fig.3B). We observed an increase in collagen in the lungs of Treg-depleted/ inactivated STAT6−/− mice (14.66±0.38%) to levels comparable to those found in STAT6×RAG2−/− mice (12.35±0.35%).
Figure 3. Airway Remodeling in Treg-depleted/ inactivated STAT6−/− mice.
STAT6−/− and STAT6×RAG2−/− mice were subjected to the allergic asthma protocol as described in Figure 1. (A) Masson's Trichrome stain was applied to paraffin embedded lung sections of each mouse. Collagen stains blue; keratin, muscle fibers, and erythrocytes stain red. The cytoplasm stains reddish pink. Collagen deposition is shown in photographs at magnifications of 10x, and 40x as indicated, and 100x. (B) NIH Image J software was used to quantify total collagen in the lung. The average percent area of collagen ± SEM (stains blue) is represented graphically. *p<0.001; n.s. indicates non-statistical significance (p > 0.001;n = 30-50 airways per group. (C) The airway smooth muscle (ASM) layer thickness was evaluated in of H&E stained lung sections from each mouse group (40x). The cross-sectional thickness of the ASM layer is delineated by arrows. (D) The transverse distance between the inner- and outermost border of the ASM layer was measured at 3 points adjacent to each airway using NIH Image J software analysis. The average diameter of airway smooth muscle layer thickness (μm ± SEM) is represented graphically. *p<0.001; n.s. indicates non-statistical significance (p > 0.001). n = 30-50 airways per group.
The transverse diameter of the airway smooth muscle (ASM) layer surrounding the airway was quantified using Image J software analysis of H&E stained lung sections (Fig.3C,D). OVA-priming and challenge increased the ASM thickness in all experimental groups. However, the average smooth muscle cell thickness in the Treg-depleted/ inactivated STAT6−/− mice was increased 2-fold as compared to control STAT6−/− mice given IgG (Fig.3D) (39, 47, 48) . This smooth muscle layer thickness was similar to that observed in STAT6×RAG2−/− mice (Fig.3D). These results show that STAT6−/− mice can be made more susceptible to airway remodeling by the depletion/ inactivation of their Tregs.
Treg depletion/ inactivation in STAT6−/− mice allows for increased T cell migration and recruitment to the lung during allergic inflammation
Previous studies have shown that Tregs are able to block egress of Teffector cells from the site of immunization and prevent them from migrating into the tissue targeted for inflammation (55). Additionally, Tregs have demonstrated their ability to suppress adhesion between endothelial cells and Teffector cells and to modulate T cell recruitment (56). Therefore, we analyzed the number of CD3+ T-cells that migrated into the lungs of STAT6×RAG2−/− mice and STAT6−/− mice by immunohistochemistry (Fig. 4). IgG-treated STAT6−/− mice had very low numbers of CD3+ T-cells in the areas adjacent to their airways (Fig. 4). These results are consistent with previous studies showing that T-cells did not migrate to lungs of STAT6−/− mice in response to OVA-challenge (38). However, PC61-treated STAT6−/− mice whose Tregs were depleted/ inactivated demonstrated a significant increase in the number of CD3+ T-cells that were able to migrate into the their lungs after OVA-priming and challenge; this was comparable to the high number of CD3+ T cells localized to the lungs of STAT6×RAG2−/− mice (Fig. 4). These results suggest that the 2-fold increase in Treg numbers in STAT6−/− mice can suppress T-cell migration to the lung.
Figure 4. Treg depletion during the induction of allergic lung inflammation enhances CD3+ T- cell migration into the lung.
STAT6−/− and STAT6×RAG2−/− mice were subjected to the allergic asthma protocol as described in Figure 1. Lung sections were stained with antibodies to CD3. (A) CD3+ cells appear brown in 10x, 40x, and 100x magnified representative images of lung sections from OVA-primed mice. (B) Graphical representation of immunohistochemistry data. The number of CD3+ cells in each lung section was quantified and graphed. Data represented as cell counts ± SEM. *p<0.05; n.s. indicates non-statistical significance (p > 0.05). HPF: high power field, 100x.
Adoptive Transfer of Transgenic, GFP-labeled CD4+CD25+Foxp3+ nTregs Suppresses Allergic Lung Inflammation
We have previously shown that when provided with WT Th2 effectors, STAT6×RAG2−/− mice were able to develop moderate levels of eosinophilic allergic lung inflammation while STAT6−/− mice were not (34). STAT6×RAG2−/− mice lack Tregs due to their inability to develop mature T lymphocytes while STAT6−/− mice had twice as many Tregs as compared to STAT6+/+ mice (34). Therefore, the ability of Th2 cells to drive allergic lung inflammation in STAT6×RAG2−/− mice may be due to their complete absence of Tregs. To test this possibility, we enriched CD4+CD25+ nTregs from untreated D011.10 Foxp3-GFPKI mice. We also prepared CD4+CD25− cells from these mice to use as negative controls. The enriched Treg population was 76% CD4+CD25+, while the control T-cell population was only 4% CD4+CD25+. As expected, >90% of CD4+CD25+ Treg cells expressed Foxp3 at high levels while most (>95%) CD4+CD25− cells were Foxp3− (Fig.S4A).
The CD4+CD25+Foxp3+ nTregs or, as a control for cell transfer, CD4+CD25− T cells were adoptively co-transferred to STAT6×RAG2−/− mice with OVA specific Th2 cells at a 1(Tregs) : 2 (CD4 Th2 effector cells) ratio. Twenty four hours later, the mice were immunized with OVA/Alum twice. All mice were challenged with aerosolized OVA twice and two days later, the degree of allergic lung inflammation was assessed (Fig.5A).
Figure 5. Adoptive transfer of nTregs into STAT6×RAG2−/− mice suppresses Th2-driven airway eosinophilia.
(A) STAT6−/− and STAT6×RAG2−/− mice received in vivo primed D011.10 CD4+ Th2 effectors and were immunized twice with Alum or OVA/Alum and challenged with OVA on two occasions 6 days apart. STAT6×RAG2−/− mice also received CD4+CD25+ Tregs or CD4+CD25− T cells prepared from DO11.10×Foxp3-GFPKI at a 1:2 ratio with the Th2 effectors (1 Treg: 2 T eff). Experimental analysis was performed 48 h following the last challenge. (B) Allergic lung inflammation was induced in STAT6−/− and STAT6×RAG2−/− mice as described above. The STAT6×RAG2−/− mice received CD4+CD25+ nTregs or CD4+CD25− T-cells in addition to the Th2 effectors as indicated. Eosinophils in the BAL were analyzed by differential counting. The average percentage of eosinophils (B) and absolute number of eosinophils (C) are depicted here in bar graphs ± SEM (n=2-3 Alum-treated mice group, n=2-4 OVA-treated mice/group). *unpaired t-test p<0.05; n.s. indicates non-statistical significance (p > 0.05). (D) BAL fluid IL-5 was measured by ELISA. Data depicted in graphical representation ± SEM (n=2-4 mice/group) ¶ ANOVA single variance p<0.05; n.s. indicates non-statistical significance (p > 0.05).
To evaluate the effectiveness of adoptive cell transfer, lung-draining lymph nodes and spleens were collected from the mice following the completion of the experiment. Supplemental Figure 4B shows flow cytometric analysis of donor KJ126+ T-cells obtained from STAT6×RAG2−/− mice. Only 1.5% of lung-draining lymph node cells from STAT6×RAG2−/− mice who received CD4+CD25− T cells expressed Foxp3. In contrast, STAT6×RAG2−/− mice that received CD4+CD25+ nTregs contained a higher proportion of donor CD4+Foxp3+ T cells (5.73%) in their lung-draining lymph nodes. The similar difference was also observed in the spleen. The percentage of CD4+Foxp3+ splenocytes increased 10-fold from ~3.8% in STAT6×RAG2−/− mice who received CD4+CD25− T cells to ~38% in STAT6×RAG2−/− mice given Tregs.
STAT6×RAG2−/− mice that received CD4+CD25− T cells along with Th2 effectors contained a high proportion of eosinophils in their BAL in both the alum- (15%) and OVA-primed (38%) groups (Fig.5B). However, the eosinophilic inflammatory component was significantly reduced when Tregs and Th2 effectors were co-transferred to STAT6×RAG2−/− mice (10%). Furthermore, the relatively elevated level of eosinophilia observed in the alum-primed, OVA-challenged group was suppressed by the Treg transfer. In addition, the transfer of Tregs to STAT6×RAG2−/− mice reduced the total number of BAL eosinophils 13 fold from 92,468 to 7,965 cells in the OVA-primed group; the reduction in eosinophil number was even more evident in the alum-primed group. No eosinophils were recovered from the BAL of STAT6−/− mice in this experiment (Fig.5C).
The Th2 cytokine IL-5 modulates eosinophil development and mediates its activation (57, 58). Additionally, IL-5 induces the release of necessary signals to allow eosinophil migration and infiltration into the airway after exposure to an allergen (58, 59). To determine if the transferred nTregs could suppress airway IL-5 production by the transferred Th2 effectors, we assessed the concentration of IL-5 in BAL fluid recovered from all experimental mice (Fig. 5D). Ova-immunized STAT6×RAG2−/− mice given CD25− T cells demonstrated a high concentration of airway IL-5 production. The amount of IL-5 recovered was substantially reduced when Ova-sensitized STAT6×RAG2−/− mice were infused with nTregs. Likewise, STAT6−/− mice treated with Ova did not elicit increased airway IL-5 secretion.
To determine the extent of eosinophilic inflammation in lung tissue sections, H&E staining was performed and the amount of inflammation was quantified (Fig. 6). Enlarged images can be seen in Fig. S4C. Histological analysis revealed extensive eosinophilic infiltration of the airway in the lungs of control OVA-primed and challenged STAT6×RAG2−/− mice who received CD4+CD25− T cells (Fig.6B). The transfer of CD4+CD25+ nTregs to STAT6×RAG2−/− mice led to an approximate 3-fold reduction in the proportion of eosinophils adjacent to the airway from 37% to 12% (Fig.6A). As expected, very few eosinophils were observed surrounding the airways of STAT6−/− mice (9±0.9%). Accumulations of eosinophils were observed around the pulmonary vasculature and in the lung interstitium in STAT6×RAG2−/− mice who were given CD4+CD25− T cells (57±5.4%) (Fig.6A). The transfer of nTregs to these mice reduced lung eosinophilia by >50%. Similarly, a low degree of pulmonary eosinophilia was observed in STAT6−/− mice (9±2.2%). Thus, eosinophilic lung inflammation in STAT6×RAG2−/− mice can be suppressed by the transfer of nTregs to levels comparable to STAT6−/− mice. PAS staining of lung sections did not demonstrate mucus production by epithelial cells in OVA-primed STAT6−/− or STAT6×RAG2−/− mice (Fig.S3B), similar to the PC61 studies.
Figure 6. Adoptive transfer of nTregs into STAT6×RAG2−/− mice suppresses Th2-driven Allergic Lung Inflammation.
Allergic lung inflammation was induced in STAT6−/− and STAT6×RAG2−/− mice as described above. The STAT6×RAG2−/− mice received CD4+CD25+ nTregs or CD4+CD25− T-cells in addition to the Th2 effectors as indicated. Lung sections were stained with H&E. (A) The percentage of eosinophils surrounding airways or blood vessels was quantified by differential counting in 9-15 HPF/group. Percentages are represented graphically ± SEM (n=3-5 mice/group). *p<0.05; n.s. indicates non-statistical significance (p > 0.05). HPF: high power field. Data is representative of two independent experiments. (B) Representative H&E images of lung sections from OVA-primed mice adjacent to the airway lumen (left) or bordering the pulmonary vasculature (right) are shown at 10x, 40x, and 100x. Arrow heads identify eosinophils surrounding the airway or lung vasculature.
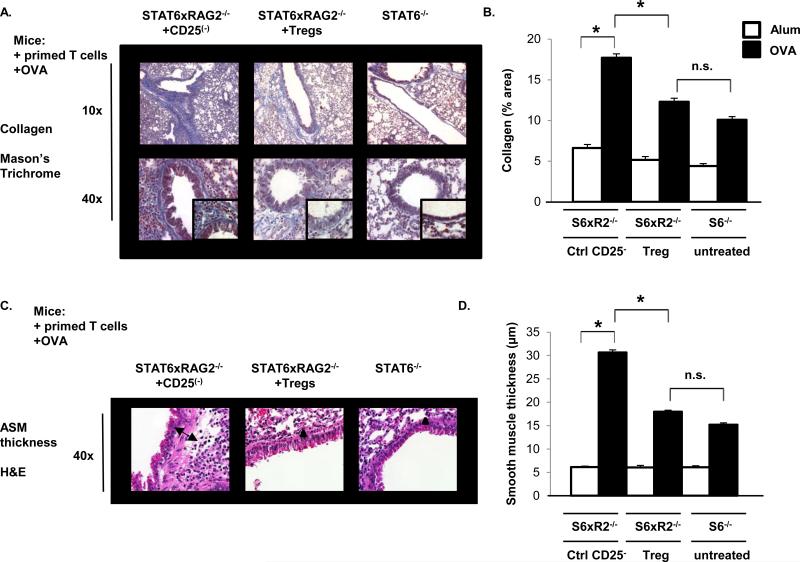
nTregs Suppress Allergen-Induced Collagen Deposition and Airway Smooth Muscle Layer Thickening in STAT6×RAG2−/− mice
To determine if airway remodeling correlated with the patterns of eosinophilic inflammation, collagen deposition and airway smooth muscle thickness were measured, as previously described (39, 47, 48), in STAT6×RAG2−/− mice who received CD4+CD25+ nTregs or CD4+CD25− T cells along with Th2 effectors and STAT6−/− mice (given Th2 effectors) (Fig.7). Collagen deposition in the larger main and lobar bronchi was present in all OVA-sensitized mouse groups, but there were differential amounts of collagen production deeper into the respiratory tree surrounding the smaller and intermediate sized terminal bronchiolar airways (Fig.7A). Dense deposits of collagen were present neighboring the airways of STAT6×RAG2−/− mice who received CD4+CD25− T cells (17.7±0.48%) (Fig.7B). In contrast, when STAT6×RAG2−/− mice were provided with nTregs, bronchiolar collagen deposition was reduced to 12±0.42% (Fig. 7B). Likewise, STAT6−/− mice also demonstrated a low degree of collagen deposition (10±0.4%) (Fig.7B). These results were mirrored by airway smooth muscle thickness measurements (Fig. 7C). Control STAT6×RAG2−/− mice who received CD4+CD25− T cells produced the highest ASM thickness and the cross-sectional width was decreased by ~50% when STAT6×RAG2−/− mice were given Tregs (Fig.7D). ASM measurements were comparable between Treg-treated STAT6×RAG2−/− mice and STAT6−/− mice (17.9 μm vs. 15.1 μm, respectively) (Fig.7D).
Figure 7. Airway Remodeling after the Transfer of Tregs into STAT6×RAG2−/− mice.
STAT6−/− and STAT6×RAG2−/− mice were subjected to the allergic asthma protocol as described in Figure 4. (A) Masson's Trichrome stain was applied to paraffin embedded lung sections of each mouse. Collagen stains blue; keratin, muscle fibers, and erythrocytes stain red. The cytoplasm stains reddish pink. Collagen deposition is shown in representative photographs at magnifications of 10x, and 40x as indicated, and 100x (inset). (B) NIH Image J software was used to quantify total collagen in the lung. The average percent area of collagen ± SEM (stains blue is represented graphically. *p<0.001; n.s. indicates non-statistical significance (p > 0.001). n = 20-40 airways per group. (C) The airway smooth muscle (ASM) layer thickness was evaluated using H&E stained lung sections (40x) from each mouse group. The cross-sectional thickness of the ASM layer is illustrated by arrows. (D) The transverse distance between the inner- and outermost border of the ASM layer was measured at 3 points adjacent to each airway using NIH Image J software analysis. The average diameter of airway smooth muscle layer thickness (μm ± SEM) is represented graphically. *p<0.001; n.s. indicates non-statistical significance (p > 0.001). n = 30-50 airways per group.
The Effect of Treg adoptive transfer on T-cell migration into the lungs of STAT6×RAG2−/− mice during allergic lung inflammation
To determine if the transferred Tregs could suppress T cell migration into the lung, we quantified the number of CD3+ T-cells in the lungs of STAT6×RAG2−/− mice and STAT6−/− mice (Figure 8). STAT6×RAG2−/− mice infused with Th2 effectors and CD25− cells had the highest numbers of CD3+ T-cells in their lungs. This amount was significantly reduced when STAT6×RAG2−/− mice were given Th2 effectors and CD4+CD25+ nTregs (27 ±1.4 cells/HPF vs. 7 ±1.3 cells/HPF, respectively) (Fig. 8B). In fact, there was a similar number of CD3+ T-cells seen in the lungs of STAT6−/− mice and Treg-infused STAT6×RAG2−/− mice (7 ±1.3 cells/HPF vs. 5 ±1.3 cells/ HPF, respectively) in both the alum-primed, OVA-challenged and OVA-primed, OVA-challenged groups. These results demonstrate that the adoptive transfer of nTregs prevents the migration of T-cells to the lungs in response to OVA-inhalation.
Figure 8. Adoptive transfer of nTregs into STAT6×RAG2−/− mice suppresses CD3+ T-cell migration into the lung during Allergic Lung Inflammation.
STAT6−/− and STAT6×RAG2−/− mice were subjected to the allergic asthma protocol as described in Figure 5. Lung sections were stained with antibodies to CD3. (A) CD3+ cells appear brown in 10x, 40x, and 100x magnified representative images of lung sections adjacent to the airway lumen from OVA-primed mice. (B) Graphical representation of immunohistochemistry data. The number of CD3+ cells in each lung section was quantified and graphed. Data represented as cell counts ± SEM. *p<0.05; n.s. indicates non-statistical significance (p > 0.05). HPF: high power field, 100x.
DISCUSSION
The studies reported here show that STAT6−/− mice are highly resistant to Th2-driven allergic lung inflammation in part because they have increased numbers of regulatory T-cells. Furthermore, they provide evidence that STAT6 suppresses Tregs in vivo during allergic lung inflammation. STAT6 is important for the propagation of IL-4-mediated allergic inflammation (10, 13, 35, 37). Indeed, Shimoda and colleagues have shown that STAT6 plays a pivotal role in Th2 cell differentiation (13). Therefore, initial observations that STAT6−/− mice were highly resistant to allergic lung inflammation were not unexpected (34, 35). However, when STAT6−/− mice repeatedly failed to elicit allergic inflammation after receiving WT Th2 cells or WT bone marrow, it became evident that the role of STAT6 spans beyond Th2 cell differentiation and migration (34). Interestingly, when STAT6−/− mice were bred into a lymphopenic genetic background (RAG2−/− mice) and resulting STAT6×RAG2−/− mice were subjected to the same treatment conditions, eosinophilic lung inflammation was induced (34).
The search for a RAG2-dependent cell type that could suppress allergic lung inflammation in STAT6−/− mice led to a list of several candidate hematopoietic cells, such as: B cells, Th1 cells, NKT cells, and Tregs. We have previously shown that STAT6−/− mice have increased levels of Tregs in their lungs and spleen, when compared to WT mice (34). Additionally, it has been shown previously that allergic airway inflammation in general can be suppressed by Tregs (14, 43, 60-63). Strickland et al. demonstrated that the transfer of CD4+CD25+ Tregs (>73% were Foxp3+) from tolerized rats into recipient rats abrogated OVA-induced airway hypperreactivity (62). Moreover, adoptively transferred iTregs generated in vitro reduced allergic lung inflammation and DC localization to lymphoid tissue in mice (45).
Our study involved the use of the PC61 clone of anti-CD25 to deplete/ inactivate Tregs from STAT6−/− mice. We found that STAT6−/− mice treated with PC61 showed a 50% reduction in their proportion of CD4+FoxP3+Tregs. All STAT6−/− mice were given the same number of CD4+ Th2 effectors. Our studies indicate that a 2 fold change in the number or percentage of Tregs can influence the outcome of allergic disease. STAT6−/− mice, who are resistant to allergic airway inflammation, have 2X more Tregs than wildtype mice (34). When we reduced the proportion of Tregs in STAT6−/− mice by half using PC61, allergic lung inflammation was exacerbated. Several independent studies have demonstrated that administration of PC61 causes the depletion of Tregs (43, 49, 64, 65). However, studies by other research groups suggest that PC61 inactivates, but does not deplete Tregs (66, 67). Kohm's study indicates a mechanism whereby PC61causes Tregs to shed IL-2Rα from their surface and functionally inactivates Tregs (66). Whether or not PC61 depletes or inactivates Tregs (by blocking IL-2 signal transduction necessary for Treg survival and function) is still under debate (50, 66). In vivo and in vitro findings by various research groups indicate that IL-2 signaling is required for Treg function (17, 66, 68, 69). Therefore, our results with PC61 are in line with the general notion that PC61treatment effectively decreases the immunosuppressive activity of Tregs in vivo.
Previous in vitro studies support the notion of an opposing relationship between STAT6 and Foxp3 in iTregs (32, 34). Dardalhon et al. showed that IL-4 inhibited TGF-β-induced expression of Foxp3 in naïve T cells in vitro by a STAT6-dependent mechanism (32). Furthermore, Pillemer et al. found that constitutive STAT6 activation rendered T cells resistant to regulation by Foxp3+ Tregs (30). Additionally, Takaki and colleagues demonstrated that STAT6 can bind directly to the Foxp3 promoter and suppress TGF-β-dependent induction of Foxp3 (33). These findings indicate that IL-4-induced activation of STAT6 can prevent the induction of Foxp3 in naïve CD4+ T-cells and therefore prevents induction of iTregs in vitro.
However, the potential for in vivo modulation of regulatory T-cells by STAT6 in the context of allergic lung inflammation was unclear. In this study, we used antibody-mediated depletion/ inactivation of Tregs and adoptive transfer of nTregs in a previously established model of Th2-driven allergic lung inflammation (34). In this model STAT6−/− and STAT6×RAG2−/− mice were provided with in vivo primed, OVA-specific CD4+ Th2 cells. Th2-driven allergic lung inflammation was partially restored in STAT6−/− mice treated with PC61 to levels observed in STAT6×RAG2−/− mice. Parameters of airway remodeling were also increased by Treg depletion/ inactivation. Adoptive transfer of CD4+CD25+Foxp3+ nTregs but not CD4+CD25−Foxp3− naïve T-cells, at a 1:2 ratio with Th2 cells caused a substantial reduction in eosinophilic inflammation and suppressed airway remodeling in STAT6×RAG2−/− mice to levels comparable to those in STAT6−/− mice. Taken together, these data demonstrate that STAT6−/− mice are highly resistant to Th2-driven lung inflammation in part because of their enhanced Treg population. Furthermore, they suggest that in addition to promoting Th2 differentiation, STAT6 suppresses Tregs in vivo in order to promote allergic airway inflammation.
Our study showed that the restoration of allergic lung inflammation in STAT6−/− mice was accompanied by enhanced T cell migration into the lung, thus indicating a role for Treg modulation of T-cell access to the lung. Furthermore, this study suggests that in addition to regulating allergic inflammation by suppressing downstream effector action of inflammatory cells (eosinophilia, airway remodeling, IL-5 production), Tregs have another checkpoint where they also block T cell recruitment and homing into the antigen-bearing tissue. This concept aligns with findings from recent studies that demonstrated the ability of Tregs to modulate Teffector cell trafficking in the context of EAE disease and diabetes (55, 70). Davidson's study utilized polyclonal Tregs in a system using an autoimmune disorder in response to a self-Ag (55). However, we illustrated that adoptively transferred monoclonal nTregs can suppress T cell trafficking into the lungs to help prevent allergic inflammation in STAT6×RAG2−/− mice. Therefore, our study reveals that regulation of T cell migration into inflamed tissue also strongly influences disease outcome for an immune-mediated disorder in response to a foreign antigen. Thus, Treg control of pathogenic Teffector cell recruitment may be a universal mechanism utilized in disorders of immune dysfunction. A study by Battaglia et al. showed that Trl cells block Teffector cell migration into tissue by producing IL-10, which prompts Teffector cells to reduce their expression of intracellular adhesion molecule 1(ICAM1) (71). Additionally, Treg-mediated sequestration of antigen-bearing tissue from Teffector cells may provide a more efficient method of regulating allergic lung inflammation. This mechanism would be an alternative to containing /controlling inflammation within the lung after pathogenic cells have released their contents and have possibly caused more tissue damage and antigen exposure. Our analysis of the total T-cell numbers in the lungs (CD3 staining) did not distinguish infiltrating effector vs. regulatory T-cells. Future studies to carefully analyze Teffector and Treg localization and migration will be necessary to clarify the mechanism by which nTregs modulate Teffector recruitment.
The relative roles of natural vs. inducible Tregs during allergic lung inflammation are still not well understood. TCR repertoire studies suggest that nTregs have TCRs that are mainly designed to recognize self antigens and to prevent autoimmune diseases (22, 23). The main cognate Ag pool for iTreg TCR is still unclear. Some studies suggest that iTregs have TCRs that can recognize both self and foreign Ags, such as allergens, to prevent immune-mediated disease (22, 23). Lewkowich et al. showed that nTregs depletion/ inactivation in mice during the immunization phase of the allergic asthma response suppressed dendritic cell activation and lead to elevated house dust mite-induced allergic airway inflammation. This study indicates that nTregs play an important role in suppressing IL-4-driven allergic lung inflammation (17). Interestingly, studies by Pandiyan and colleagues revealed a positive role for IL-4 in supporting nTregs in vitro; IL-4 was able to maintain Foxp3 and CD25 expression in nTregs (72).
However, our study establishes that in vivo, IL-4-activated STAT6 inhibits nTregs and allows for the propagation of Th2-driven lung inflammation. Our adoptive transfer of control OVA-specific CD25− cells did not show a high level of conversion of iTregs in vivo in the presence of Th2 cells. Thus, in our adoptive transfer model, the suppression of allergic inflammation and remodeling was likely mediated by the nTregs. Based on our in vitro studies showing an equipotent suppression of T-cell proliferation by nTregs prepared from STAT6+/+ and STAT6−/− mice (34), we transferred STAT6+/+ nTregs in this experiment. In future studies, it will be interesting to test whether these nTregs would have different regulatory potencies in vivo.
The definitive role of in vivo STAT6 modulation of iTregs during allergic lung inflammation has not been completely resolved. In a transgenic murine model that lacked natural Tregs, Lafaille et al. demonstrated that adaptive or inducible Tregs alone reduced chronic, but not acute, lung inflammation induced by intranasal treatment with OVA, thus, indicating that iTregs are sufficient to suppress allergic lung inflammation (73). The protocol we utilized for Treg depletion/ inactivation would affect both nTregs and iTregs and therefore does not distinguish between the suppressive roles of these two cell subsets during allergic lung inflammation. Additionally, a study by Josefowicz et al. revealed that compared to their WT counterparts, C57BL/6 mice devoid of CNS1-dependent iTregs (but have intact nTregs) experienced spontaneous Th2-type airway inflammation, suggesting a non-redundant role for iTregs to block mucosal allergic inflammation in the lung (74). Furthermore, Xu and colleagues demonstrated that the adoptive transfer of in vitro generated iTregs to sensitized mice, before or during OVA challenge, can reduce allergic lung inflammation and improve lung function (29).
One specific type of regulatory T-cell capable of producing large amounts of IL-10, Tr1, has been shown to significantly affect human allergic asthma (75-77). There is an inverse relationship between the levels of IL-10-secreting T cells and the severity of allergic asthma in patients. Furthermore, Akbari et al. found that respiratory tolerance from allergic inflammation in mice involved induction of IL-10-secreting Tregs (27). Therefore, studies that elucidate the interaction between IL-4 activation of STAT6 and Tr1 induction would be of both scientific and clinical interest. Further investigation into the relative role of STAT6 in modulating nTregs, iTregs, and Tr1 during the allergic response has the potential to make significant scientific contributions to the field of allergy and immunology, but to also aid in the development of novel therapeutic clinical strategies to combat and/or prevent this immune-mediated illness in humans.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms. Xiulan Qi for outstanding technical assistance and Dr. Preeta Dasgupta for advice on experimental design and analysis. We also thank Ms. Leisha Coates for facilitation of tail injections, and Dr. C. Colin Brinkman for aiding in pulmonary lymph node identification and Dr. Jonathan Bromberg for helpful and enlightening scientific discussions.
Abbreviations used in this article
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- BM
bone marrow
- CNS1
conserved non-coding sequence 1
- DC
dendritic cell
- IHC
immunohistochemistry
- iTreg
inducible Treg
- nTreg
natural Treg
- Treg
regulatory T cell
- Th
T helper cell
- Th 9
T helper type 9 cell
- Tr1
IL-10-Treg cell
- WT
wild-type
- Teff
effector T cell
- Ag
Antigen
Footnotes
This work was supported by National Institutes of Health Grants AI038985 (to A.D.K.), DK068342 (to D.W.S.) and by T32HL06798 and T32AI007540 and UNCF/Merck Science Initiative (to N.J.D.)
Disclosures
The authors have no financial conflicts of interest
REFERENCES
- 1.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. The Journal of allergy and clinical immunology. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. The New England journal of medicine. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 4.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annual review of immunology. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 5.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. The Journal of experimental medicine. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poston RN, Chanez P, Lacoste JY, Litchfield T, Lee TH, Bousquet J. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. The American review of respiratory disease. 1992;145:918–921. doi: 10.1164/ajrccm/145.4_Pt_1.918. [DOI] [PubMed] [Google Scholar]
- 8.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. The Journal of allergy and clinical immunology. 1991;88:935–942. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 9.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 11.Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. Journal of immunology. 2004;172:4545–4555. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- 12.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annual review of immunology. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadeiba H, Locksley RM. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. Journal of immunology. 2003;170:5502–5510. doi: 10.4049/jimmunol.170.11.5502. [DOI] [PubMed] [Google Scholar]
- 17.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. The Journal of experimental medicine. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. The Journal of clinical investigation. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunological reviews. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 22.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature reviews. Immunology. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 23.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. The Journal of clinical investigation. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nature medicine. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 28.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. Journal of immunology. 2004;172:3842–3849. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou X, Wang J, Fan H, Yan CS, Kuang JL, Warburton D, Togbe D, Ryffel B, Zheng SG, Shi W. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PloS one. 2012;7:e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. Journal of immunology. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace L, Rizzo S, Palombi C, Brombacher F, Doria G. Cutting edge: IL-4-induced protection of CD4+CD25− Th cells from CD4+CD25+ regulatory T cell-mediated suppression. Journal of immunology. 2006;176:3900–3904. doi: 10.4049/jimmunol.176.7.3900. [DOI] [PubMed] [Google Scholar]
- 32.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. The Journal of biological chemistry. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapoval SP, Dasgupta P, Smith EP, DeTolla LJ, Lipsky MM, Kelly-Welch AE, Keegan AD. STAT6 expression in multiple cell types mediates the cooperative development of allergic airway disease. Journal of immunology. 2011;186:2571–2583. doi: 10.4049/jimmunol.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. The Journal of experimental medicine. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomkinson A, Kanehiro A, Rabinovitch N, Joetham A, Cieslewicz G, Gelfand EW. The failure of STAT6-deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5. American journal of respiratory and critical care medicine. 1999;160:1283–1291. doi: 10.1164/ajrccm.160.4.9809065. [DOI] [PubMed] [Google Scholar]
- 37.Hoshino A, Tsuji T, Matsuzaki J, Jinushi T, Ashino S, Teramura T, Chamoto K, Tanaka Y, Asakura Y, Sakurai T, Mita Y, Takaoka A, Nakaike S, Takeshima T, Ikeda H, Nishimura T. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. International immunology. 2004;16:1497–1505. doi: 10.1093/intimm/dxh151. [DOI] [PubMed] [Google Scholar]
- 38.Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. The Journal of experimental medicine. 2001;193:1087–1096. doi: 10.1084/jem.193.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasgupta P, Chapoval SP, Smith EP, Keegan AD. Transfer of in vivo primed transgenic T cells supports allergic lung inflammation and FIZZ1 and Ym1 production in an IL-4Ralpha and STAT6 dependent manner. BMC immunology. 2011;12:60. doi: 10.1186/1471-2172-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. The Journal of experimental medicine. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skupsky J, Zhang AH, Su Y, Scott DW. B-cell-delivered gene therapy induces functional T regulatory cells and leads to a loss of antigen-specific effector cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:1527–1535. doi: 10.1038/mt.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapoval SP, Nabozny GH, Marietta EV, Raymond EL, Krco CJ, Andrews AG, David CS. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. The Journal of clinical investigation. 1999;103:1707–1717. doi: 10.1172/JCI6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. American journal of physiology. Lung cellular and molecular physiology. 2009;296:L307–319. doi: 10.1152/ajplung.00521.2007. [DOI] [PubMed] [Google Scholar]
- 44.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 46.Leech MD, Benson RA, De Vries A, Fitch PM, Howie SE. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. Journal of immunology. 2007;179:7050–7058. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- 47.Rangan GK, Tesch GH. Quantification of renal pathology by image analysis. Nephrology. 2007;12:553–558. doi: 10.1111/j.1440-1797.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 48.Henderson WR, Jr., Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. American journal of respiratory and critical care medicine. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 49.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. Journal of immunology. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 50.Stephens LA, Anderton SM. Comment on “Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells”. Journal of immunology. 2006;177:2036, 2037–2038. doi: 10.4049/jimmunol.177.4.2036. author reply. [DOI] [PubMed] [Google Scholar]
- 51.Faustino L, da Fonseca DM, Takenaka MC, Mirotti L, Florsheim EB, Guereschi MG, Silva JS, Basso AS, Russo M. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. Journal of immunology. 2013;190:2614–2621. doi: 10.4049/jimmunol.1202354. [DOI] [PubMed] [Google Scholar]
- 52.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. The Journal of clinical investigation. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003;123:417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 54.Halwani R, Al-Muhsen S, Hamid Q. Airway remodeling in asthma. Current opinion in pharmacology. 2010;10:236–245. doi: 10.1016/j.coph.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Davidson TS, Shevach EM. Polyclonal Treg cells modulate T effector cell trafficking. European journal of immunology. 2011;41:2862–2870. doi: 10.1002/eji.201141503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maganto-Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK, Croce KJ, Luscinskas FW, Lichtman AH, Grabie N. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. Journal of immunology. 2011;187:3521–3529. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffer PJ, Schweizer RC, Dubois GR, Maikoe T, Lammers JW, Koenderman L. Analysis of signal transduction pathways in human eosinophils activated by chemoattractants and the T-helper 2-derived cytokines interleukin-4 and interleukin-5. Blood. 1998;91:2547–2557. [PubMed] [Google Scholar]
- 58.Shi H, Qin S, Huang G, Chen Y, Xiao C, Xu H, Liang G, Xie Z, Qin X, Wu J, Li G, Zhang C. Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. American journal of respiratory cell and molecular biology. 1997;16:220–224. doi: 10.1165/ajrcmb.16.3.9070605. [DOI] [PubMed] [Google Scholar]
- 59.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. The Journal of experimental medicine. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nature reviews. Drug discovery. 2009;8:645–660. doi: 10.1038/nrd2653. [DOI] [PubMed] [Google Scholar]
- 61.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. The Journal of clinical investigation. 2004;114:1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. The Journal of experimental medicine. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umetsu DT, Akbari O, Dekruyff RH. Regulatory T cells control the development of allergic disease and asthma. The Journal of allergy and clinical immunology. 2003;112:480–487. 488. quiz. [PubMed] [Google Scholar]
- 64.Saito K, Torii M, Ma N, Tsuchiya T, Wang L, Hori T, Nagakubo D, Nitta N, Kanegasaki S, Hieshima K, Yoshie O, Gabazza EC, Katayama N, Shiku H, Kuribayashi K, Kato T. Differential regulatory function of resting and preactivated allergen-specific CD4+ CD25+ regulatory T cells in Th2-type airway inflammation. Journal of immunology. 2008;181:6889–6897. doi: 10.4049/jimmunol.181.10.6889. [DOI] [PubMed] [Google Scholar]
- 65.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. European journal of immunology. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 66.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. Journal of immunology. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 67.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. Journal of immunology. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulhankova K, Rouse T, Nasr ME, Field EH. Dendritic cells control CD4+CD25+ Treg cell suppressor function in vitro through juxtacrine delivery of IL-2. PloS one. 2012;7:e43609. doi: 10.1371/journal.pone.0043609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. Journal of immunology. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 70.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. Journal of immunology. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 71.Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, Roncarolo MG. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes. 2006;55:1571–1580. doi: 10.2337/db05-1576. [DOI] [PubMed] [Google Scholar]
- 72.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biology direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. The Journal of allergy and clinical immunology. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 76.Lim S, Crawley E, Woo P, Barnes PJ. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 1998;352:113. doi: 10.1016/S0140-6736(98)85018-6. [DOI] [PubMed] [Google Scholar]
- 77.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. The Journal of experimental medicine. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.