Abstract
The invasion and stimulation of normally non-phagocytic host cells, such as epithelial and endothelial cells, is a key step in the pathogenesis of many fungal infections. In most cases, host cell invasion and/or stimulation of a pro-inflammatory response is induced when proteins or carbohydrates on the fungal cell surface bind to receptors on the host cell. While many of these fungal–host cell interactions have only been investigated in vitro, the therapeutic efficacy of blocking the host cell receptors for Candida albicans and Rhizopus oryzae has been demonstrated in experimental animal models of infection. Here we summarize recent studies of the fungal receptors on normally non-phagocytic host cells and the therapeutic implications of blocking these receptors.
Keywords: epithelial cells, endothelial cells, fungi, receptor, pathogenesis
Therapeutic efficacy of blocking host cell receptors
Many intracellular microbial pathogens, including viruses, bacteria, parasites, and fungi, invade host cells by binding to one or more receptors on the host cell surface. Receptor binding can activate host cell endocytosis mechanisms, causing the host cell to internalize the pathogen. It can also stimulate the host cell to produce inflammatory mediators, which can be either beneficial or harmful. Because microbial binding to host cell receptors is an essential step in the pathogenesis of many infections, inhibiting this process is a promising approach to prevent and/or treat many infectious diseases. Also, because receptor blocking therapies target the host rather than the microbial pathogen, resistance may be less likely to occur.
Perhaps the best example of a therapeutic strategy directed at blocking the interaction of a pathogen with its host cell receptor is use of antiretroviral drugs to block the binding of HIV to C-C chemokine receptor type 5 (CCR5). It was discovered that HIV enters host cells by binding to CCR5, which is the receptor for CCL5 (RANTES), CCL3 (macrophage inflammatory protein [MIP]1α), and CCL4 (MIP-1β) [1]. The importance of this interaction during the initiation of infection was demonstrated by the finding that humans who are homozygous for a 32 base pair deletion in the CCR5 gene are highly resistant to HIV infection [2]. Of note, these individuals do not appear to have increased susceptibility to many other types of infection, suggesting that the lack of functional CCR5 does not have deleterious effects on immune function. This information led to the development of the antiretroviral drug, maraviroc, which blocks CCR5 and is highly effective for the treatment of HIV infection [3].
Over the past decade, numerous host cell receptors for medically important fungi have been identified. This review will focus on the fungal receptors that are expressed by normally non-phagocytic host cells and the therapeutic potential of blocking fungal interactions with these receptors.
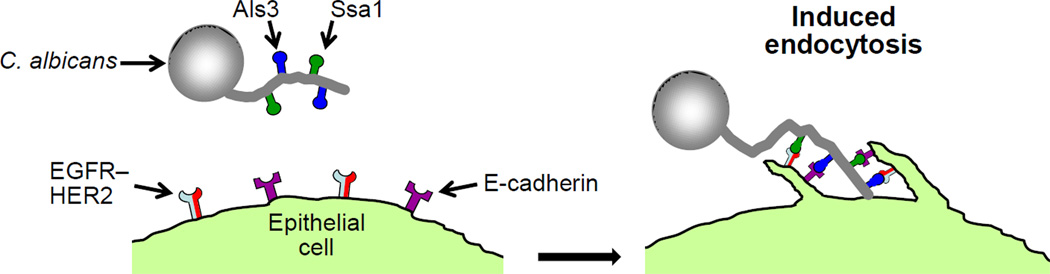
Candida albicans binds to multiple receptors on oral epithelial cells and vascular endothelial cells
C. albicans causes superficial mucosal infections, such as oropharyngeal and vulvovaginal candidiasis [4]. This fungus also causes hematogenously disseminated candidiasis, a serious health care-associated infection that is associated with a mortality of approximately 40% [5]. C. albicans invasion of normally non-phagocytic host cells plays a key role in the pathogenesis of both oropharyngeal and hematogenously disseminated infection. For example, C. albicans invades oral epithelial cells during oropharyngeal candidiasis, and it invades endothelial cells as it escapes from the vasculature during the initiation of hematogenously disseminated candidiasis [6–10]. The mechanisms by which C. albicans invades oral epithelial cells and vascular endothelial cells have been investigated extensively. This organism can invade both types of host cells by two different mechanisms. The first is active penetration, in which progressively elongating hyphae physically push their way into the host cells [11]. This process depends on the C. albicans signaling pathways that govern hyphal formation and elongation; the host cell is passive in this process. The second mechanism is induced endocytosis, in which C. albicans stimulates its own endocytosis by the host cell [7, 10, 12]. C. albicans hyphae have been found to express on their surface two different invasins that bind to host cell receptors and induce endocytosis. One of these invasins is Als3, which is a member of the agglutinin-like sequence family of adhesin/invasin proteins [13]. The other invasin is Ssa1, which is a 70 kDa heat shock protein [14]. Both of these cell surface proteins bind to receptors on epithelial cells and endothelial cells and induce endocytosis.
Oral epithelial cells
C. albicans induces its own endocytosis by oral epithelial cells when Als3 and Ssa1 interact with E-cadherin and a heterodimer composed of the epidermal growth factor receptor (EGFR) and HER2 (Table 1, Figure 1) [13, 15]. Studies in which human E-cadherin was heterologously expressed in Chinese hamster ovary cells, which lack both E-cadherin and HER2–EGFR, demonstrate that E-cadherin is sufficient to mediate endocytosis. Similarly, heterologous expression of HER2 and EGFR in the NIH3T3 fibroblast cell line, which does not express E-cadherin, results in significantly increased endocytosis of C. albicans. Therefore, both E-cadherin and HER2–EGFR can independently mediate the endocytosis of this organism. Nevertheless, E-cadherin appears to function in the same signaling pathway as EGFR–HER2 because small interfering RNA (siRNA) knockdown of E-cadherin combined with blockade of HER2 function does not inhibit C. albicans invasion of epithelial cells more than inhibition of HER2 alone. Binding to E-cadherin activates the clathrin-dependent endocytosis mechanism, leading to rearrangement of the actin cytoskeleton and the formation of pseudopods, which surround the organism and pull it into the epithelial cell [16]. It is highly likely, but not yet proven, that the binding of C. albicans to EGFR–HER2 also stimulates the clathrin-dependent endocytosis process.
Table 1.
Summary of host cell receptors and their fungal ligands
| Fungus | Host cell | Receptor | Fungal ligand | Host cell response |
In vivo studies |
Refs |
|---|---|---|---|---|---|---|
| Candida albicans | Oral epithelial cells | E-cadherin | Als3, Ssa1 | Endocytosis | No | [13, 14] |
| Oral epithelial cells | EGFR–HER2 | Als3 | Endocytosis | Yes | [15] | |
| Human umbilical vein endothelial cells | N-cadherin | Als3, Ssa1 | Endocytosis | No | [13, 14, 17] | |
| Brain microvascular endothelial cells | Gp96 | Als3 | Endocytosis | Yes | [20, 21] | |
| Candida glabrata | Oral epithelial cells | Lactosylceramide (CDw17) | Unknown | GM-CSF secretion | No | [24] |
| Cryptococcus neoformans | Pulmonary epithelial cells | CD14 | Glucuronoxylomannan | IL-8 secretion | No | [27] |
| Brain microvascular endothelial cells | CD44 | Hyaluronic acid | Endocytosis | Yes | [33, 36–38] | |
| Brain microvascular endothelial cells | Receptor of hyaluronan-mediated motility (RHAMM) | Hyaluronic acid | Endocytosis | Yes | [36] | |
| Rhizopus oryzae | Human umbilical vein endothelial cells | Grp78 | Unknown | Endocytosis; host cell damage | Yes | [44] |
| Aspergillus fumigatus | Pulmonary epithelial cells | Dectin-1 | β-glucans | Endocytosis; stimulation of TNF, IL-8, HBD2, and HBD9 mRNA expression | Yes | [49–51] |
| Pulmonary epithelial cells | E-cadherin | Unknown | Adherence; endocytosis | No | [53] | |
| Pneumocystis carinii | Alveolar epithelial cells | IL-1 receptor | Unknown | Secretion of CCL2 and CXCL2 | No | [60] |
| Alveolar epithelial cells | Lactosylceramide (CDw17) | β-glucans | Secretion of TNF and CXCL2 | No | [61, 62] |
Figure 1.
Schematic diagram of Candida albicans invasion of an oral epithelial cell by induced endocytosis. C. albicans hyphae express the invasins, Als3 and Ssa1, which interact with the epidermal growth factor (EGFR)–HER2 complex and E-cadherin on the epithelial cell surface. These interactions induce the epithelial cell to form pseudopods that surround the hypha and pull it into the epithelial cell.
The interactions of C. albicans with EGFR and HER2 have been studied in the corticosteroid-treated mouse model of oropharyngeal candidiasis. In these mice, oral infection with C. albicans stimulates the tyrosine phosphorylation of EGFR and HER2 in the epithelial cell lining of the tongue, which is evidence that these receptors are activated by the presence of the organism. Treatment of the mice with GW2974, an orally bioavailable inhibitor of both EGFR and HER2 kinase activity, reduces the phosphorylation of EGFR and HER2. Moreover, treatment with GW2974 significantly decreases the severity of oropharyngeal infection, causing a 33-fold reduction in oral fungal burden and a marked decrease in the number and size of the fungal lesions seen on histopathological analysis [15]. These results demonstrate the importance of the interactions of C. albicans with EGFR and HER2 during the pathogenesis of oropharyngeal infection. Moreover, these data provide an example of the therapeutic potential of blocking the host cell receptors for this fungus. Although both monoclonal antibodies and small molecule inhibitors of EGFR and HER2 have been developed for the treatment of cancer, the current ones in clinical use are too toxic to be employed for the treatment or prevention of oropharyngeal candidiasis. However, it is possible that a topical, poorly absorbed inhibitor of EGFR–HER2 might be less toxic while retaining therapeutic efficacy against this disease.
Vascular endothelial cells
C. albicans utilizes different receptors to invade endothelial cells in different vascular beds. Studies with human umbilical vein endothelial cells, which are likely representative of most systemic endothelial cells, demonstrate that N-cadherin functions as a receptor for C. albicans on these cells [17]. Binding of C. albicans to endothelial cell N-cadherin activates the clathrin-dependent endocytosis pathway [16]. However, there must be additional receptors for C. albicans because siRNA knockdown of N-cadherin expression only reduces endothelial cell uptake of C. albicans by 34% [17]. In addition, the role of N-cadherin in endothelial cell invasion by C. albicans has only been studied in vitro. Mouse studies to determine the role of N-cadherin during hematogenously disseminated candidiasis cannot be performed using endothelial cell-specific N-cadherin knockout mice because these animals die in utero [18]. However, N-cadherin plays a role in the growth of cancer cells, and ADH-1, a cyclic pentapeptide inhibitor of N-cadherin, has been developed for the treatment of cancer patients. ADH1 has been tested in a phase I trial in cancer patients [19]. Therefore, it would be informative to determine if ADH-1 could prevent or ameliorate disseminated candidiasis in experimental animal models.
Forming the blood-brain barrier, the endothelial cells that line the blood vessels in the brain have uniquely tight intercellular junctions. Furthermore, brain endothelial cells express surface proteins, such as gp96, that are not expressed on the surface of endothelial cells in other vascular beds. C. albicans Als3 binds to gp96 on the surface of human brain microvascular endothelial cells, and this binding induces the endocytosis of the organism. The importance of the Als3-gp96 interaction during hematogenously disseminated candidiasis is demonstrated by the finding that C. albicans mutants that lack either Vps51 or Slr1 have increased surface expression of Als3 [20, 21]. Both of these mutants have increased capacity to invade human brain microvascular endothelial cells in vitro. Also, Als3-mediated invasion of these endothelial cells is mediated by gp96 because C. albicans invasion can be blocked by an anti-gp96 monoclonal antibody or siRNA knockdown of gp96 [20]. Als3 is important for brain invasion during hematogenously disseminated candidiasis because, when injected into mice, the vps51Δ/Δ and slr1Δ/Δ mutants have enhanced trafficking to the brain [20, 21]. Moreover, homozygous deletion of ALS3 in the vps51Δ/Δ mutant significantly reduces the neurotropism of this mutant [20]. Collectively, these results indicate that, during a hematogenously disseminated infection, C. albicans traffics to and invades the brain in part by binding to gp96 that is uniquely expressed on the surface of the brain endothelial cells. Whether N-cadherin also mediates brain endothelial cell invasion is not yet known. These data have important therapeutic implications because they suggest that C albicans utilizes different endothelial cell receptors to invade different vascular beds. Thus, it is possible that a therapeutic strategy designed to block C albicans invasion of endothelial cells would have to target multiple endothelial cell receptors to be effective.
Lactosylceramide CDw17 is required for epithelial cell stimulation by Candida glabrata
Although C albicans is still the causative organism in the majority of cases of mucosal candidiasis, this Candida species now causes less than half of cases of candidemia [22, 23]. Candida glabrata, Candida parapsilosis, and Candida tropicalis are increasingly common causes of candidemia, but relatively little is known about how these organisms invade normally non-phagocytic host cells. However, Li and Dongari-Bagtzoglou [24] discovered that C glabrata is internalized by oral epithelial cells and activates the NF-κB transcription factor, thereby stimulating the secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF). C glabrata-induced GM-CSF production is not mediated by Toll-like receptor 4 (TLR4), even though oral epithelial cells express this receptor on their surface. However, an antibody against the glycosphingolipid, lactosylceramide (CDw17), inhibits NF-κB activation and significantly decreases the production of GM-CSF. Acting as a pattern recognition receptor, CDw17 is known to form membrane microdomains that can directly interact with microbial pathogens. Binding of a pathogen to CDw17 causes clustering of Src family signaling molecules, leading to downstream signaling events within the host cell. In addition, CDw17 enriched microdomains contain glycosylphosphatidyl inositol (GPI)-anchored proteins, such as integrins, that can also function as receptors for microbial pathogens [25].
Many details of the interaction between C. glabrata and CDw17 remain to be elucidated and include determining whether this organism binds directly to CDw17 or to a receptor associated with this glycosphingolipid, the identity of the C. glabrata surface structure that binds to CDw17 microdomains, and whether CDw17 also mediates the endocytosis of the organism. Moreover, the exact role of GM-CSF in the pathogenesis of oropharyngeal candidiasis is uncertain. On one hand, GM-CSF may help recruit leukocytes to foci of infection and enhance their fungicidal activities, thereby reducing oral fungal burden. On the other hand, because some of the tissue damage during oropharyngeal candidiasis may be due to an over exuberant inflammatory response, it is possible that the pro-inflammatory effects of GM-CSF may actually exacerbate the disease. For these reasons, it is unclear whether blockade of CDw17 would enhance or inhibit the extent of oropharyngeal infection caused by C. glabrata. Furthermore, it is currently unknown whether CDw17 functions as an endothelial cell receptor for C. glabrata and contributes to the pathogenesis of hematogenously disseminated candidiasis.
Invasion of pulmonary epithelial cells and brain endothelial cells by Cryptococcus neoformans
It is estimated that each year there are approximately one million cases of cryptococcal meningitis and over half of these patients die [26]. The majority of cases of cryptococcal meningitis occur in patients with impaired cell-mediated immunity, especially those with HIV/AIDS. Patients contract this disease by inhaling either spores or desiccated yeast from the environment. After an initial, often asymptomatic pulmonary infection, the organism is carried by the bloodstream to the brain, where it typically causes a lymphocytic meningitis.
Epithelial cells
The epithelial cells that line the airways and alveoli are some of the first host cells to come in contact with C. neoformans. In vitro studies with the A549 pulmonary epithelial cell line indicate that glucuronoxylomannan (GXM) in the cryptococcal capsule binds to CD14 on the surface of epithelial cells. Binding of GXM to CD14 results in increased C. neoformans adherence and enhanced epithelial cell secretion of interleukin (IL) 8 [27]. Although C. neoformans is also endocytosed by and damages A549 cells [28], it is not yet known whether CD14 or other epithelial cell receptors mediate these pathogenic interactions.
Endothelial cells
There are three potential mechanisms by which C. neoformans can traverse the blood-brain barrier and infect the brain. The first is a Trojan horse mechanism in which the organism is phagocytosed by a macrophage, which then diapedeses across the blood-brain barrier, transporting the fungus with it into the brain [29]. The second is the proteolytic degradation of intercellular tight junctions, which facilitates the passage of the organism between the endothelial cells via a paracellular route [30, 31]. The third is the binding of C. neoformans to receptors on the endothelial cell, triggering the uptake and transcytosis of the organism [32, 33]. It is probable that all three mechanisms mediate traversal of the blood-brain barrier by C. neoformans.
Because invasion of the brain is central to the pathogenesis of cryptococcal meningitis, multiple investigators have studied this process, both in vivo and in vitro. A standard approach to investigate mechanisms of brain endothelial cell invasion in vivo is to inject mice intravenously with C. neoformans and then image the blood vessels in the brains of either live or killed animals. The advantage of this approach is that, following injection of a large inoculum, it is relatively easy to detect organisms that are in the process of traversing the blood-brain barrier. This method is appropriate for studying brain invasion by the paracellular and transcellular route. However, it is less useful for investigating brain invasion by the Trojan horse mechanism because the injected organisms are not taken up by macrophages before they enter the cerebral circulation. The optimal approach to investigating the Trojan horse mechanism of endothelial cell invasion is to image C. neoformans invasion of brain as the organisms disseminates from a pulmonary infection. However, such an experiment would be technically difficult to perform because in this model, the organisms infect the brain asynchronously and in low numbers.
Two different groups used histopathologic analysis to investigate how blood-borne C. neoformans traverses the blood-brain barrier in mice [34, 35]. Both groups found that following intravenous inoculation, C. neoformans cells were visible within the brain endothelial cells of the infected mice. These results suggest that C. neoformans can invade the blood-brain barrier by either paracellular or transcellular route. More recently, Shi et al. [31] performed real-time imaging of the cerebral blood vessels of mice infected with fluorescently labeled C. neoformans cells. They found that both live and killed fungal cells formed microemboli that lodged in the cerebral capillaries. Interestingly, only live organisms were able to subsequently traverse the endothelial cell lining of capillaries and enter the brain in this model. In the real-time imaging studies, a C. neoformans mutant that did not produce urease had markedly reduced capacity to traverse brain endothelial cells. Also, mice infected with this mutant or treated with a urease inhibitor had a 1- to 2-day increase in median survival and approximately 3-fold decrease in brain fungal burden. Although it is not completely understood why C. neoformans urease is necessary for maximal traversal across the blood-brain barrier, it is possible that urease participates in the enzymatic digestion of the tight junctions between endothelial cells and thus facilitates brain invasion via the transcellular route. However the finding that either genetic or pharmacologic reduction in urease activity incompletely inhibits brain invasion suggests that C. neoformans must utilize additional mechanisms to traverse the blood-brain barrier.
The importance of the transcellular mechanism of brain invasion in vivo was demonstrated by Tseng et al, who investigated the capacity of 109 selected C. neoformans mutants to infect the brain in the mouse model of meningitis [32]. They identified a rub1 Δ mutant with increased capacity to infect the brain and an fnx1 Δ mutant with reduced capacity to infect the brain. Consistent with these in vivo results, the rub1Δ mutant had increased transcytosis across brain endothelial cells in vitro, whereas the fnx1Δ mutant had reduced transcytosis across these cells. These results indicate that C. neoformans can also invade the brain by a transcellular route.
The transcellular route of endothelial cell invasion is induced by the binding of a fungal ligand to one or more receptors on the endothelial cell. It has been found that the binding of hyaluronic acid in the C. neoformans capsule to the CD44 hyaluronic acid receptor on the surface of brain endothelial cells plays a key role in cryptococcal transcellular invasion of brain endothelial cells, both in vitro and in vivo [33, 36]. Hyaluronic acid in the C. neoformans capsule is synthesized by the hyaluronic acid synthase, Cps1 [37]. A cps1Δ mutant has reduced capsular hyaluronic acid, impaired capacity to invade brain microvascular endothelial cells in vitro, and markedly attenuated virulence in mice, thus demonstrating the important of hyaluronic acid in brain endothelial cell invasion [38]. The endothelial cell receptor for hyaluronic acid is CD44, which is located in lipid rafts/caveolae on the endothelial cell surface [39, 40]. When hyaluronic acid in the C. neoformans capsule binds to CD44, a signaling pathway containing protein kinase Cα (PKCα) and dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3) is activated [39, 41]. This signaling pathway causes rearrangement of the actin cytoskeleton, leading to the formation of pseudopods that surround the organism and pull it into the endothelial cell. The role of CD44 in the pathogenesis of cryptococcal meningitis has been investigated using CD44−/− mice [36]. Following intravenous inoculation with C. neoformans, these mice have modestly improved survival and approximately a 2-fold reduction in brain fungal burden compared to wild-type mice. One reason that CD44−/− mice are not more resistant to cryptococcal infection may be that receptor of hyaluronan-mediated motility (RHAMM), is still functional in these mice. In support of this model, siRNA knockdown of CD44 plus RHAMM inhibits C. neoformans invasion of brain microvascular endothelial cells in vitro more than knockdown of either receptor alone. These results suggest that a therapeutic approach to inhibit receptor-mediated brain invasion by C. neoformans would have to target both CD44 and RHAMM. Although CD44 and RHAMM likely activate different signaling pathways, both have been found to be associated with lipid rafts [42]. Interestingly, pretreatment of mice with simvastatin, which inhibits the function of receptors in lipid rafts, decreases brain cryptococcal fungal burden by approximately 6-fold [36]. However, whether simvastatin also prolongs survival in mice with cryptococcal meningitis or inhibits C. neoformans invasion and transcytosis of brain endothelial cells mediated by CD44 and RHAMM remains to be determined. It would also be interesting to investigate whether combined therapy with simvastatin and a urease inhibitor provides additive protection from cryptococcal meningitis.
GRP78 is an endothelial cell receptor for Rhizopus oryzae
Mucormycosis is caused by fungi of the order Murcorales, most commonly Rhizopus oryzae. Risk factors for this infection include diabetic ketoacidosis (DKA) and hematologic malignancy. Patients acquire this infection by inhaling conidia from the environment, which typically induce either an invasive sinusitis or pneumonia. Mucormycosis is characterized by angioinvasion, in which the fungus invades the blood vessels, causing thrombosis and tissue necrosis. In vitro, R. oryzae invades human umbilical vein endothelial cells by induced endocytosis, which results in significant endothelial cell damage [43].
Liu et al. [44] discovered that glucose-regulated protein 78 (GRP78) functions as an endothelial cell receptor for R. oryzae. Multiple clinical isolates of R. oryzae, along with Rhizopus microsporus and Cunnighamella spp. bind to GRP78 in extracts of endothelial cell membrane proteins. In addition, both an anti-GRP78 antibody and short hairpin RNA (shRNA) knockdown of GRP78 inhibit endothelial cell endocytosis of R. oryzae and protect endothelial cells from fungal damage. Exposure of endothelial cells to elevated glucose in vitro results in enhanced GRP78 mRNA expression, increased endocytosis of R. oryzae, and elevated endothelial cell damage. Similarly, mice with DKA have increased GRP78 mRNA expression in their sinuses, lung, and brain, as compared to normal mice. Notably, an anti-GRP78 antiserum protects mice in DKA against lethal R. oryzae pneumonia. These results demonstrate the potential therapeutic utility of blocking GRP78 in preventing mucormycosis.
Aspergillus fumigatus interacts with dectin-1 and E-cadherin on the surface of pulmonary epithelial cells
The mold Aspergillus fumigatus causes pneumonia predominantly in patients who are immunocompromised or have underlying airway disease. This disease is initiated by inhalation of A fumigatus conidia, which are deposited along the airways and in the alveoli. After this organism comes in contact with pulmonary epithelial cells, three different types of interactions occur, epithelial cell invasion, damage, and stimulation of cytokine and defensin production. The response of nasal, tracheobronchial, and alveolar epithelial cells to both intact A. fumigatus cells and culture filtrates has recently been reviewed in detail [45]. To summarize, this organism is internalized by respiratory epithelial cells and causes damage to at least some of types of these cells [46]. In addition, A. fumigatus culture filtrates and intact cells stimulate respiratory epithelial cells to produce proinflammatory cytokines, chemokines, and defensins, including tumor necrosis factor (TNF), IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and human β defensin (HBD) 2, and HBD9. Thus, rather than acting as passive bystanders, respiratory epithelial cells play an active role in orchestrating the host inflammatory response to A. fumigatus.
In vitro studies demonstrate that the C-type lectin receptor, dectin-1 is required for both maximal uptake of A. fumigatus by epithelial cells and stimulation of proinflammatory response by these host cells. Swollen conidia of A fumigatus express β glucans on their surface, which is recognized by dectin-1 on both leukocytes and epithelial cells [47, 48]. When swollen conidia come in contact with the A549 pulmonary epithelial cell line, they bind to dectin-1, which activates phospholipase D and induces the host cell to endocytose the conidia [49]. Furthermore, A. fumigatus infection of human bronchial epithelial cells activates dectin-1 signaling, which stimulates the production of TNF, IL-8, HBD2, and HBD9. Dectin-1 signaling in these epithelial cells is dependent on TLR2 [50]. Collectively, these data suggest that while therapeutic inhibition of dectin-1 might be beneficial because it would reduce the uptake of A. fumigatus by respiratory epithelial cells, it could also be detrimental because it would decrease the production of pro-inflammatory mediators by these cells.
Determining the role of dectin-1 in the host defense against invasive pulmonary aspergillosis is complicated because dectin-1 is a key receptor for A. fumigatus on both epithelial cells and leukocytes. However, Cunha et al. [51] were able to differentiate the effects of dectin-1 deficiency on myeloid versus non-myeloid cells in the susceptibility of both mice and humans to invasive aspergillosis. They transplanted hematopoietic stem cells from dectin-1−/− mice into wild-type mice, to generate animals with a myeloid-specific defect in dectin-1 expression. These mice were actually less susceptible to invasive aspergillosis than were control mice that had received hematopoietic stem cells from wild-type mice. In contrast, when dectin-1−/− mice received hematopoietic stem cells from wild-type mice, these mice were more susceptible to invasive aspergillosis than the control mice. These results suggest that dectin-1 expression in non-myeloid cells, probably pulmonary epithelial cells, is important for the host defense against invasive aspergillosis in mice.
These investigators used a similar approach to investigate the myeloid-specific effects of dectin-1 deficiency on the susceptibility of patients who underwent hematopoietic stem cell transplantation. Some humans have a naturally occurring Y238X polymorphism in the DECTIN1 gene that results in a premature stop codon. Individuals who are homozygous for this polymorphism have no detectable dectin-1 on the surface of their cells, and individuals who are heterozygous for this polymorphism have an intermediate level of dectin-1 cell surface expression [52]. Cunha et al. [51] reported the effects of the Y238X DECTIN1 polymorphism in the incidence of invasive aspergillosis in hematopoietic stem cell transplant patients. They found that there was a significant increase in invasive aspergillosis when the Y238X polymorphism was present in both the donor and recipient. However, when this polymorphism was present only in the donor or only in the recipient, there was a non-significant trend towards increased aspergillosis. These results suggest that, in humans, partial dectin-1 deficiency in non-myeloid cells does not lead to significantly increased risk of aspergillosis. On the other hand, partial deficiency of dectin-1 does not appear to be protective against this infection. Therefore, blocking the interactions of A. fumigatus with dectin-1 on respiratory epithelial cells is unlikely to be an effective strategy to prevent invasive aspergillosis.
Recently, E-cadherin has been found to function as a second receptor for A. fumigatus conidia on A549 pulmonary epithelial cells. However, maximal inhibition of E-cadherin using a combination of siRNA and a neutralizing antibody only reduced the uptake of A. fumigatus cells by 30% [53]. Whether the simultaneous inhibition of both E-cadherin and dectin-1 result in additive inhibition of endocytosis remains to be determined.
Pneumocystis stimulation of pulmonary epithelial cells requires CDw17
Pneumocystis jirovecii is one of the most common causes of pneumonia in patients with impaired cell-mediated immunity, such as transplant patients and those with HIV/AIDS. This organism adheres tightly to the pulmonary epithelial cells, but does not appear to invade them [54]. However, binding to these cells induces the production of inflammatory chemokines, including CCL2 (monocyte chemoattractant protein 1) and CXCL2 (macrophage inflammatory protein 2) [55, 56]. The importance of this response in the pathogenesis of Pneumocystis pneumonia is supported by the finding that treatment with corticosteroids to suppress the host inflammatory response prevents respiratory deterioration and markedly improves survival in AIDS patients with this disease [57, 58]. Binding of either intact Pneumocystis cells or Pneumocystis cell wall β-glucans to alveolar epithelial cells activates the JNK, MAPK, NF-κB and PKC-a signaling pathways, leading to chemokine secretion [56, 59–61]. The IL-1 receptor is required for induction of chemokine secretion, although it is not known whether Pneumocystis binds to this receptor directly, or activates it via a paracrine mechanism by inducing the alveolar epithelial cells to secrete IL-1 [60]. Interestingly, although the MyD88 adapter protein is also necessary for maximal chemokine secretion, TLR-2 and TLR-4 are not. Furthermore, Pneumocystis cell wall β-glucans bind to CDw17, which stimulates both NF-κB and PKC, and results in chemokine secretion [61, 62]. These results suggest the possibility that a therapeutic strategy to inhibit the binding of Pneumocystis to CDw17 might be useful to prevent or treat infections caused by this organism.
Concluding remarks and future directions
In the past, anti-infective strategies have focused almost exclusively on drugs that inhibit the growth of the pathogen or vaccines that enhance the host immune response. While these approaches are still highly useful, recent experimental animal studies of oropharyngeal candidiasis and mucormycosis demonstrate the therapeutic efficacy of blocking the host cell receptors to which fungi bind. Implementing such an approach requires a thorough understanding of the pathogenesis of the fungal infection. For example, while blocking pulmonary epithelial cell receptors to inhibit cytokine production would likely be beneficial for preventing or treating Pneumocystis pneumonia, such an approach would probably be deleterious in a patient with invasive pulmonary aspergillosis. Another concern is that some receptors, such as dectin-1 and CDw17 are expressed by both normally non-phagocytic cells and phagocytes. Thus, blocking these receptors might impair phagocyte-mediated clearance of the pathogen, which could possibly outweigh the benefits of inhibiting host cell invasion. For this reason it is important to focus therapeutic strategies on receptors that are either expressed predominantly on normally non-phagocytic cells or that are functionally redundant on phagocytes.
Because some fungi bind to more than one host cell receptor, it may be necessary to develop strategies to block multiple receptors. However, an alternative approach would be to block the downstream signaling pathway(s) that is active when a fungus binds to its host cell receptors. To date, therapeutic inhibition of signaling pathways has been used for the treatment of cancer and autoimmune diseases [63, 64]. The remarkably low level of toxicity of these agents suggests that they could also be used for the treatment of infectious diseases. Indeed, mouse studies with the ABL tyrosine kinase inhibitor, imatinib, demonstrate that this compound inhibits the entry of Mycobacterium tuberculosis into macrophages and reduces intracellular survival. Importantly, imatinib acts synergistically with standard anti-tuberculosis drugs and could be a valuable adjunctive therapy for tuberculosis [65]. In the future, this type of approach should be developed for the treatment of fungal infections.
Highlights.
Many fungi bind to receptors on host cells and thereby induce their own uptake.
Inhibiting Candida albicans interaction with EGFR-HER2 reduces oropharyngeal infection.
Blocking binding of Rhizopus oryzae to grp78 protects against lethal mucormycosis.
Targeting host cell receptors for fungi is a promising therapeutic strategy.
Acknowledgements
This work was funded by National Institutes of Health grants R01AI054928, R01AI073829, and R01DE017088.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer statement
S.F. is a co-founder and shareholder of NovaDigm Therapeutics.
References
- 1.Alkhatib G, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 3.Gulick RM, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG, et al. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 2004;38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, et al. Inflammatory response and clinical course of adult patients with nosocomial bloodstream infections caused by Candida spp. Clin Microbiol Infect. 2006;12:170–177. doi: 10.1111/j.1469-0691.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- 6.Grubb SE, et al. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect. Immun. 2008;76:4370–4377. doi: 10.1128/IAI.00332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotrosen D, et al. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J. Infect. Dis. 1985;152:1264–1274. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- 8.Montes LF, Wilborn WH. Ultrastructural features of host-parasite relationship in oral candidiasis. J. Bacteriol. 1968;96:1349–1356. doi: 10.1128/jb.96.4.1349-1356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichart PA, et al. Pseudomembranous oral candidiasis in HIV infection: ultrastructural findings. J. Oral Pathol. Med. 1995;24:276–281. doi: 10.1111/j.1600-0714.1995.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 10.Park H, et al. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell. Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 11.Zakikhany K, et al. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell. Microbiol. 2007;9:2938–2954. doi: 10.1111/j.1462-5822.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 12.Filler SG, et al. Penetration and damage of endothelial cells by Candida albicans . Infect. Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phan QT, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JN, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W, et al. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. USA. 2012;109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Ruiz E, et al. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell. Microbiol. 2009;11:1179–1189. doi: 10.1111/j.1462-5822.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan QT, et al. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J. Biol. Chem. 2005;280:10455–10461. doi: 10.1074/jbc.M412592200. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J. Cell Biol. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarom N, et al. Phase I clinical trial of Exherin (ADH-1) in patients with advanced solid tumors. Curr Clin Pharmacol. 2013;8:81–88. [PubMed] [Google Scholar]
- 20.Liu Y, et al. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 2011;7:e1002305. doi: 10.1371/journal.ppat.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariyachet C, et al. SR-like RNA-binding protein Slr1 affects Candida albicans filamentation and virulence. Infect. Immun. 2013;81:1267–1276. doi: 10.1128/IAI.00864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller M, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004ȓ2008. Diagn Microbiol Infect Dis. 2012;74:323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland AA, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008ȓ2011. Clin. Infect. Dis. 2012;55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Dongari-Bagtzoglou A. Epithelial GM-CSF induction by Candida glabrata . J. DentRes. 2009;88:746–751. doi: 10.1177/0022034509341266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwabuchi K, et al. Membrane microdomains in immunity: glycosphingolipid-enriched domain-mediated innate immune responses. Biofactors. 2012;38:275–283. doi: 10.1002/biof.1017. [DOI] [PubMed] [Google Scholar]
- 26.Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa FM, et al. Binding of glucuronoxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin Vaccine Immunol. 2007;14:94–98. doi: 10.1128/CVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbosa FM, et al. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006;8:493–502. doi: 10.1016/j.micinf.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Charlier C, et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans . Infect. Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stie J, Fox D. Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiology. 2012;158:240–258. doi: 10.1099/mic.0.051524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi M, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Investig. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng HK, et al. Identification of genes from the fungal pathogen Cryptococcus neoformans related to transmigration into the central nervous system. PLoS One. 2012;7:e45083. doi: 10.1371/journal.pone.0045083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jong A, et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 2008;10:1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 34.Chang YC, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chretien F, et al. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 2002;186:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- 36.Jong A, et al. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J. Biol. Chem. 2012;287:15298–15306. doi: 10.1074/jbc.M112.353375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jong A, et al. Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryot. Cell. 2007;6:1486–1496. doi: 10.1128/EC.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YC, et al. CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans . Infect. Immun. 2006;74:3930–3938. doi: 10.1128/IAI.00089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang SH, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3) J. Biol. Chem. 2011;286:34761–34769. doi: 10.1074/jbc.M111.219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long M, et al. Lipid raft/caveolae signaling is required for Cryptococcus neoformans invasion into human brain microvascular endothelial cells. J Biomed Sci. 2012;19:19. doi: 10.1186/1423-0127-19-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jong A, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-alpha activation. Cell. Microbiol. 2008;10:1854–1865. doi: 10.1111/j.1462-5822.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilarski LM, et al. Potential role for hyaluronan and the hyaluronan receptor RHAMM in mobilization and trafficking of hematopoietic progenitor cells. Blood. 1999;93:2918–2927. [PubMed] [Google Scholar]
- 43.Ibrahim AS, et al. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect. Immun. 2005;73:778–783. doi: 10.1128/IAI.73.2.778-783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu M, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010;120:1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osherov N. Interaction of the pathogenic mold Aspergillus fumigatus with lung epithelial cells. Front Microbiol. 2012;3:26. doi: 10.3389/fmicb.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ejzykowicz DE, et al. Role of Aspergillus fumigatus DvrA in host cell interactions and virulence. Eukaryot. Cell. 2010;9:1432–1440. doi: 10.1128/EC.00055-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steele C, et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus . PLoS Pathog. 2005;1:9. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohl TM, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han X, et al. beta-1,3-Glucan-induced host phospholipase D activation is involved in Aspergillus fumigatus internalization into type II human pneumocyte A549 cells. PLoS ONE. 2011;6:8. doi: 10.1371/journal.pone.0021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun WK, et al. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:2755–2764. doi: 10.1007/s10096-012-1624-8. [DOI] [PubMed] [Google Scholar]
- 51.Cunha C, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 52.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu XY, et al. E-cadherin mediates adhesion and endocytosis of Aspergillus fumigatus blastospores in human epithelial cells. Chin Med J. 2012;125:617–621. [PubMed] [Google Scholar]
- 54.Long EG, et al. Attachment of Pneumocystis carinii to rat pneumocytes. Lab. Invest. 1986;54:609–615. [PubMed] [Google Scholar]
- 55.Hahn PY, et al. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 2003;278:2043–2050. doi: 10.1074/jbc.M209715200. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, et al. Pneumocystis stimulates MCP-1 production by alveolar epithelial cells through a JNK-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2007;292:16. doi: 10.1152/ajplung.00452.2006. [DOI] [PubMed] [Google Scholar]
- 57.Bozzette SA, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N. Engl. J. Med. 1990;323:1451–1457. doi: 10.1056/NEJM199011223232104. [DOI] [PubMed] [Google Scholar]
- 58.Gagnon S, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N. Engl. J. Med. 1990;323:1444–1450. doi: 10.1056/NEJM199011223232103. [DOI] [PubMed] [Google Scholar]
- 59.Carmona EM, et al. Pneumocystis cell wall beta-glucan stimulates calcium-dependent signaling of IL-8 secretion by human airway epithelial cells. Respir. Res. 2010;11:1465–9921. doi: 10.1186/1465-9921-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bello-Irizarry SN, et al. The alveolar epithelial cell chemokine response to Pneumocystis requires adaptor molecule MyD88 and interleukin-1 receptor but not toll-like receptor 2 or 4. Infect. Immun. 2012;80:3912–3920. doi: 10.1128/IAI.00708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans SE, et al. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol. 2005;32:490–497. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans SE, et al. Primary alveolar epithelial cell surface membrane microdomain function is required for Pneumocystis beta-glucan-induced inflammatory responses. Innate Immun. 2012;18:709–716. doi: 10.1177/1753425912436763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flaherty KT, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kremer JM, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–981. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 65.Napier RJ, et al. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10:475–485. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]