Abstract
Qualitative aspects of diet may affect body composition and propensity for weight gain or loss. We tested the hypothesis that consumption of a relatively low glycemic load (GL) diet would reduce total and visceral adipose tissue under both eucaloric and hypocaloric conditions. Participants were 69 healthy overweight men and women. Body composition was assessed by DXA and fat distribution by CT scan at baseline, after 8 weeks of a eucaloric diet intervention, and after 8 weeks of a hypocaloric (1000 kcal/d deficit) diet intervention. Participants were provided all food for both phases, and randomized to either a low GL diet (≤45 points per 1000 kcal; n=40) or high GL diet (>75 points per 1000 kcal, n=29). After the eucaloric phase, participants who consumed the low GL diet had 11% less intra-abdominal fat (IAAT) than those who consumed the high GL diet (P<0.05, adjusted for total fat mass and baseline IAAT). Participants lost an average of 5.8 kg during the hypocaloric phase, with no differences in the amount of weight loss with diet assignment (P=0.39). Following weight loss, participants who consumed the low GL diet had 4.4% less total fat mass than those who consumed the high GL diet (P<0.05, adjusted for lean mass and baseline fat mass). Consumption of a relatively low GL diet may affect energy partitioning, both inducing reduction in IAAT independent of weight change, and enhancing loss of fat relative to lean mass during weight loss.
Introduction
The concept that diet quality may have metabolic effects unique from caloric content has been gaining momentum. In particular, glycemic index (GI), the extent to which a food increases serum glucose concentrations, has been proposed as potentially affecting weight change or body composition. By increasing insulin to a greater extent or for a longer time, foods with a relatively high GI may affect specific metabolic processes, such as lipolysis, lipogenesis, or substrate oxidation1–3. These processes in turn may affect hunger, satiety, food intake, or energy expenditure, factors that could impact energy balance and body composition.
In addition to affecting body weight per se, diet quality also may affect energy partitioning; i.e., the amount of fat mass relative to fat-free mass that is deposited or lost. Consumption of high GL diets, and subsequent elevated insulin response, may selectively preserve fat mass due to the lipogenic actions of insulin. Further, diet quality also may affect the specific location of the fat that is deposited or mobilized. Results of some studies have indicated that relatively greater consumption of lower GI foods is associated with a smaller waist circumference4; although a sex-effect has been reported4–6. Males and females are generally thought to distribute weight differently, such that males have an android or centripetal fat distribution pattern, whereas females have greater lipid storage to the gluteo-femoral region. Whether sex affects the impact of diet quality on body composition and fat distribution under weight maintenance conditions has not been extensively examined.
The objective of this study was to test the hypothesis that consumption of a relatively low GL diet would reduce total and regional adipose tissue during both weight maintenance and weight loss conditions. A secondary aim was to determine if there is a sexual dimorphism in outcomes of interest.
Methods and procedures
Subjects
Participants were 69 healthy overweight or obese (BMI>25) African American and European Americans (52% European American; 45% male), aged 21–50 years. Females were all premenopausal. Race was self reported during a telephone screen. Inclusion and exclusion criteria have been described elsewhere7. In brief, participants were relatively sedentary (<2 hr/wk activity), non-diabetic, non-smokers, and weight stable for 6 months prior to enrolling in the study (i.e. no weight change greater than 2.29 kg). The protocol was approved by the Institutional Review Board for Human Use at UAB, and all subjects signed an informed consent prior to testing.
Procedures
Participants completed a 4 day food record (3 week days, 1 weekend day) for assessment of typical, free-living, nutrient intake prior to beginning the 16-wk dietary intervention. After completing the food record, all subjects consumed the same diet for habituation (3 days). The dietary intervention included 2 phases: 8-wks under eucaloric conditions followed by 8-wks under hypocaloric conditions. Dual-energy X-ray absorptiometry (DXA) and computed tomography (CT) scans were acquired for all participants at baseline, following the 8-wk eucaloric phase, and following the subsequent 8-wk hypocaloric phase. For the duration of the intervention, participants reported to the General Clinical Research Center each weekday morning to be weighed, eat breakfast, and collect food for their remaining meals. On Fridays, participants picked up food for Saturday and Sunday to consume at home. All food was provided by the General Clinical Research Unit (GCRC) Metabolic Kitchen. Body weight was recorded five times weekly to monitor weight maintenance and weight loss.
Diets
Participants were blinded to an assigned diet which was either the low GL diet (≤45 points per 1000 kcal; 43% CHO, 18% protein, 39% fat, n=40) or the high GL diet (≥75 points per 1000 kcal, 59% CHO, 18% protein, 27% fat, n=29). The GL of the low GL diet was achieved by both reducing the % of total kcal derived from CHO and incorporating low GI foods. The average GI of the foods included on the low GL diet was 49 points and the high GL diet was 60 points. Macronutrient composition, average GI points, and GL points for the intervention diets were identical for both the eucaloric and hypocaloric phases. Intervention diet menus were designed using Nutrition Data System for Research (NDSR) software versions 2006 and 2007 (Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN). Diet composition and sample menus were previously reported elsewhere7;8. In brief, both the low GL diet and the high GL diet included foods typical to an American diet. Breakfast menus on the low GL diet included (but were not limited to) items such as: oatmeal or rye bread, bacon, eggs, and fruit. Breakfast menus on the high GL diet included (but were not limited to) items such as: pancakes, waffles, or cereal with milk or yogurt, and fruit juice. Lunch and dinner menus on both diets generally consisted of a main entrée supplemented with items such as a roll with margarine and vegetables (e.g. green beans, broccoli, or salad). Main entrée items for lunch and dinner were either frozen packaged meals such as roasted turkey, lasagna, or chicken with pasta by Lean Cuisine (Stouffer’s Nestle, Solon, OH) or Healthy Choice (ConAgra Foods, Omaha, NE) or entrées prepared by the metabolic kitchen staff (e.g. sandwich or grilled chicken breast). Glucose was used as the reference for determining GL points. Energy requirements were determined by the Harris Benedict equation with an activity factor of 1.35 for females and 1.5 for males during the eucaloric phase. Energy intake was adjusted if necessary to maintain body weight within 2 kg of baseline weight. The addition or reduction in calories to the assigned diets did not affect the macronutrient compositions or GL. Estimated energy requirements established during the eucaloric phase were reduced by 1000 kcal to achieve a 1–2 lb weight reduction per wk during the hypocaloric phase of the study. Subjects were asked to maintain their baseline physical activity level throughout the intervention time period.
Body composition and fat distribution
Total body fat mass and lean mass were measured by DXA using a Lunar Prodigy densitometer (GE-Lunar Corporation, Madison, WI, software version 12.3). Participants were required to wear light clothing, remove all metal objects from their body, and lie supine with arms at their sides while undergoing a total body scan. Intra-abdominal adipose tissue (IAAT), subcutaneous abdominal adipose tissue (SAAT), thigh muscle, thigh subcutaneous adipose tissue (SAT), thigh perimuscular adipose tissue (PMAT), and thigh intermuscular adipose tissue (IMAT) were determined by computed tomography (CT) scanning. A five millimeter axial scan at the level of the umbilicus (approximately the L4–L5 intervertebral space) and another at mid-thigh were taken. Scans were later analyzed for cross-sectional area (cm2) of adipose tissue and muscle tissue using SliceOmatic image analysis software (version 4.3: Tomovision, Montreal, Canada). The abdomen scan was used to analyze IAAT and SAAT. Thigh IMAT and PMAT were separated from thigh SAT by manually drawing a line along the fascia lata surrounding the thigh muscle. Subsequently, IMAT was partitioned from PMAT by manually drawing a line around the muscle itself to capture adipose tissue located directly between and within muscle groups9;10. All scans were analyzed by the same image analyst (AG).
Statistical methods
Descriptive statistics were computed for all variables of interest. Variables known to deviate from a normal distribution were log 10 transformed prior to statistical analysis. All statistical tests were two-sided and were performed using a type I error rate of 0.05. Statistical analyses were performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC). Two-way repeated measures analysis of variance was used to examine the effects of time (baseline to follow-up), diet group (high GL vs low GL diets) and time x diet group interaction for measures of body composition and fat distribution from both eucaloric and hypocaloric diet phases.
Analysis of covariance (ANCOVA) was used to determine the effect of diet on changes in individual adipose tissue depots after adjusting for change in total fat mass during the eucaloric phase. ANCOVA was also used to determine the effect of diet on change in total fat mass independent of total lean mass during the hypocaloric phase. Dependent variables were 8-wk (post-intervention) outcomes, and baseline outcome measures were used as covariates. For subgroup analyses by sex, paired t-tests were used to examine changes in body composition and fat distribution from baseline to follow-up during the eucaloric phase. Subgroup analysis by sex was not conducted following the hypocaloric phase due to subgroup sample size limitations during this phase of the study.
Results
Descriptive information on the subject population is shown in Table 1. By study design, subjects were overweight or obese at baseline of the eucaloric phase (BMI 25–46.9 kg/m2). BMI was significantly higher in the high GL diet group at baseline of both phases. At baseline of the eucaloric phase, average weight and age did not statistically differ by diet group, and the low GL group had significantly greater total fat mass (P=0.02) and greater thigh SAT (p≤0.05). There were no other significant differences in regional adiposity by diet group at baseline of the eucaloric or hypocaloric phase.
Table 1.
Baseline characteristics of study population by diet group during eucaloric and hypocaloric phases
| Variable | Diet | Eucaloric Phase | Hypocaloric Phase |
|---|---|---|---|
|
| |||
| n | High GL | 29 | 28 |
| Low GL | 40 | 31 | |
|
| |||
| Sex(% male) | High GL | 48% (n=14) | 46% (n=13) |
| Low GL | 43% (n=17) | 45% (n=14) | |
|
| |||
| Race (% EA) | High GL | 48% (n=14) | 50% (n=14) |
| Low GL | 55% (n=22) | 58% (n=18) | |
|
| |||
| Age (yr) | High GL | 34.6 ± 8.11 | 34.7 ± 8.11 |
| Low GL | 35.6 ± 8.51 | 35.9 ± 8.41 | |
|
| |||
| BMI (kg/m2)2 | High GL | 31.4 ± 4.41 | 30.9 ± 4.51 |
| Low GL | 33.5 ± 4.31 | 32.4 ± 4.11 | |
Data reported as mean ± SD; EA, European American; BMI, body mass index
P≤0.05 (2-sample t test for significant differences between diet groups at baseline of both phases).
Eucaloric phase
Although each subject’s daily energy intake was calculated on an individual basis to maintain body mass during the eucaloric phase, fluctuations in body mass occurred over the 8 week intervention period. On average, a change of −1.03% (−1.0 kg) in body mass (range = −2.10% to +4.05%; −2.07 kg to +4.00 kg) was observed, which did not statistically differ with diet assignment.
Changes in body composition and fat distribution variables over the 8-week eucaloric dietary intervention period are reported in Table 2. Significant time effects were observed for SAAT such that it decreased in both diet groups over the 8-wk eucaloric period. A significant time by group effect was observed for IAAT, such that a greater loss over the eucaloric intervention period was observed in the low GL group relative to the high GL group. IAAT remained significantly lower (11%) after the 8-wk eucaloric intervention in the low GL diet group compared to the high GL diet group after adjustment for baseline IAAT and 8-wk total fat mass (P<0.05).
Table 2.
Fat distribution and body composition outcomes for eucaloric phase by diet
| Variable | Diet | Baseline (mean ± SD) | Follow-up (mean ± SD) | Change (%) | Time P† | Group P† | Group*time P† |
|---|---|---|---|---|---|---|---|
| Weight (kg) | High GL | 96.1±20.3 | 95.2±20.7 | −1.6±0.4 | 0.47 | 0.36 | 0.39 |
| Low GL | 99.8±18.1 | 98.6±17.9 | −1.9±2.2 | ||||
| Total Lean | High GL | 56.4±1.6 | 56.2±1.5 | −0.4±0.4 | 0.60 | 0.99 | 0.73 |
| Low GL | 55.7±1.3 | 55.3±1.3 | −0.5±0.5 | ||||
| Total Fat (kg) | High GL | 36.8±7.9 | 35.1±8.1 | −4.7±0.8 | 0.07 | 0.02 | 0.69 |
| Low GL | 41.3±8.7 | 39.4±9.7 | −5.0±1.0 | ||||
| IAAT (cm2) | High GL | 80.6±48.3 | 82.4±57.9 | −1.3±3.5 | 0.12 | 0.80 | 0.03 |
| Low GL | 89.5±46.3 | 81.5±49.4 | −10.9±3.0 | ||||
| SAAT (cm2) | High GL | 409.9±125.3 | 384.5±118.6 | −6.1±1.5 | 0.02 | 0.33 | 0.66 |
| Low GL | 426.0±112.0 | 404.2±121.6 | −6.1±1.2 | ||||
| Thigh SAT(cm2) | High GL | 241.7±89.1 | 229.5±87.9 | −3.9±3.2 | 0.82 | 0.02 | 0.26 |
| Low GL | 298.2±110.6 | 276.5±109.8 | −8.3±8.3 | ||||
| Thigh IMAT | High GL | 14.1±6.9 | 13.0±6.7 | −5.6±4.7 | 0.40 | 0.36 | 0.98 |
| Low GL | 15.0±6.4 | 13.8±6.4 | −10.1±3.2 | ||||
| Thigh PMAT | High GL | 18.2±5.5 | 18.0±6.0 | −0.9±3.3 | 0.45 | 0.20 | 0.15 |
| Low GL | 20.7±7.9 | 19.4±8.2 | −5.2±3.6 | ||||
| Thigh muscle | High GL | 317.1±93.5 | 322.9±75.4 | −0.5±1.0 | 0.17 | 0.70 | 0.12 |
| Low GL | 333.4±72.6 | 325.7±12.5 | −2.2±1.1 |
The percent change from baseline is reported as mean ± SEM. P, p-value; IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SAT, subcutaneous adipose tissue; IMAT, intermuscular adipose tissue; PMAT, perimuscular adipose tissue.
p†, P-value for 2-way ANOVAfor unadjusted data
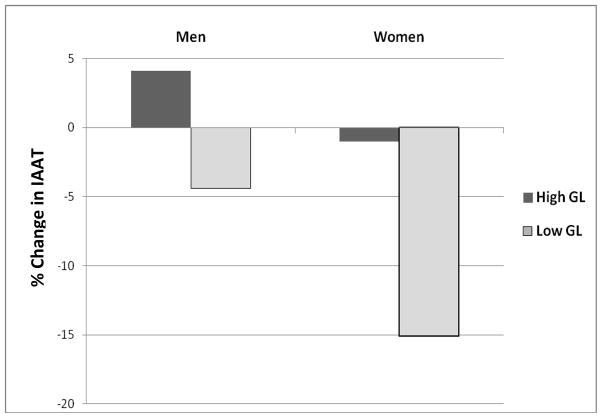
Subgroup analysis by sex indicated all groups lost total fat mass from baseline to 8 wks of the eucaloric phase, such that men lost 4.2% on the high GL diet (P<0.001) and 6.3% on the low GL diet (P<0.001) and women lost 4.9% on the high GL diet (P<0.01) and 3.1% on the low GL diet (P<0.05). Only women in the low GL group lost IAAT, such that on average women in this group lost 15.1% (P=0.001), while women in the high GL diet group lost 1% (P=0.82), men in the high GL diet group gained 4.2% (P=0.55), and men in the low GL diet group lost 4.3% (P=0.44) (Figure 1). All subgroups, except the women in the low GL diet group, lost SAAT, such that men on the high GL diet lost 5.7%(P<0.01), men on the low GL diet lost 8%(P<0.001), women on the high GL diet lost 6.9%(P<0.01), and women on the low GL diet lost 1% (P<0.12). Women on both the low GL diet and high GL diet lost thigh SAT (5.8%, 9.8%, respectively, P<0.01) and thigh IMAT (9.4%, 11.9%, respectively, P<0.05). Only men in the low GL diet group lost thigh SAT (10.4%) and thigh PMAT (15.5%) (P< 0.01 for both; data not shown). Race did not have an effect on outcomes of interest and was equally distributed across subgroups by sex.
Figure 1.
Mean % change in IAAT (cm2) following consumption of the eucaloric high GL and low GL diets by gender. Men consuming the high GL diet gained 4.1% (NS) and men consuming the low GL diet lost 4.4% (NS) IAAT. Women consuming the high GL diet lost 1% (NS) and women consuming the low GL diet lost 15.1% (P<0.01) IAAT.
Hypocaloric phase
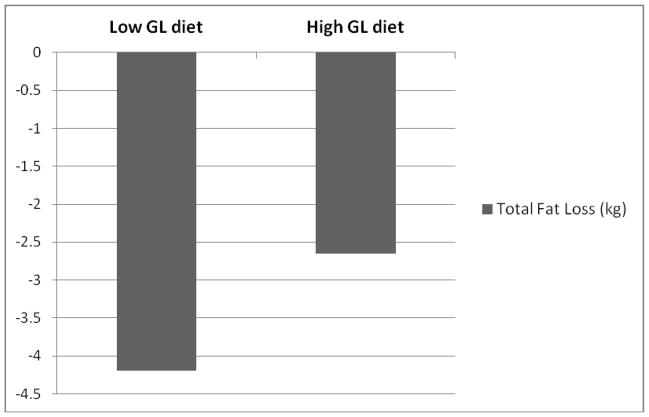
Changes in body composition and fat distribution resulting from the 8-wk hypocaloric diet intervention phase are reported in Table 3. Significant time effects were observed for weight, total lean, and IAAT reflecting that these outcomes decreased in both groups over the 8-wk intervention. A significant time by group effect was observed for total fat, such that the low GL diet group lost more fat mass over the hypocaloric intervention period. No group effects were observed for the hypocaloric diet phase. Total fat mass remained significantly lower after the 8-wk hypocaloric intervention in the low GL diet group compared to the high GL diet group after adjustment for baseline total fat mass and follow up total lean mass (P<0.05) (Figure 2).
Table 3.
Fat distribution and body composition outcomes for hypocaloric phase by diet
| Variable | Diet | Baseline (mean ± SD) | Follow-up (mean ± SD) | Change (%) | Time P† | Group P† | Group*time P† |
|---|---|---|---|---|---|---|---|
| Weight (kg) | High GL | 94.3±20.4 | 89.4±20.9 | −4.3±0.8 | <0.001 | 0.52 | 0.41 |
| Low GL | 98.4±17.9 | 92.9±18.1 | −6.1±3.9 | ||||
| Total Lean | High GL | 55.5±1.5 | 53.7±1.5 | −3.3±0.7 | 0.01 | 0.87 | 0.36 |
| Low GL | 55.3±1.3 | 54.1±1.3 | −2.2±0.5 | ||||
| Total Fat (kg) | High GL | 34.9±8.2 | 32.4±9.4 | −8.3±1.4 | 0.16 | 0.13 | 0.02 |
| Low GL | 39.1±9.7 | 35.3±9.8 | −10.5±1.2 | ||||
| IAAT (cm2) | High GL | 86.6±59.3 | 71.1±50.0 | −13.6±3.3 | 0.05 | 0.92 | 0.45 |
| Low GL | 84.5±50.4 | 73.6±45.5 | −13.8±3.3 | ||||
| SAAT (cm2) | High GL | 378.4±116.6 | 342.7±131.3 | −10.8±1.6 | 0.07 | 0.60 | 0.37 |
| Low GL | 404.8±107.7 | 358.0±99.3 | −11.7±8.0 | ||||
| Thigh SAT(cm2) | High GL | 225.4±89.1 | 207.2±81.6 | −7.9±2.3 | 0.34 | 0.06 | 0.31 |
| Low GL | 285.6±104.9 | 253.7±102.4 | −9.2±1.4 | ||||
| Thigh IMAT | High GL | 12.9±6.8 | 10.5±5.5 | −17.4±3.6 | 0.39 | 0.36 | 0.99 |
| Low GL | 14.3±6.2 | 11.8±5.3 | −17.5±1.7 | ||||
| Thigh PMAT | High GL | 17.8±5.8 | 16.6±6.6 | −0.08±3.3 | 0.21 | 0.37 | 0.55 |
| Low GL | 19.1±7.8 | 18.4±7.3 | −2.6±2.6 | ||||
| Thigh muscle | High GL | 320.2±71.9 | 312.4±70.3 | −2.3±0.7 | 0.11 | 0.95 | 0.90 |
| Low GL | 324.8±35.7 | 317.4±73.2 | −2.1±0.6 |
The percent change from baseline is reported as mean±SEM. P, p-value; IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SAT, subcutaneous adipose tissue; IMAT, intermuscular adipose tissue; PMAT, perimuscular adipose tissue.
p†, P-value for 2-way ANOVAfor unadjusted data
Figure 2.
Change in total fat mass (kg) adjusted for total lean mass (kg) following the 8-wk hypocaloric phase by diet. Low GL diet group had significantly less total fat mass relative to lean mass following the hypocaloric phase (P<0.05)
Discussion
The goal of the present study was to test the hypothesis that consumption of a relatively low GL diet compared to a high GL diet would result in preferential visceral fat loss and greater total fat loss following both weight maintenance and weight loss conditions. We also aimed to examine if there were sex-specific differences in outcomes of interest. Following the eucaloric phase, we found participants who consumed the low GL diet had 11% less IAAT after adjustment for total fat mass than those who consumed the high GL diet. However, in subgroup analysis, loss of IAAT in the low GL group was specific to women, who lost an average of 15.1% IAAT. Following the hypocaloric phase, we found participants who consumed the low GL diet had 4.4 % less total fat mass than those who consumed the high GL diet (P<0.05, follow-up fat mass adjusted for lean mass and baseline fat mass). Our findings suggest that consuming a low GL diet may promote loss of abdominal fat, even with little or no change in weight, and may also promote greater loss of total body fat during weight loss when compared to a high GL diet.
Our results indicate that consuming a low GL diet may promote loss of IAAT, even under weight maintenance conditions. Several cross-sectional studies have linked greater intake of low GI foods to smaller waist circumference, a proxy measure of visceral adiposity4–6. However, to our knowledge this is the first tightly controlled dietary intervention including a robust, direct measure of body fat distribution to report a significant reduction in IAAT as the result of a low GL diet in healthy overweight and obese subjects. The precise mechanisms leading to preferential IAAT loss during weight maintenance conditions following the consumption of a low GL diet are not clear, however may be related to insulin secretion. We previously reported, in this same population, that consumption of a low GL diet for 8 weeks relative to a high GL diet resulted in a lower insulin secretory response to a fixed meal challenge7. The reduced postprandial insulin response following consumption of a low GL diet may be permissive to increased fatty acid mobilization from adipose tissue within the abdominal cavity, as has been observed with total body fat11.
We found the reduction in IAAT during eucaloric conditions to be specific to women on the low GL diet, such that this was the only subgroup to significantly lose IAAT. Our findings are in congruence with other studies linking the consumption of lower GI foods to a smaller waist circumference specifically in women4–6. Halkjaer et al found that CHO energy intake from fruits and vegetables was inversely associated with change in waist circumference over a 5 year period, and conversely, CHO energy intake from all other food groups was positively associated with change in waist circumference5. Further, these associations were significantly stronger in women than in men. Similarly, high intake of refined grains was associated with gain in waist circumference adjusted for BMI over 6 years in women but not men4. A dietary counseling study among men and women with type 2 diabetes reported consumption of a moderately reduced CHO diet resulted in preferential visceral adipose tissue loss among women but not men12. Taken together, findings from these studies suggest macronutrient composition of the diet and CHO quality may have an effect on fat distribution that is specific to women. However, the mechanisms regulating specific loss of IAAT among women following a low GL diet are unknown. Further investigation is warranted to explore whether interactions between the changes in postprandial insulin dynamics and the sex hormone environment may underlie sex differences in adipose distribution.
The reason for sexual dimorphic results in response to the diets is not clear; however it is possible that a repartitioning of lipid played a role. Women on the low GL diet tended to have more thigh SAT (P=0.09) and SAAT (P<0.05) than women on the high GL diet at the end of the eucaloric phase (data not shown; adjusted for baseline value, and changes in total fat and IAAT), which may suggest that triglyceride was preferentially stored in subcutaneous adipose tissue in women consuming the low GL diet. Greater circulating estrogen in women may promote deposition of lipid in the hip/thigh area13, an effect that may have been facilitated by the low GL diet.
Following the 8-wk hypocaloric diet phase, participants who consumed the low GL diet had significantly greater total body fat loss than those who consumed the high GL diet. Other studies have shown inconsistent findings in regards to the effectiveness of low GL diets yielding greater weight loss and total fat loss compared to other dietary approaches14. These inconsistencies may be due to differences in methodology, underlying physiological differences in study populations, and other confounding factors affecting diet adherence and efficacy. To our knowledge this is the first tightly controlled dietary intervention study to report a significant difference in total body fat loss after 8 wks of consuming a low GL diet with only a modest reduction in % CHO when compared to a high GL.
Greater fat loss resulting from consumption of the hypocaloric low GL diet may be related to effects of glycemic load on fat oxidation and energy expenditure. Animal and human studies have demonstrated impaired metabolic flexibility and reduced fat oxidation resulting from consumption of a high CHO diet15;16. The observed changes in fat oxidation in these studies may be related to a greater postprandial insulin response following consumption of a diet with high GI or CHO content. Evidence also suggests a low GL vs. high GL diet may increase postprandial energy expenditure, also known as diet-induced thermogenesis (DIT), by reducing the rate of CHO absorption and disposal17–19. However, in the present study, it is also possible the higher fat content on the low GL diet influenced energy metabolism during weight loss. Both the percentage fat from omega-3 and oleic acid and the absolute amount of omega-3 and oleic acid were higher in the low GL diet7, Data from animal and human studies indicate long chain omega-3 fatty acids may induce body fat loss by influencing fat oxidation and energy expenditure20. Therefore, it seems possible the difference in fat and/or CHO content between the low and high GL diet may have affected the outcomes. The effects of elevated postprandial energy expenditure in conjunction with an increased propensity to oxidize fat may have contributed to greater fat mass loss among those consuming the low GL diet in our study. Further research is needed to determine if the here-observed greater total body fat loss under weight loss conditions on a low GL diet vs. high GL diet is attributable to greater fatty acid oxidation or postprandial energy expenditure, and whether these effects were induced by CHO or fat content in a low GL diet.
Strengths of this study included control of subject intake by supplying all food over the study period; use of a eucaloric diet arm, which avoided confounding by large changes in energy balance; use of robust measures to determine body composition and fat distribution; diets comprised of foods that may be practically consumed and a macronutrient profile with only a modest reduction in CHO. Limitations to this study included a relatively small sample size in subgroups by gender and inability to determine independent effects of dietary CHO vs. fat. Also, observed changes in total fat and fat distribution during the eucaloric phase may have hindered observation of further changes during the hypocaloric phase of the intervention.
In conclusion, consumption of a relatively low GL diet may induce loss of IAAT during weight maintenance conditions, especially in women. During weight loss, consumption of a low GL diet may affect energy partitioning, enhancing loss of fat relative to lean mass compared to a high GL, low-fat diet. Further studies are needed to identify mechanisms linking low GL diet to loss of visceral and total fat, and for gender-specific effects.
Acknowledgments
Funding
This work was supported by R01DK67538, M01-RR-00032, UL1RR025777, P30-DK56336, P60DK079626.
The authors gratefully acknowledge the help of Maryellen Williams and Cindy Zeng of the UAB Metabolism Core Laboratory (Nutrition Obesity Research Center, Diabetes Research and Training Center, Center for Clinical and Translational Science) with laboratory analyses, and of Betty Darnell and Suzanne Choquette of the UAB Center for Clinical and Translational Science with experimental design and diet development.
Footnotes
Disclosure
The authors had no conflicts of interest to disclose
Reference List
- 1.Ludwig DS. Dietary glycemic index and the regulation of body weight. Lipids. 2003;38:117–121. doi: 10.1007/s11745-003-1040-x. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armendariz-Anguiano AL, Jimenez-Cruz A, Bacardi-Gascon M, et al. Effect of a low glycemic load on body composition and Homeostasis Model Assessment (HOMA) in overweight and obese subjects. Nutr Hosp. 2011;26:170–175. [PubMed] [Google Scholar]
- 4.Halkjaer J, Sorensen TI, Tjonneland A, et al. Food and drinking patterns as predictors of 6-year BMI-adjusted changes in waist circumference. Br J Nutr. 2004;92:735–748. doi: 10.1079/bjn20041246. [DOI] [PubMed] [Google Scholar]
- 5.Halkjaer J, Tjonneland A, Thomsen BL, et al. Intake of macronutrients as predictors of 5-y changes in waist circumference. Am J Clin Nutr. 2006;84:789–797. doi: 10.1093/ajcn/84.4.789. [DOI] [PubMed] [Google Scholar]
- 6.Romaguera D, Angquist L, Du H, et al. Dietary determinants of changes in waist circumference adjusted for body mass index - a proxy measure of visceral adiposity. PLoS One. 2010;5:e11588. doi: 10.1371/journal.pone.0011588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goree LL, Chandler-Laney P, Ellis AC, et al. Dietary macronutrient composition affects beta cell responsiveness but not insulin sensitivity. Am J Clin Nutr. 2011;94:120–127. doi: 10.3945/ajcn.110.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gower BA, Goree LL, Chandler-Laney PC, et al. A higher-carbohydrate, lower-fat diet reduces fasting glucose concentration and improves beta-cell function in individuals with impaired fasting glucose. Metabolism. 2011 doi: 10.1016/j.metabol.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song MY, Ruts E, Kim J, et al. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 11.Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 12.Sasakabe T, Haimoto H, Umegaki H, et al. Effects of a moderate low-carbohydrate diet on preferential abdominal fat loss and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2011;4:167–174. doi: 10.2147/DMSO.S19635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbers JM, Asscheman H, Seidell JC, et al. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276:E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- 14.Esfahani A, Wong JM, Mirrahimi A, et al. The application of the glycemic index and glycemic load in weight loss: A review of the clinical evidence. IUBMB Life. 2011;63:7–13. doi: 10.1002/iub.418. [DOI] [PubMed] [Google Scholar]
- 15.Bray MS, Tsai JY, Villegas-Montoya C, et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes(Lond) 2010;34:1589–1598. doi: 10.1038/ijo.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isken F, Klaus S, Petzke KJ, et al. Impairment of fat oxidation under high- vs low-glycemic index diet occurs before the development of an obese phenotype. Am J Physiol Endocrinol Metab. 2010;298:E287–E295. doi: 10.1152/ajpendo.00515.2009. [DOI] [PubMed] [Google Scholar]
- 17.Agus MS, Swain JF, Larson CL, et al. Dietary composition and physiologic adaptations to energy restriction. Am J Clin Nutr. 2000;71:901–907. doi: 10.1093/ajcn/71.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira MA, Swain J, Goldfine AB, et al. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 19.Scazzina F, Del RD, Benini L, et al. The effect of breakfasts varying in glycemic index and glycemic load on dietary induced thermogenesis and respiratory quotient. Nutr Metab Cardiovasc Dis. 2011;21:121–125. doi: 10.1016/j.numecd.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Baillie RA, Takada R, Nakamura M, et al. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids. 1999;60:351–356. doi: 10.1016/s0952-3278(99)80011-8. [DOI] [PubMed] [Google Scholar]