Abstract
Research on alcohol and drug dependence has shown that the development of addiction depends on a complex interplay of psychological factors, genetic or epigenetic predisposing factors, and neurobiological adaptations induced by drug consumption. A greater understanding of the mechanisms leading to alcohol abuse will allow researchers to identify genetic variation that corresponds to a specific biological vulnerability to addiction, thus defining robust endophenotypes that might help deconstruct these complex syndromes into more tractable components. To this end, it is critical to develop a translational framework that links alterations at the molecular level, to changes in neuronal function, and ultimately to changes at the behavioral and clinical levels. Translational phenotypes can be identified by the combination of animal and human studies designed to elucidate the neurofunctional, anatomical and pharmacological mechanisms underlying the etiology of alcohol addiction. The present article offers an overview of medication development in alcoholism with a focus on the critical aspect of translational research. Moreover, significant examples of promising targets from neuropeptidergic systems, namely nociceptin/orphanin FQ and neuropeptide S are given.
Keywords: Alcoholism, Drug abuse, Addiction, Translational Medicine, Brain Imaging
Introduction
Alcoholism is one of the most widespread form of addiction and has one of the highest negative social, medical and economical impact on our societies In recent years several approaches have been investigated to help alcohol abusers to not only control alcohol drinking but also alcohol cravings and relapse. Medications such as disulfiram, naltrexone (injectable or oral), and acamprosate have been developed and approved for the treatment of alcoholism [1]. While all of these medications have demonstrated effectiveness in reducing alcohol abuse, there are limitations associated with each option, such as limited efficacy, occurrence of side effects and high dropout rates Clearly, the continued development of effective pharmacotherapies for alcohol dependence is needed. Drug development, which is classically carried out by the pharmaceutical industry is a complex process that requires a multilevel approach, is extremely expensive and takes several years. Compared to other disease areas the pharmaceutical industry has historically invested limited resources in drug development programs for alcoholism, which may explain, at least in part, the paucity of approved medication so far available. Several factors may explain the lack of interest in developing medication for alcoholism by private industry. First is the stigma that is still associated with alcoholism and addiction in general. There are problems associated with the coverage of the medication costs by the private insurance or the public health systems and, differently from other diseases, little desire to treatment by a substantial proportion of patients that is unable to recognize alcoholism as a medical condition.
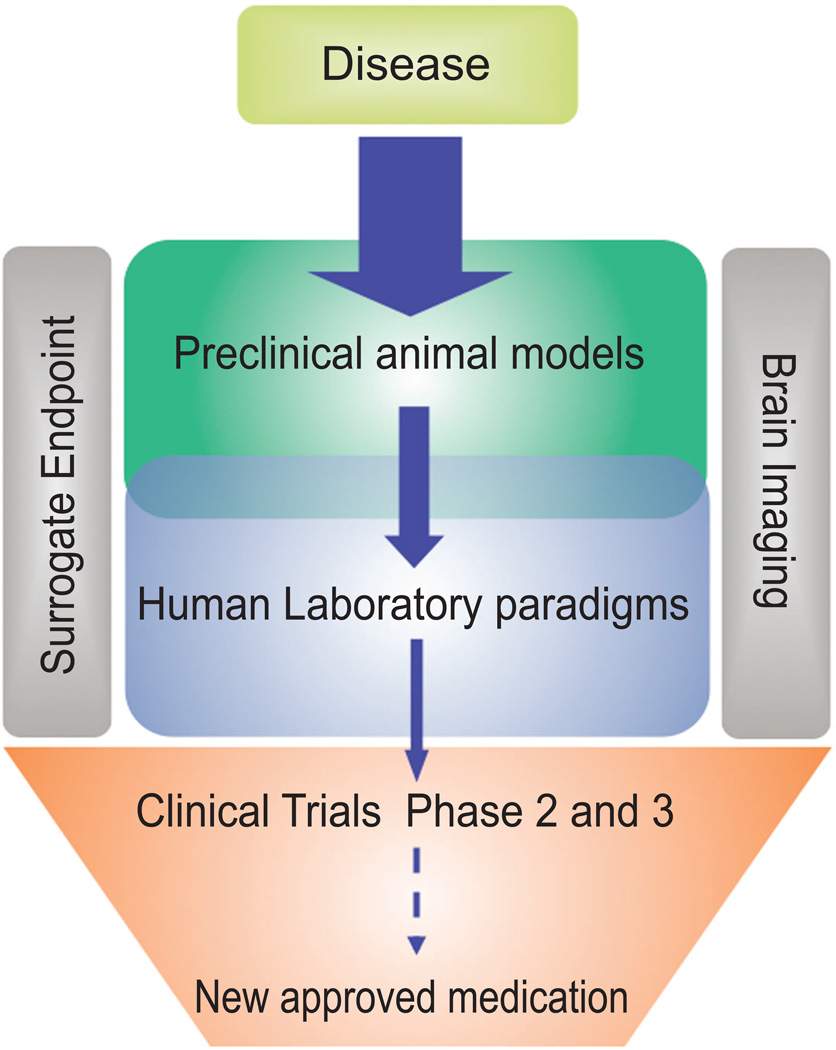
A second important limitation is the complexity of the disease which dramatically reduce the expectation of the industry to successfully develop a medication from lab to marketplace. Over the years, preclinical research has identified a large number of promising biological targets for alcoholism and several promising molecules are available. However, the limited number of medication successfully developed so far, as well as the lack of a well validated development path severely limit the interest of the pharmaceutical industry in this area. The development of a clear and well structured translational approach for medication development is a major challenge in alcohol addiction research (Fig.1). The present article offers an overview of the-state-of-art in medication development in alcoholism focusing on critical aspect of translational research. Moreover, significant examples of promising targets from neuropeptidergic systems, namely nociceptin/orphanin FQ (N/OFQ) and neuropetide S will be presented.
Figure 1.
Schematic representing the critical steps of translational research. A relatively large number of biological targets and promising chemical entities are available for preclinical investigation. The availability of well validated animal models is critical to screen these molecules. A limited number of molecules with satisfactory pharmacological and toxicological profile are moved into the clinic. Human laboratory paradigms can be used to provide initial evidence of efficacy in humans. The utilization of appropriate surrogate markers that possibly overlap preclinical endpoints (i.e, alcohol intake, cue- and stress- induced alcohol craving) is fundamental to translate preclinical findings into meaningful clinical information. Brain imaging techniques play a critical role in bridging preclinical and clinical research: their use provides an unprecedented help in new medication development .
Brain Imaging Technologies a Bridge Between Preclinal and Clinical Research
Neuroimaging methods have been extensively applied to study the human brain and its structural and functional organization in healthy and disease states. Imaging techniques enable the researcher to explore endophenotypes that are more proximal to the biological mechanisms underlying the risk for the development of alcohol use disorders. An important advantage of the neuroimaging approach is that the output does not rely on subjective reports of an effect, but rather measure a biologically-based expression of the phenotype. Recent developments have extended this approach to animal models, thus paving the way to a translational use of neuroimaging techniques to bridge clinical and preclinical research.
Among the various imaging modalities, two have emerged as particularly impactful in addressing psychiatric disorders like addiction and alcohol dependence, and amenable to application in both humans and laboratory animals: Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET).
Brain imaging techniques have been extensively used to investigate morphological, metabolic and functional changes associated with alcohol abuse in humans. Morpho-anatomical studies have revealed reduced grey matter (GM) volume in alcoholic patients, in the frontal lobes, the cerebellum and the limbic system showing the most pronounced abnormalities [2–6]. Such alterations have been recently demonstrated to be predictive of relapse risk, suggesting a significant role for grey matter shrinkage in clinical outcomes in alcoholism [•5]. White matter abnormalities as well as numerous functional and neuro-metabolic deficits (reviewed by [2]) have also been reported in heavy consumers of alcohol [7,8]. Reduced resting-state metabolism in frontal-parietal, orbitofrontal cortex and striatal areas in active and abstinent alcohol-abusers has also been reported [9–11].
An unanswered question in alcohol research is whether these alterations are the sole consequence of chronic alcohol use, or also represent an innate factor contributing to biological propensity toward ethanol addiction. Recent neuorimaging studies have begun to address this question. Individuals at high-risk for alcohol dependence have been shown to have altered sensitivity of the reward circuitry [12–14], and reductions in cortical and thalamic grey matter volumes [15], two features commonly observed in abstinent alcoholic patients. Importantly, the presence of shared fronto-striatal abnormalities has also been recently reported in drug-naïve siblings of psycho- stimulant drug abusers [16]. These preliminary findings highlight a putative role for inborn morpho-functional brain abnormalities in the aetiology of alcohol-dependence.
Neuroimaging studies in preclinical species exploring the role of heritable brain abnormalities as a vulnerability factor for alcoholism have only very recently started appearing in the literature [••17]. The genetically selected alcohol preferring msP rat was chosen as an established selection-based model for the investigation of the neurobiology of alcoholism closely mimicking several fundamental aspects of human disease such as the occurrence of binge-like ethanol drinking [18], psychological withdrawal symptoms escalating alcohol intake upon abstinence and highvulnerability to stress-mediated relapse [19]. Importantly, the model also reproduces important comorbid states pervasively associated with alcoholism, such as increased sensitivity to stress, anxious phenotype and depressive-like symptoms [18,19].
Structural and functional MRI was applied to study alterations in brain morphometry and basal metabolism in this model. msP rats exhibited reduced grey matter volume in the thalamus, ventral tegmental area, insular and cingulate cortex, consistent with observations in abstinent alcoholics and in individuals at high risk of alcohol dependence [••17]. As the animals imaged in this study were alcohol-naïve, this work suggests that some of the morpho-functional alterations documented in alcoholics may reflect a pre-existing endophenotype predisposing to alcohol addiction. Recent clinical data lends preliminary evidence to this hypothesis.
While MRI approaches will give information about the morphoanatomical alterations related to alcohol dependence, and can help establish a link between behavior and brain circuits, they do not provide specific information about its neurochemical determinants. To this end, molecular PET-imaging represents a powerful means to explore the neurochemistry of addiction, and the specific receptor and neurotransmitter systems involved. However, PET imaging relies on the availability of selective radiotracer ligands. Currently, no more than 25 targets can be quantified in the human brain, and dopamine is the only system for which transmitter-sensitive radioligands have been extensively used. With regard to peptidergic neurotransmission the only PET ligand available until recently was [11C]carfentanil which allowed the exploration of mu opioid receptor (MOP) receptor function in brain diseases including addiction [20].
A new advancement in the study of opioid peptide neutrasmission is the very recent development of PET radioligands for the kappa opioid receptor (KOP) and the N/OFQ receptor (NOP) [••21,••22]. The availability of these new tools will allow investigators to better determine the role of these receptors in psychiatric research. For example, referring to alcoholism, they could be used to investigate if KOP and NOP receptors may have an abnormal distribution in alcoholic patients, thus providing evidence of the involvement of abnormal peptidergic neurotransmission in the aetiology of alcohol dependence; but avalibility to these ligands will also facilitate the development of drugs targeting this system.
Neuropeptide systems as a target of novel medication for alcoholism
Neuropeptides have always received much attention in the alcohol field, the main reason being the early discovery of the key role of opioid neurotransmission in mediating alcohol reward, withdrawal-induced dysphoria and relapse [23,24]. Over the years, in addition to opioids, the involvement of several other neuropetidergic systems in the physiophathology of alcoholism has been documented. Some of these neurotransmitter systems are now under deep scrutiny because they are considered as highly promising targets for medication development. For some of these neuropeptide targets (i.e., Corticotrophin Releasing Factor receptor 1 and Neurokinin receptor 1), clinical stage molecules are already available and initial studies in humans have been already carried out or are underway. Medication development programs and translational approaches related to these targets have been recently covered by comprehensive studies and will not be the focus in the present review (for review see: [25,26]. Here, we will focus instead on two less explored but highly promising peptidergic systems, the nociceptin/orphanin FQ and the neuropeptide S, that are currently being subjected to intense exploration and are considered highly promising targets for medication development in alcohol addiction.
Nociceptin/Orphanin FQ System
Nociceptin/orphanin FQ is a 17 amino acid neuropeptide, structurally related to the opioid peptide dynorphin A and binds to its cognate receptor opioid receptor-like1 (ORL1) now named NOP receptor.
N/OFQ and NOP receptors are widely distributed in the brain, where they are largely co-expressed .. Despite being opioid-like, N/OFQ acts in the brain to produce functional anti-opioid effects. For instance, it blocks opioid-induced supraspinal analgesia [27], morphine-induced conditioned place preference [28,29] and morphine induced increases in extracellular dopamine levels in the nucleus accumbens [30].
Moreover, activation of NOP receptors by N/OFQ or by synthetic agonists produces anxiolytic-like effects [31,32] that appear to be particularly robust under stressful conditions, such as e.g. during alcohol withdrawal [•33]. This may depend upon the ability of N/OFQ to act as a functional antagonist for extrahypothalamic actions of Corticotrophin Releasing Factor (CRF) and CRF1R receptor activation [34,35].
Consistent with the anti-opioid nature of N/OFQ it has been shown that activation of the NOP receptors blunts the reinforcing and motivational effects of alcohol across a range of behavioral measures, including alcohol intake [36], conditioned place preference [37] and relapse to alcohol seeking triggered by alcohol associated cues [38]. Whereas, in agreement with its antiCRF properties it has been shown that N/OFQ administration prevents foot-shock stress-induced reinstatement of alcohol seeking in the rat [39].
Studies in msP rats have shown that they are particularly sensitive to suppression of alcohol drinking and relapse by N/OFQ and N/OFQ analogues [36,38,40]. msP rats exhibit high innate sensitivity to stress, and high measures of both anxiety- and depression-like behaviors that are ameliorated by alcohol consumption [18]. Hence, N/OFQ effects in msP rats are in part likely due to its ability to alleviate a negative emotional state that otherwise provides an incentive for negatively reinforced alcohol consumption. If this hypothesis is correct, one could predict that a NOP agonist might be particularly efficacious in alcoholic patient that drink to self-medicate from a negative affective state or for tension reduction purposes.
From the translational point of view, an exciting recent development was the discovery that buprenorphine, a drug currently employed for pain management and heroin addiction treatment, in addition to its ability to activate MOP and to block KOP receptors at higher dose it also activates NOP receptors [41,42]. Interestingly, similar to prototypical MOP agonists, buprenorphine at low doses increased ethanol consumption in the rat, an effect that was blocked by co-administration of the MOP preferential antagonist naltrexone [43–45]. At higher doses, buprenorphine markedly reduced alcohol intake and this effect was blocked by the selective NOP antagonist UFP 101 but not by naltrexone. These findings indicate that at low doses buprenorphine increases alcohol intake via stimulation of classic opioid receptors, while at higher doses reduces it via activation of NOP receptors [•46]. An intriguing finding from studies on heroin addicts was that treatment with buprenorphine also attenuates alcohol consumption in these patients [47,48] whereas methadone, the other opioid agonist used to treat heroin addiction, appears to be less efficacious on alcohol and in some studies was even shown to increase drinking [47,49]. Although these findings point to the possibility that NOP receptor activation by buprenorphine is responsible for these effects on alcohol in the absence of clinically available N/OFQ antagonists this hypothesis hard to demonstrate.
Very recently, 11C-NOP-1A, a new radioligand for the nociceptin/orphanin FQ peptide receptor, with high affinity (Ki, 0.15 nM) and adequate lipophilicity (measured logD, 3.4) for PET brain imaging has been developed [••21]. Using this ligand, it is possible to evaluate if the high doses of buprenorphine that attenuates alcohol and cocaine consumption will displace 11C-NOP-1A from NOP receptors. This study will help to further clarify the potential of NOP receptors as a treatment target for alcoholism and possibly other forms of addiction opening new vistas for drug development programs on this peptidergic system. Non-peptide, orally available and brain penetrant NOP receptor agonists have been developed, and seem to have acceptable safety and tolerability. Some of these are in relatively advanced stages of development, and may soon become ready for clinical evaluation (Table 1).
Table 1.
Compounds targeting the N/OFQ system, relative developmental stage and effects on addiction.
| Agonist | Chemical entity | Effects on drug taking and relapse | Dev. phase | Ref. |
|---|---|---|---|---|
| N/OFQ | peptidic | ↓ Alcohol intake | Preclinic | 36, 38 |
| Ro 64-6198 | small molecule | ↓ ↑ Alcohol intake | Preclinic | 70, 82 |
| Ro 64-6570 | small molecule | Not tested | Preclinic | 71 |
| W212393 | small molecule | Not tested | Preclinic | 72 |
| GRT6005 | small molecule | Not tested | Clinic | NCT01725087 |
| SCH 655842 | small molecule | Not tested | Preclinic | 74 |
| SCH 221510 | small molecule | Not tested | Preclinic | 75 |
| UFP-112 | peptidic | ↓ Alcohol intake | Preclinic | 70 |
| UFP-102 | peptidic | ↓ Alcohol intake | Preclinic | 70 |
| OS-462 | peptidic | ↓ Alcohol intake | Preclinic | 70 |
| Buprenorphine * | small molecule | ↓ Alcohol intake | Clinic | 46 |
| SCH 486757 | small molecule | Not tested | Clinic | 73 |
| NCT00230230 | ||||
| Antagonist | ||||
| UFP-101 | peptidic | — Alcohol intake | Preclinic | 46 |
| J113397 | small molecule | Not tested | Preclinic | 76 |
| NiK-21273 | small molecule | Not tested | Preclinic | 77 |
| Compound 24 | small molecule | Not tested | Preclinic | 78 |
| SB-612111 | small molecule | Not tested | Preclinic | 79 |
| Nphe | peptidic | — Alcohol intake | Preclinic | 80 |
| GF-4 | peptidic | Not tested | Preclinic | 81 |
Buprenorphine-induced alcohol drinking reduction is mediated by NOP.
Neuropeptide S system
A new interesting area of research in the field of neuropeptides is offered by the relatively recent deorphanization of the G-protein coupled receptor 154 (GPR 154), currently named the NPS receptor (NPSR), and that is activated by a 20 aa peptide named neuropeptide S (NPS) [50]]. NPS precursor mRNA is expressed in about 500 cells localized only in the brainstem [51,52]. Whereas NPSR is widely expressed in brain areas important in regulating affective responses, emotions and cognition such as the amygdala the hippocampus and the hypothalamus [51–53].
Recent preclinical findings suggest a strong role for the NPS system in drug abuse (see for review [54]). For example neurochemical studies have shown that central injection of NPS facilitates corticomesolimbic DA neurotransmission, a hallmark of reward [55,56]. But, ICV NPS administration induced neither place preference nor aversion [57], suggesting that NPS is devoid of direct rewarding properties. When given to rats trained to lever press for cocaine NPS did not influence drug self-administration [•58]. Cocaine self-administration was also unaffected by the selective NPSR antagonist SHA 68 [•58–60]. Central administration of NPS has also been found to leave alcohol self-administration unaffected in non-dependent Wistar rats. However, NPS decreased alcohol drinking in alcohol-preferring (P) rats but not in the non-preferring (NP) control line [61]. Similar results were found in msP alcohol preferring rats [54]. The P and the msP rat are both highly stress-reactive, and show increased measures of anxiety-like behavior. It has been hypothesized that their escalated alcohol drinking is in part negatively reinforced by alcohol’s ability to relieve negative emotional states [18,19,62]. Hence it is possible that, in alcohol preferring rats, NPS decreases alcohol consumption through its anxiolytic-like properties.
One of the most striking features of NPS pharmacology in relation to addiction is its ability to promote relapse to drug seeking. For instance, it was shown that NPS, given ICV or into the LH potentiated relapse to alcohol seeking induced by cues; an effect apparently mediated by activation of the orexin-1 (OX1) receptor system [54].
Studies on cocaine have confirmed the permissive role of NPS in relapse to drug seeking. [•58,63]. Whereas administration of the NPSR antagonists reduced reinstatement of cocaine seeking [•58,60].
A link between the NPS system and alcohol withdrawal has been also described. Over-expression of NPSR transcript was observed at 12 hours and at one week after completion of a five day alcohol intoxication cycle [64]. Accordingly, anxiolytic-like effects of NPS were more pronounced in rats with a history of alcohol dependence than in controls [64]. This finding was confirmed in another study in which it was shown that the anxiolytic and anti-depressant effects of NPS are enhanced in abstinent previously alcohol exposed mice [•65]. Overall, these data suggest that elevation of NPSR expression following a history of alcohol dependence may represent a neuroadaptive mechanism that attempts to compensate for the increased anxiety in animals. This neuroadaptation may set up a dynamic in which increased NPS neurotransmission, initially induced to compensate for withdrawal anxiety persists and promotes relapse during later stages of abstinence.
Hence, of particular interest is the possibility that NPSR antagonists may be useful in the treatment of drug craving and relapse in dependent individuals. Development of selective heterocyclic brain penetrant small molecules are underway. At present NPSR antagonists that can be used as tools to probe the biology of the NPS system have been developed [59]. Hopefully, in the near future compounds for clinical evaluation will be available to be tested in addicted patients (Table 2).
Table 2.
Compounds targeting the NPS system, relative developmental stage and effects on addiction.
| Agonist | Chemical entity | Effects on drug taking and relapse | Dev. Phase | Ref. |
|---|---|---|---|---|
| NPS | peptidic | — Alcohol intake | Preclinic | 61 |
| ↑ Alcohol cue-induce reinstatement | 83 | |||
| ↑Cocaine cue-induce reinstatement | 63 | |||
| Antagonist | ||||
| SHA68 | small molecule | — Cocaine self-administration | Preclinic | 58 |
| ↓ Cocaine cue-induced reinstatement | ||||
| [tBu-D-Gly5]NPS | peptidic | Not tested | Preclinic | 66 |
| [D-Cys(tBu)5]NPS | peptidic | ↓ Cocaine cue-induced reinstatement | Preclinic | 60 |
| [D-Val5]NPS | peptidic | Not tested | Preclinic | 66 |
| PI1 | small molecule | Not tested | Preclinic | 67 |
| QA1 | small molecule | — Cocaine self-administration | Preclinic | 60 |
| ↓ Cocaine cue-induced reinstatement | ||||
| ML 154 | small molecule | Not tested | Preclinic | 68 |
| RT-118 | small molecule | ↓ Cocaine self-administration and relapse | Preclinic | 69 |
Conclusions and Remarks
Processes involved in the development of alcoholism are thought to reside largely in the brain and they are the result of complex interactions between genetic and environmental determinants. To successfully move new drugs in alcoholism from lab to patient it is important to establish appropriate drug development strategies and to delineate a clear path for the development. Availability of well validated animal models and human laboratory paradigms with surrogate markers predictive of clinical efficacy are two important conditions. While neuroimaging methods can provide a novel and powerful tool to investigate and define a translational phenotype for alcohol dependence in preclinical species and in humans, a major excitement in the field of alcohol addiction is the preclinical characterization of a number of biological targets of potential interests; among those several neuropeptidergic systems, including N/OFQ and NPS. Novel imaging tracers selective for neuropeptide-sensitive receptors are currently being developed. Their availability will provide further possibilities to study the implication of these neuropeptides in the aetiology of alcohol addiction and will be of fundamental importance for the development of new compounds aimed at targeting these systems.
Highlights.
Traslational medicine has a major significance in drug development on alcoholism.
Brain imaging techniques are fundamental in bridging between preclinical and clinical research.
Neuropeptidergic systems are promising for drug development in alcoholism.
Acknowledgements
We thank Lydia Ayanwuyi for her help with manuscript preparation. This work was supported by the National Institutes of Health, grant RO1 AA017447 and RO1 AA014351 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 3.Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 4.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 5. Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. • This study is the first to demonstrate that gray matter volume deficits in medial frontal and posterior parietal-occipital brain regions are predictive of early relapse, suggesting a significant role for gray matter atrophy in poor clinical outcomes in alcohol dependent patients.
- 6.Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP. Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. Am J Psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- 11.Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzemann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–829. [PubMed] [Google Scholar]
- 12.Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O'Malley S, Book GA, Reynolds B, et al. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O'Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 16.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 17. Gozzi A, Agosta F, Massi M, Ciccocioppo R, Bifone A. Reduced limbic metabolism and fronto-cortical volume in rats vulnerable to alcohol addiction. Neuroimage. 2013;69:112–119. doi: 10.1016/j.neuroimage.2012.12.015. •• In this study magnetic resonance imaging in genetically selected alcohol preferring msP rats demonstrated the presence of inborn grey matter and metabolic abnormalities in these animals remarkably similar to those mapped in abstinent alcoholics and subjects at high risk for alcohol dependence. High drinking in msP rats is at least in part explained by their attempt to self-medicate from negative affect. Notably, some morphological abnormalities observed in this rat strain are also similar to those observed in patient suffering from anxiety and depression.
- 18.Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction Biology. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadzot B, Mayberg HS, Frost JJ. Imaging opiate receptors in the human brain with positron emission tomography. Potential applications for drug addiction research. Acta Psychiatr Belg. 1990;90:9–19. [PubMed] [Google Scholar]
- 21. Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, Hong J, Zoghbi SS, Fujita M, Toledo MA, et al. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J Med Chem. 2011;54:2687–2700. doi: 10.1021/jm101487v. •• This is the first PET ligand for NOP receptors tested in rats, monkey and humans.
- 22. Zheng MQ, Nabulsi N, Kim SJ, Tomasi G, Lin SF, Mitch C, Quimby S, Barth V, Rash K, Masters J, et al. Synthesis and Evaluation of 11C-LY2795050 as a kappa-Opioid Receptor Antagonist Radiotracer for PET Imaging. J Nucl Med. 2013 doi: 10.2967/jnumed.112.109512. •• This is the first developed KOP antagonist tracer for PET imaging that has been successfully advanced into clinic.
- 23.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 24.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett S, Heilig M. Translational approaches to medication development. Curr Top Behav Neurosci. 2013;13:543–582. doi: 10.1007/7854_2011_132. [DOI] [PubMed] [Google Scholar]
- 26.Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- 28.Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- 29.Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- 30.Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- 31.Gavioli EC, Calo G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:319–330. doi: 10.1007/s00210-006-0035-8. [DOI] [PubMed] [Google Scholar]
- 32.Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 2005;182:132–143. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- 33. Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. • Stimulation of NOP receptors attenuates not only alcohol intake and relapse but also the somatic and affective signs of alcohol withdrawal in dependent rats.
- 34.Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- 35.Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- 37.Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- 38.Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- 40.Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, et al. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wnendt S, Kruger T, Janocha E, Hildebrandt D, Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol. 1999;56:334–338. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]
- 42.Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- 43.Hubbell CL, Czirr SA, Hunter GA, Beaman CM, LeCann NC, Reid LD. Consumption of ethanol solution is potentiated by morphine and attenuated by naloxone persistently across repeated daily administrations. Alcohol. 1986;3:39–54. doi: 10.1016/0741-8329(86)90070-4. [DOI] [PubMed] [Google Scholar]
- 44.Hubbell CL, Mankes RF, Reid LD. A small dose of morphine leads rats to drink more alcohol and achieve higher blood alcohol concentrations. Alcohol Clin Exp Res. 1993;17:1040–1043. doi: 10.1111/j.1530-0277.1993.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 46. Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. •• Anti addictive properties of buprenorphine may in part depend upon activation of NOP receptors. This study provides a proof-of-concept for the potential of NOP agonists as a treatment for addiction.
- 47.Nava F, Manzato E, Leonardi C, Lucchini A. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1867–1872. doi: 10.1016/j.pnpbp.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 49.Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34:215–223. doi: 10.1016/j.jsat.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y-L, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y-L, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–8102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Zeng J, Zhou A, Theodorsson E, Fahrenkrug J, Reinscheid RK. Molecular fingerprint of neuropeptide S-producing neurons in the mouse brain. J Comp Neurol. 2011;519:1847–1866. doi: 10.1002/cne.22603. [DOI] [PubMed] [Google Scholar]
- 53.Leonard SK, Ring RH. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience. 2011;172:153–163. doi: 10.1016/j.neuroscience.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Cannella N, Kallupi M, Ruggeri B, Ciccocioppo R, Ubaldi M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Progress in Neurobiology. 2012 doi: 10.1016/j.pneurobio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Si W, Aluisio L, Okamura N, Clark SD, Fraser I, Sutton SW, Bonaventure P, Reinscheid RK. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J Neurochem. 2010;115:475–482. doi: 10.1111/j.1471-4159.2010.06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mochizuki T, Kim J, Sasaki K. Microinjection of neuropeptide S into the rat ventral tegmental area induces hyperactivity and increases extracellular levels of dopamine metabolites in the nucleus accumbens shell. Peptides. 2010;31:926–931. doi: 10.1016/j.peptides.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Li W, Gao YH, Chang M, Peng YL, Yao J, Han RW, Wang R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides. 2009;30:234–240. doi: 10.1016/j.peptides.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 58. Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci U S A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. • The relapse facilitating properties of NPS are blocked by OX1R blockade. This indicates that NPS is an upstream regulator of the orexin system.
- 59.Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydrooxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kallupi M, de Guglielmo G, Cannella N, Li HW, Calo G, Guerrini R, Ubaldi M, Renger JJ, Uebele VN, Ciccocioppo R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2013;226:347–355. doi: 10.1007/s00213-012-2910-y. [DOI] [PubMed] [Google Scholar]
- 61.Badia-Elder NE, Henderson AN, Bertholomey ML, Dodge NC, Stewart RB. The effects of neuropeptide S on ethanol drinking and other related behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res. 2008;32:1380–1387. doi: 10.1111/j.1530-0277.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- 62.Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- 63.Paneda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J Neurosci. 2009;29:4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruggeri B, Braconi S, Cannella N, Kallupi M, Soverchia L, Ciccocioppo R, Ubaldi M. Neuropeptide S receptor gene expression in alcohol withdrawal and protracted abstinence in postdependent rats. Alcohol Clin Exp Res. 2010;34:90–97. doi: 10.1111/j.1530-0277.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 65. Enquist J, Ferwerda M, Madhavan A, Hok D, Whistler JL. Chronic ethanol potentiates the effect of neuropeptide s in the basolateral amygdala and shows increased anxiolytic and anti-depressive effects. Neuropsychopharmacology. 2012;37:2436–2445. doi: 10.1038/npp.2012.102. • NPS shows enhanced anxiolytic and anti-depressant effects in acute ethanol abstinent mice indicating that this peptidergic system is subjected to neuroadaptive changes following exposure to alcohol.
- 66.Guerrini R, Camarda V, Trapella C, Calo G, Rizzi A, Ruzza C, Fiorini S, Marzola E, Reinscheid RK, Regoli D, et al. Further studies at neuropeptide s position 5: discovery of novel neuropeptide S receptor antagonists. Journal of Medicinal Chemistry. 2009;52:4068–4071. doi: 10.1021/jm900604g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trotter BW, Nanda KK, Manley PJ, Uebele VN, Condra CL, Gotter AL, Menzel K, Henault M, Stocco R, Renger JJ, et al. Tricyclic imidazole antagonists of the Neuropeptide S Receptor. Bioorg Med Chem Lett. 2010;20:4704–4708. doi: 10.1016/j.bmcl.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 68.Patnaik S, Marugan J, Liu K, Zheng W, Thorsell A, Eskay R, Southall N, Heilig M, Inglese J, Austin C. Identification of Small Molecule Antagonists of the Neuropeptide-S Receptor. In. Probe Reports from the NIH Molecular Libraries Program. 2010 Edited by. [PubMed] [Google Scholar]
- 69.Schmoutz CD, Zhang Y, Runyon SP, Goeders NE. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2012;103:332–337. doi: 10.1016/j.pbb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Economidou D, Fedeli A, Fardon RM, Weiss F, Massi M, Ciccocioppo R. Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides. 2006;27:3299–3306. doi: 10.1016/j.peptides.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kotlinska J, Rafalski P, Talarek S, Dylag T, Rolka K, Wichmann J, Silberring J. Is the nociceptin (NOP) receptor involved in attenuation of the expression of sensitization to morphine-induced hyperlocomotion in mice? Behav Pharmacol. 2005;16:101–106. doi: 10.1097/00008877-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Teshima K, Minoguchi M, Tounai S, Ashimori A, Eguchi J, Allen CN, Shibata S. Nonphotic entrainment of the circadian body temperature rhythm by the selective ORL1 receptor agonist W-212393 in rats. Br J Pharmacol. 2005;146:33–40. doi: 10.1038/sj.bjp.0706311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLeod RL, Tulshian DB, Bolser DC, Varty GB, Baptista M, Fernandez X, Parra LE, Zimmer JC, Erickson CH, Ho GD, et al. Pharmacological profile of the NOP agonist and cough suppressing agent SCH 486757 (8-[Bis(2-Chlorophenyl)Methyl]-3-(2-Pyrimidinyl)-8-Azabicyclo[3.2.1]Octan-3-Ol) in preclinical models. Eur J Pharmacol. 2010;630:112–120. doi: 10.1016/j.ejphar.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu SX, Higgins GA, Hodgson RA, Hyde LA, Del Vecchio RA, Guthrie DH, Kazdoba T, McCool MF, Morgan CA, Bercovici A, et al. The anxiolytic-like profile of the nociceptin receptor agonist, endo-8-[bis(2-chlorophenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octane-3-carboxami de (SCH 655842): comparison of efficacy and side effects across rodent species. Eur J Pharmacol. 2011;661:63–71. doi: 10.1016/j.ejphar.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 75.Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, Zhang H, Fawzi AB, Graziano MP, Ho GD, et al. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510) J Pharmacol Exp Ther. 2008;326:672–682. doi: 10.1124/jpet.108.136937. [DOI] [PubMed] [Google Scholar]
- 76.Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397) J Med Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- 77.Marti M, Mela F, Budri M, Volta M, Malfacini D, Molinari S, Zaveri N, Ronzoni S, Petrillo P, Calo G, et al. Acute and chronic antiparkinsonian effects of the novel nociceptin/orphanin FQ receptor antagonist NiK-21273 in comparison with SB-612111. Br J Pharmacol. 2013;168:863–879. doi: 10.1111/j.1476-5381.2012.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao YY, Trapella C, Chiou LC. 1-Benzyl-N-[3-[spiroisobenzofuran-1(3H),4'-piperidin-1-yl]propyl]pyrrolidine-2-ca rboxamide (Compound 24) antagonizes NOP receptor-mediated potassium channel activation in rat periaqueductal gray slices. Eur J Pharmacol. 2009;606:84–89. doi: 10.1016/j.ejphar.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 79.Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calo G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrah ydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther. 2007;321:968–974. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- 80.Ciccocioppo R, Polidori C, Antonelli L, Salvadori S, Guerrini R, Massi M. Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides. 2002;23:117–125. doi: 10.1016/s0196-9781(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 81.Volta M, Marti M, McDonald J, Molinari S, Camarda V, Pela M, Trapella C, Morari M. Pharmacological profile and antiparkinsonian properties of the novel nociceptin/orphanin FQ receptor antagonist 1-[1-cyclooctylmethyl-5-(1-hydroxy-1-methyl-ethyl)-1,2,3,6-tetrahydro-pyridin-4-y l]-3-ethyl-1,3-dihydro-benzoimidazol-2-one (GF-4) Peptides. 2010;31:1194–1204. doi: 10.1016/j.peptides.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- 83.Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]