Abstract
Background
While ACL reconstruction is the treatment gold standard for ACL injury, it does not reduce the risk of post-traumatic osteoarthritis. Therefore, new treatments that minimize this postoperative complication are of interest. Bio-enhanced ACL repair, in which a bioactive scaffold is used to stimulate healing of an ACL transection, has shown considerable promise in short term studies. The long-term results of this technique and the effects of the bio-enhancement on the articular cartilage have not been previously evaluated in a large animal model.
Hypothesis
1) The structural (tensile) properties of the porcine ACL at 6 and 12 months after injury are similar when treated with bio-enhanced ACL repair, bio-enhanced ACL reconstruction, or conventional ACL reconstruction, and all treatments yield results superior to untreated ACL transection. 2) After one year, macroscopic cartilage damage following bio-enhanced ACL repair is similar to bio-enhanced ACL reconstruction and less than conventional ACL reconstruction and untreated ACL transection.
Study Design
Controlled laboratory study (porcine model)
Methods
Sixty-two Yucatan mini-pigs underwent ACL transection and randomization to four experimental groups: 1) no treatment, 2) conventional ACL reconstruction, 3) “bio-enhanced” ACL reconstruction using a bioactive scaffold, and 4) “bio-enhanced” ACL repair using a bioactive scaffold. The biomechanical properties of the ligament or graft and macroscopic assessments of the cartilage surfaces were performed after 6 and 12 months of healing.
Results
The structural properties (i.e., linear stiffness, yield and maximum loads) of the ligament following bio-enhanced ACL repair were not significantly different from bio-enhanced ACL reconstruction or conventional ACL reconstruction, but were significantly greater than untreated ACL transection after 12 months of healing. Macroscopic cartilage damage after bio-enhanced ACL repair was significantly less than untreated ACL transection and bio-enhanced ACL reconstruction, and there was a strong trend (p=.068) that it was less than conventional ACL reconstruction in the porcine model at 12 months.
Conclusions
Bio-enhanced ACL repair produces a ligament that is biomechanically similar to an ACL graft and provides chondroprotection to the joint following ACL surgery.
Clinical Relevance
Bio-enhanced ACL repair may provide a new less invasive treatment option that reduces cartilage damage following joint injury.
Keywords: Anterior cruciate ligament, collagen, platelet, reconstruction, osteoarthritis
INTRODUCTION
Patients presenting with an ACL tear are at high risk for developing post-traumatic osteoarthritis whether or not they undergo ACL reconstruction surgery. This risk has been reported to be as high as 78% within 14 years of injury.38 The reason for this is unknown but is likely due to the inflammatory response, impact damage, abnormal joint kinematics and abnormal stresses within the cartilage that predispose the menisci and articular surfaces to premature breakdown.2,7,10
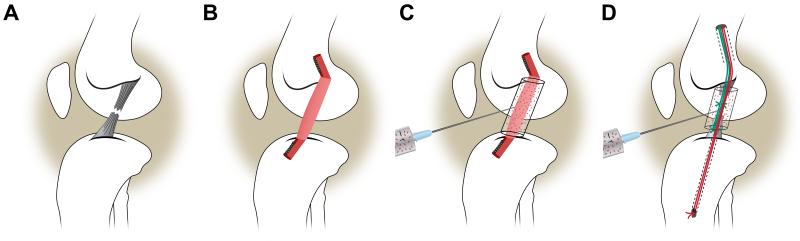
Because the rates of graft failure and post-traumatic osteoarthritis are significant problems for ACL injured patients, there is a need to develop novel approaches to improve outcomes. One such approach is bio-enhanced ACL repair, in which a bioactive scaffold is placed between the torn ends of the ligament to stimulate healing with suture repair. 12,13,18,21,22,24-27,37 The bioactive scaffold, which is based on the extracellular matrix proteins found in the normal ACL, activates the platelets in the patient’s own blood to release anabolic growth factors including PDGF, FGF-2 and TGF-β into the wound site (Fig. 1).14,17,28 The bio-enhanced ACL repair technique has shown efficacy in animal models as the structural properties of the repaired ligament are similar to that of an ACL reconstruction graft after 3 months.37 Another approach is bio-enhanced ACL reconstruction (Fig. 1), in which a similar bioactive scaffold is placed around an autograft or allograft to enhance healing.13,33 Preliminary short-term data have reported that the scaffold-platelet composite can stimulate healing of ACL autografts and allografts in pre-clinical models.13,33 The long-term results of bio-enhanced ACL repair and reconstruction and the effects of the bio-enhancement on the articular cartilage have not been evaluated.
Fig. 1.
Four treatment groups were evaluated in this study: A) ACL transection, B) conventional ACL reconstruction, C) bio-enhanced ACL reconstruction, and D) bio-enhanced ACL repair.
It has been previously demonstrated that the use of a bioactive scaffold can significantly improve the results of a traditional ACL suture repair,18 and that the structural properties for the bio-enhanced ACL repairs are equivalent to those of conventional ACL reconstruction in the porcine model at 3 months.37 The first aim of this study was to determine whether the results previously reported at 3 months would persist 6 and 12 months after surgery. The second aim was to determine if the bioactive scaffold would reduce macroscopic cartilage damage after ACL injury and treatment. We hypothesized that the structural properties of the ligament following bio-enhanced ACL repair would be equal to bio-enhanced ACL reconstruction, conventional ACL reconstruction, and superior to untreated ACL transection. We also hypothesized that the macroscopic cartilage damage following bio-enhanced ACL repair would be equal to bio-enhanced ACL reconstruction and less than conventional ACL reconstruction and untreated ACL transection.
MATERIALS AND METHODS
Study Design
Institutional Animal Care and Use Committee approvals were obtained. Sixty four Yucatan mini-pigs in late adolescence (with closed tibial and femoral physes) [age (mean±SD): 15.0±0.95 months; weight: 58.6±7.9 kg] underwent ACL transection and were randomized to one of four experimental groups: 1) no treatment, 2) conventional ACL reconstruction with bone-patellar tendon-bone (BPTB) allograft,37 3) bio-enhanced ACL reconstruction with BPTB allograft using a bioactive scaffold,13 and 4) bio-enhanced ACL repair using a bioactive scaffold of the same material and sutures (Fig. 1).25 Half of the animals within each treatment group were allowed to heal for 6 and 12 months, respectively.
Preparation of the extra-cellular matrix scaffold
The bioactive scaffolds (MIACH, Boston Children’s Hospital, Boston MA) were manufactured as previously described.25 A slurry of extracellular matrix proteins was produced by solubilizing bovine connective tissue. The collagen concentration was adjusted to a minimum of 10 mg/ml and lyophilized. For the bio-enhanced ACL reconstruction group, the scaffold was a porous hollow cylinder with an outer diameter of 22 mm, inner diameter of 10 mm, and length of 30 mm.25 For the bio-enhanced ACL repair group, the scaffolds were solid porous cylinders 22 mm in diameter and 30 mm long.13 All scaffolds were stored at -20°C until the day of surgery. When implanted in the joint, the scaffolds were bio-activated with the addition of blood containing platelets.
Surgical technique: ACL transection
A medial arthrotomy was created and the fat-pad partially resected to expose the ACL. The ACL was cut between the proximal and middle thirds of the ligament. A Lachman test was performed to verify complete transection. The knee was then irrigated with 500 cc of normal saline. For those animals assigned to receive no treatment, the incision was then closed,37 and the ligament was allowed to heal naturally.
Surgical Technique: ACL reconstruction and Bio-enhanced ACL reconstruction
Following ACL transection in the animals assigned to conventional ACL reconstruction, fresh-frozen BPTB allografts harvested from age, weight, and gender matched donors were performed as previously described.13 The entire patellar tendon (~10 mm in width) was used for the soft tissue portion of the graft while the bone plugs were trimmed to 7 mm diameter. Femoral graft fixation was achieved with a 6×20 mm bio-absorbable interference screw (Biosure; Smith & Nephew, Andover, MA). The graft was manually pre-conditioned in tension twenty times and firmly tensioned with the knee in maximal extension (~30° for the pig). The distal block was secured in the tibia using a second 6 mm interference screw backed up with an extracortical tibial button.
For the animals in the bio-enhanced ACL reconstruction group, the same ACL reconstruction procedure was performed; however, just after femoral graft fixation, the hollow cylindrical extracellular matrix based scaffold was threaded onto the graft and positioned to cover the intra-articular soft tissue portion. The distal bone plug was seated retrograde into the tibial tunnel and fixed to the tibia using a 6 mm interference screw backed with an extracortical tibial button. Three cubic centimeters of autologous blood were used to saturate and activate the scaffold in situ. The scaffold-blood composite was confined within the intercondylar notch and did not extend over the articular surfaces. The composite was allowed to set for a minimum of 10 minutes before completing the surgical procedure.
Surgical Technique: Bio-enhanced ACL repair
For the animals in the bio-enhanced ACL repair group, the repair was performed as previously described.25 In brief, an Endobutton carrying three looped sutures was passed thru a 4 mm femoral tunnel and flipped. Two of the sutures were threaded through the scaffold, into a predrilled tibial tunnel and fixed extracortically using a button with the knee in maximum extension. The remaining suture was tied to a Kessler suture of #1 Vicryl (Ethicon, Somerville, NJ), which had been placed in the tibial stump of the ACL (Fig. 1).11 Three cubic centimeters of autologous blood were used to saturate and activate the scaffold. The scaffold-blood composite was allowed to set for a minimum of 10 minutes before completion.
All incisions were closed in layers.37 Following surgery, all animals were housed for four weeks in individualized pens and were then shipped to a farm for long-term care (Coyote Consulting Corporation Inc, Douglas, MA). After 6 and 12 months of healing, the animals were euthanized, the limbs harvested and immediately frozen at -20°C until mechanical testing.
Biomechanical testing
The knees were prepared for biomechanical testing as previously described.13 The biomechanical testing procedures (i.e., AP knee laxity, structural properties) were performed using a servohydraulic load frame and custom fixtures (MTS Systems Corporation, Eden Prairie, MN).13 All investigators were blinded to the treatment group during preparation and testing. AP knee laxity was measured with the joint capsule intact at 30°, 60°, and 90° of flexion by applying anterior-posterior directed shear loads of ±40N at 0.0833 Hz for 12 cycles.11 The structural properties of the ligaments and grafts were then determined by tensile failure testing after the capsule and other ligaments were removed.18 The femur was lowered until a 5N compression load was applied followed by a tensile ramp of 20 mm/min. The load-displacement data were recorded and the linear stiffness, yield and failure loads were calculated.19 Prior to tensile testing, the cross sectional areas of the healing ACLs or grafts were measured with vernier calipers and the cross section estimated assuming they were elliptical.
Ligament Histology
After mechanical testing, the knees were cut in the sagittal plane through the ACL tissue (intact, repaired or graft). All tissue was fixed in formalin, decalcified (DELTA-Cal, Delta Products Group, Aurora, IL) and sagittally cut through the ACL tissue mass. The knee sections were dehydrated, embedded in paraffin, and microtomed into 7 micron sections. These sections were mounted on slides (Corning 75×50 mm Plain Microscope Slides, Corning Incorporated, Corning, NY), and stored at 4° C until staining with hematoxylin and eosin or α-smooth muscle actin antibodies. H&E was used to determine cell density and collagen formation, while α-smooth muscle actin immunohistochemistry was used to determine vascularity. Qualitative analyses of the cell density and vascular density were performed by an observer blinded to the treatment groups and time point of the photomicrographs.
Macroscopic Cartilage Assessment
After tissue harvest, the length and width of all visible lesions in four regions of interest (i.e., the medial and lateral femoral condyles and the medial and lateral tibial plateaus) of the surgically treated and contralateral ACL intact knees were measured using calipers following the application of India ink to highlight surface irregularities. Lesion areas were estimated assuming elliptical fits. The lesion areas for each region were summed to give the total lesion area for each knee joint. Two independent examiners, who were blinded to the leg and treatment group, performed all measurements. The values for each examiner were averaged. In addition, the specimens were scored using a commonly used macroscopic scoring method (Appendix).32
Statistical Analysis
Generalized estimating equations were used to model the structural properties, cross sectional areas, AP knee laxities, total cartilage lesion areas and macroscopic cartilage scores. Models contained main effects and all interactions involving experimental condition, limb, and post-surgical time of sacrifice. The nesting of surgical and intact limbs within animal was modeled as having correlated error using a heterogeneous compound symmetry variance-covariance matrix, block diagonal by group. The choice of the distribution and corresponding link function was chosen based on measurement scaling and model residual diagnostics. When no clear winner emerged, conventional treatment was chosen. Classical sandwich estimation was further used to adjust for model misspecification. Hypotheses were tested as orthogonal linear estimates and adjusted (padj) to maintain alpha at 0.05 using the Holm test. The initial family of comparisons between treatment groups determined if the contralateral limbs were affected by experimental condition, which would invalidate their use for normalization of surgical limbs. It was determined that normalization was not appropriate for analyzing the cartilage lesion/scoring data as the data for the uninjured knee were dependent on the animal’s treatment group assignment.
RESULTS
Animal welfare
One animal died on induction of anesthesia. Full weight bearing status was achieved within 48-72 hours for all other animals. Two animals (both in the ACL transected group) developed subcutaneous abscesses near the jaw that were treated with short-term oral antibiotics, and an animal in the bio-enhanced ACL reconstruction group (6 month) died of a respiratory infection within 1 week of the planned euthanasia date. These three animals were included in the study as there was no visual evidence of joint synovitis at the time of dissection. One animal in the bio-enhanced repair group (12 month) was shipped to the external facility at two weeks rather than four weeks post-operatively, a deviation in the post-operative rehabilitation, and another in the ACL reconstruction group (6 month) was euthanized at 2 rather than 6 months due to identification error. Both of these animals were excluded from the analysis. At 6 months, the total number of animals in each group was 8, except for the ACL transected group (n=7). At 12 months, the total number of animals in each group was 8, except for the ACL transected group (n=7) and bio-enhanced ACL repair group (n=7).
Ligament Biomechanics – Structural Properties
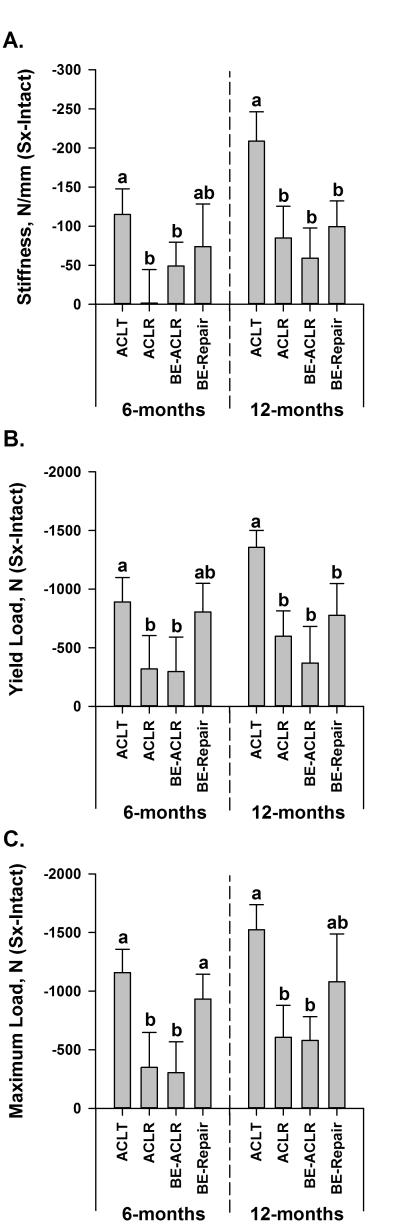
The mean structural property data for the surgical and control knees at 6 and 12 months are presented in Table 1. At 6 months, the mean normalized linear stiffness values between the three surgical treatment groups were not significantly different from each other (padj>.2890; Fig. 2A). After 12 months of healing, the normalized linear stiffness values for the three treatment groups were all significantly greater than the ACL transected group (padj<.0005; Fig. 2A). The normalized linear stiffness values for the ligament or graft following bio-enhanced ACL repair, bio-enhanced ACL reconstruction and conventional ACL reconstruction were equivalent to each other (padj>.5746). The same overall patterns across groups were observed in the yield load (Fig. 2B) and maximum failure load (Fig. 2C) data sets. The cross sectional areas of the bio-enhanced ACL repairs and both grafts were highly variable and not significantly different (p>.14; Table 1).
Table 1.
The mean (95% Confidence Limits) for the linear stiffness (stiffness), yield load and maximum load and AP laxity at 30° (AP30), 60° (AP60), and 90° (AP90) of flexion for the four treatment groups; ACL transection (ACLT), ACL reconstruction (ACLR), bio-enhanced ACL reconstruction (BE-ACLR), and bio-enhanced ACL repair (BE-repair), at 6 and 12 months after surgery.
| 6 Month | ACLT | ACLR | BE-ACLR | BE-repair | ||||
|---|---|---|---|---|---|---|---|---|
| Surgical | Intact | Surgical | Intact | Surgical | Intact | Surgical | Intact | |
| Stiffness (kN/mm) |
0.09 (0.06-0.12) |
0.20 (0.19-0.22) |
0.22 (0.19-0.24) |
0.22 (0.21-0.25) |
0.18 (0.16-0.20) |
0.23 (0.21-0.25) |
0.12 (0.08-0.16) |
0.20 (0.16-0.23) |
| Yield Load (kN) |
0.37 (0.26-0.48) |
1.26 (1.13-1.39) |
1.18 (0.90-1.46) |
1.49 (1.29-1.71) |
1.20 (0.96-1.43) |
1.50 (1.39-1.61) |
.56 (0.36-0.75) |
1.36 (1.23-1.49) |
| Maximum Load (kN) |
0.38 (0.27-0.49) |
1.54 (1.43-1.64) |
1.26 (0.97-1.55) |
1.61 (1.43-1.79) |
1.29 (1.02-1.55) |
1.59 (1.45-1.73) |
0.60 (0.43-0.78) |
1.54 (1.42-1.65) |
| CSA (mm2) |
36 (20-65) |
34 (26-43) |
84 (75-94) |
29 (27-32) |
78 (57-107) |
31 (22-45) |
54 (38-76) |
31 (28-34) |
| AP30 (mm) |
4.7 (3.7-6.1) |
2.8 (2.2-3.6) |
2.2 (1.8-2.5) |
2.4 (1.8-3.2) |
2.5 (1.8-3.4) |
1.7 (1.2-2.4) |
3.6 (2.5-5.1) |
2.7 (2.3-3.2) |
| AP60 (mm) |
8.8 (7.4-10.4) |
3.5 (3.2-3.8) |
7.7 (6.7-8.8) |
3.0 (2.8-3.3) |
6.5 (5.0-8.4) |
3.0 (2.5-3.6) |
8.5 (7.4-9.8) |
3.0 (2.7-3.4) |
| AP90 (mm) |
6.9 (5.7-8.2) |
2.7 (2.4-3.1) |
8.1 (6.4-10.2) |
2.1 (1.9-2.4) |
7.8 (6.4-9.5) |
2.1 (1.8-2.5) |
7.1 (6.4-7.7) |
2.2 (2.1-2.4) |
| 12 Month | ||||||||
| Stiffness (kN/mm) |
0.07 (0.04-0.11) |
.28 (.27-.29) |
0.18 (0.13-0.22) |
0.26 (0.23-0.28) |
0.21 (0.17-0.25) |
0.27 (0.25-0.28) |
0.15 (0.12-0.18) |
0.25 (0.23-0.27) |
| Yield Load (kN) |
0.20 (0.11-0.29) |
1.56 (1.43-1.67) |
0.93 (0.67-1.18) |
1.52 (1.30-1.74) |
0.96 (0.72-1.21) |
1.33 (1.12-1.54) |
0.57 (0.42-0.72) |
1.35 (1.16-1.53) |
| Maximum Load (kN) |
0.26 (0.14-0.38) |
1.78 (1.62-1.95) |
1.17 (1.31-2.19) |
1.78 (1.65-1.90) |
1.17 (0.90-1.43) |
1.74 (1.59-1.90) |
0.71 (0.46-0.97) |
1.79 (1.58-2.00) |
| CSA (mm2) |
22 (7-69) |
26 (23-29) |
66 (48-91) |
30 (25-35) |
55 (43-68) |
30 (24-36) |
59 (39-89) |
29 (23-37) |
| AP30 (mm) |
6.0 (4.4-8.1) |
2.2 (1.8-2.8) |
2.8 (2.2-3.5) |
2.4 (2.1-2.7) |
2.8 (1.9-4.1) |
2.4 (1.9-3) |
4.2 (2.7-6.6) |
2.9 (2.3-3.7) |
| AP60 (mm) |
9.9 (8.8-11.2) |
3.0 (2.6-3.5) |
6.2 (4.6-8.4) |
3.1 (2.8-3.4) |
6.6 (4.8-9.2) |
2.8 (2.3-3.4) |
8.8 (8.1-9.7) |
3.2 (2.7-3.9) |
| AP90 (mm) |
7.5 (6.5-8.8) |
1.9 (1.7-2.2) |
8.9 (7.2-11.0) |
2.4 (2.2-2.6) |
7.2 (5.2-10) |
2.6 (2.2-3) |
8.0 (7.5-8.7) |
2.3 (2-2.6) |
Fig. 2.
The mean differences between limbs (Surgical-Intact) for A) linear stiffness, B) yield load, and C) maximum load for the four experimental groups (ACLT=ACL transection, ACLR=ACL reconstruction, BE-ACLR=bio-enhanced ACL reconstruction and BE-Repair=bio-enhanced ACL repair) at 6 and 12 months. The mean data are plotted with the 95% confidence intervals. A value of zero indicates that the yield or maximum failure loads are equal between legs. Means that do not differ between groups after Holm adjustment within each time point have the same lower case letter (a or b).
Knee Biomechanics – AP Laxity
The AP laxity data for both the surgical and contralateral uninjured knees are provided in Table 1. At 6 months, the ratios (surgical/intact) of mean AP laxity values at 30° flexion were similar between the ACL reconstructed, bio-enhanced ACL reconstruction, and bio-enhanced ACL repair groups at 6 months (padj>.2801). Only the mean AP laxity value for the ACL reconstructed group was significantly lower than the ACL transected group (padj=.0433). After 12 months of healing, the mean AP laxity values at 30° flexion for all three surgical treatment groups were significantly lower than the ACL transected group (padj<.0269). No other significant differences in AP laxity were found.
Ligament Histology
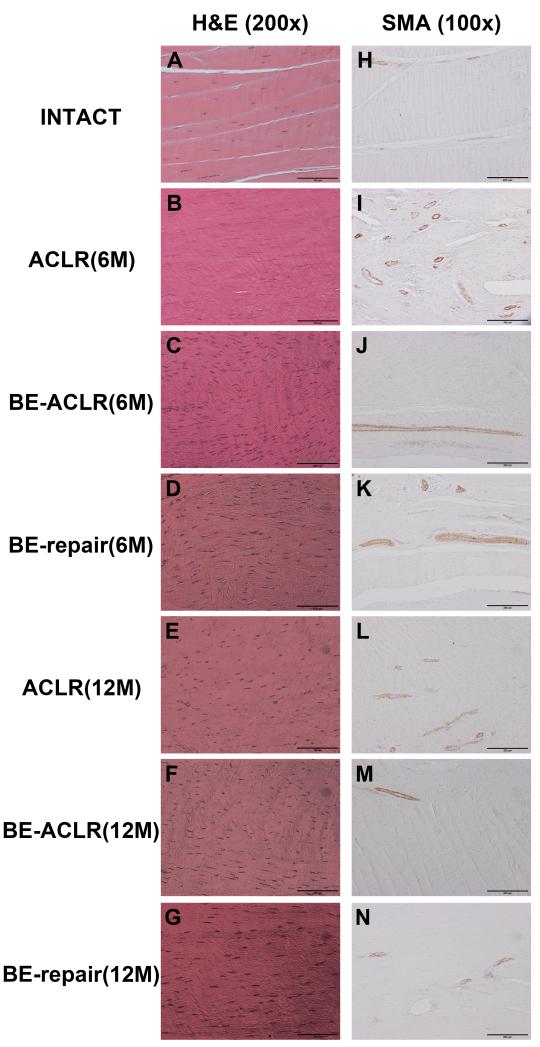
The intact ACLs had relatively sparse distributions of cells and vasculature (Fig. 3). Six months after surgery, the ligament repair and reconstruction groups had much greater cellularity throughout the ACL tissue (Fig. 3). The hypercellularity in all groups decreased by 12 months, but the treatment groups still appeared to have a greater number of cells within the tissue than the intact ACL knee. In terms of vascularity, the bio-enhanced ACL reconstructed group had a pattern similar as seen in the intact ACL at both 6 and 12 months, while the bio-enhanced repair group and the ACL reconstruction group were hypervascular at both time points.
Fig. 3.
Histological examination of the ACL and replacement tissues 6 and 12 months after surgery. The intact ACLs had relatively sparse cells and vasculature (A & H). In contrast, all three experimental groups had a much higher cell density throughout the ACL tissue 6 months post-opeartively (B, C and D). The hypercellularity in all groups decreased by 12 months, but the experimental groups still appeared to have a greater number of cells within the tissue than the intact ACL knee (E, F & G). In terms of vascularity, the bio-enhanced ACLR group had a vascularity pattern similar as seen in the intact ACL at both 6 and 12 months (Figure J and M), while the bio-enhanced repair group and the ACL reconstruction group were hypervascular at both time points (I, K, L, & N).
Cartilage Assessment
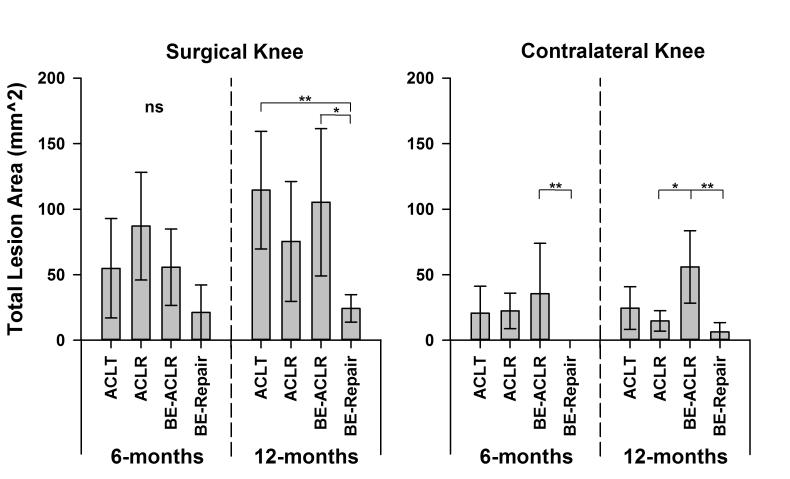
There were no significant differences in the lesion areas of the surgical limbs between the four experimental groups 6 months post-operatively (padj>.380; Fig 4). However, at 12 months, the mean lesion area for the bio-enhanced ACL repair knees was significantly less than the ACL transected knees (padj=.0017) and the bio-enhanced ACL reconstructed knees (padj=.0198). There was a strong trend indicating that the lesion areas in the bio-enhanced ACL repaired knees were less than the ACL reconstructed knees (padj=.068). There were no other statistical differences (padj>.6821) 12 months post-operatively. Comparisons between the total macroscopic scores between groups and knees followed similar trends to those of the lesion area measurements (Appendix; Fig. S1).
Fig. 4.
The mean total lesion areas for the four experimental groups (ACLT = ACL transection, ACLR = ACL reconstruction, BE-ACLR = bio-enhanced ACL reconstruction and BE-Repair = bio-enhanced ACL repair) at 6 and 12 months for the surgical and the ACL intact knee. The mean data are plotted with the 95% confidence intervals. Means that are significantly different after Holm adjustment are highlighted with *(padj<.05) or **(padj<.01). It should be noted that it was not appropriate to normalize the findings to the contralateral knee as there were significant differences in the contralateral knee between treatment groups both at 6 and 12 months (B).
DISCUSSION
The results support the first hypothesis that the structural properties of the ligament following bio-enhanced ACL repair were similar to those of bio-enhanced ACL reconstruction, conventional ACL reconstruction, and superior to those following untreated ACL transection after 12 months of healing. The linear stiffness and yield load values for all three surgically treated groups were similar at both 6 and 12 months. Although there was no difference between the untreated ACL transected ligaments and the bio-enhanced ACL repaired ligaments at 6 months, the yield load of the bio-enhanced repair procedure continued to improve and became significantly different from the transection control at 12 months (Fig. 2). On average, the mean AP laxity values of the surgically treated limbs were greater than the contralateral uninjured limb (Table 1). The AP laxity values at 30° flexion for the surgically treated knees were significantly less than the untreated ACL transected limbs at 12 months. However, no other differences in AP laxity were observed.
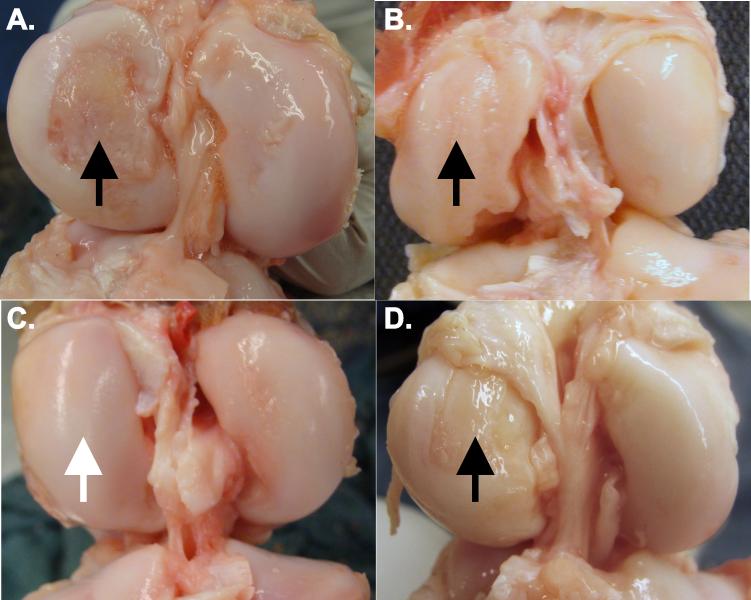
The results partially supported our second hypothesis that the macroscopic cartilage damage of the tibiofemoral joint following bio-enhanced ACL repair was less than untreated ACL transection, and there was a strong trend to be less than conventional ACL reconstruction (p=0.068) at 12 months (Fig. 4&5). ACL transection and ACL reconstruction both resulted in increased chondral damage over time, a finding previously reported in human patients.38 However, our results did not support the portion of the hypothesis related to bio-enhanced ACL reconstruction, where the macroscopic cartilage damage was greater than expected and not significantly different from ACL reconstructed or ACL transected knees. No differences in macroscopic cartilage damage were observed in the operative knees between treatment groups at 6 months. This finding may reflect the longer time period required to allow the damage to progress that is measurable using gross macroscopic techniques.
Fig. 5.
The distal femur cartilage 1-year after A) an untreated ACL rupture, B) after conventional ACL reconstruction, C) after bio-enhanced ACL repair, and D) after bio-enhanced ACL reconstruction. Note the damage to the medial femoral condyle in the untreated, ACL reconstructed knees, and bio-enhanced ACL reconstructed knees (black arrows), and the lack of damage in the medial femoral condyle in the bio-enhanced ACL repair and bio-enhanced ACL reconstructed knees (white arrow).
Furthermore, macroscopic cartilage damage was evident in the contralateral uninjured knees (Fig. 4B). The macroscopic cartilage scores in the contralateral knee of the bio-enhanced ACL reconstructed animals were significantly greater than the other treatment groups. Because the animals were randomized to the treatment groups, it is unlikely that this effect was due to different baseline conditions between treatments. The treatment group differences in the contralateral knee questions its use as a normal control in studies evaluating of post-traumatic cartilage integrity, at least in the porcine model. This affect has been noted in other large animal models.4 In future studies it would be valuable to include a sham control group to isolate the source of this contralateral effect.
The bio-enhanced ACL repair technique resulted in protection of the articular cartilage in the porcine model. The mechanism behind this is unknown and the focus of ongoing research. Following ACL injury it is well known that joint kinematics, and hence cartilage loading, are altered,2 which in turn could negatively affect cartilage metabolism. Although ACL reconstruction and repair procedures attempt to restore joint kinematics, evidence suggests that this attempt is not completely successful.3,30,35 Examination of the AP laxity values from the four experimental groups demonstrates that normal kinematics were not restored in the present study (Table 1). Other factors besides joint kinematics must therefore play a role in chondroprotection following bio-enhanced ACL repair.
It seems reasonable to assume that altering joint inflammation and subsequent release of degradative enzymes post-injury and post-surgery could preserve articular cartilage integrity. It is possible that the bioactive scaffold containing the platelets to stimulate ligament healing in bio-enhanced ACL repair also affected cartilage biology. In vitro studies have shown that growth factors released from platelets stimulate chondrocyte proliferation and biosynthesis of cartilage extra-cellular matrix,1,8,34 and diminish deleterious IL-1β effects on human arthritic chondrocytes.36 Recently, an antigen-induced porcine arthritis model determined that the intra-articular injection of platelets reduced several inflammatory cytokines in both cartilage and synovial fluid, reduced synovial hypertrophy, and increased the synthesis of proteoglycan and collagen type II.20 These data suggest that the chondroprotection following the bio-enhanced ACL repair procedure may be due, at least in part, to the activation of platelets in the bioactive scaffold. However, the bio-enhanced ACL reconstruction procedure did not have this effect. Future research will be aimed at figuring out the mechanisms to explain these divergent findings.
The placement of the bioactive scaffold during surgical ACL repair resulted in a ligament that was biomechanically similar to the graft after ACL reconstruction in the late adolescent model. These data support previous findings in the juvenile model where bio-enhanced ACL repair was also similar to ACL reconstruction.37 However, the structural properties of the bio-enhanced ACL reconstruction group were not significantly different from conventional ACL reconstruction in the late adolescent model. This is contrary to previous findings in juvenile pigs at 3 months,13 but supported by those found in the adult goats 6 weeks post-operatively.33 A likely explanation is that the healing response in late adolescent and adult animals is diminished when compared to juvenile animals,25 thus the effects of bio-enhanced ACL reconstruction may be less obvious.
The structural properties following ACL reconstruction and bio-enhanced ACL reconstruction were not significantly different from one another after 12 months of healing with the maximum loads reaching 65% and 67% of the contralateral knee, respectively. Likewise, the linear stiffness values reached 69% and 77% of the contralateral knee, respectively. The structural properties obtained in our study are similar or better than those previously reported for patellar tendon grafts in ovine and caprine models after 12 months.6,15 Complete restoration of the structural properties of the ACL was not expected as other animal models of ACL reconstruction have shown that the histology, gross morphometry and biomechanical properties of healing ACL grafts are inferior to the ACL intact knee.9,16,31
The pig model has some limitations common to all animal models of ACL surgery. It is a quadruped and therefore does not fully represent the human condition. Post-operative rehabilitation is particularly difficult to control in any animal model. Nonetheless, the porcine model has specific advantages for this study because the porcine platelet profile and wound healing characteristics more closely match the human as compared to the goat or sheep.23,29 The porcine model is also well suited for this application as many anatomical and biomechanical similarities between the pig and human knee have been noted.5,29,39
There are other limitations to consider. The ACL injury was created using a scalpel to transect the ligament in its midsubstance. It is possible that a more frayed ligament would heal differently with bio-enhanced ACL repair. For the ACL reconstructed treatment groups, fresh frozen allografts were used instead of autografts. In the porcine model, harvesting the patellar tendon graft would compromise the extensor mechanism and soft tissue autografts are not readily available. It is possible that autografts would provide better outcomes. Nonetheless, the structural properties of allografts in our pig model were similar to those reported for autografts using other quadruped models.9,16,31 Finally, it would have been advantageous to include a sham control group (i.e., no ACL transection) since treatment group assignment affected the cartilage integrity of the contralateral knee.
In this paper, we compared the performance of conventional ACL reconstruction, bio-enhanced ACL reconstruction and bio-enhanced primary repair at time points up to a year after surgery using a late adolescent animal model. While none of the treatment methods were able to restore normal AP laxity of the porcine knee, all treatments had similar biomechanical performance with one year of healing. Furthermore, the group treated with bio-enhanced ACL repair had significantly less post-traumatic osteoarthritis than the other experimental groups. The biomechanical similarities and improved cartilage outcomes for bio-enhanced repair suggest further work to translate this technique to clinical trial may be warranted.
Supplementary Material
ACKNOWLEDGEMENTS
This publication was made possible by Grant Numbers 1RO1-AR056834, 1RO1-AR056834S1 (ARRA), and 2R01-AR054099 from NIAMS/NIH and the Lucy Lippitt Endowed Professorship. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or the NIH.
The authors gratefully acknowledge the assistance of many colleagues with this project: Jason T. Machan, Ph.D. directed and performed the statistical analyses; Patrick Vavken, M.D., Benedict Proffen, M.D., Carla Haslauer, Ph.D., Alison Biercevicz, B.S. and Elise Magarian, B.S. for all their help with the animal surgeries, joint harvests, and post-operative testing; David Paller, M.S., Sarath Koruprolu, B.S., and Ryan Rich for performing the biomechanical testing (RIHOF); and Matthew Shalvoy and Natalie Inoue for assisting with the cartilage scoring.
REFERENCES
- 1.Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14:1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Johnson RJ, Naud S, et al. Accelerated versus nonaccelerated rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. Am J Sports Med. 2011;39:2536–2548. doi: 10.1177/0363546511422349. [DOI] [PubMed] [Google Scholar]
- 4.Bleedorn JA, Greuel EN, Manley PA, et al. Synovitis in dogs with stable stifle joints and incipient cranial cruciate ligament rupture: a cross-sectional study. Vet Surg. 2011;40:531–543. doi: 10.1111/j.1532-950X.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 5.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J Orthop Res. 2011;29:641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 6.Buma P, Kok HJ, Blankevoort L, Kuijpers W, Huiskes R, Van Kampen A. Augmentation in anterior cruciate ligament reconstruction-a histological and biomechanical study on goats. Int Orthop. 2004;28:91–96. doi: 10.1007/s00264-003-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 8.Drengk A, Zapf A, Sturmer EK, Sturmer KM, Frosch KH. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2009;189:317–326. doi: 10.1159/000151290. [DOI] [PubMed] [Google Scholar]
- 9.Dustmann M, Schmidt T, Gangey I, Unterhauser FN, Weiler A, Scheffler SU. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: A comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16:360–369. doi: 10.1007/s00167-007-0471-0. [DOI] [PubMed] [Google Scholar]
- 10.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in synovial fluids from patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anterioposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28:703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fufa D, Shealy B, Jacobson M, Kevy S, Murray MM. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzmuller W, Rehm KE, Perren SM. Mechanical properties of PDS-augmented patellar tendon transplants in reconstruction of the anterior cruciate ligament. Unfallchirurg. 1992;95:306–310. [PubMed] [Google Scholar]
- 16.Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson M, Fufa D, Abreu EL, Kevy S, Murray MM. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16:370–378. doi: 10.1111/j.1524-475X.2008.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi S, Mastrangelo A, Magarian E, Fleming BC, Murray MM. Collagen-Platelet Composite enhances biomechanical and histologic healing of the porcine ACL. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsuragi R, Yasuda K, Tsujino J, Keira M, Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47–56. doi: 10.1177/03635465000280012001. [DOI] [PubMed] [Google Scholar]
- 20.Lippross S, Moeller B, Haas H, et al. Intraarticular injection of platelet-rich plasma reduces inflammation in a pig model of rheumatoid arthritis of the knee joint. Arthritis Rheum. 2011;63:3344–3353. doi: 10.1002/art.30547. [DOI] [PubMed] [Google Scholar]
- 21.Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38:2528–2534. doi: 10.1177/0363546510377416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29:1002–1007. doi: 10.1002/jor.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller XM, Tevaearai HT, Jegger D, Tucker O, von Segesser LK. Are standard human coagulation tests suitable in pigs and calves during extracorporeal circulation? Artif Organs. 2001;25:579–584. doi: 10.1046/j.1525-1594.2001.025007579.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;29:S49–S57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92:2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: An in vivo study. J Orthop Res. 2009;27:639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 28.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 29.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:469–476. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43:1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheffler SU, Schmidt T, Gangey I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24:448–458. doi: 10.1016/j.arthro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Schinhan M, Gruber M, Vavken P, et al. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2011 doi: 10.1002/jor.21521. [DOI] [PubMed] [Google Scholar]
- 33.Spindler KP, Murray MM, Carey JL, Zurakowski D, Fleming BC. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631–638. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spreafico A, Chellini F, Frediani B, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem. 2009;108:1153–1165. doi: 10.1002/jcb.22344. [DOI] [PubMed] [Google Scholar]
- 35.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 36.van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–2370. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 37.Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672–680. doi: 10.1016/j.arthro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: A study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Engin. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.