Abstract
We aimed to determine the effects of vitamin B1 deficiency on vitamin contents of urine, liver, and blood. In the current study, rats were divided into 3 groups (n = 5, each group): the first was freely fed a complete diet (ad lib-fed control group); the second freely fed a vitamin B1-free diet (vitamin B1 deficient group); and the third pair-fed a complete diet with the same amounts of the vitamin B1 deficient group (pair-fed control group). The experimental period was for 15 days. The blood concentrations of vitamin B2, PLP, vitamin B12, folic acid, and biotin were lower in the pair-fed control than in the ad lib-fed control and those of nicotinamide and pantothenic acid were the same. We conclude that Vitamin B1 deficiency did not affect concentrations of the other B-group vitamins.
Keywords: vitamins, urine, vitamin B1, deficiency, content
Introduction
It is known that B-group vitamins play a conservative role in energy production and the biosyntheses of many physiologically important molecules in cells. However, information about how deficiency in one vitamin affects the concentrations of other vitamin is scant. Therefore, it is necessary to study the effect of single vitamin deficiencies on other B-group vitamin levels. We previously reported the effects of vitamin B12-deficiency on the contents of seven other B-group vitamins, in which the contents of vitamin B1, folate, and biotin in the liver, and vitamin B1 and folate in kidney were decreased, while the contents of vitamin B6 and biotin were increased, by feeding rats a vitamin B12-free diet.1 It is known that both vitamin B12 and folate deficiencies are associated with neurological disorders,2,3 so the previous report1 implies the physiological correlation between vitamin B12 and vitamin B1. In other words, the possible adverse relationship of these vitamins must be considered when neurological disorders appear.
Outbreaks of beriberi, a vitamin B1 deficiency, have been reported even in recent years.4–13 All of these reported that thiamin therapy produced complete, rapid clinical recovery in all cases. One paper stated: “the patient was administered intramuscular thiamin and this resulted in rapid and dramatic improvement of his condition within hours, including resolution of edema and cardiac symptoms.”13 These reports imply that the vitamin B1 deficiency would not affect the other B-group vitamins, including vitamin B12.4–13 We could not find papers examining the influence of vitamin B1-deficiency on the contents of other B-group vitamins. A literature search did not reveal any systematic investigation of the influence of vitamin B1 deficiency upon the status of the rest of the B-group vitamins. Therefore, we compared the contents of B-group vitamins in the urine, liver, and blood among vitamin B1 deficient, pair-fed control and ad lib-fed control rats.
Methods
Chemicals
Vitamin-free milk casein, sucrose, and l-methionine were purchased from Wako Pure Chemical Industries (Osaka, Japan). Corn oil was purchased from Ajinomoto (Tokyo, Japan). Gelatinized cornstarch, a mineral mixture (AIN-93G mineral mixture),14 and a vitamin mixture (nicotinic acid-free AIN-93 vitamin mixture containing 25% choline bitartrate)14 were obtained from Oriental Yeast Co., Ltd. (Tokyo, Japan).
Thiamin hydrochloride (C12H17ClN4OS-HCl; MWt = 337.27), riboflavin (C17H20N4O6; MWt = 376.37), pyridoxine hydrochloride (C8H11NO3-HCl; MWt = 205.63), pyridoxal 5′-phosphate (PLP) monohydtrate (C8H10NO6P-H2O = 265.16), cyanocobalamin (C63H88CoN14O14P; MWt = 1355.40), nicotinamide (C6H6N2O; MWt = 122.13), calcium pantothenate (C18H32N2O10-Ca; MWt = 476.54), folic acid (pteroylmonoglutamic acid, C19H19N7O6; MWt = 441.40) and d(+)-biotin (C10H16N2O3S; MWt = 244.31) were purchased from Wako Pure Chemical Industries. 4-Pyridoxic acid (4-PIC, C8H9NO4; MWt = 183.16) was made by ICN Pharmaceuticals (Costa Mesa, CA, USA) and obtained through Wako Pure Chemical Industries.
N1-Methylnicotinamide (MNA) chloride (C7H9N2O-HCl; MWt = 159.61) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). N1-Methyl-2-pyridone-5-carboxamide (2-Py, C7H8N2O2; MWt = 152.15) and N1-methyl-4-pyridone-3-carboxamide (4-Py, C7H8N2O2; MWt = 152.15) were synthesized by the methods of Pullman and Colowick15 and Shibata et al,16 respectively. All other chemicals used were of the highest purity available from commercial sources.
Animals and treatment
The care and treatment of the experimental animals conformed to the University of Shiga Prefecture guidelines for the ethical treatment of laboratory animals. The room temperature was maintained at around 22 °C and about 60% humidity with a 12/12 light/dark cycle (06:00–18:00/18:00–06:00).
Male 3-week-old Wistar rats obtained from CLEA Japan (Tokyo) were divided into three groups (n = 5 per group) and each rat was housed in a metabolic cage CT-10 (CLEA Japan). Group 1 was freely fed a conventional chemically-defined complete diet (Table 1) and used as an ad lib-fed control. Group 2 was freely fed the complete diet without vitamin B1 (vitamin B1-free diet) (Table 1) and used as a vitamin B1-deficient group. Group 3 was fed the complete diet (Table 1), but the daily food amount was equal to the amount of the vitamin B1-deficient group and used as a pair-fed control group. The 24-h urine samples were collected at 09:00–09:00 of the last day in amber bottles containing 1 mL of 1 mol/L HCl and were stored at −25 °C until needed. The rats were sacrificed at around 09:00; blood was collected and tissues were taken to measure the weights and the contents of B-group vitamins in the urine, liver, and blood. The removed livers were preserved at −25 °C until needed.
Table 1.
Compositions of the diets.
| Complete diet (control diet) (%) | Vitamin B1-free diet (%) | |
|---|---|---|
| Vitamin-free milk casein | 20.0 | 20.0 |
| L-methionine | 0.2 | 0.2 |
| Sucrose | 70.3 | 70.3 |
| Corn oil | 5.0 | 5.0 |
| Mineral mixture (AIN-93-G-MX) | 3.5 | 3.5 |
| Vitamin mixture* (AIN-93-VX) | 1.0 | 0 |
| Vitamin mixture (vitamin B1-free)* (AIN-93-VX) | 0 | 1.0 |
Note:
Reeves, R. G.: Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1998;127:838S–41S.
Measurement of B-group vitamins in urine, liver and blood
Preparation and measurement of the extracts of B-group vitamins from urine and blood were as previously described.17,18 The concentrations of B-group vitamins in liver were measured as follows.
Vitamin B1: Frozen liver samples, ≈0.5 g, were thawed, minced, and then added to 10 volumes of cold 5% trichloroacetic acid and homogenized with a Waring blender. The acidified homogenate was centrifuged at 10,000 × g for 10 minutes (min) at 4 °C and the supernatant was retained and used for measurement of vitamin B1.19
Vitamin B2: Frozen liver samples, ≈0.5 g, were thawed, minced, and then added to 10 volumes of 50 mmol/L KH2PO4-K2HPO4 buffer (pH 7.0) and homogenized with a Waring blender. To 0.1 mL of the homogenate, 0.44 mL of water and 0.26 mL of 0.5 mol/L H2SO4 was added and then kept at 80 °C for 15 min. After being cooled, 0.2 mL of 10% trichloroacetic acid was added and centrifuged at 10,000 × g for 3 min at 4 °C. From the supernatant obtained, 0.2 mL was withdrawn and added to 0.2 mL of 1 mol/L NaOH. The alkalized mixture was irradiated at a height of 20 cm from the liquid with 2 fluorescent lamps (20 W) for 30 min at room temperature20 and then 0.02 mL of glacial acetic acid was added to the mixture. The neutralized mixture was passed through a 0.45 μm microfilter and the filtrate was injected directly into the HPLC system for measuring lumiflavin.21
Vitamin B6: Frozen liver samples, ≈0.5 g, were thawed, minced, and then added to 90 mL of 55 mmol/L HCl and homogenized with a Waring blender. The homogenate was autoclaved at 121 °C for 3 hours (h) to convert vitamin B6 coenzyme to free-form of vitamin B6. After being cooled, the mixture was adjusted to pH 5.0 with 1 mol/L NaOH and then made up to 100 mL with water. The solution was filtered with qualitative filter No. 2 (ADVANTEC, Inc.). The filtrate was used for measuring vitamin B6 as previously described.22
Vitamin B12: Frozen liver samples, ≈0.5 g, were thawed, minced, and then added to 2.5 mL of 0.57 mol/L acetic acid-sodium acetate buffer (pH 4.5) plus 5 mL of water and 0.1 mL of 0.05% KCN. The suspension was homogenized with a Waring blender. The homogenate was then put into a boiling water bath for 5 min. After being cooled, 0.15 mL of 10% metaphosphoric acid was added and made up to 10 mL with water. The solution was filtered with qualitative filter No. 2 (ADVANTEC, Inc.). The filtrate was used for measuring vitamin B12 as previously described.23
Nicotinamide: Frozen liver samples, ≈0.6 g, were thawed, minced, and then added to 5 volumes of 0.1 g/mL isonicotinamide. The suspension was homogenized with a Waring blender. The homogenate (1 mL) was withdrawn and added to 4 mL of water, then autoclaved at 121 °C for 10 min to convert pyridine nucleotide coenzymes to nicotinamide. After being cooled, the mixture was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was retained and the precipitated materials were extracted again with 5 mL of water and the supernatant was retained. Both retained supernatants were combined and the extract was used for measuring nicotinamide as described.16
Pantothenic acid: Frozen liver samples, ≈0.2 g, were thawed, minced, and then added to 10 volumes of 50 mmol/L KH2PO4-K2HPO4 buffer (pH 7.0). The suspension was homogenized with a Teflon/glass homogenizer. The homogenate was incubated at 37 °C overnight to convert free pantothenic acid from the bound type of pantothenate compounds by using endogenous pantetheinase in the homogenate. The reaction was stopped by putting the sample into a boiling water bath for 5 min. After being cooled, the mixture was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was retained and the precipitated materials were extracted again with 2 mL of water and the supernatant was retained. Both retained supernatants were combined, and the extract was used for measuring pantothenic acid as previously described.24
Folate: Frozen liver samples, ≈0.5 g, were thawed, minced, and then added to 10 volumes of 0.1 mol/L KH2PO4-K2HPO4 buffer, pH 6.1. The suspension was homogenized with a Waring blender. The homogenate was autoclaved at 121 °C for 5 min. After being cooled, 2.5 mL of proteinase MS (200 U/mL of water; Kaken Pharmaceutical Co., Ltd., Tokyo, Japan) was added and then incubated at 37 °C for 3 h to digest proteins and then release polyglutamated folates from the protein-bound types. The reaction was stopped by putting the sample into a boiling water bath for 10 min. After being cooled, 0.5 mL of conjugase (extract from porcine kidney acetone powder, Type II, Sigma-Aldrich, St Louis, MO, USA) was added and the mixture incubated at 37 °C overnight to convert polyglutamated folates to monoglutamated folates. The reaction was stopped by putting the sample into a boiling water bath for 10 min. After being cooled, the mixture was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was retained, and the precipitated materials were extracted again with 3 mL of water and the supernatant was retained. Both retained supernatants were combined, and the extract was used for measuring folate as previously described.25 The conjugase solution was made as follows: 60 mL of 50 mmol/L KH2PO4-K2HPO4 buffer, pH 7.0 was added to 20 g of porcine kidney acetone powder and stirred for 30 min at 4 °C. The suspension was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was dialyzed against a large amount of 50 mmol/L KH2PO4-K2HPO4 buffer (pH 7.0) to remove endogenous folate of the kidney acetone powder. The dialyzed conjugase solution was used.
Biotin: Frozen liver samples, ≈0.5 g, were thawed, minced, and then added to 2 volumes of 2.25 mol/L H2SO4 and then homogenized with a Waring blender. The suspension was autoclaved at 121 °C for 1 h to convert the bound form biotin to the free form of biotin. After being cooled, the suspension was centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatant was used for measuring biotin as previously described.26
B-group vitamin analyses
The measurements of B-group vitamins except for vitamin B6 were measured as previously described.18 The urinary excretion of 4-pyridoxic acid, a catabolite of vitamin B6, was measured according to Gregory and Kirk.27
Statistical analysis
Statistical significance was determined by ANOVA followed by Bonferroni’s multiple comparison tests; P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA).
Results
Body weight, food intake, and water intake
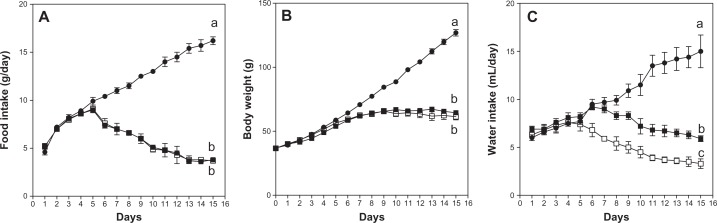
Rats were divided into 3 groups. Group 1 was ad libitumfed a conventional chemically defined purified diet (complete diet) (Table 1) and used as an ad lib-fed control. Group 2 was ad libitumfed the purified diet without vitamin B1 (vitamin B1-free diet) (Table 1) and used as a vitamin B1-deficient group. Group 3 was fed the complete diet (Table 1), but the daily food amount was equal to the amount of the vitamin B1-deficient group and used as a pair-fed control group. Group 1, ad lib-fed control was used as a gold standard. The body weight, food intake and water intake were reasonably higher in the ad lib-fed control than in the vitamin B1-free diet group and the pair-fed group (P < 0.05). The body weight gains, food intakes, and water intakes during the experiment are shown in Figure 1. The body weight gain was observed at beginning of the experiment even in the rats fed the vitamin B1-free diet as in the rats of ad-lib and pair-fed control groups. However, the weight gain stopped from day 6 (Fig. 1B) with concomitantly decreased food intake (Fig. 1A) and water intake (Fig. 1C).
Figure 1.
Comparison of the food intake (A), body weight gain (B), and water intake (C) among the rats that were fed the control (ad lib-fed), control (pair-fed), and vitamin B1-free diet., •, control (ad lib-fed); ▪, control (pair-fed); □ vitamin B1-free.
Notes: Values are mean ± SEM for 5 rats. Values at the last day of the experiment that do not share the same superscripted letters are significantly different by one-way ANOVA with Bonferroni’s multiple comparison tests (P < 0.05).
Tissue weights
Table 2 shows the basic parameters such as tissue weights among the 3 groups. The basic parameters in the ad lib-fed control rats were reasonably higher than those in the pair-fed control and vitamin B1-free diet group. These parameters were almost the same between the pair-fed control and vitamin B1-free diet group.
Table 2.
Comparison of various tissue weights among the rats that were fed the complete (ad lib-fed), complete (pair-fed), and vitamin B1-free diets.
|
Complete diet (control diet)
|
Vitamin B1-free diet | ||
|---|---|---|---|
| ad lib-fed | pair-fed | ||
| Initial body weight (g) | 36.9 ± 0.6 | 36.8 ± 0.7 | 36.7 ± 0.9 |
| Final body weight (g) | 126.9 ± 2.5a | 64.5 ± 0.5b | 61.4 ± 2.5b |
| Body weight gain (g/15 days) | 90.0 ± 1.9a | 27.7 ± 0.6b | 24.7 ± 1.9b |
| Food intake during the urine collection (g/days) | 16.2 ± 0.4a | 3.7 ± 0.2b | 3.6 ± 0.1b |
| Liver (g) | 6.43 ± 0.13a | 2.09 ± 0.05b | 2.31 ± 0.11b |
| Kidney (g) | 1.22 ± 0.02a | 0.69 ± 0.01b | 0.73 ± 0.02b |
| Heart (g) | 0.62 ± 0.02a | 0.29 ± 0.01b | 0.30 ± 0.01b |
| Spleen (g) | 0.63 ± 0.02a | 0.15 ± 0.00b | 0.16 ± 0.01b |
| Testis (g) | 1.11 ± 0.02a | 0.87 ± 0.02b | 0.72 ± 0.07b |
| Brain (g) | 1.22 ± 0.02 | 1.13 ± 0.00 | 1.11 ± 0.02 |
| Lung (g) | 0.86 ± 0.02a | 0.59 ± 0.03b | 0.56 ± 0.03b |
Notes: Values are means ± SEM for five rats. Values within the same row that do not share the same superscripted letters are significantly different by one-way ANOVA with Bonferroni’s multiple comparison tests (P < 0.05).
Urinary excretion of B-group vitamins
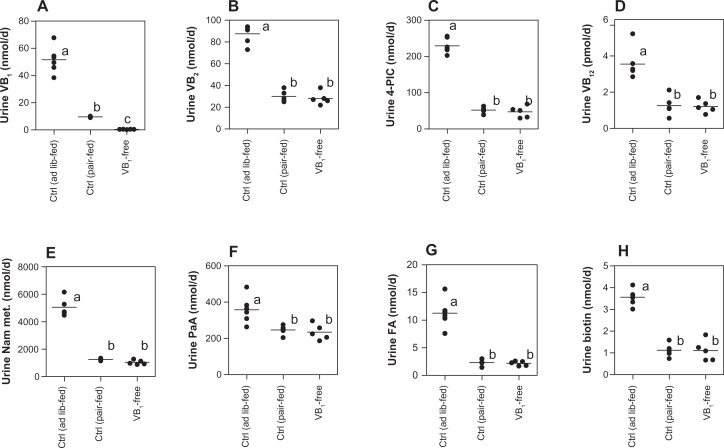
Urinary excretion of water-soluble vitamin generally means surplus over necessity of vitamin need of body. The urinary excretions of each of the B-group vitamins in the ad lib-fed control rats were reasonably higher than those in the pair-fed control and vitamin B1-free diet group. The urinary amount of each vitamin was almost the same between the pair-fed control and vitamin B1-free diet group, except for vitamin B1, as shown in Figure 2.
Figure 2.
Comparison of the B-group vitamins of urine among the rats that were fed the control (ad lib-fed), control (pair-fed), and vitamin B1-free diets. (A) Vitamin B1; (B) vitamin B2; (C) vitamin B6; (D) vitamin B12; (E) nicotinamide (Nam); (F) pantothenic acid (PaA); (G) folate (FA); (H) biotin.
Notes: Each dot represents a value for each rat. Horizontal line is the average value of five rats of the same group. Values within the same figure that do not share the same superscripted letters are significantly different by one-way ANOVA with Bonferroni’s multiple comparison tests (P < 0.05).
Liver concentrations of B-group vitamins
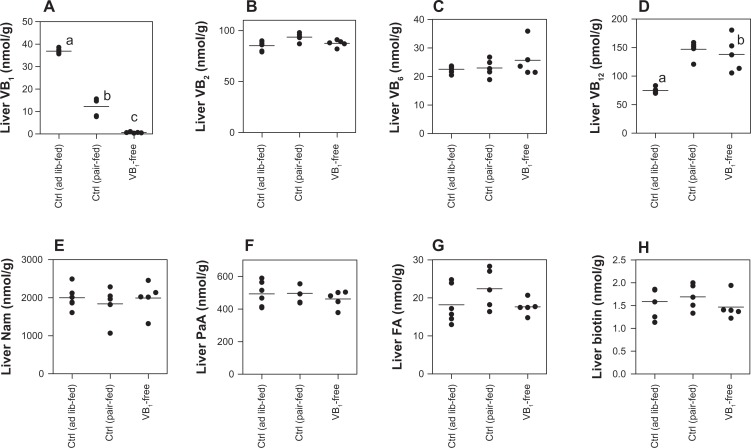
Liver concentration of water-soluble vitamin generally means the level of body stores of vitamin. The liver concentration of each B-group vitamin in the ad lib-fed control rats was not higher than that in the pair-fed control and vitamin B1-free diet group; therefore, the concentration of each B-group vitamin was almost the same among the three groups except for vitamins B1 and B12 as shown in Figure 3. The scale of the liver concentration of vitamin B1 was ad-lib control rats > pair-fed control rats > vitamin B1 deficient rats. The liver concentration of vitamin B12 was lower in the ad-lib control rats than in the pair-fed and vitamin B1 deficient rats.
Figure 3.
Comparison of the B-group vitamins of liver among the rats that were fed the control (ad lib-fed), control (pair-fed), and vitamin B1-free diets. (A) Vitamin B1; (B) vitamin B2; (C) vitamin B6; (D) vitamin B12; (E) nicotinamide (Nam); (F) pantothenic acid (PaA); (G) folate (FA); (H) biotin.
Notes: Each dot represents a value for each rat. Horizontal line is the average value of five rats of the same group. Values within the same figure that do not share the same superscripted letters are significantly different by one-way ANOVA with Bonferroni’s multiple comparison tests (P < 0.05).
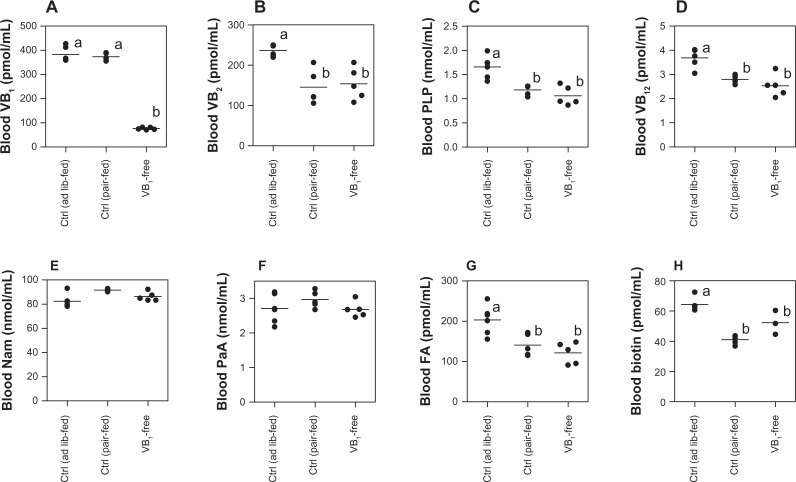
Blood concentrations of B-group vitamins
Blood concentration of water-soluble vitamin generally means the amount of available vitamin for the body’s use. The blood concentration of each B-group vitamin in the ad lib-fed control rats was higher (over vitamin B1, vitamin B2, PLP, vitamin B12, folate, and biotin) and/or almost the same (over nicotinamide and pantothenic acid) compared to the vitamin B1-free diet group as shown in Figure 4. The blood concentrations of all of the water-soluble vitamins except for vitamin B1 were almost the same between the vitamin B1-free diet group and the pair-fed control. The blood concentration of each vitamin in the rats which were belonging with the ad lib-fed control and pair-fed control were not the same even when they were administered the same diet. The concentrations of vitamin B2, PLP, vitamin B12, folate, and biotin were lower in the pair-fed control than in the ad lib-fed control. These vitamin concentrations in blood might be affected by food restriction.
Figure 4.
Comparison of the B-group vitamins of blood among the rats that were fed the control (ad lib-fed), control (pair-fed), and vitamin B1-free diets. (A) Vitamin B1; (B) vitamin B2; (C) vitamin B6; (D) vitamin B12; (E) nicotinamide (Nam); (F) pantothenic acid (PaA); (G) folate (FA); (H) biotin.
Notes: Each dot represents a value for each rat. Horizontal line is the average value of five rats of the same group. Values within the same figure that do not share the same superscripted letters are significantly different by one-way ANOVA with Bonferroni’s multiple comparison tests (P < 0.05).
Discussion
Each of the 8 kinds of B-group vitamins take part in many metabolic pathways. For example, NAD+, TDP, FAD, and CoA are involved in the respiratory chain reaction (from glucose to CO2 H2O), and PLP, and CoA, NAD+, TDP, FAD, biotin, and adenosylcobala-min are involved in the catabolism of the branched-chain amino acids. In summary, B-group vitamins play a major role in cells. As stated previously, we could not find papers that described the vitamin-vitamin interactions of B-group vitamins. We reported previously the effects of vitamin B12-deficiency on the contents of 7 other B-group vitamins, in which the concentrations of vitamin B1 in liver and kidney were lower in the vitamin B12-deficient group than in the pair-fed control group. As the next experiment, we investigated whether vitamin B1 deficiency affects the contents of other B-group vitamins in the urine, liver, and blood. Generally, the urinary excretion of water-soluble vitamins indicates a surplus amount of body store,28–31 the liver content indicates the amount of body storage, and the blood concentration indicates the amount of available circulation of the vitamins. TDP, the coenzyme form of vitamin B1, catalyzes two general types of reactions: (1) the formation of ketones as catalyzed by transketolase and (2) the oxidative decarboxylation of 2-oxoacids catalyzed by dehydrogenase complexes. The reaction of the dehydrogenase complexes—for example, the pyruvate dehydrogenase complex—involves a large multi-enzyme structure that contains 3 enzyme activities: pyruvate dehydrogenase, also known as pyruvate decarboxylase, dihydrolipoyl transacetylase, and dihydrolipoyl dehydrogenase. These enzyme complexes require several vitamins such as vitamin B1, vitamin B2, niacin, and pantothenic acid. This implies that vitamin B1 deficiency affects the contents of other B-group vitamins. The vitamin B1 deficiency beriberi occurs even in recent years in poor countries that consume rice as their staple food.4–13 Vitamin B1 deficiency manifests with a severe decrease in appetite, decrease in growth, and peripheral neurologic symptoms of paresthesias. In the present rat experiment, similar phenomena were observed. At day 15 of the experiment, the nutritional status of the rats fed the vitamin B1-free diet had severely decreased. Thus, we decided to end the experiment. Although 1 group of the 3 groups was administered the vitamin B1-free diet, the food intake and body weight gains among the 3 groups were almost the same during the first 5 days. The food intake in the vitamin B1-free diet group decreased at day 6 of the experiment with concomitant reduction in body weight compared to the ad-lib control group. These phenomenon means that the weaning rats (3-weeks old) used in the present experiment each had a 5-day store of vitamin B1. In our previous reports,33 the 5-week-old rats each had a 10-day store of vitamin B1. The difference in number of days until the vitamin B1-deficiency appeared is probably due to the difference in bodily vitamin B1 stores. It is evidence that infants can easily to fall into nutritional insufficiency and deficiency compared with young people and adults.
We reported that vitamin B12 deficiency exerts a remarkable influence on the storage and circulation of other B-group vitamins.1 Surprisingly, the vitamin B1 deficiency had little effect on the other B-group vitamin concentrations in the liver and blood in comparison with the pair-fed control. In summary, vitamin B1 deficiency causes a severe decrease in appetite, water intake, and growth, but concentrations of other B-group vitamins in the blood and liver, and the urinary excretory amounts are not affected by a vitamin B1-free diet in comparison with the pair-fed control rats. These results could be evidence over the fact that the administration of thiamin resulted in rapid and dramatic improvement of beriberi patient conditions within hours, including resolution of edema and cardiac symptoms.13
Although it was not a major objective in the present study, some characteristic phenomena were observed between the ad lib-fed and pair-fed controls. The rats belonging in these two groups were administered the exactly same diet, but the food intake of the rats in the pair-fed group were restricted and pair-fed with the rats in the vitamin B1-free diet. Therefore, this effect might be due to severe food or energy restriction, but not due to vitamin B1 deficiency. The blood concentrations of vitamin B2, PLP, vitamin B12, folic acid, and biotin were lower in the pair-fed control than in the ad lib-fed control, while those of nicotinamide and pantothenic acid were the same. The different phenomena among the vitamins would be attributable to the existence of the specific metabolism of each vitamin when animals are restricted in terms of energy intake; eg, the rats can coin a necessary amount of nicotinamide from an amino acid tryptophan under normal conditions,34 and the conversion of nicotinamide from tryptophan increases severe food restriction.35
It is reasonable that the vitamin B1 concentration in the livers of rats fed the vitamin B1-free diet was lower than in the ad-lib and pair-fed control rats. An interesting finding was obtained: a differences of vitamin B1 concentration in liver was observed between the groups of ad lib-fed control and pair-fed control, although the rats in the two groups were administered the exactly same diet. The lower content of vitamin B1 in liver and the same concentrations in blood between ad lib-fed and pair-fed controls suggests higher turnover, and higher elimination to extra-hepatic tissues from a storage tissue liver compared to other B-group vitamins. This might be one of the reasons why deficiency in B1 is the most rapid compared to other B-group vitamins.
Another interesting phenomenon is that the liver concentration of vitamin B12 was higher in vitamin B1-free diet group than in the ad lib-fed control. The same phenomenon was observed between the pair-fed control and ad lib-fed control groups. Accordingly, the higher concentration of vitamin B12 in liver is not caused by a vitamin B1-decifiency, but it might be affected by food restriction, or energy restriction. On the contrary, the blood concentration of vitamin B12 was lower in the pair-fed control and vitamin B1-free diet group than in the ad-lib control. The energy restriction might suppress the transport of vitamin B12 from liver to blood. As described above, the vitamin B1 concentrations in liver and kidney and the urinary excretion of vitamin B1 were lower in vitamin B12- deficient rats than in the pair-fed control rats.1 It is probable that intestinal absorption of vitamin B1 is impaired by vitamin B12-deficiency. On the contrary, vitamin B1-deficiency did not cause to impair intestinal absorption of vitamin B12 because the concentrations of vitamin B12 in liver and blood, and the urinary excretion of vitamin B12 were not different between the vitamin B1-free diet and pair-fed control groups. The blood concentrations of vitamin B2, PLP, folate, and biotin were lower in the pair-fed control and vitamin B1-free diet groups than in the ad-lib control group, although the liver concentrations of these vitamins were almost the same among the 3 groups; this indicates that energy restriction suppresses the delivery of B-group vitamins from liver to blood. The exceptions were nicotinamide and pantothenic acid, for which concentrations in the liver were much more higher compared to the vitamin B2, PLP, vitamin B12, folate, and biotin. This might be a reason why the blood concentrations of nicotinamide and pantothenic acid were not reduced by energy restriction.
In conclusion, vitamin B1 deficiency did not affect concentrations of the other B-group vitamins under the same energy intake. Severe food restriction caused impairment in the absorption, distribution, and elimination of B-group vitamins.
Footnotes
Funding
This investigation was part of the project “Studies on the Dietary Reference Intakes for Japanese” (principal investigator, Sinkan Tokudome), which was supported by a Research Grant for Comprehensive Research on Cardiovascular and Life-Style Related Diseases from the Ministry of Health, Labour and Welfare of Japan.
Author Contributions
Designed the study: KS, TF. Drafted the manuscript: KS. Performed the experiment: AS. All authors reviewed and approved the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
References
- 1.Shibata K, Kawata T, Ishida K, et al. The contents of B-group vitamins in various tissues, serum, and urine of vitamin B12-deficient rats. Vitamins (Japan) 2011;85:18–22. [Google Scholar]
- 2.Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;1996;54:382–90. doi: 10.1111/j.1753-4887.1996.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 3.Fava M, Borus JS, Alpert JE, Nirtrnberg AA, Rosenbaum JF, Bottinglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–8. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 4.Tang CM, Rolfe M, Wells JC, Cham K. Outbreak of beri-beri in The Gambia. Lancet. 1989;22:206–7. doi: 10.1016/s0140-6736(89)90383-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen KT, Chioi ST, Chang YC, Pan WH, Twu SJ. Cardiac beriberi among illegal mainland Chinese immigrants. J Int Med Res. 2001;29:37–40. doi: 10.1177/147323000102900106. [DOI] [PubMed] [Google Scholar]
- 6.de Montmollin D, MacPhail J, McMahon J, Coninx R. Outbreak of beri-beri in a prison in West Africa. Tro Doct. 2002;32:234–6. doi: 10.1177/004947550203200419. [DOI] [PubMed] [Google Scholar]
- 7.Chen KT, Twu SJ, Chiou ST, Pan WH, Chang HJ, Serdula MK. Outbreak of beriberi among illegal mainland Chinese immigrants at a detention center in Taiwan. Pub Health Rep. 2003;118:59–64. doi: 10.1016/S0033-3549(04)50217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattal-Valevski A, Kesler A, Sela BA, et al. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics. 2005;115:e233–8. doi: 10.1542/peds.2004-1255. [DOI] [PubMed] [Google Scholar]
- 9.Doung-ngern P, Kesornsukhon S, Kanlayanaphotprn J, Wanadurongwan S, Songchitsomboon S. Beriberi outbreak among commercial fishermen, Thailand 2005. South Asian J Trop Med Public Health. 2007;38:130–5. [PubMed] [Google Scholar]
- 10.Ahoua L, Etienne W, Fermon F, et al. Outbreak of beriberi in a prison in Côte d’lvoire. Food Nutr Bull. 2007;28:283–90. doi: 10.1177/156482650702800304. [DOI] [PubMed] [Google Scholar]
- 11.Cerroni MP, Barrado JCS, Nobrega AA, et al. Outbreak of beriberi in an Indian population of the upper Amazon region, Roraima State, Brazil, 2008. Am J Trp Met Hyg. 2010;83:1093–7. doi: 10.4269/ajtmh.2010.10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima HC, Porto EA, Marins JR, et al. Outbreak of beriberi in the state of Maranhão, Brazil: revisiting the mycotoxin aetiologic hypothesis. Trop Doct. 2010;40:95–7. doi: 10.1258/td.2009.090439. [DOI] [PubMed] [Google Scholar]
- 13.Watson JT, El Bushra H, Lebo EJ, et al. Outbreak of beriberi among African Union troops in Mogadishu, Somalia. PLoS ONE. 2011;6:e28345. doi: 10.1371/journal.pone.0028345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–41S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 15.Pullman ME, Colowick SP. Preparation of 2- and 6-pyridones of N1- methylnicotinamide. J Biol Chem. 1954;206:121–7. [PubMed] [Google Scholar]
- 16.Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-3-pyridone-4-carboxamide, by high-performance liquid chromatography. J Chromatogr. 1988;424:23–8. doi: 10.1016/s0378-4347(00)81072-5. [DOI] [PubMed] [Google Scholar]
- 17.Fukuwatari T, Wada H, Shibata K. Age-related alterations of B-group vitamin contents in urine, blood and liver from rats. J Nutr Sci Vitaminol. 2008;54:357–62. doi: 10.3177/jnsv.54.357. [DOI] [PubMed] [Google Scholar]
- 18.Shibata K, Fukuwatari T, Ohta M, et al. Values of water-soluble vitamins in blood and urine of Japanese young men and women consuming a semi-purified diet based on the Japanese Dietary Reference Intakes. J Nutr Sci Vitaminol (Tokyo) 2005;51:319–28. doi: 10.3177/jnsv.51.319. [DOI] [PubMed] [Google Scholar]
- 19.Fukuwatari T, Suzuura C, Sasaki R, Shibata K. Action site of bisphenol A as metabolic disruptor lies in the tryptophan-nicotinamide conversion pathway. Shokuhin Eiseigaku Zasshi. 2004;45:231–8. doi: 10.3358/shokueishi.45.231. [DOI] [PubMed] [Google Scholar]
- 20.Yagi K. Sources of light used for the decomposition of vitamin B2 in alkaline medium. Vitamins (Japan) 1953;7:493–6. [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. A simple method for micro-determination of flavin in human serum and whole blood by high-performance liquid chromatography. Biochem Int. 1982;4:187–94. [Google Scholar]
- 22.Cunniff P, editor. Official Methods of Analysis of AOAC International. Arlington: AOAC International; 1995. [Google Scholar]
- 23.Watanabe F, Abe K, Katsura H, et al. Biological activity of hydroxo-vitamin B12 degradation product formed during microwave heating. J Agric Food Chem. 1998;46:5177–80. [Google Scholar]
- 24.Skeggs H, Wright LD. The use of Lactobacillus arabinosus in the microbiological determination of pantothenic acid. J Biol Chem. 1944;156:21–6. [Google Scholar]
- 25.Aiso K, Tamura T. Trienzyme treatment for food folate analysis: optimal pH and incubation time for a-amylase and protease treatment. J Nutr Sci Vitaminol (Tokyo) 1998;44:361–70. doi: 10.3177/jnsv.44.361. [DOI] [PubMed] [Google Scholar]
- 26.Fukui T, Iinuma K, Oizumi J, Izumi Y. Agar plate method using Lactobacillus plantarum for biotin determination in serum and urine. J Nutr Sci Vitaminol. 1994;40:491–8. doi: 10.3177/jnsv.40.491. [DOI] [PubMed] [Google Scholar]
- 27.Gregory JF, 3rd, Kirk JR. Determination of urinary 4-pyridoxicacidusing high performance liquid chromatography. Am J Clin Nutr. 1979;32:879–83. doi: 10.1093/ajcn/32.4.879. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Twenty-four-hour urinary water-soluble vitamin levels correlate with their intakes in free-living Japanese school children. Public Health Nutr. 2011;14:327–33. doi: 10.1017/S1368980010001904. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Twenty-four-hour urinary water-soluble vitamins correlate to vitamin intakes in free-living Japanese university students. Eur J Clin Nutr. 2010;64:800–7. doi: 10.1038/ejcn.2010.72. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Urinary excretion of vitamin B1, B2, B6, niacin, pantothenic acid, folate, and vitamin C correlates with dietary intakes of free-living elderly, female Japanese. Nutr Res. 2010;30:171–8. doi: 10.1016/j.nutres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Fukuwatari T, Shibata K. Urinary water-soluble vitamin and their metabolites contents as nutritional markers for evaluating vitamin intakes in young Japanese women. J Nutr Sci Vitaminol (Tokyo) 2008;54:223–9. doi: 10.3177/jnsv.54.223. [DOI] [PubMed] [Google Scholar]
- 32.Shibata K, Murata K. Tryptophan-NAD metabolism in thiamin-deficient rats with or without supplementation of excessive nicotinamide. Vitamins (Japan) 1985;59:555–63. [Google Scholar]
- 33.Shibata K, Kondo T, Yonezima M. Conversion ratio of tryptophan to niacin in rats fed a vitamin B1-free diet. J Nutr Sci Vitaminol (Tokyo) 1997;43:479–83. doi: 10.3177/jnsv.43.479. [DOI] [PubMed] [Google Scholar]
- 34.Shibata K, Mushiage M, Kondo T, Hayakawa T, Tsuge H. Effects of vitamin B6 deficiency on the conversion ratio of tryptophan to niacin. Biosci Biotechnol Biochem. 1995;59:2060–3. doi: 10.1271/bbb.59.2060. [DOI] [PubMed] [Google Scholar]
- 35.Shibata K, Kondo T, Miki A. Increased conversion ratio of tryptophan to niacin in severe food restriction. Biosci Biotechnol Biochem. 1998;62:580–3. doi: 10.1271/bbb.62.580. [DOI] [PubMed] [Google Scholar]