Abstract
The development of an effective immunotherapy is an attractive strategy towards cancer treatment. Tumor associated carbohydrate antigens (TACAs) are overexpressed on a variety of cancer cell surfaces, which present tempting targets for anti-cancer vaccine development. However, such carbohydrates are often poorly immunogenic. To overcome this challenge, we show here that the display of a very weak TACA, the monomeric Tn antigen, on bacteriophage Qβ virus-like particles elicits powerful humoral responses to the carbohydrate. The effects of adjuvants, antigen display pattern and vaccine dose on the strength and subclasses of antibody responses were established. The local density of antigen rather than the total amount of antigen administered was found to be crucial for induction of high Tn-specific IgG titers. The ability to display antigens in an organized and high density manner is a key advantage of virus like particles such as Qβ as vaccine carriers. Glycan microarray analysis showed that the antibodies generated were highly selective towards Tn antigens. Furthermore, Qβ elicited much higher levels of IgG antibodies than other types of virus like particles and the IgG antibodies produced reacted strongly with the native Tn antigens on human leukemia cells. Thus, Qβ presents a highly attractive platform for the development of carbohydrate based anti-cancer vaccines.
INTRODUCTION
Vaccine strategies aiming to stimulate the immune system to eradicate tumor cells are highly attractive for tumor prevention and therapy.1 One such approach is to target tumor associated carbohydrate antigens (TACAs), which are overexpressed on the surfaces of many types of malignant cells. Some cancer patients can naturally develop antibodies against TACAs and high levels of anti-TACA antibodies have been correlated with improved prognosis and survival.2 However, the induction of highly potent immune responses against TACAs is challenging.
The major difficulty in generating effective anti-TACA immunity is that TACAs are typically T cell independent B cell antigens.3 TACAs can directly interact with B cells leading to the secretion of low titers of IgM antibodies, which generally last only a short time. To elicit strong and long lasting immunity, B cell stimulation by T helper cells is required, leading to a switch of antibody isotype from IgM to IgG and the induction of immunological memory.4 In this way, when transformed cells bearing TACAs emerge in immunized hosts, the immune system can recognize the disease related carbohydrate epitopes, quickly reactivate and remove the malignant cells.
Innovative studies have been carried out to develop TACA based anti-cancer vaccines,5–7 which include glyco-engineering to improve immunogenicity,7–10 synthesis of novel adjuvants,11 enhancement of immune responses through pre-existing antibodies,12–13 as well as the development of novel carriers.14–20 We focus on the last of these approaches, since carriers can provide the necessary epitopes to promote T helper cell activation in a general way, applicable to a variety of antigens.
Immunogenic proteins such as keyhole limpet hemocyanin (KLH) and tetanus toxoid (TT) are popular choices as carriers, having been conjugated with TACAs to induce strong IgM and IgG responses in various preclinical and clinical evaluations.15, 21 These carrier proteins contain multiple T helper cell epitopes, which are important in the potentiation of antibody responses.21 This mechanistic viewpoint has inspired several elegant reductionist approaches as well, featuring synthetic multi-component vaccines bearing well defined T helper cell epitopes, TACA antigens, and adjuvants with promising results.22–23 In addition, a variety of non-protein carriers have been investigated to improve the quality of anti-TACA immune responses, including dendrimers,14, 20 polysaccharides,16 gold nanoparticles,17–18 and very small proteoliposomes.19
We have become interested in exploring virus like particles (VLPs) as TACA delivery platforms.24–26 VLPs, which are composed of subunit proteins that self-assemble in a highly ordered manner, emerge as a promising direction in vaccine development.27 Non-infectious to humans or animal hosts, VLPs are safe and highly immunogenic because of several properties,28 including their sizes (<100 nm diameter, promoting both uptake by antigen presenting cells and trafficking to lymph nodes29), repetitive structure promoting B cell recognition,30 ability to crosslink B cell receptors,31 and presentation of immunostimulatory motifs such as RNA ligands of Toll-like receptors.32
One potential limitation of using immunogenic carrier proteins is the possible diminution of the desired immune response due to pre-existing anti-carrier immunity established by prior exposure to the carrier. Such carrier induced epitopic suppression has been reported for both KLH and TT.33–34 To overcome this challenge, structurally distinct vaccine carriers are needed. This prompted us to investigate the utility of a VLP, i.e., bacteriophage Qβ as a new TACA carrier. This particle is composed of 180 copies of a 14.3-kD subunit, arranged in an icosahedral structure approximately 28 nm in diameter,35 and is very stable toward chemical manipulation. Therapeutic vaccine candidates based on Qβ have demonstrated preclinical or clinical efficacy in a variety of diseases. Herein we report Qβ as a powerful antigen delivery platform for anti-cancer vaccine development by boosting the immune response to a TACA, the Tn antigen.
The Tn antigen (GalNAc-α-O-Ser/Thr), is overexpressed on the surface of a variety of cancer cells including breast, colon, and prostate cancer,36–37 and is involved in aggressive growth and lymphatic metastasis of cancers.38 The expression level of Tn was found to correlate strongly with overall survival, nodal status and tumor size in breast cancer patients.36 However, the development of anti-Tn vaccines has been quite challenging. Presumably due to its small size, the monomeric Tn antigen did not generate strong responses in immunized mice, even after conjugation to an immunogenic carrier.20, 39–40 To overcome this, a common approach is to cluster multiple consecutive Tn together rendering it more prominent to the immunological system.20, 40 This can be synthetically challenging and time-consuming. Rather than constructing Tn clusters, we attached multiple copies of the monomeric glycan to the icosahedral Qβ scaffold, a one-step operation that can conveniently provide high-density organized displays of the desired epitope. As a weak TACA, Tn serves as a useful model and the results obtained here can provide critical insights for the rational design of effective TACA based anti-cancer vaccines.
RESULTS AND DISCUSSION
Syntheses of Qβ–Tn conjugates and control particles
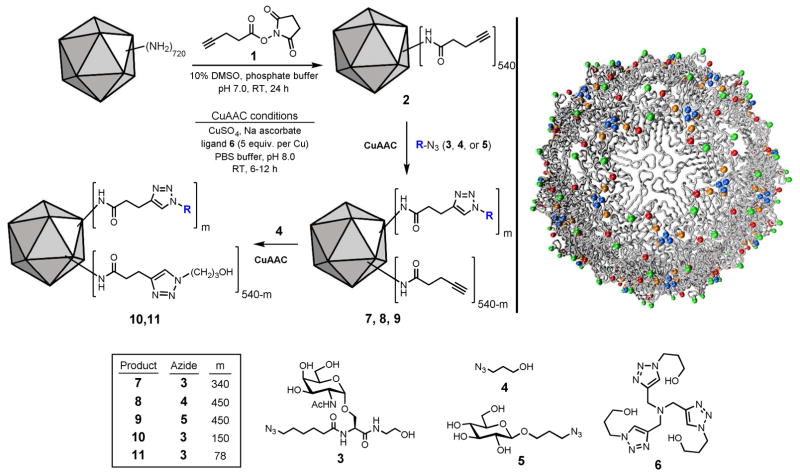
Tn ligation onto the Qβ VLP was performed with the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction, optimized for bioconjugative operations.41 This process allows high densities of decoration to be achieved on the desired biomolecular scaffold with a minimum of high-value reactants.42 Qβ capsid was first treated with a large excess of the pent-4-ynoic acid N-hydroxysuccinimide ester 1, to place alkyne groups at 540 amino groups (lysine side chains and subunit N-termini) accessible on the outer surface of the capsid (Figure 1). Following removal of the excess reagent, azide modified Tn 3 was added to the alkyne Qβ 2 in the presence of the copper catalyst using ligand 6 (THPTA).41 Depending on the reaction time and reagent concentrations, the number of Tn units attached could be varied. A “high loading” of 340 copies of Tn per particle was produced using only 5 equiv. of azide 3 per subunit (1.5–2 equiv. per alkyne), and the excess Tn could be recovered from the reaction mixture. The remaining unreacted alkyne groups on Qβ virion were capped in a second CuAAC reaction using a large excess of 3-azido 1-propanol 4 producing Qβ-Tn 7. Control particles bearing n-propanol (Qβ-propanol 8) and glucose (Qβ-glucose 9) were prepared similarly, each displaying an average of 450 ± 50 attached units per capsid (Figure 1). These controls were designed to probe the effects of capsid and the carbohydrate structural specificity of immune responses.
Figure 1.
Syntheses of Qβ conjugates 7–11. Upper right: structure of the Qβ capsid, with external amino groups marked in color: N-termini = orange, K2 = blue, K13 = red, K16 = green.
The reactive amino groups on the Qβ exterior are generally well dispersed over the protein surface (Figure 1). While K16 (green residue in figure 1) is most exposed, there are no great differences in accessibility to solution-phase species among the amine groups, except when extensive substitution is desired at the K2 residues clustered tightly around the 3-fold symmetry axis (blue residue). Thus, acylation and subsequent triazole formation should occur evenly at the exterior amine positions.43 (Interior-surface amine groups are blocked by the bacterial and plasmid RNA that is sequestered inside the particle.44) When the overall number of attached Tn moieties per particle is increased, they are expected to be arrayed in larger numbers of repetitive clustered patterns over the icosahedral capsid.
Establishment of enzyme-linked immunosorbent assay (ELISA) protocol
With Qβ-Tn and the control particles in hand, we examined the humoral responses to these particles in the presence of three different adjuvants – complete Freund’s adjuvant (CFA), alum, or TiterMax Gold. Three groups of five C57BL/6 mice were vaccinated three times at two-week intervals with Qbeta-Tn 7, Qbeta-propanol 8, or Qbeta-glucose 9 in the presence of the respective adjuvant. An additional group of mice was inoculated using Qbeta-Tn 7 without any exogenous adjuvant (PBS group). One week after the final boost, serum samples were taken and analyzed for anti-Tn antibody responses.
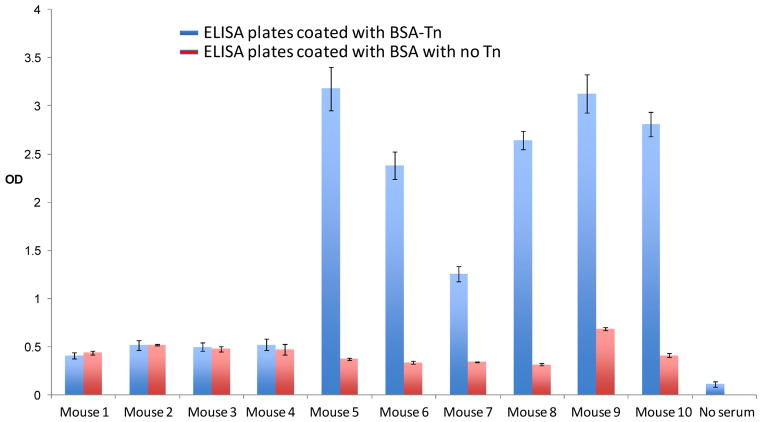
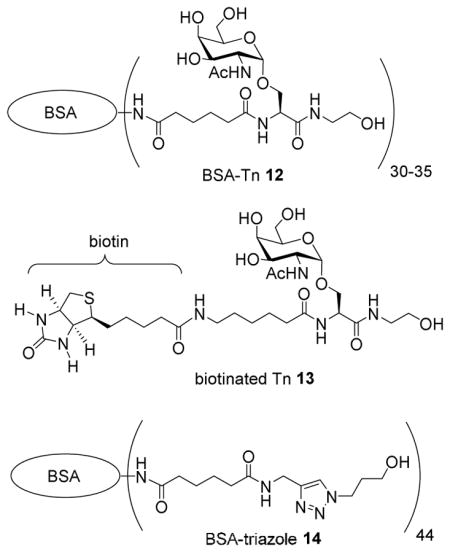
For ELISA analysis of the resulting sera, a bovine serum albumin (BSA) amide-linked conjugate of Tn was prepared with 30–35 copies of Tn units attached per protein (BSA-Tn 12). The amide connection between Tn and BSA in 12 was used to focus the assay on the Tn moiety rather than to the triazole present in the linker of the administered antigens. Sera from mice receiving Qβ-Tn 7 bound substantially better to BSA-Tn 12 than sera from mice receiving controls 8 or 9, and very little binding was observed to unmodified BSA, indicating that anti- Tn responses were obtained using this protocol (Figure 2). In contrast, no selective binding was observed to a biotinated Tn antigen (13) immobilized onto neutravidin-coated plates, which is a commonly used ELISA protocol (Figure S1).24–25, 45 These divergent ELISA results suggest that polyvalent antibody binding is required for detection since neutravidin-based display (four biotin-binding sites per neutravidin) is likely to be of substantially lower density than the BSA-Tn conjugate resulting in weak interactions and low avidities with anti-Tn antibodies.46
Figure 2.
ELISA analysis of the binding of sera from mice inoculated with antigens 7–9 against immobilized BSA-Tn conjugate 12 and BSA alone. Antigens: mice 1,2 = Qβ-glucose 9; mice 3,4 = Qβ-propanol 8; mice 5,6 = Qβ-Tn 7 (1 μg Tn); mice 7,8 = Qβ-Tn 7 (4 μg Tn); mice 9,10 = Qβ-Tn 7 (20 μg Tn). Each serum was added to four separate wells on the ELISA plate. The error bars were the standard deviations of OD values of wells incubated with a specific serum.
Effects of immuno-adjuvants and the nature of the particle on anti-Tn antibody responses
Although some intragroup variation was observed, Qβ-Tn immunized mice receiving the CFA adjuvant produced significantly more IgG antibodies than those receiving TiterMax Gold or alum (Figure 3a). Interestingly, mice immunized with Qβ-Tn in PBS (without external adjuvant) also responded. There were no statistically significant differences in IgG levels between the PBS groups and CFA groups, although the CFA groups gave higher mean values. In contrast to the IgG antibodies, the IgM responses from all the groups were similar (Figure 3b). Based on these results, CFA was selected as the adjuvant for further studies.
Figure 3.
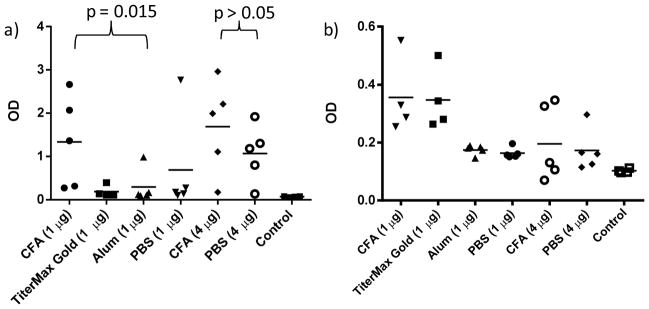
(A) IgG and (B) IgM responses against Tn antigen in immunized mice with or without exogenous adjuvants. The data shown here are measured at a serum dilution of 1:6400. Each data point represents the optical density at 450 nm measured from serum of individual mice. Lines represent the mean value for each group. Statistical analysis was performed using student t test.
For CFA immunization, CFA was only applied in the primary injection. In the two boost injections, incomplete Freund’s adjuvant (IFA), which is just mineral oil without mycobacteria components, was used to avoid over-stimulation of the immune system and toxicity of CFA. When IFA was applied as the adjuvant in both prime and boost injections with Qβ-Tn 7, a much poorer IgG response than the CFA protocol was obtained (Figure S2), suggesting the bacterial components in CFA were important in eliciting high IgG responses. The success of Qβ-Tn 7 in PBS without exogenous adjuvant to generate IgG responses, is consistent with the expectation that Qβ is of has favorable characteristics of size, structure, and costimulatory abilities.
Besides Qβ, other VLPs such as cowpea mosaic virus capsid (CPMV) and tobacco mosaic virus capsid (TMV) could be used as Tn carriers as well.24–25 Tn was linked onto CPMV and TMV with on average 120 copies of Tn on CPMV (Supporting Info, Figures S5 and S6) and greater than 2000 copies of Tn installed on TMV.24 The abilities of CPMV-Tn and TMV-Tn to potentiate anti-Tn IgG responses were compared with that of Qβ-Tn 7 under the identical immunization protocol (CFA adjuvant, 4 μg Tn). As shown in Figure S3, the IgG titers induced in mice following Qβ-Tn immunization were significantly higher compared to the levels induced by either CPMV-Tn or TMV-Tn. The underlying mechanisms behind the superior immunogenicity of Qβ remain to be fully elucidated, which is the subject of further investigation.
The effect of dose on anti-Tn immune response and antibody subtype
The dose of immunogen can significantly impact the immune responses. To establish the optimal dose, mice were immunized with Qβ-Tn 7 in amounts to provide 1, 4 and 20 μg Tn per injection following the aforementioned protocol. Determination of antibody titers by ELISA against BSA-Tn 12 showed high anti-Tn IgG titers for all Qβ-Tn immunized groups (Figure 4a). The highest average titer (263,000) was obtained from the 4 μg group although the differences between groups did not reach statistical significance. The mean IgG titers of the 4 μg group were two orders of magnitude higher than those from the control groups (Qβ-propanol and Qβ-glucose, p < 0.001). Substantial anti-Tn IgM antibodies were elicited with highest average titer (13,400) from the 20 μg group (Figure 4b). As discussed in the introduction, it has been challenging to elicit high titers of IgG antibodies using monomeric form of Tn as the antigen.39–40 The high titers obtained with Qβ-Tn highlights the advantage of Qβ as a TACA carrier.
Figure 4.
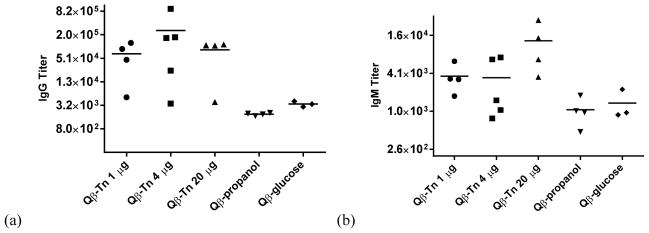
(a) IgG and (b) IgM titers against Tn in mice immunized with Qβ-Tn 7, Qβ-propanol 8 and Qβ-glucose 9. The titer numbers were calculated as the highest folds of dilution that gave three times the absorbance of normal mouse sera at 1:1600 dilution.
As discussed above, a prerequisite for potent IgG responses is the stimulation of B cells by T helper cells.4 The high IgG titers obtained following Qβ-Tn immunization indicate that Qβ contains the necessary T helper epitopes for T cell potentiation and Tn functionalization of Qβ does not significantly disrupt the functions of these epitopes. We suggest that the covalent linkage between Tn and Qβ ensures that Tn is processed and presented together with Qβ by the same B cells, thus presumably allowing potent B cell stimulation by matched activated T helper cells. The subclasses of IgG were also analyzed (Figure S4). Substantial amounts of all IgG subclasses were generated with Qβ-Tn 7, indicating the induction of a balanced IgG immune response involving a broad range of cytokines,47 as well as T helper cell independent IgG3response.48
Anti-Tn responses do not correlate with antibody levels towards competing epitopes
For each group of Qβ-Tn immunized mice, variations were observed in anti-Tn IgG titers. Immune responses to the capsid or the triazole linker may be competitive, suppressing anti-glycan immunity in some cases. To test this possibility, we analyzed the levels of antibodies against unmodified Qβ particle and the triazole-containing linker, the latter displayed on BSA as conjugate 14.
All sera, including those from mice immunized with control particles 8 and 9, showed responses to both capsid and the triazole containing linker with no correlations to the levels of anti-Tn antibodies (Figure 5 and Figure S5). This suggests that the low magnitudes of the anti-Tn IgG responses observed for some Qβ-Tn 7 immunized mice were not due to immune impairment. The anti-Qβ virion and anti-triazole antibody titers did not significantly vary with the dose or structures of Qβ constructs administered (Figure S5). The compatibility of the triazole linker with TACA based vaccine constructs is consistent with several prior reports.42, 49–51 It is known that rigid structures such as triazole can induce antibodies against themselves, which may consume the Th cells and reduce anti-glycan responses.52 Thus, it is possible that a flexible linker between Tn and Qβ can reduce the amount of anti-linker antibodies thus further improving the anti-Tn immunity, which will be explored in the future.
Figure 5.
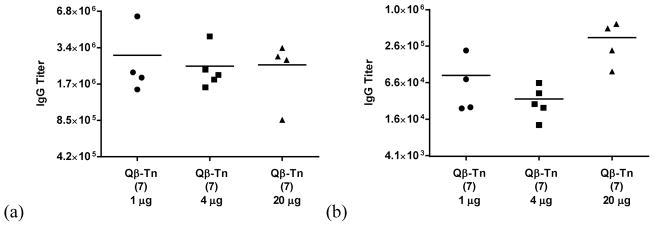
IgG titer against (a) Qβ virion and (b) BSA-triazole conjugate 14. Sera are from mice immunized with 1 μg, 4 μg, and 20 μg of Qβ-Tn 7 respectively. The titer numbers were calculated as the highest folds of dilution that gave three times the absorbance of normal mouse sera at 1:1600 dilution.
High local antigen density is critical for high antibody responses
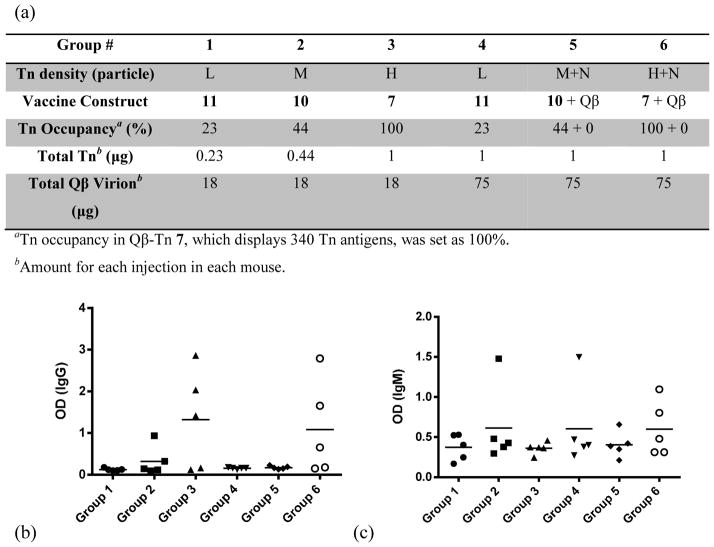
To better understand the origin of immune potentiating effect of Qβ, two additional Qβ-Tn constructs (Qβ-Tn 10 and 11, Figure 6a) were prepared with lower Tn densities by decreasing the amount of Tn-azide 3 used in the CuAAC conjugation reaction. Relative to particle 7 (high loading, designated “H”, 340 Tn per particle), particle 10 bears a medium Tn density (150 copies per particle, “M”), and 11 is lightly loaded (78 Tn molecules per particle, “L”). As each Qβ capsid contains 180 subunits, in the high density Qβ-Tn 7, there were on average close to 2 Tn per capsid with each pair of the carbohydrates expected within 5 nm of each other. Lower Tn loading on Qβ-Tn 10 and 11 provides more dispersed presentations of Tn.
Figure 6.
(a) The amounts of Tn and Qβ in six groups of mice for deciphering the importance of antigen display patterns. (b) IgG and (c) IgM responses induced by Qβ conjugated with variable Tn antigen densities (serum dilution 1:6400).
Two sets of immunization experiments were performed. First, three groups of mice (groups 1–3) were immunized with Qβ-Tn 11, 10 and 7 respectively, with the amounts of Qβ capsid kept constant within all three groups (Figure 6a). Due to the differential Tn loading on the capsid, these groups received increasing amounts Tn. ELISA analysis showed that group 3 mice generated the highest IgG antibody responses (Figure 6b). This suggested that although equal amounts of T helper epitopes were presented by the capsid, higher levels of Tn (~ 1 μg) were needed to generate strong IgG titers. Interestingly, the Tn specific IgM responses, indicative of early B cell activation, were comparable from all groups (Figure 6c). This is consistent with results by Bachmann and co-workers,53 who reported that the density of peptide antigens on Qβ did not affect IgM titers but had great influence on the T helper cell dependent IgG responses. In another study, Schiller and co-workers demonstrated that to elicit strong antibodies against tumor necrosis factor-α using papillomavirus VLP as the carrier, a high density of antigen was crucial to break B cell tolerance against this self antigen.54
In the second experiment, the amount of Tn was kept constant, and unmodified Qβ particles (designed “N” in Figure 6a) were added when necessary to equalize the total amount of particle administered. Thus, group 4 received 75 μg of particle 11 in each injection, which delivered 1 μg of attached Tn. Groups 5 and 6 received 33 μg and 17 μg of particles 10 and 7, respectively, to carry the same total amount of Tn, along with the unmodified particle to bring the total virion to 75 μg. In group 4, Tn antigen was randomly distributed on all Qβ capsids. In contrast, Tn was present only on 23% of the capsid in group 6, but with much higher local organization. If antigen display patterns were not important, groups 4, 5, and 6 should give similar anti-Tn responses. However, ELISA analysis demonstrated that only group 6 generated significant IgG responses (Figure 6b). As before, IgM response was independent of these variations (Figure 6c).
These results suggest that the organized epitope display is crucial for induction of antibody isotype switching from B cells, but not for early B cell activation. This is consistent with the fact that the spacing between many neighboring Tn molecules on Qβ-Tn 7 is approximately around 5 nm, which is suitable for strong BCR crosslinking, leading to potent B cell activation and isotype switching producing IgG antibodies.3 BCR crosslinking rather than simple binding has been proposed as a requisite signal to induce IgG production.31 Highly organized antigen geometry cannot be achieved with the traditional amorphous carriers such as KLH and tetanus toxoid (TT) highlighting a key advantage of Qβ as antigen carriers.
Groups 3 and 6 mice received the same amount of Tn antigen displayed in the same organized manner, while group 6 had four times the amount of Qβ capsids. The IgG levels generated from these two groups were comparable (Figure 6b). In addition, comparison of groups 1 and 2 with 4 and 5 demonstrated that increasing the dose of Qβ and thereby the amount of T helper epitopes available did not compensate for the inefficient IgG response resulting from poorly organized antigen display. These results together suggest excess Qβ did not significantly suppress55 or improve humoral responses under the conditions evaluated.
Glyco-microarray results confirmed the Tn selectivity in antibody recognition
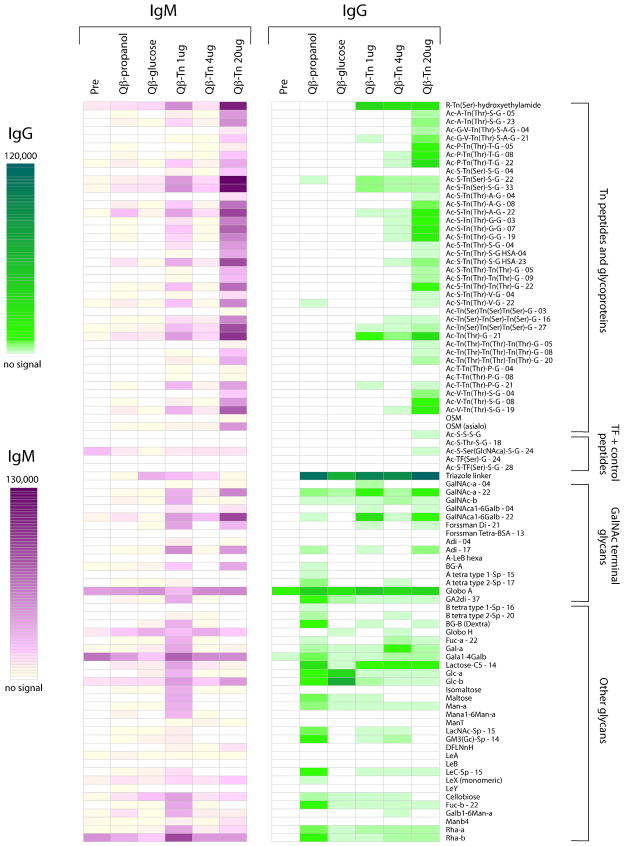
Our ELISA results demonstrated that the post-immune mouse sera contained antibodies capable of recognizing the Tn antigen. To establish the binding specificities of the antibodies, the sera from mice immunized with Qβ-Tn 7 and control particles 8 and 9 were screened against a carbohydrate microarray.56 This glycan array contained 329 members, which were prepared by attaching a variety of O-linked glycopeptides, N-linked glycopeptides, glycolipids, and glycoproteins to BSA, followed by printing the BSA conjugates on the slides (A full list of the library components is given in Table S1 in Supporting Information). After incubation with each mouse serum sample and washing, a fluorescently labeled secondary antibody was used to quantify the relative amounts of serum antibody bound to individual array components. For clarity, the average intensities from each group of mice were presented (Figure 7). Consistent with the ELISA results, sera from all mice immunized with Qβ-Tn 7 reacted well with the immunizing antigen (R-Tn(Ser)-hydroxyethylamide in Figure 7) compared to the control sera and the pre-vaccination sera. A variety of Tn containing glycopeptides and GalNAc terminated glycans were also recognized suggesting the GalNAc moiety was the major element in recognition. Both monomeric Tn and Tn clusters reacted with the Qβ-Tn post-immune sera. The microarray also contained spots with BSA bearing different densities of terminal GalNAc: GalNAc-α-04 (4 GalNAc per BSA) vs. GalNAc-α-22 (22 GalNAc per BSA) and GalNAcα1-6Galβ-04 (4 GalNAcα1-6Gal per BSA) vs. GalNAcα1-6Galβ-22 (22 GalNAcα1-6Gal per BSA) (see GalNAc terminal glycans group in Figure 7). In both cases, significantly greater binding was observed to the higher-density display, much like the aforementioned ELISA observations using BSA-Tn 12 and Tn-biotin 13.
Figure 7.
Glyco-microarray profiles comparing pre- and post-immune sera from various groups of mice. The average fluorescence signals from at least 4 mice per group are presented (IgM data shown in magenta and IgG data shown in green). Glycans are attached to BSA prior to printing on the array surface. The numbers after the glycan abbreviation correspond to the average number of glycans per molecule of albumin [for example, Ac-A-Tn(Thr)-S-G-05 is a neoglycoprotein with an average of 5 Tn peptides per molecule of albumin]. Selected array results are shown and full results can be found in supporting information.
Although the dose of Qβ-Tn did not significantly impact the titers against the immunizing Tn antigen 3, interesting dose dependent effects were observed on antibody selectivity and specificity. Both IgG and IgM antibodies from the 20 μg group bound strongest with Tn containing array components compared to antibodies from the groups receiving lower doses (Figure 7). Furthermore, antibodies from the 20 μg group showed more specificity towards GalNAc and less cross-reactivities with other glycans. In other words, the higher glycopeptide antigen dose induced a more focused immune response toward the glycan antigen, suggesting the potential advantage of using high antigen dose for immunization.
The induced anti-Tn antibodies reacted with “native” Tn epitopes on tumor cells
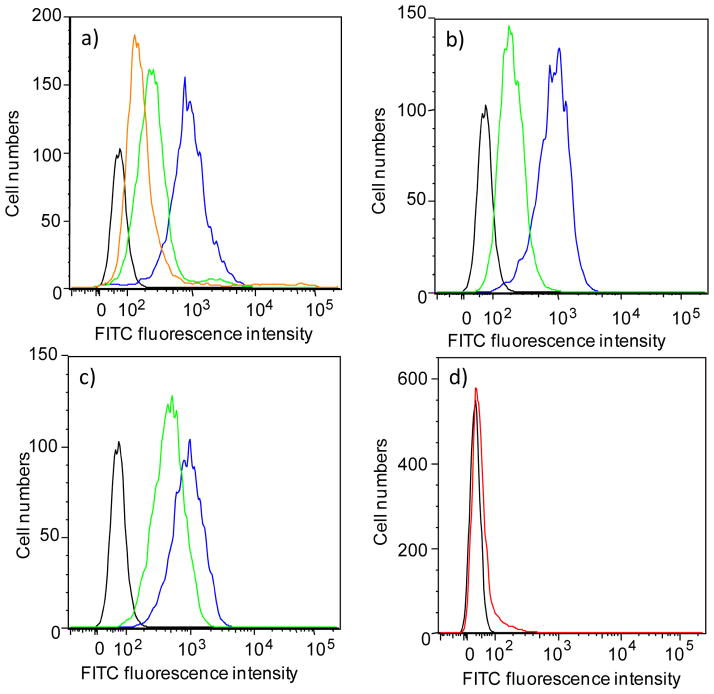
For an effective immunotherapy, it is important that anti-TACA antibodies generated by the synthetic vaccine can recognize the antigens present in the native environments such as the surfaces of cancer cells. To test this, we analyzed the binding of sera showing high IgG titers by ELISA to Jurkat cells, a human leukemia cell line expressing Tn antigens on the surface (Figure 8).57 Significant cell recognition by these serum antibodies was observed using flow cytometry, whereas serum from mice given control particle 8 or 9 showed no binding (Figure 8d). Consistent with ELISA data, the percentages of cells exhibited positive reactivities with Qβ-Tn in FACS analysis were higher than those from TMV-Tn (Figure S6).
Figure 8.
Flow cytometry analysis of Jurkat cell binding by sera (1:10 dilution) from representative mice immunized with increasing amounts of Qβ-Tn 7 (a) 1 μg Tn, (b) 4 μg Tn, (c) 20 μg Tn, and (d) control mouse immunized with 8. Black curve (negative control) represents the fluorescence obtained with FITC conjugated anti-mouse IgG antibodies in the absence of serum. Colored curves represent results for different mice in the same group.
CONCLUSION
We demonstrate here that the bacteriophage Qβ capsid is a powerful antigen delivery platform capable of boosting the humoral immune responses to a very weak TACA, the Tn antigen. The addition of external Freund’s adjuvant to Qβ-Tn conjugates produced higher IgG titers, although strong immune responses were also obtained without exogenous adjuvants. The IgG antibodies produced were highly selective towards Tn antigen binding and reacted strongly with native Tn antigens on human leukemia cells demonstrating the physiological relevance of the IgG antibodies induced by the Qβ-Tn vaccine construct. Once the density of Tn antigen administered passed a threshold level, the pattern of Tn antigen display, rather than the total amount of Tn antigen administered appeared to be crucial for the induction of high anti-Tn IgG titers. The knowledge gained from the current study will facilitate the rational design and optimization of carbohydrate based anti-cancer vaccines using virus-like particles such as Qβ as a powerful antigen delivery system.
Methods
General procedures for mouse immunization
Pathogen-free C57BL/6 female mice age 6–10 weeks were obtained from Charles River and maintained in the University Laboratory Animal Resources facility of Michigan State University. All animal care procedures and experimental protocols have been approved by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University. Groups of five C57BL/6 mice were injected subcutaneously under the scruff on day 0 with 0.1 mL various Qβ constructs as emulsions in complete Freund’s adjuvant (Fisher), TiterMax Gold adjuvant (Sigma Aldrich), Imject Alum (Thermo Scientific), or Incomplete Freund’s adjuvant (Fisher) according to the manufacturer’s instructions. Boosters were given subcutaneously under the scruff on days 14 and 28 (for complete Freund’s adjuvant group, incomplete Freund’s adjuvant was used). Serum samples were collected on days 0 (before immunization), 7 and 35. The final bleeding was done by cardiac bleed.
Procedures for ELISA
A 96-well microtiter plate was first coated with a solution of BSA-Tn, BSA-triazole, or wide type Qβ viron in PBS buffer (10 μg mL−1) and then incubated at 4 °C overnight. The plate was then washed four times with PBS/0.5% Tween-20 (PBST), followed by the addition of 1% (w/v) BSA in PBS to each well and incubation at room temperature for one hour. The plate was washed again with PBST and mice sera were added in 0.1% (w/v) BSA/PBS. The plate was incubated for two hours at 37 °C and washed. A 1:2000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, IgM, IgG1, IgG2b, IgG2c, or IgG3 respectively (Jackson ImmunoResearch Laboratory) in 0.1% BSA/PBS was added to each well. The plate was incubated for one hour at 37 °C, washed and a solution of 3,3,5′,5′-tetramethylbenzidine (TMB) was added. Color was allowed to develop for 15 min and then a solution of 0.5 M H2SO4 was added to quench the reaction. The optical density was then measured at 450 nm. The titer was determined by regression analysis with log10 dilution plotted with optical density. The titer was calculated as the highest dilution that gave three times the absorbance of normal mouse sera diluted at 1:1600 (about 0.1 for all sera).
Supplementary Material
Acknowledgments
We are grateful to the National Cancer Institute (R01CA149451-01A1) and the Marie Curie Actions (VLPsiRNA-FP7-PEOPLE-2009-IOF-254069 M. C.-A.) for financial support of our work. This work was also supported in part by the intramural research program of the NIH, NCI. We thank T. Zhang and S. Kim for helpful discussions, Z. Polonskaya and B. Laufer for assistance with Qβ conjugates, and T. Carter for assistance with the graphics.
Footnotes
Supporting Information: Information on synthesis, Qβ, CPMV conjugation and characterization. Procedures for glycan microarray and FACS analysis and full array data. Supporting figures and NMR spectra. This information is available free of charge via the Internet at http://pubs.acs.org/.
Notes: The authors declare no competing financial interest.
Contributor Information
M.G. Finn, Email: mgfinn@scripps.edu.
Xuefei Huang, Email: xuefei@chemistry.msu.edu.
References
- 1.Finn OJ. Cancer vaccines: Between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K. Cancer vaccines and carbohydrate epitopes. Vaccine. 2011;29:8802–8826. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mond JJ, Lees A, Snapper CM. T Cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 4.Goldsby RA, Kindt TJ, Osborne BA. Kuby immunology. Freeman; New York: 2000. pp. 461–464. [Google Scholar]
- 5.Yin Z, Huang X. Recent development in carbohydrate based anticancer vaccines. J Carbohydr Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CC, Ye XS. Carbohydrate-based cancer vaccines: target cancer with sugar bullets. Glycoconjugate J. 2012;29:259–271. doi: 10.1007/s10719-012-9399-9. [DOI] [PubMed] [Google Scholar]
- 7.Guo ZW, Wang QL. Recent development in carbohydrate-based cancer vaccines. Curr Opin Chem Biol. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann-Röder A, Johannes M. Synthesis of a MUC1-glycopeptide–BSA conjugate vaccine bearing the 3′-deoxy-3′-fluoro-Thomsen Friedenreich antigen. Chem Commun. 2011;47:9903–9905. doi: 10.1039/c1cc13184b. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 9.Yang F, Zheng XJ, Huo CX, Wang Y, Zhang Y, Ye XS. Enhancement of the immunogenicity of synthetic carbohydrate vaccines by chemical modifications of STn antigen. ACS Chem Biol. 2011;6:252–259. doi: 10.1021/cb100287q. [DOI] [PubMed] [Google Scholar]
- 10.Kuberan B, Sikkander SA, Tomiyama H, Linhardt RJ. Synthesis of a C-glycoside analogue of sTn: an HIV- and tumor-associated antigen. Angew Chem Int Ed. 2003;42:2073–2075. doi: 10.1002/anie.200351099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chea EK, Fernández-Tejada A, Damani P, Adams MM, Gardner JR, Livingston PO, Ragupathi G, Gin DY. Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J Am Chem Soc. 2012;134:13448–13457. doi: 10.1021/ja305121q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Gu L, Zhang W, Motari E, Cai L, Styslinger TJ, Wang PG. L-rhamnose antigen: a promising alternative to alpha-gal for cancer immunotherapies. ACS Chem Biol. 2011;6:185–191. doi: 10.1021/cb100318z. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Lombardo SA, Herner DN, Talan RS, Wall KA, Sucheck SJ. Synthesis of a single-molecule L-rhamnose-containing three-component vaccine and evaluation of antigenicity in the presence of anti-L-rhamnose antibodies. J Am Chem Soc. 2010;132:17236–17246. doi: 10.1021/ja107029z. [DOI] [PubMed] [Google Scholar]
- 14.Heegaard PMH, Boas U, Sorensen NS. Dendrimers for vaccine and immunostimulatory uses. A review Bioconjugate Chem. 2010;21:405–418. doi: 10.1021/bc900290d. [DOI] [PubMed] [Google Scholar]
- 15.Zhu JL, Warren JD, Danishefsky SJ. Synthetic carbohydrate-based anticancer vaccines: the Memorial Sloan-Kettering experience. Expert Rev Vaccines. 2009;8:1399–1413. doi: 10.1586/erv.09.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J Am Chem Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 17.Brinas RP, Sundgren A, Sahoo P, Morey S, Rittenhouse-Olson K, Wilding GE, Deng W, Barchi JJ. Design and synthesis of multifunctional gold nanoparticles bearing tumor-associated glycopeptide antigens as potential cancer vaccines. Bioconjugate Chem. 2012;23:1513–1523. doi: 10.1021/bc200606s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojeda R, de Paz JL, Barrientos AG, Martin-Lomas M, Penades S. Preparation of multifunctional glyconanoparticles as a platform for potential carbohydrate-based anticancer vaccines. Carbohydr Res. 2007;342:448–459. doi: 10.1016/j.carres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Estevez FCA, Solorzano L, Valiente O, Mesa C, Barroso O, Sierra GV, Fernandez LE. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP) Vaccine. 1999;18:190–197. doi: 10.1016/s0264-410x(99)00219-4. [DOI] [PubMed] [Google Scholar]
- 20.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser A, Gaidzik N, Westerlind U, Kowalczyk D, Hobel A, Schmitt E, Kunz H. A synthetic vaccine consisting of a tumor-associated sialyl-Tn MUC1 tandem-repeat glycopeptide and tetanus toxoid: induction of a strong and highly selective immune response. Angew Chem Int Ed. 2009;48:7551–7555. doi: 10.1002/anie.200902564. [DOI] [PubMed] [Google Scholar]
- 22.Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, Boons GJ. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci, USA. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P, BenMohamed L. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Z, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, Miermont A, Gildersleeve JC, Wang Q, Huang X. Tobacco mosaic virus as a new carrier for tumor associated carbohydrate antigens. Bioconjugate Chem. 2012;23:1694–1703. doi: 10.1021/bc300244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miermont A, Barnhill H, Strable E, Lu XW, Wall KA, Wang Q, Finn MG, Huang X. Cowpea mosaic virus capsid: A promising carrier for the development of carbohydrate based antitumor vaccines. Chem-Eur J. 2008;14:4939–4947. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, Finn MG, Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBiochem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 27.Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 29.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 30.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 32.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 33.Di John D, Wasserman SS, Torres JR, Cortesia MJ, Murillo J, Losonsky GA, Herrington DA, Sturcher D, Levine MM. Effect of priming with carrier on response to conjugate vaccine. Lancet. 1989;334:1415–1418. doi: 10.1016/s0140-6736(89)92033-3. [DOI] [PubMed] [Google Scholar]
- 34.Herzenberg LA, Tokuhisa T. Epitope-specific regulation. I Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J Exp Med. 1982;155:1730–1740. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golmohammadi R, Fridborg K, Bundule M, Valegard K, Liljas L. The crystal structure of bacteriophage Qβ at 3.5Å resolution. Structure. 1996;4:543–554. doi: 10.1016/s0969-2126(96)00060-3. [DOI] [PubMed] [Google Scholar]
- 36.Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010;12:204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Anver MR, Butcher DO, Gildersleeve JC. Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther. 2009;8:971–979. doi: 10.1158/1535-7163.MCT-08-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi T, Takazawa H, Imai S, Morimoto J, Watanabe T, Kanno M, Igarashi S. Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer cells in relation to lymphatic metastasis: is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res Treat. 2006;98:31–43. doi: 10.1007/s10549-005-9115-6. [DOI] [PubMed] [Google Scholar]
- 39.Buskas T, Ingale S, Boons GJ. Towards a fully synthetic carbohydrate-based anticancer vaccine: Synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated Tn antigen. Angew Chem Int Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 40.Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, Clausen H, Danishefsky SJ, Livingston PO. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol Immunother. 2005;54:424–430. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew Chem Int Ed Engl. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Astronomo RD, Kaltgrad E, Udit AK, Wang SK, Doores KJ, Huang CY, Pantophlet R, Paulson JC, Wong CH, Finn MG, Burton DR. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem Biol. 2010;17:357–370. doi: 10.1016/j.chembiol.2010.03.012. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasuhn DE, Jr, Singh P, Strable E, Brown S, Manchester M, Finn MG. Plasma clearance of bacteriophage Qβ particles as a function of surface charge. J Am Chem Soc. 2008;130:1328–1334. doi: 10.1021/ja075937f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau JL, Baksh MM, Fiedler JD, Brown SD, Kussrow A, Bornhop DJ, Ordoukhanian P, Finn MG. Evolution and protein packaging of small molecule RNA aptamers. ACS Nano. 2011;5:7722–7729. doi: 10.1021/nn2006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: Synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjugate Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 46.Blixt O, Lavrova OI, Mazurov DV, Cló E, Kraaun SK, Bovin NV, Filatov AV. Analysis of Tn antigenicity with a panel of new IgM and IgG1 monoclonal antibodies raised against leukemic cells. Glycobiology. 2012;22:529–542. doi: 10.1093/glycob/cwr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckman MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 48.Snapper CM, McIntyre TM, Mandler R, Pecanha LMT, Finkelman FD, Lees A, Mond JJ. Induction of IgG3 secretion by interferon γ: a model for for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992;175:1367–1371. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Zhou Z, Tang S, Guo Z. Carbohydrate-monophosphoryl lipid A conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem Biol. 2012:235–240. doi: 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bay S, Fort S, Birikaki L, Ganneau C, Samain E, Coic YM, Bonhomme F, Deriaud E, Leclerc C, Lo-Man R. Induction of a melanoma-specific antibody response by a monovalent, but not a divalent, synthetic GM2 neoglycopeptide. ChemMedChem. 2009;4:582–587. doi: 10.1002/cmdc.200900032. [DOI] [PubMed] [Google Scholar]
- 51.Lipinski T, Luu T, Kitov PI, Szpacenko A, Bundle DR. A structurally diversified linker enhances the immune response to a small carbohydrate hapten. Glycoconjugate J. 2011;28:149–164. doi: 10.1007/s10719-011-9331-8. [DOI] [PubMed] [Google Scholar]
- 52.Costantino P, Rappuoli R, Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Discov. 2011;6:1045–1066. doi: 10.1517/17460441.2011.609554. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 53.Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol. 2002;32:3305–3314. doi: 10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 54.Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated Papillomavirus virus-like particles. J Immunol. 2002;169:6120–6126. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 55.Dagan R, Eskola J, Leclerc C, Leroy O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect Immun. 1998;66:2093–2098. doi: 10.1128/iai.66.5.2093-2098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oyelaran O, Gildersleeve JC. Glycan arrays: recent advances and future challenges. Curr Opin Chem Biol. 2009;13:406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakada H, Inoue M, Tanaka N, Numata Y, Kitagawa H, Fukui S, Yamashina I. Expression of the Tn antigen on T-lymphoid cell line Jurkat. Biochem Biophys Res Commun. 1991;179:762–767. doi: 10.1016/0006-291x(91)91882-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.