Abstract
Using a rodent model of ischemia [permanent middle cerebral artery occlusion (pMCAO)], previous studies demonstrated that whisker stimulation treatment completely protects the cortex from impending stroke when initiated within 2 h following pMCAO. When initiated 3 h post-pMCAO, the identical treatment exacerbates stroke damage. Rats in these studies, however, were anesthetized with sodium pentobarbital, whereas human stroke patients are typically awake. To overcome this drawback, our laboratory has begun to use the anesthetic isoflurane, which allows rats to rapidly recover from pMCAO within minutes, to test stimulation treatment in awake rats and to determine whether isoflurane has an effect upon the pMCAO stroke model. We found no difference in infarct volume between pMCAO in untreated controls under either sodium pentobarbital or isoflurane, and the primary finding was that rats that received treatment immediately post-pMCAO maintain cortical function and no stroke damage, whereas rats that received treatment 3 h post-pMCAO exhibited eliminated cortical activity and extensive stroke damage. The only difference between anesthetics was the broad extent of evoked cortical activity observed during both functional imaging and electrophysiological recording, suggesting that the extent of evoked activity evident under isoflurane anesthesia is supported by underlying neuronal activity. Given the high degree of similarity with previous data, we conclude that the pMCAO stroke model is upheld with the use of isoflurane. This study demonstrated that the isoflurane-anesthetized rat pMCAO model can be used for cerebrovascular studies, and allows for highly detailed investigation of potential novel treatments for ischemic stroke using awake, behaving animals.
Keywords: isoflurane, protection, rat, rodent model, stimulation treatment, stroke
Introduction
Using a rodent model of ischemia [permanent middle cerebral artery occlusion (pMCAO)], previous studies have shown that single whisker stimulation treatment can completely protect the cortex from impending stroke damage when initiated within 2 h of pMCAO, but when initiated 3 h post-pMCAO, treatment resulted in significant stroke damage (for a recent review see Frostig et al., 2012) (Lay et al., 2010; Davis et al., 2011; Lay et al., 2011; Frostig et al., 2012; Lay et al., 2012). Although these findings demonstrate the translational potential of a drug-free, non-invasive, protective treatment from acute ischemic stroke, subjects in this study, similar to the vast majority of other stroke models, were anesthetized, whereas human stroke patients are typically awake. A better animal model of human stroke should therefore be based on studying the consequences of pMCAO in awake, behaving animals, following only brief anesthesia exposure necessary for the pMCAO procedure. However, the previously described studies were performed using sodium pentobarbital anesthesia, rendering animals unconscious for hours after anesthetic cessation. In this situation, awake, behaving studies, following even brief use of sodium pentobarbital anesthesia for the pMCAO procedure, would be impossible to conduct, as the great majority of experimental subjects would recover from even brief anesthesia beyond the 2 h `window of opportunity' for sensory-induced protection. Employment of the volatile anesthetic isoflurane, from which rats recover within minutes of anesthetic cessation, seems to be the optimal solution for the shift toward studies of awake, behaving animals following pMCAO, which must be performed under anesthesia.
A potential problem, however, for the use of isoflurane for an animal model of ischemic stroke has emerged from investigations that have assessed the impact of isoflurane on ischemic outcome (Warner et al., 1986; Nehls et al., 1987; Baughman et al., 1988; Gelb et al., 1989; Ishikawa et al., 1989; Kawaguchi et al., 2000; Warner, 2000; Sakai et al., 2007; Makaryus et al., 2011). Results have been discrepant, with many conflicting reports regarding the protective ability of this anesthetic (Warner et al., 1986; Warner, 2000; Dirnagl, 2010). As a result, whether the use of isoflurane positively or negatively affects ischemic outcome remains an open question.
In the present study, we sought to determine if isoflurane can be used effectively within our ischemic stroke model, and whether isoflurane has positive or negative effects on outcome following pMCAO. Our strategy to assess this issue includes a two-pronged approach: (i) the comparison of infarct volume in rats that undergo pMCAO and never receive stimulation treatment under both types of anesthesia, and (ii) replication of our previous findings on the protective or harmful effects of sensory-based treatment obtained under sodium pentobarbital. Functional imaging was conducted on the first day of each experiment prior to pMCAO and stimulation treatment, and again the following day. At the conclusion of day 2 of experimentation, post-mortem histology was then performed (Fig. 1). The rationale of these studies was that, if we found no difference between animals using both approaches, then the experimental ischemic stroke model would be upheld with the use of isoflurane.
Fig. 1.

Schematic of the experimental design. For both experimental groups, vertical lines indicate the time of pMCAO, time of treatment, and the following 24 h reassessment.
Materials and methods
All procedures were in compliance with NIH guidelines and approved by UC Irvine Animal Care and Use Committee (protocol no. 1997–1608, assurance ID no. A3416.01).
Surgical preparation and anesthesia
Experimental subjects (295–400 g male Sprague Dawley rats) were individually housed in standard cages. All subjects were anesthetized using the inhalational anesthetic isoflurane (halogenated ether 2-chloro-2-difluoromethoxy-1,1,1-trifluoro-ethane), and maintained at 1.0–2.0% (E-Z Anesthesia Machine) isoflurane in 100% oxygen. The current experiments using isoflurane anesthesia were conducted alongside previously published experiments (Lay et al., 2010, 2011), which had used sodium pentobarbital anesthesia. An `imaging' area (~5 × 6 mm) of the skull over the left somatosensory cortex was thinned. Dextrose (5%; 3 mL) was administered initially and subsequently every 6 h during the experiment. Body temperature was maintained at 37 °C. In a subset of animals, arterial oxygen saturation (min-max, mean±SE) (77–99, 92±3% oxygen saturation), respiration (21–90, 63±4 RPM), pulse distension (a proxy for blood pressure; 5–64, 25±8% vessel distention), and heart rate (275–413, 336±15 BPM) were measured using pulse oximetry (Starr Life Sciences, Allison Park, PA, USA) to ensure that any observed changes following pMCAO were not due to variability in vital parameters. Previous studies in our laboratory have shown that pMCAO and stimulation treatment do not alter systemic vital parameters (Lay et al., 2010, 2011), and we again did not observe any significant alterations in vital parameters in this study.
Baseline data collection was followed by pMCAO [double ligature and transection of the stem (primary branch of the middle cerebral artery segment; just distal to the lenticulostriate branch) of the left proximal middle cerebral artery] (Lay et al., 2010; Frostig et al., 2012).
Histology (2,3,5-triphenyltetrazolium chloride staining for infarct)
At the conclusion of each experiment, the brain was removed, sectioned into 2 mm coronal slices, and incubated in 2% 2,3,5-triphenyltetrazolium chloride at 37 °C for 20 min in the dark (Bederson et al., 1986). The infarct volume was determined by an observer blind to experimental condition. A small amount of damage occasionally produced at the surgical site was excluded from infarct analysis (Tamura et al., 1981). Infarct volume comparisons were performed by employing two-sample t-tests.
Stimulation treatment
As in previous studies (Lay et al., 2010; Davis et al., 2011; Lay et al., 2011, 2012), a total of 1280 whisker deflections, delivered in 256 events (five whisker deflections per event in a 5 Hz pattern) at varying intervals of 21±5 s between onsets of consecutive events, was distributed over 120 min.
Intrinsic signal optical imaging and analysis
We used intrinsic signal optical imaging (ISOI) to assess the evoked functional response to single whisker stimulation (whisker functional representation). For a recent review of ISOI see Frostig & Chen-Bee (2009). A detailed description of ISOI (Grinvald et al., 1986; Frostig et al., 1990; Ts'o et al., 1990) data acquisition and analysis can be found elsewhere (Chen-Bee et al., 2000; Chen-Bee et al., 2007). Briefly, a charge-coupled device camera was used for imaging with red light illumination. Post-stimulus ratio images were created by calculating the fractional change (FC) values relative to activity collected immediately before stimulus onset. The first two phases of evoked functional representation, the `initial dip' and `overshoot', were analyzed. The ratio image containing the maximum areal extent was quantified at a threshold level of 5.0 × 10−4 away from zero. Although previous work has utilized 2.5 × 10−4 FC, the higher 5.0 × 10−4 FC threshold was chosen here to achieve areal extent values that were comparable to the previous studies. Peak amplitude was quantified in FC units from the pixel with peak activity within the maximum areal extent for each of the two phases.
As there were no responses to quantify in animals that received whisker stimulation 3 h after ischemic onset (+3 h), the post-pMCAO imaging evoked area and amplitude were converted to difference score values (post-occlusion – baseline), with values away from 0 signifying a change from baseline. A constant was added in order to allow for ANOVA, scores were transformed with a natural log function to better satisfy the assumptions of an ANOVA, and inferential statistics were performed on the transformed data. The alpha level was set to 0.05 and Bonferroni adjustments were applied to account for multiple contrasts. Separate ANOVAs followed by respective contrasts were performed for the two phases of the whisker functional representation. The alpha level was set to 0.05 and Bonferroni adjustment applied to account for the two contrasts (p=0.05/2=0.025).
Electrophysiology: extracellular recording and analysis
We used extracellular recording to confirm that the extent of activity observed during functional imaging was reflective of whisker-evoked subthreshold/suprathreshold cortical activity. ISOI was performed to identify the location of peak optical activity evoked by whisker stimulation in order to guide the proper placement of electrodes for subsequent neuronal recording (Masino et al., 1993; Brett-Green et al., 2001; Frostig et al., 2008). Neural activity was filtered and amplified simultaneously from an in-line straight array of seven low-impedance (1–2 mΩ) tungsten microelectrodes (Microprobe) using a multi-channel acquisition system (Alpha Omega). The electrode array was positioned with the second electrode placed at the peak of ISOI activity. Suprathreshold [multi-unit activity (MUA)] and subthreshold [local field potential (LFP)] evoked neuronal activity was obtained from a depth of ~300–400 μm (supragranular layer) below the cortical surface. Recorded signals were amplified and bandpass (1–3000 Hz) filtered to allow the simultaneous capture of MUA and LFP from the same electrode, and then digitized at a rate of 24 kHz. Recording sessions consisted of the same whisker stimulation parameters as used during ISOI. Spike2 software was used for the off-line extraction of MUA and LFP by refiltering the collected data in either the 1–300 Hz range (LFP) or 300–3000 Hz range (MUA) for the subsequent analysis. A single average LFP waveform or MUA response in 1 ms bins was generated from 64 stimulation trials. LFP and MUA magnitudes were then calculated based on the first peak `on' response minus the mean obtained from a 1 s duration of pre-stimulus data.
Results
Isoflurane does not alter infarct volume post-permanent middle cerebral artery occlusion in untreated rats
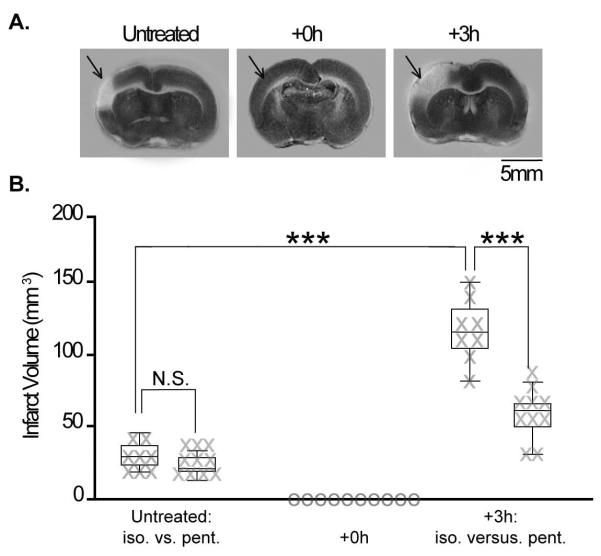
In order to establish whether isoflurane by itself has an effect upon the damage sustained following pMCAO, our first goal was to determine whether rats anesthetized with isoflurane (Fig. 2A) sustain a similar degree of infarct volume compared with those anesthetized with sodium pentobarbital. Untreated controls (rats underwent pMCAO but never received stimulation treatment; n=8) were compared with an identical untreated control group anesthetized with sodium pentobarbital (n=10, previously published data) (Lay et al., 2010, 2011). We found that untreated controls anesthetized with isoflurane sustained the same degree of infarct [range, 19.4–45.9 mm3, 30.6±3.2 mm3 (mean ±SEM)] as those anesthetized with sodium pentobarbital [previously published data; range, 13.0–35.0 mm3, 23.2±2.3 mm3 (mean ±SEM); t16 = −1.94, p=0.07; Fig. 2B, left] (Lay et al., 2010). Under these conditions, isoflurane did not influence infarct volume following pMCAO, and is therefore not protective in this experimental model.
Fig. 2.
Box-and-whisker plots, with individual data plotted, of the volume of infarct sustained by animals that underwent: pMCAO (no-stimulation controls), pMCAO and single whisker stimulation immediately (+0 h), or 3 h (+3 h) post-occlusion as assessed via 2,3,5-triphenyltetrazolium chloride assay for infarct. No-stimulation controls never received whisker stimulation, yet underwent anesthetic and surgical procedures (including pMCAO) that were identical to those of the experimental groups. Significant difference in infarct volume between groups (***p<0.0001).
Cortical function is completely protected as a result of immediate stimulation treatment
After confirming that isoflurane does not alter the ischemic challenge placed upon the cortex by pMCAO, we proceeded by studying the effects of stimulation treatment. All subjects underwent pMCAO, and were randomly assigned to one of two experimental groups (n=8 per group). Following pMCAO, whisker stimulation treatment (designed to mimic the rodent's natural whisker use) was delivered either immediately (+0 h group), or 3 h (+3 h group) after ischemic onset (Fig. 1).
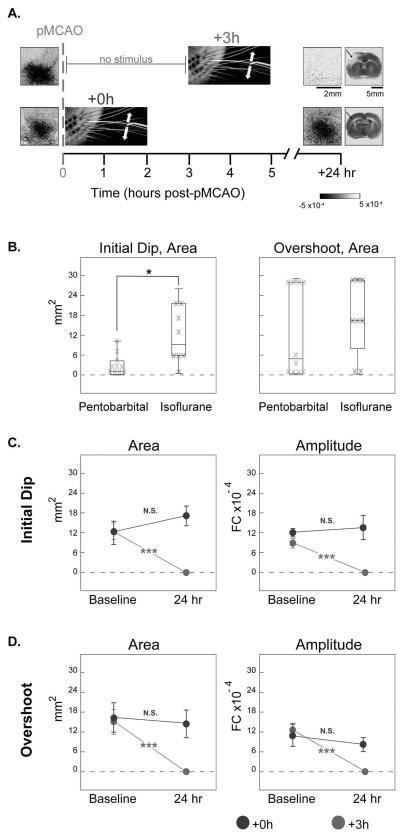
The ISOI revealed that rats that received treatment immediately after pMCAO (+0 h) maintained whisker functional representation 24 h post-pMCAO compared with baseline, and did not sustain stroke damage (Fig. 3A, bottom row). In contrast, treatment delivered 3 h post-pMCAO (+3 h) failed to restore whisker functional representation, and resulted in a substantial infarct (Fig. 3A, top row). Importantly, we also observed a large increase in the initial dip (t16 = −2.27, p=0.04) at baseline for all rats anesthetized with isoflurane compared with previous studies in which rats were anesthetized with sodium pentobarbital (Fig. 3B, left). In addition, the overshoot phase frequently extended beyond the region of the cortex being monitored (maximum area of cortex monitored was 28.04 mm2), artificially limiting the full areal extent of overshoot activity. As a result, there was also a trend for the overshoot to increase compared with rats anesthetized with sodium pentobarbital (Fig. 3B, right), although the difference was not significant.
Fig. 3.
Isoflurane is an effective alternative to sodium pentobarbital for use in sensory-induced neuroprotective studies. (A) Representative data from ISOI of the initial dip for +0 h and +3 h groups, and from 2,3,5-triphenyltetrazolium chloride (TTC) staining of +0 h and +3 h groups for comparison. All +0 h subjects regained whisker functional representation and did not sustain infarct, whereas +3 h rats never demonstrated any post-pMCAO cortical activity and sustained infarct larger than that of untreated controls (staining indicates healthy tissue, lack of staining would indicate ischemic infarct). Linear grayscale bar indicates intrinsic signal strength ×10−4. Scale bar below imaging data indicates 2 mm, the bar below TTC indicates 5 mm, and arrows indicate approximate region vulnerable to pMCAO infarct. (B) Box-and-whisker plots, with individual data plotted, for quantitative comparison of whisker functional representation observed at baseline in +0 h animals anesthetized with isoflurane (black) or sodium pentobarbital (gray). Both the area of the initial dip (left) and overshoot (right) are included for comparison. Significant difference between the area of the initial dip evoked while under sodium pentobarbital vs. isoflurane anesthesia (*p<0.05). Quantitative analysis of whisker functional representation in terms of area (left) and amplitude (right) of the initial dip (C) and overshoot (D). Group baseline and 24 h post-pMCAO data are plotted in each graph. A value of zero indicates no response. Means and SEs are provided for the area and amplitude of the whisker functional representation. Significant differences between group baseline and 24 h values (***p<0.001).
Given the increase in activity, we chose to quantify all ISOI data (Fig. 3C and D) at a higher threshold of analysis to achieve areal extent values that were comparable to the previous studies (Fig. 4). The area and amplitude of evoked activity were quantified for the first two phases (initial dip and overshoot) of the whisker functional representation. Between-subject ANOVAs of imaging data were conducted between +0 h and +3 h rats at baseline, and at 24 h following treatment. At baseline, there were no between-group differences in the initial dip (area: F1,14= 0.01, p=0.91; amplitude: F1,14= 3.40, p= 0.09; ANOVA), or overshoot phases (area: F1,14= 0.07, p= 0.80; amplitude: F1,14= 0.07, p=0.79; ANOVA).
Fig. 4.
The whisker functional representation is quantified at a higher threshold of analysis under isoflurane anesthesia. Each phase of the whisker functional representation may be rendered three-dimensionally by plotting FC along the z-axis and its two-dimensional areal extent may be visualized and quantified at incrementally higher thresholds. The threshold used in this study is 5.0 × 10−4 FC (used to quantify the area of activity). Although previous work has utilized 2.5 × 10−4 FC, the higher 5.0 × 10−4 FC threshold was chosen here to achieve areal extent values that were comparable to the previous studies. Outlined in white, a +0 h subject's initial dip is visualized at 2.5 × 10−4 FC under sodium pentobarbital anesthesia (left), and a +0 h subject's initial dip is visualized at 5.0 × 10−4 FC under isoflurane (right).
At 24 h after pMCAO, differences were found between groups for both the initial dip and overshoot (initial dip area: F1,14= 16.37, p=0.001; amplitude: F1,14= 22.58, p= 0.0003; overshoot amplitude: F1,14= 5.73, p= 0.03; ANOVA). In the +0 h group, every subject's entire whisker functional representation remained at baseline levels, and the entire whisker functional representation for every subject in the +3 h group had been reduced to zero (initial dip area: F1,14= 21.69, p=0.0004; amplitude: F1,14= 33.62, p= 0.00005; overshoot amplitude: F1,14= 18.58, p= 0.0007; Fig. 3C and D).
Although the overshoot area shared the same trends as the initial dip (+0 h maintain the whisker functional representation, whereas the whisker functional representation is reduced to 0 in the +3 h group, for subjects anesthetized with isoflurane or pentobarbital), a between-group ANOVA performed on the overshoot area did not find a significant difference (F1,14= 3.54, p= 0.08; ANOVA). Given the strong qualitative difference observed between rats that maintained the overshoot area (+0 h) and rats that lost the overshoot area (+3 h), however, we sought to statistically evaluate this observation with two paired t-tests, performed separately on each group. The change in overshoot area after pMCAO was significant in the +3 h group (t7 = 4.34, p=0.003), but remained unchanged in the +0 h group (t7 = 0.35, p=0.74).
In summary, when stimulation treatment was delivered immediately (+0 h) after pMCAO, whisker functional representation was re-established to levels equivalent to baseline and there was no infarct 24 h post-pMCAO. Treatment delivered 3 h post-pMCAO (+3 h) failed to restore whisker functional representation and resulted in substantial infarct (Fig. 3A, C and D).
Stimulation treatment results in an increase in cortical damage when delivered 3 h post-permanent middle cerebral artery occlusion
An additional critical factor of stimulation treatment 3 h following pMCAO (+3 h) was that treatment at this time increased the degree of infarct sustained compared with controls (Lay et al., 2010; Davis et al., 2011). In order to determine whether the same increase in stroke damage occurred under isoflurane, we compared the infarct volume sustained by +3 h subjects with untreated controls. We found that +3 h animals anesthetized with isoflurane sustained infarct volumes averaging 119.3±8.1 mm3 (range, 83.1–153.7 mm3; Fig. 2B, right). This value was significantly greater than that of untreated controls also anesthetized with isoflurane (t14 = 10.20, p=7.3 ×10−8; Fig. 2B). This result is in agreement with previous findings, which have shown that sensory stimulation delivered 3 h post-pMCAO exacerbates ischemic damage (Lay et al., 2010; Davis et al., 2011).
Interestingly, differences in infarct volume were also observed between +3 h subjects anesthetized with isoflurane and +3 h subjects anesthetized with sodium pentobarbital (Fig. 2B, right). We found that +3 h subjects anesthetized with isoflurane sustained larger infarcts than those of +3 h subjects anesthetized with sodium pentobarbital [previously published data; range, 30.4–82.6 mm3; mean, 59.2±4.4mm3 (mean ±SEM); t16 = −6.90, p=3.6 ×10−6 (Lay et al., 2010, 2011).
Neural recordings support the extent of activity observed during functional imaging
What might explain the increase in the volume of infarct sustained by +3 h subjects anesthetized with isoflurane vs. those anesthetized with sodium pentobarbital? Previously, we have argued that cortical activation may play a key role in determining the extent of infarct sustained by +3 h subjects (Lay et al., 2010; Davis et al., 2011; Frostig et al., 2012). Given that +3 h subjects under isoflurane demonstrate a greater extent of cortical activation (measured here as whisker functional representation) in response to single whisker stimulation when compared with those under sodium pentobarbital, we wished to confirm that the increase in whisker functional representation was truly reflective of evoked cortical subthreshold and suprathreshold activity. We therefore corroborated our ISOI data by directly measuring neuronal activity.
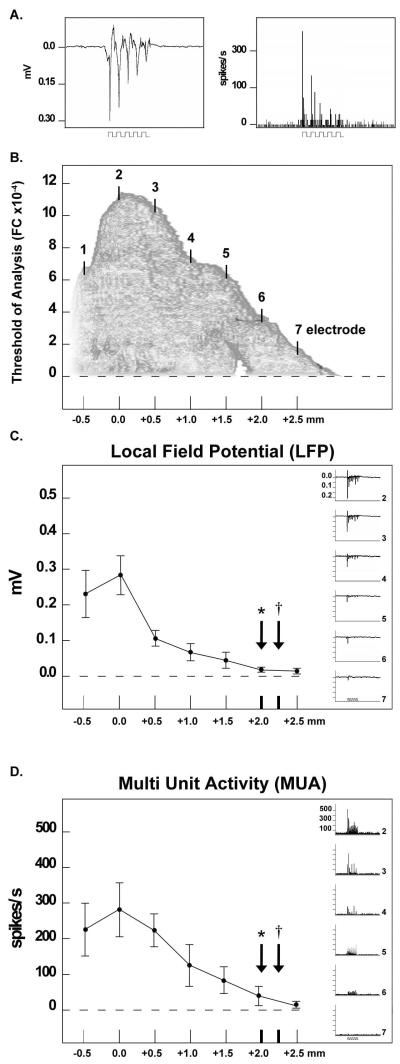
The LFP (subthreshold activity) and MUA (suprathreshold activity) were recorded from control rats (n=5, Fig. 5) using a whisker stimulation paradigm that was identical to that used during imaging. Two primary observations were made. First, unlike the neuronal response to whisker stimulation under sodium pentobarbital, LFP and MUA activity undergo a `fast-adapting' response to whisker stimulation such that evoked activity rapidly decreases across each of the five whisker deflections during stimulation (Fig. 5A, left and right). Second, in order to compare the extent of LFP, MUA, and whisker functional representation, the seven-electrode array was positioned so that electrode 2 was located within the peak of the initial dip (Fig. 5B). Both LFP (Fig. 5C) and MUA (Fig. 5D) met and surpassed the average radius of the initial dip and overshoot phases of the whisker functional representation, which were collected for each rat prior to neural recording and analyzed at the 5.0×10−4 (FC) threshold of analysis used in this study (Fig. 5C and D). These results served as confirmation that our imaging data reflected the extent of whisker-evoked activity. Moreover, it should be noted that the whisker functional representation is most typically quantified at 2.5×10−4 (FC), half that of the threshold of analysis employed here. Even the seven-electrode array, which covered a line of 2.5 mm between the electrode located at the peak of the initial dip (electrode 2) and the furthest electrode (electrode 7), does not record the full extent of evoked activity, and therefore under-represents the striking degree of cortical activation that occurs as a result of whisker stimulation in rats anesthetized with isoflurane.
Fig. 5.
Evoked neuronal activity underlies the whisker functional representation. (A) Representative LFP (measured in mV) and MUA (measured in spikes/s) responses recorded from a control rat anesthetized with isoflurane. Stepping function indicates stimulus delivery. Note the fast adaptation of both the LFP and MUA response to whisker stimulation. (B) Approximate placement of the seven-electrode array superimposed upon the initial dip. The X-axis represents the distance from the peak of the initial dip. The Y-axis represents the amplitude of whisker-evoked initial dip activity at a given distance from the peak. In this example, the initial dip is an average of the five rats used for extracellular recording. Evoked LFP (C) and MUA (D) activity plotted along the electrode array. Mean and SE are plotted for each recording site. The X-axis represents the distance from the peak of whisker-evoked activity, and arrows along the X-axis represent the average radius of the initial dip (arrows with asterisks) and overshoot (arrows with daggers) observed in recording subjects during ISOI mapping. Insets: representative LFP and MUA data from recording electrodes 2 (peak activity) to 7. Stepping function indicates stimulus delivery.
Furthermore, the observed increase in whisker functional representation, LFP, and MUA all coincided with an increase in infarct sustained in +3 h subjects. This result further strengthens our previous suggestion that the extent of evoked cortical activity plays a critical role in determining the extent of ischemic infarct. The finding that activity plays a central role in the evolution of infarct is perhaps surprising given that, currently in both humans and rats, dogma states that the area of the brain that sustains infarct is dictated solely by the spatial architecture of the vasculature that it is supplied by (Coyle, 1986; 1987; Wei et al., 1995; Wei et al., 1998; Dirnagl et al., 1999; Caplan, 2009). In contrast, however, we have suggested that cortical activity may play a central role in determining the extent of protection conferred during early treatment, and the extent of infarct sustained during late treatment (3 h) following pMCAO (Lay et al., 2010; Davis et al., 2011; Lay et al., 2011; Frostig et al., 2012).
Discussion
This study characterized the impact of isoflurane use upon our laboratory's experimental model of stroke (pMCAO), and employed a two-pronged approach to determine whether isoflurane has positive or negative effects following pMCAO. First, we found that isoflurane does not affect the volume of ischemic damage sustained by adult rats that undergo pMCAO vs. those anesthetized with sodium pentobarbital. We then characterized the effects of single whisker stimulation treatment (stimulation was intermittently delivered 256 times, with random intervals averaging 21 s, totaling 4.27 min of stimulation, over the course of 2 h), and found that the adult rat cortex responds to protective (+0 h) treatment, and harmful (+3 h) treatment in a manner similar to previously published research utilizing sodium pentobarbital as the anesthetic (Lay et al., 2010; Davis et al., 2011; Frostig et al., 2012; Lay et al., 2012). Finally, we found that there was an increase in the extent of the cortex activated by single whisker stimulation, and report a strong correspondence between functional imaging (ISOI) and electrophysiological recordings taken under isoflurane administration. Together, these results demonstrate that sensory-induced protection from ischemic stroke is not affected by the use of isoflurane, and allow for highly detailed investigation of potential novel treatments for ischemic stroke using awake, behaving animals.
In addition to the data presented here, a large amount of pre-clinical study has gone into the investigation of isoflurane as a neuroprotective agent. It has been reported that rats having undergone hemispheric ischemia had smaller infarct volumes after being exposed to isoflurane vs. nitrous oxide sedation (Baughman et al., 1988). However, in a study by Warner et al. (1986), rats showed no benefit from isoflurane pre-treatment vs. untreated controls vs. transient carotid artery occlusion, although this discrepancy could be due to the location or method of infarct. Baboons under isoflurane had a worse outcome vs. thiopental or fentanyl groups following a transient MCAO (Nehls et al., 1987), and macaques received no benefit from isoflurane vs. halothane (Gelb et al., 1989). In a human study, subjects maintained neuronal electrical activity at significantly lower levels of cerebral blood flow during isoflurane anesthesia vs. halothane controls (Michenfelder et al., 1987). When considering this body of work in totality, the two major challenges to making effective between-group comparisons are: the use of many forms of anesthesia between studies, and the lack of standardization of isoflurane delivery (isoflurane concentration, oxygen, oxygen/nitrogen mixes). Whether (and in what cases) isoflurane is capable of effective neuroprotection remains unclear. In our hands, pMCAO results in a consistent infarct size, and stimulation treatment remains effective in completely protecting both the function and structure of the cortex under isoflurane administration when delivered in 100% oxygen, compared with sodium pentobarbital-anesthetized rats.
With respect to our laboratory's utilization of isoflurane vs. sodium pentobarbital, two critical differences readily became apparent. (i) Even relatively low concentrations (1–1.5%) of isoflurane can impact neuroimaging (Berger et al., 2007; Alkire, 2008; Alkire et al., 2008; Schummers et al., 2008). Whisker stimulation resulted in a large area of cortical activation under isoflurane, and necessitated the use of a higher threshold of analysis, double our typical threshold used for quantifying the whisker functional representation of animals anesthetized with sodium pentobarbital. (ii) Whereas untreated controls sustained equal infarct volumes when anesthetized with either anesthesia, rats that received stimulation treatment 3 h post-pMCAO (+3 h) sustained greater infarct volumes when anesthetized with isoflurane than those anesthetized with sodium pentobarbital.
Is there a relationship between these two key differences? Using ISOI to assess evoked cortical function, we found a significant increase in the extent of the initial dip under isoflurane when compared with functional imaging conducted using sodium pentobarbital. Using extracellular recording, we found that both subthreshold and suprathreshold neural activity underlie the large extent of evoked activity observed. Previous work in our laboratory has demonstrated that an increase in the extent of cortical activation 3 h post-stroke onset results in an increase in infarct volume sustained (Davis et al., 2011). In this case, an increase in cortical activation extent was achieved by stimulating the entire whisker array rather than a single C2 whisker. We therefore hypothesized that an isoflurane-induced increase in cortical activation would similarly result in an increase in infarct volume amongst +3 h rats compared with +3 h rats anesthetized with sodium pentobarbital. This hypothesis was supported by the finding that +3 h rats anesthetized with isoflurane sustained a significantly greater infarct volume (119.3±8.1 mm3), which is nearly double the average infarct volume sustained by +3 h subjects under sodium pentobarbital anesthesia (61.8±3.7 mm3). Furthermore, in order to obtain comparable whisker functional representation extent values, the threshold of analysis needed to quantify our functional imaging data was also twice the normal threshold (2.5 ×10−4 to 5.0 ×10−4 FC), further suggesting that an even more explicit relationship exists between the area of activation and infarct volume sustained. Together, these findings further underscore the powerful role that cortical activity plays within the context of cortical ischemia.
Although differences in the amplitude and areal extent of whisker functional representation were observed under isoflurane vs. sodium pentobarbital, several key similarities were also observed that allowed us to resolve the issue of whether this anesthetic is an appropriate and logical alternative to sodium pentobarbital.
First, although fiercely debated, isoflurane administration has been found to be neuroprotective in multiple animal models of stroke (Kawaguchi et al., 2000; Warner, 2000; Sakai et al., 2007). Our laboratory employs a permanent occlusion of the middle cerebral artery (pMCAO), and our data showed that rats that undergo pMCAO and never receive whisker stimulation sustained equal infarct volumes when sedated with either isoflurane or sodium pentobarbital. Therefore, in our model, we concluded that isoflurane is not neuroprotective. Second, stimulation treatment delivered immediately following pMCAO resulted in the complete protection of cortical function and structure as investigated via ISOI and 2,3,5-triphenyltetrazolium chloride histology. The finding that this level of protection occurs irrespective of a particular anesthetic state considerably widens the applicability of this potential treatment strategy.
We have shown previously that every young adult and aged rat that receives stimulation treatment immediately post-pMCAO also demonstrates fully intact behavioral capability and cortical structure when assessed 1 week following ischemic onset (Lay et al., 2010, 2012). We have also demonstrated that the whisker functional representation, in addition to blood flow, physical capability, and tissue remain completely intact up to 4 months post-pMCAO and immediate stimulation treatment (Hancock, 2012). Although these findings strongly indicate that immediate stimulation treatment administered under isoflurane would also confer a similarly long-lasting protective effect, this question is beyond the scope of the current study.
Finally, +3 h rats anesthetized with isoflurane lose all cortical function and sustain exacerbated infarct in a manner that is very similar to +3 h rats anesthetized with sodium pentobarbital. The finding that the identical stimulation treatment is protective when delivered early (immediate treatment) and harmful when delivered too late (3 h treatment) indicates that the effects of sensory-induced activity occur irrespective of anesthesia type, and reflects protective processes innate to brain function. This would support the notion that the same brain processes described here may be applicable to the human stroke sufferer.
Given the high degree of similarity between these results and those that used sodium pentobarbital, we conclude that, despite the changes in cortical activation patterns compared with sodium pentobarbital, the use of isoflurane anesthesia does not affect outcome in our particular stroke model, at least by 24 h following stroke onset. In our hands, pMCAO results in a consistent infarct size under isoflurane administration. With the addition of stimulation treatment, we found that animals treated immediately post-pMCAO were completely protected from cortical dysfunction and infarct, whereas those treated 3 h post-pMCAO lost all function and sustained significant ischemic damage. Finally, we found a strong correspondence between functional imaging with subthreshold and suprathreshold recordings, and noted a large extent of evoked cortical activation with both techniques. Together, these findings further support the dynamic nature of sensory-induced protection from ischemic stroke, underscore its independence upon a particular anesthetic state, and allow for future studies to more accurately reflect the state of stroke victims by providing the possibility for awake, behaving study.
Acknowledgements
We thank Quynh Vu for her contribution to the experiments herein. This work was supported by the American Heart Association Predoctoral Fellowship 788808-41910, NIH-NINDS NS-066001 and NS-055832, and The Center for Hearing Research NIH Training Grant 1T32DC010775-01.
Abbreviations
- +0 h
whisker stimulation treatment delivered immediately following ischemic onset
- +3 h
whisker stimulation treatment delivered 3 h post-ischemic onset
- FC
fractional change
- ISOI
intrinsic signal optical imaging
- LFP
local field potential
- MUA
multi-unit activity
- pMCAO
permanent middle cerebral artery occlusion
Footnotes
Conflicting Interests The authors declare no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article.
References
- Alkire MT. Probing the mind: anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. doi: 10.1038/clpt.2008.75. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman VL, Hoffman WE, Miletich DJ, Albrecht RF, Thomas C. Neurologic outcome in rats following incomplete cerebral ischemia during halothane, isoflurane, or N2O. Anesthesiology. 1988;69:192–198. doi: 10.1097/00000542-198808000-00007. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Berger T, Borgdorff A, Crochet S, Neubauer FB, Lefort S, Fauvet B, Ferezou I, Carleton A, Luscher HR, Petersen CC. Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol. 2007;97:3751–3762. doi: 10.1152/jn.01178.2006. [DOI] [PubMed] [Google Scholar]
- Brett-Green BA, Chen-Bee CH, Frostig RD. Comparing the functional representations of central and border whiskers in rat primary somatosensory cortex. J Neurosci. 2001;21:9944–9954. doi: 10.1523/JNEUROSCI.21-24-09944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LR. Caplan's Stroke, A Clinical Approach. Saunder's & Elsevier; Phildelphia: 2009. [Google Scholar]
- Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Bee CH, Polley DB, Brett-Green B, Prakash N, Kwon MC, Frostig RD. Visualizing and quantifying evoked cortical activity assessed with intrinsic signal imaging. J Neurosci Methods. 2000;97:157–173. doi: 10.1016/s0165-0270(00)00180-1. [DOI] [PubMed] [Google Scholar]
- Coyle P. Different susceptibilities to cerebral infarction in spontaneously hypertensive (SHR) and normotensive Sprague-Dawley rats. Stroke. 1986;17:520–525. doi: 10.1161/01.str.17.3.520. [DOI] [PubMed] [Google Scholar]
- Coyle P. Spatial relations of dorsal anastomoses and lesion border after middle cerebral artery occlusion. Stroke. 1987;18:1133–1140. doi: 10.1161/01.str.18.6.1133. [DOI] [PubMed] [Google Scholar]
- Davis MF, Lay CC, Chen-Bee CH, Frostig RD. Amount but not pattern of protective sensory stimulation alters recovery after permanent middle cerebral artery occlusion. Stroke. 2011;42:792–798. doi: 10.1161/STROKEAHA.110.607135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Chen-Bee CH. Visualizing adult cortical plasticity using intrinsic signal optical imaging. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. CRC Press; 2009. pp. 255–287. [PubMed] [Google Scholar]
- Frostig RD, Lay CC, Davis MF. A rat's whiskers point the way toward a novel stimulus-dependent, protective stroke therapy. Neuroscientist. 2012 doi: 10.1177/1073858412462607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U.S.A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Xiong Y, Chen-Bee CH, Kvasnak E, Stehberg J. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J Neurosci. 2008;28:13274–13284. doi: 10.1523/JNEUROSCI.4074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb AW, Boisvert DP, Tang C, Lam AM, Marchak BE, Dowman R, Mielke BW. Primate brain tolerance to temporary focal cerebral ischemia during isoflurane- or sodium nitroprusside-induced hypotension. Anesthesiology. 1989;70:678–683. doi: 10.1097/00000542-198904000-00023. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hancock AM, Lay CC, Davis MF, Frostig RD. Neuroscience 2012. Society for Neuroscience; New Orleans, LA, USA: 2012. Protection of Rodent Cortex from Ischemic Stroke by Mild Sensory Stimulation Remains Stable Over Time. [Google Scholar]
- Kawaguchi M, Kimbro JR, Drummond JC, Cole DJ, Kelly PJ, Patel PM. Isoflurane delays but does not prevent cerebral infarction in rats subjected to focal ischemia. Anesthesiology. 2000;92:1335–1342. doi: 10.1097/00000542-200005000-00023. [DOI] [PubMed] [Google Scholar]
- Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS One. 2010;5:e11270. doi: 10.1371/journal.pone.0011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation reestablishes cortical function during the acute phase of ischemia. J Neurosci. 2011;31:11495–11504. doi: 10.1523/JNEUROSCI.1741-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation protects the aged rodent from cortical ischemic stroke following permanent middle cerebral artery occlusion. Journal of the American Heart Association Cardiovascular and Cerebrovascular Disease. 2012 doi: 10.1161/JAHA.112.001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Kwon MC, Dory Y, Frostig RD. Characterization of functional organization within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proc Natl Acad Sci U.S.A. 1993;90:9998–10002. doi: 10.1073/pnas.90.21.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michenfelder JD, Sundt TM, Fode N, Sharbrough FW. Isoflurane when compared to enflurane and halothane decreases the frequency of cerebral ischemia during carotid endarterectomy. Anesthesiology. 1987;67:336–340. doi: 10.1097/00000542-198709000-00010. [DOI] [PubMed] [Google Scholar]
- Nehls DG, Todd MM, Spetzler RF, Drummond JC, Thompson RA, Johnson PC. A comparison of the cerebral protective effects of isoflurane and barbiturates during temporary focal ischemia in primates. Anesthesiology. 1987;66:453–464. doi: 10.1097/00000542-198704000-00002. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. discussion 98–10. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Ts'o DY, Frostig RD, Lieke EE, Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990;249:417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- Warner DS. Isoflurane neuroprotection: a passing fantasy, again? Anesthesiology. 2000;92:3. [PubMed] [Google Scholar]
- Warner DS, Deshpande JK, Wieloch T. The effect of isoflurane on neuronal necrosis following near-complete forebrain ischemia in the rat. Anesthesiology. 1986;64:19–23. doi: 10.1097/00000542-198601000-00004. [DOI] [PubMed] [Google Scholar]
- Wei L, Craven K, Erinjeri J, Liang GE, Bereczki D, Rovainen CM, Woolsey TA, Fenstermacher JD. Local cerebral blood flow during the first hour following acute ligation of multiple arterioles in rat whisker barrel cortex. Neurobiol Dis. 1998;5:142–150. doi: 10.1006/nbdi.1998.0199. [DOI] [PubMed] [Google Scholar]
- Wei L, Rovainen CM, Woolsey TA. Ministrokes in rat barrel cortex. Stroke. 1995;26:1459–1462. doi: 10.1161/01.str.26.8.1459. [DOI] [PubMed] [Google Scholar]