Abstract
Background:
Coffee has been reported to be rich in antioxidants, with both acute and chronic consumption leading to enhanced blood antioxidant capacity. High-fat feeding is known to result in excess production of reactive oxygen and nitrogen species, promoting a condition of postprandial oxidative stress.
Methods:
We tested the hypothesis that coffee intake following a high-fat meal would attenuate the typical increase in blood oxidative stress during the acute postprandial period. On 3 different occasions, 16 men and women consumed a high-fat milk shake followed by either 16 ounces of caffeinated or decaffeinated coffee or bottled water. Blood samples were collected before and at 2 and 4 hours following intake of the milk shake and analyzed for triglycerides (TAG), malondialdehyde (MDA), hydrogen peroxide (H2O2), and Trolox equivalent antioxidant capacity (TEAC).
Results:
Values for TAG and MDA (P < 0.001), as well as for H2O2 (P < 0.001), increased significantly following milk shake consumption, with values higher at 4 hours compared with 2 hours post consumption for TAG and H2O2 (P < 0.05). TEAC was unaffected by the milk shake consumption. Coffee had no impact on TAG, MDA, H2O2, or TEAC, with no condition or interaction effects noted for any variable (P > 0.05).
Conclusions:
Acute coffee consumption following a high-fat milk shake has no impact on postprandial oxidative stress.
Keywords: coffee, caffeine, triglyceride, reactive oxygen species
Introduction
Reactive oxygen and nitrogen species (RONS) is an umbrella term that encompasses oxygen radicals, nitrogen radicals, and their nonradical derivatives.1 Although RONS can perform useful functions such as initiating cellular signaling and defending against harmful agents, an overexpression of this species can cause damage to lipids, protein, DNA, and other molecules.2 To prevent this, organisms are equipped with antioxidant defense systems that scavenge RONS. However, the balance between RONS expression and antioxidant scavenging can become disturbed if RONS production is increased and/or the antioxidant defense system is weakened.3 This undesirable phenomenon, which is referred to as “oxidative stress,” has been linked to over one hundred diseases as well as the aging process.2,4
Several conditions have been demonstrated to induce oxidative stress, including physical exercise5 and exposure to various pollutants such as cigarette smoke,6 radiation,7 and ozone.8 Another stressor that may be a more frequent cause of oxidative stress for many individuals is the metabolism of a high-fat and/or high-carbohydrate meal.9 Such metabolism floods the circulatory and peripheral tissues with substrate, and the processing of these substrates overwhelms the mitochondrial electron transport chain. This accelerates electron leakage and subsequent superoxide formation, which in turn initiates a cascade of events that culminates in additional RONS formation.10 The excessive RONS formation and subsequent oxidative damage caused by this metabolism of foodstuff is termed “postprandial oxidative stress.” Such dysmetabolism is thought to be involved in the pathogenesis of human disease, including insulin resistance11 and cardiovascular disease.12,13
Antioxidant intake is thought to decrease oxidative stress, with foods and nutritional supplements containing antioxidants often recommended for this purpose. The reduction in oxidative stress following antioxidant intake has been shown to occur both in the fasted14 and postprandial state.15 Coffee is a beverage that has been reported to increase blood antioxidant capacity following both acute16 and chronic intake.17 Coffee is rich in antioxidants,18 including chlorogenic acid, melanoidins, and hydroxycinnamic acids.19 This may help explain the j-shaped relationship between daily coffee intake and cardiovascular risk observed in some studies (reviewed in Di Castelnuovo et al20) and may indicate a potential for coffee to attenuate postprandial oxidative stress.21–23
Although all types of coffee contain relatively high concentrations of antioxidants,24,25 caffeinated coffee may possess even greater antioxidant activity. Specifically, caffeine is noted to have antioxidant properties,26,27 and caffeinated coffee has been recently reported to attenuate lipid and protein oxidation in rat tibialis anterior muscle following exercise.28 That said, caffeine intake is also known to acutely elevate blood fatty acid concentrations,29 which may lead to increased lipid peroxidation in the presence of high concentrations of RONS (as observed following high-fat meal intake).30 Hence, it is possible that any potential antioxidant effect of caffeine may be counteracted by the increased propensity for lipids to be oxidized following high-fat feeding.
The purpose of this study was to determine the effect of coffee intake on postprandial oxidative stress. We hypothesized that intake of both caffeinated and decaffeinated coffee would attenuate oxidative stress. Considering that individuals often consume coffee in conjunction with meals that are high in fat and/or simple sugar—such as eggs, bacon, bagels, donuts, and other breakfast and dessert foods—the potential ability of coffee intake to minimize postprandial oxidative stress may have implications for many people.
Materials and Methods
Subjects and screening (first laboratory visit)
Subjects were recruited by word of mouth and recruitment flyers from the University of Memphis campus and the local Memphis community. Subjects were required to be healthy at the time of enrollment, without a history of cardiovascular or metabolic disease. In addition, no subject smoked cigarettes, nor did any subject use medications or dietary supplements throughout the study period that may have impacted the outcome measures. No requirements were placed on subjects with regard to daily coffee consumption, as we were unaware of data indicating differing antioxidant effects of coffee intake in habitual versus nonhabitual coffee drinkers.
A total of 16 healthy and physically active men (n = 8) and women (n = 8) participated in this study, 9 of whom were light coffee drinkers (mean daily intake, 14 ounces) and 7 of whom did not consume coffee. All subjects completed a medical history and physical activity questionnaire prior to being enrolled. Subjects’ height (using a wall-mounted stadiometer), body weight (using a medical-grade scale), body fat percentage (using Lange calipers and a 7-site skinfold test), waist and hip circumference (using a tension-regulated tape measure), heart rate (via 60 second palpation on the radial artery), and blood pressure (via upper arm blood pressure cuff and auscultation with a stethoscope) were measured and recorded. Subject characteristics are presented in Table 1. Subjects also received diet logs and were provided with instructions on how to record food and beverage intake during the 2 days prior to each test day. During the initial visit to the lab, subjects completed all paperwork and provided written informed consent. All experimental procedures were performed in accordance with the Helsinki Declaration, and The University of Memphis Human Subjects Committee approved all experimental procedures (protocol number 062111–772).
Table 1.
Descriptive characteristics of subjects.
| Variable | Data |
|---|---|
| Age (years) | 29.2 ± 14.4 |
| Height (cm) | 173.8 ± 10.4 |
| Body Weight (kg) | 70.7 ± 11.7 |
| BMI (kg m−2) | 23.3 ± 2.2 |
| Waist (cm) | 79.1 ± 9.1 |
| Hip (cm) | 100.3 ± 3.9 |
| Waist: Hip | 0.79 ± 0.08 |
| Body Fat (%) | 17.2 ± 7.2 |
| Heart Rate (beats·minute−1) | 67.2 ± 10.7 |
| Systolic Blood Pressure (mm Hg) | 108.3 ± 9.8 |
| Diastolic Blood Pressure (mm Hg) | 64.2 ± 12.2 |
| Years anaerobic exercise training | 3.0 ± 5.3 |
| Hours per week anaerobic exercise | 1.6 ± 1.9 |
| Years aerobic exercise training | 9.7 ± 16.8 |
| Hours per week aerobic exercise | 2.7 ± 2.8 |
Data were expressed as mean ± SD.
Testing (second, third, and fourth laboratory visits)
Subjects reported to the lab on 3 different occasions separated by 5 to 8 days in a 10-hour fasted state and without having consumed caffeine during the prior 24 hours. Following the measurement of resting heart rate (60-second palpation) and blood pressure (standard auscultation procedures using a cuff and stethoscope), a fasting blood sample was taken. Subjects then consumed the test meal (milk shake) as described below.
The test meal consisted of a milk shake made with a combination of whole milk, ice cream (Breyers “all natural” vanilla), and heavy whipping cream. As we have done previously,31,32 the size (dietary energy) of the milk shake was determined based on subjects’ body weight, providing 0.8 grams of fat, 1.0 gram of carbohydrate, and 0.25 grams of protein per kilogram of body weight. This provided approximately 12.2 kilocalories/kilogram of body weight (eg, a subject weighing 70 kilograms would consume a milk shake containing 854 kilocalories), yielding an energy content similar to milk shakes provided in many commercial establishments. The lipid content of the milk shake was similar to what we have used in 2 prior studies of postprandial oxidative stress31,32 and less than that used in some of our other work.33,34
Subjects were allowed a maximum of 15 minutes to completely consume the milk shake. One subject who was mildly lactose intolerant was given a standard dose (9000 FCC lactase units) of lactase enzyme. Blood was again collected from subjects at 2 and 4 hours following intake of the milk shake (time started as soon as subjects began consuming the milk shake). Heart rate and blood pressure was also measured at these times. Subjects remained in the lab during this 4-hour period and expended little energy (ie, watched movies, worked on the computer, etc). No additional meals or calorie-containing beverages were allowed during this period. However, water was allowed ad libitum, in addition to the 16 ounces of water (or coffee) provided below.
Following milk shake consumption on each of the 3 visits, subjects consumed either 16 ounces of freshly brewed caffeinated or decaffeinated coffee (black, unsweetened) or 16 ounces of bottled water. The order of assignment on the 3 days of testing was random, using a crossover design, that is, each subject received all 3 conditions (caffeinated coffee, decaffeinated coffee, water) over the course of the study period. The caffeinated coffee and decaffeinated coffee provided approximately 175 mg and 15 mg of caffeine, respectively. Medium roast coffee beans (Eight O-Clock Coffee Company, Montvale, NJ) were ground (approximate total of 30 grams of beans per 16 ounce serving of coffee) and each serving of coffee was brewed fresh (using bottled water) and separately in an effort to maintain a similar brew-to-consume time for each subject. Although results vary across studies,25 and all forms of coffee beans appear to be rich in antioxidants, it has been reported that medium roast coffee beans have the highest antioxidant content.35 This may be due in part to the fact that naturally occurring antioxidants are not significantly depleted in the roasting process and newly formed antioxidants are gained by roasting through the Maillard reaction.36
Subjects were provided with the freshly brewed coffee 15 minutes following brewing completion—a time when the coffee’s temperature did not provide discomfort to subjects. Subjects were not told whether they were given caffeinated or decaffeinated coffee on a given day; of course, many of them were able to infer which condition they received based on their bodies’ response to the caffeine intake. The coffee or water was completely consumed within 15 minutes following the consumption of the milk shake.
Blood collection and biochemistry
Venous blood samples were taken from an antecubital vein via needle and Vacutainer before and at 2 and 4 hours following intake of the milk shake. Blood triglyceride (TAG) is highly responsive to high-fat feeding, and our prior work involving healthy adults indicates that the peak TAG response to a high-fat milk shake occurs at 2 hours post ingestion, with oxidative stress biomarkers peaking between 2 and 4 hours post ingestion.34,37 Blood samples that were collected in tubes containing EDTA were immediately centrifuged at 1500 g for 15 minutes at 4°C for collection of plasma. Blood samples that were collected in tubes containing no additives were allowed to clot at room temperature for 30 minutes and then separated by centrifugation at 1500 g for 15 minutes at 4°C for collection of serum. Plasma and serum samples were stored in multiple aliquots at −70°C until analyzed for the following variables.
Triglycerides were analyzed in serum following standard enzymatic procedures as described by the reagent manufacturer (Thermo Electron Clinical Chemistry). Malondialdehyde (MDA) was analyzed in plasma following the procedures of Jentzsch et al38 using reagents purchased from Northwest Life Science Specialties (Vancouver, WA). Hydrogen peroxide (H2O2) was analyzed in plasma using the Amplex Red reagent method as described by the manufacturer (Molecular Probes, Invitrogen Detection Technologies, Eugene, OR) and used as a gross surrogate measure of radical production. Trolox equivalent antioxidant capacity (TEAC) was analyzed in serum according to the procedures outlined by the reagent provider (Sigma Chemical, St. Louis, MO) and used to represent total antioxidant capacity of the serum. Assays were performed in duplicate on first thaw.
Dietary intake and physical activity
Subjects were instructed to maintain their usual dietary intake during the entire study period. Diet records reflecting the 48-hour periods that preceded each test day were analyzed (Food Processor SQL, version 9.9, ESHA Research, Salem, OR). Subjects were asked to refrain from strenuous physical activity for the 48 hours prior to each test day, but they were instructed to otherwise maintain their usual physical activity during the entire course of the study.
Statistical analysis
Data were analyzed using a 3 (condition) × 3 (time) analysis of variance. Significant effects were further analyzed using Tukey post hoc tests. All analyses were performed using JMP statistical software (version 4.0.3, SAS Institute, Cary, NC). Statistical significance was set at P < 0.05 (two tailed). Data are presented as mean ± standard error of the mean (SEM).
Results
A total of 16 subjects successfully completed all aspects of the study. However, we did not include the data from one male subject in our analysis because this subject had a mean fasting TAG concentration that was an outlier compared with the values of the remaining subjects (573 mg dL−1). No component of dietary intake was different for subjects across conditions (P > 0.05, Table 2). Heart rate and blood pressure were not impacted by the milk shake and not different for any condition (P > 0.05, Fig. 1 panels A–C).
Table 2.
Dietary data of subjects during the 2 days before each test visit.
| Variable | Caffeinated coffee (n = 16) | Decaffeinated coffee (n = 16) | Water (n = 16) |
|---|---|---|---|
| Kilocalories | 1819.6 ± 143.4 | 1977.4 ± 157.4 | 1931.4 ± 190.4 |
| Protein (g) | 84.1 ± 8.7 | 88.0 ± 10.1 | 90.5 ± 9.8 |
| Carbohydrate (g) | 230.6 ± 23.2 | 235.1 ± 22.7 | 233.5 ± 22.5 |
| Fat (g) | 64.6 ± 6.7 | 74.3 ± 9.1 | 66.3 ± 8.7 |
| Vitamin C (mg) | 102.8 ± 25.2 | 93.6 ± 18.9 | 97.3 ± 22.9 |
| Vitamin E (mg) | 8.5 ± 2.4 | 7.9 ± 2.4 | 6.1 ± 1.4 |
| Vitamin A (RE) | 607.3 ± 182.7 | 680.0 ± 192.3 | 674.5 ± 172.0 |
| Selenium (μg) | 43.7 ± 8.2 | 56.1 ± 11.9 | 57.3 ± 12.3 |
Data were expressed as mean ± SEM.
No differences noted between conditions for any variable (P > 0.05).
Figure 1.
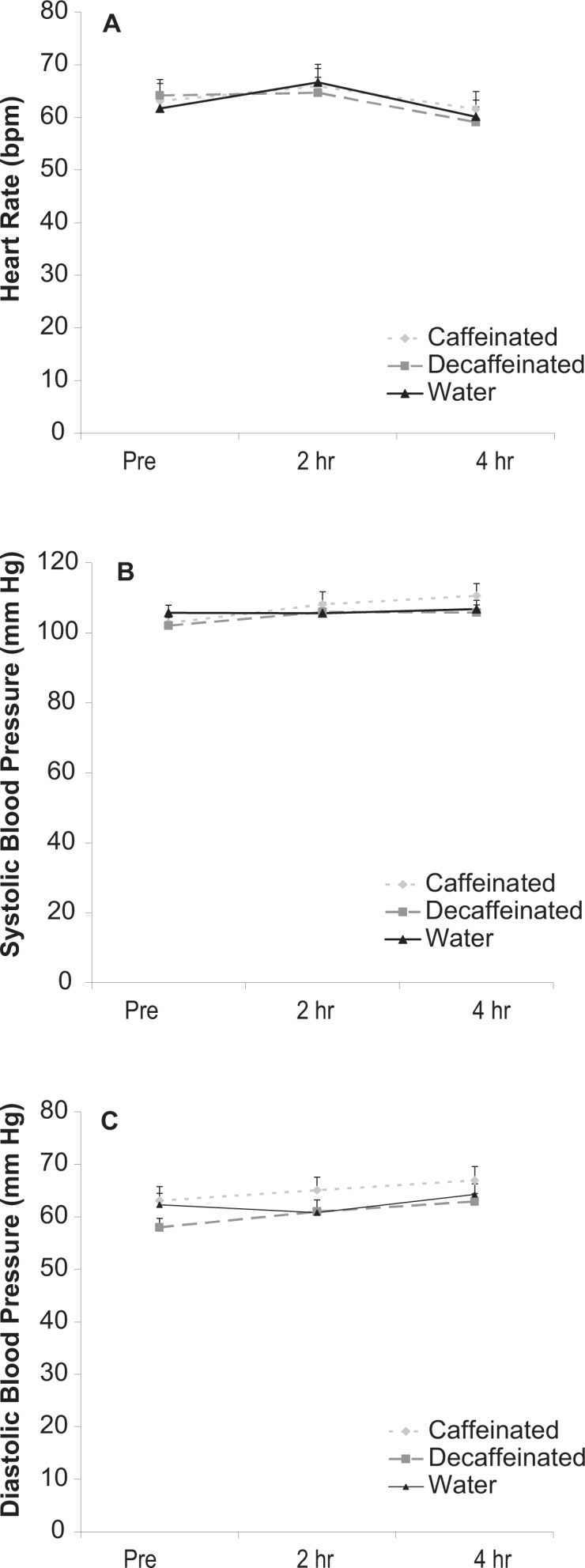
Heart rate (A) and systolic (B) and diastolic (C) blood pressure of subjects before and after intake of a high-fat milk shake followed by coffee or water.
Data were expressed as mean ± SEM.
No differences noted for any variable (P > 0.05).
TAG values increased significantly over time in response to the milk shake (P < 0.001). Values were higher at 2 and 4 hours post milk shake ingestion compared with pre and at 4 hours post ingestion compared with 2 hours (P < 0.05). No condition (P = 0.70) or interaction effects (P = 0.67) were noted for TAG. Data are presented in Figure 2 panel A.
Figure 2.
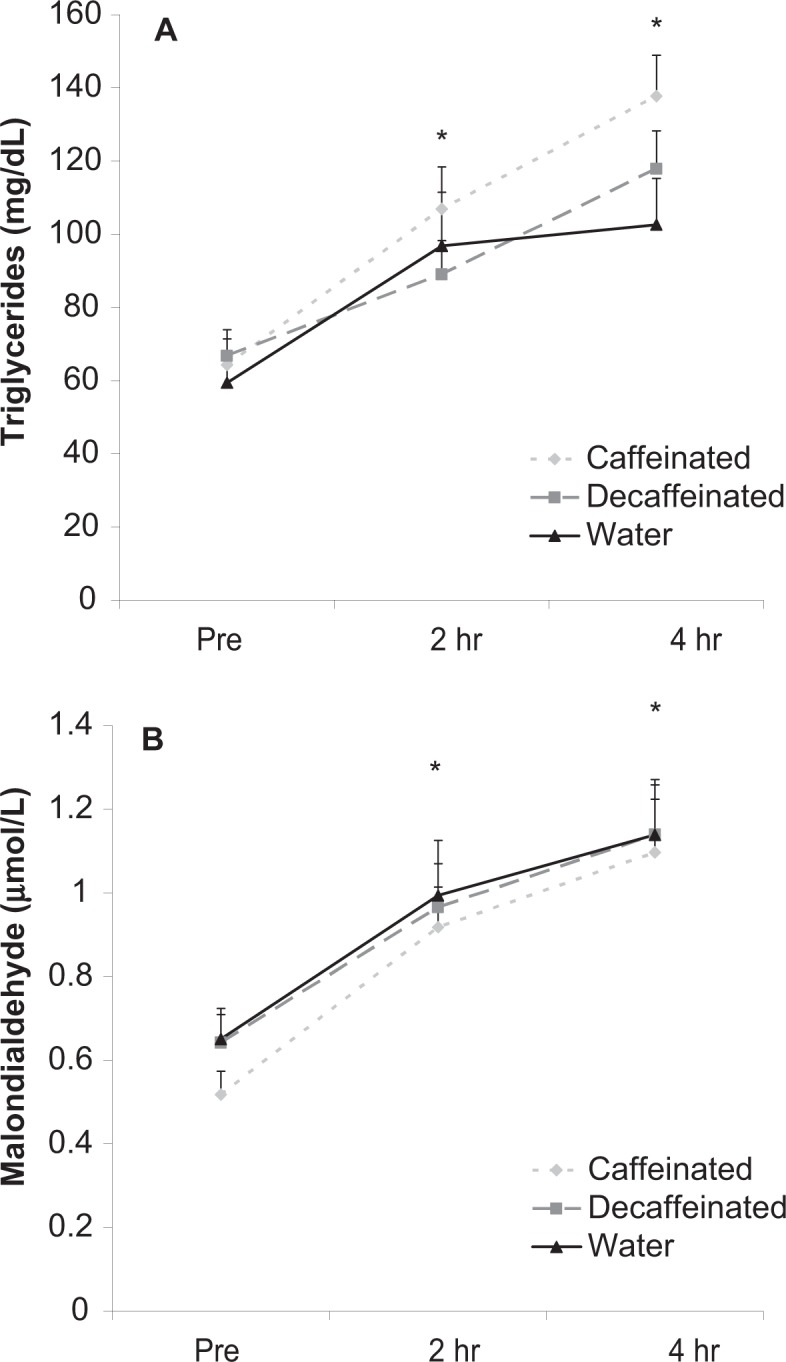
Blood triglycerides (A) and malondialdehyde (B) before and after intake of a high-fat milk shake followed by coffee or water.
Data were expressed as mean ± SEM.
*Time effect for triglycerides (P < 0.001); 2 hr and 4 hr greater than pre; 4 hr greater than 2 hr. No other differences noted for triglycerides (P > 0.05).
*Time effect for malondialdehyde (P < 0.001); 2 hr and 4 hr greater than pre. No other differences noted for malondialdehyde (P > 0.05).
MDA values increased significantly over time in response to the milk shake (P < 0.001). Values were higher at 2 and 4 hours post milk shake ingestion compared with pre (P < 0.05). No condition (P = 0.22) or interaction effects (P = 0.95) were noted for MDA. Data are presented in Figure 2 panel B.
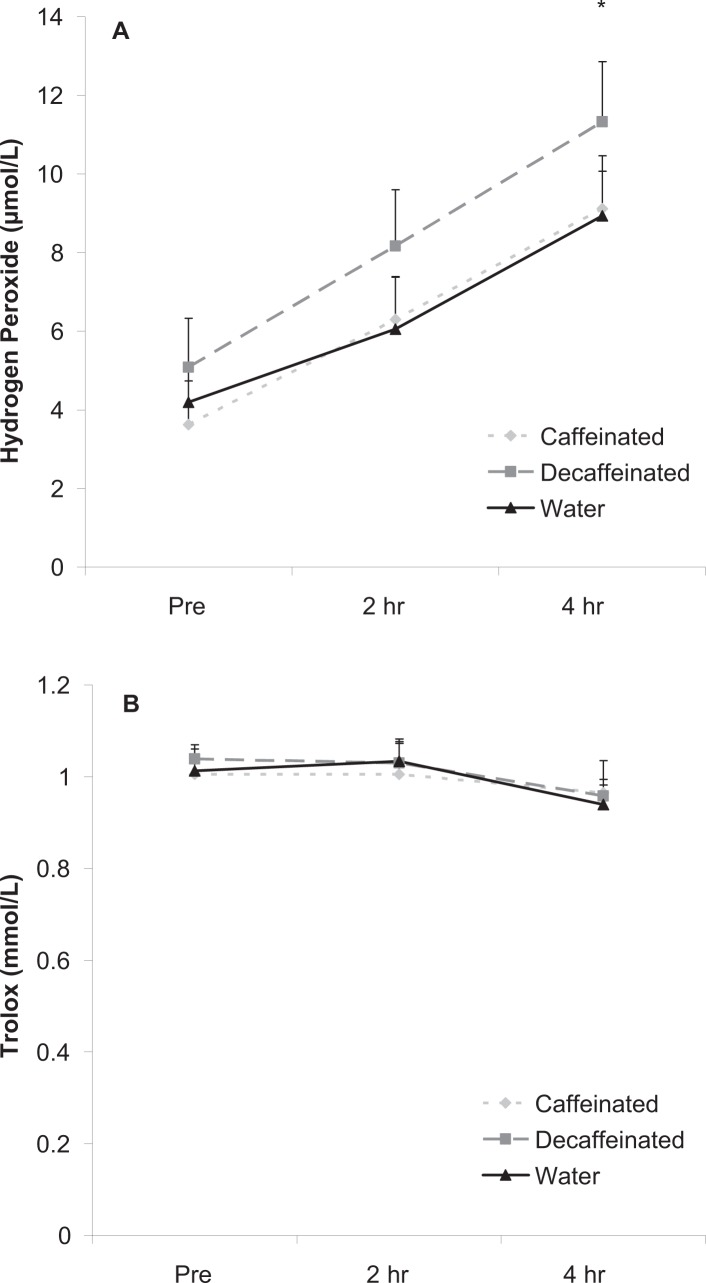
H2O2 values increased significantly over time in response to the milk shake (P < 0.001). Values were higher at 4 hours post milk shake ingestion compared with pre and 2 hours (P < 0.05). No condition (p = 0.22) or interaction effects (P = 0.94) were noted for H2O2. Data are presented in Figure 3 panel A.
Figure 3.
Blood hydrogen peroxide (A) and Trolox equivalent antioxidant capacity (B) before and after intake of a high-fat milk shake followed by coffee or water.
Data were expressed as mean ± SEM.
*Time effect for hydrogen peroxide (P < 0.001); 4 hr greater than pre and 2 hr.
No other differences noted for hydrogen peroxide (P > 0.05).
No differences noted for Trolox equivalent antioxidant capacity (P > 0.05).
TEAC was unaffected by the milk shake and not different across conditions. No time (P = 0.36), condition (P = 0.86), or interaction effects (P = 0.94) were noted. Data are presented in Figure 3 panel B.
Discussion
Our findings indicate that acute consumption of coffee has no effect on TAG, MDA, H2O2, or TEAC for up to 4 hours following the consumption of a high-fat milk shake. Whether a larger volume of coffee (and hence, antioxidant content) and/or a smaller volume of milk shake would yield different results remains unknown. Likewise, coffee ingestion at a time prior to milk shake consumption may have yielded different findings. Future study is needed to generate answers to these questions. Moreover, the inclusion of additional antioxidant biomarkers (eg, glutathione, superoxide dismutase, and catalase) and oxidative stress biomarkers (eg, isoprostanes and protein carbonyls) may have strengthened the study.
As we have reported in prior studies, intake of a high-fat milk shake causes an increase in the concentrations of both TAG and oxidative stress biomarkers.31,33,34,37 Surprisingly, the response of all variables for subjects in the present study was somewhat different than what we have noted in recent work,34,37 with values for TAG, MDA, and H2O2 continuing to increase 4 hour postmeal rather than peaking 2 hours postmeal. This delayed TAG and oxidative stress response likely cannot be explained by the addition of coffee to the study design, as we observed a similar response for the water condition (with the exception of the TAG data, for which water resulted in similar TAG values at 2 and 4 hours postmeal). Our failure to include a longer time course of measurement in the current design is a limitation of this work, as it is possible (but not probable) that we may have observed between-group differences in TAG and oxidative stress responses at time points greater than 4 hours following milk shake ingestion.
Some prior studies have reported an attenuation in postprandial oxidative stress following acute anti-oxidant intake.39–43 These findings led us to hypothesize that coffee consumption would also attenuate postprandial oxidative stress, as coffee has been reported in one study to provide 66% of dietary antioxidant intake,18 and is abundant in chlorogenic acid, melanoidins, and hydroxycinnamic acids.19 On the other hand, although we do not know the exact antioxidant potential of coffee compared with antioxidant supplements, it is possible that the antioxidant content of a 16-ounce serving of coffee was too low, relative to the content of a typical high-potency antioxidant supplement, to affect our outcome variables.
We measured heart rate and blood pressure in the present study because we hypothesized that the RONS production induced by the milk shake would promote an acute state of endothelial dysfunction44 and a concomitant increase in blood pressure, and we wanted to determine if the hypothesized attenuation in oxidative stress following coffee intake would prevent this rise. However, neither heart rate nor blood pressure was affected by the milk shake; hence, testing the ability of coffee to alter this response was not possible. Our line of questioning may have been more appropriately addressed if we were to include a sample of hypertensive individuals within the research design.45 As can be viewed in Table 1, the subjects’ baseline resting heart rate and blood pressure were both within healthy norms.
Conclusion
We report for the first time that acute consumption of coffee does not affect TAG, MDA, H2O2, or TEAC for up to 4 hours following the consumption of a high-fat milk shake. Our findings suggest that coffee intake is not adequate to counteract the massive increase in RONS generated by consumption of a high-fat meal. Rather than attempting to counteract the increase in RONS following high-fat consumption with antioxidant intake, it may be more effective to reduce the oxidative insult by reducing the meal’s fat content.31,37 Future work may (1) extend the time frame of measurement beyond the 4-hour postprandial period, (2) include a meal that is lower in fat content (so as to not overwhelm the system with RONS), (3) provide coffee at a time prior to intake of the high-fat meal, and/or (4) consider the inclusion of a sample of subjects with known chronic disease (eg, diabetes or hypertension). Such additional experiments may help to more fully elucidate the role of coffee on attenuating postprandial oxidative stress.
Footnotes
Author Contributions
Study design and manuscript preparation: RJB, JFT. Data collection, blood collection and processing, and data entry: JFT, TMF. Biochemical work and statistical analyses: RJB. All authors read and approved of the final manuscript.
Funding
Funding for this work was provided by The University of Memphis. The funding source had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report or in the decision to submit the article for publication.
Competing Interests
JFT and TMF disclose no competing interests. RJB has received consulting fees from Bergstrom Nutrition, OmniActive Health, Sigmatau HealthScience and Purity Products, speaking fees or royalties from Bergstrom Nutrition, Miami Research Associates and Formulife, and his institution has received grants or has pending grants from USPlabs, Kaneka Nutrients, Miami Research Associates, Sigma-tau Health Science, Bergstrom Nutrition, Advanced Oral Technologies, Purity Products, Life Extension Clinical Research and Danisco.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
References
- 1.Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Skrha J. Effect of caloric restriction on oxidative markers. Adv Clin Chem. 2009;47:223–247. [PubMed] [Google Scholar]
- 4.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 5.Bloomer RJ. Effect of exercise on oxidative stress biomarkers. Adv Clin Chem. 2008;46:1–50. doi: 10.1016/s0065-2423(08)00401-0. [DOI] [PubMed] [Google Scholar]
- 6.Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14(suppl 1):90–96. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- 7.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- 10.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 11.Fisher-Wellman K, Bloomer RJ. Macronutrient specific postprandial oxidative stress: relevance to the development of insulin resistance. Curr Diabetes Rev. 2009;5:228–238. doi: 10.2174/157339909789804369. [DOI] [PubMed] [Google Scholar]
- 12.Tushuizen ME, Diamant M, Heine RJ. Postprandial dysmetabolism and cardiovascular disease in type 2 diabetes. Postgrad Med J. 2005;81:1–6. doi: 10.1136/pgmj.2004.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Node K, Inoue T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol. 2009;8:23. doi: 10.1186/1475-2840-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigo R, Prat H, Passalacqua W, Araya J, Bachler JP. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin Sci (Lond) 2008;114:625–634. doi: 10.1042/CS20070343. [DOI] [PubMed] [Google Scholar]
- 15.Masterjohn C, Mah E, Guo Y, Koo SI, Bruno RS. Gamma-Tocopherol abolishes postprandial increases in plasma methylglyoxal following an oral dose of glucose in healthy, college-aged men. J Nutr Biochem. 2012;23:292–298. doi: 10.1016/j.jnutbio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Moura-Nunes N, Perrone D, Farah A, Donangelo C. Plasma antioxidant capacity but not endogenous non-enzymatic antioxidant levels increases after acute coffee intake. 22nd International Conference on Coffee Science; September 14–19, 2008; Campinas, Brazil: ASIC; pp. 127–133. [Google Scholar]
- 17.Yukawa GS, Mune M, Otani H, et al. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry (Mosc) 2004;69:70–74. doi: 10.1023/b:biry.0000016354.05438.0f. [DOI] [PubMed] [Google Scholar]
- 18.Svilaas A, Sakhi AK, Andersen LF, et al. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr. 2004;134:562–567. doi: 10.1093/jn/134.3.562. [DOI] [PubMed] [Google Scholar]
- 19.Esquivel P, Jiménez VM. Functional properties of coffee and coffee by products. Food Res Int. 2012;46:488–495. [Google Scholar]
- 20.Di Castelnuovo A, di Giuseppe R, Iacoviello L, de Gaetano G. Consumption of cocoa, tea and coffee and risk of cardiovascular disease. Eur J Intern Med. 2012;23:15–25. doi: 10.1016/j.ejim.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–914. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echeverri D, Montes FR, Cabrera M, Galan A, Prieto A. Caffeine’s Vascular Mechanisms of Action. Int J Vasc Med. 2010;2010:834–844. doi: 10.1155/2010/834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yukawa GS, Mune M, Otani H, et al. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry (Mosc) 2004;69:70–74. doi: 10.1023/b:biry.0000016354.05438.0f. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-González I, Jiménez-Escrig A, Saura-Calixto F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter) Food Chem. 2005;90:133–139. [Google Scholar]
- 25.Vignoli J, Bassoli D, Benassi M. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011;124:863–868. [Google Scholar]
- 26.Abreu RV, Silva-Oliveira EM, Moraes MF, Pereira GS, Moraes-Santos T. Chronic coffee and caffeine ingestion effects on the cognitive function and antioxidant system of rat brains. Pharmacol Biochem Behav. 2011;99:659–664. doi: 10.1016/j.pbb.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Azam S, Hadi N, Khan NU, Hadi SM. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci Monit. 2003;9:BR325–330. [PubMed] [Google Scholar]
- 28.Viana AL, Fonseca M, Meireles EL, Duarte SM, Rodrigues MR, Paula FB. Effects of the consumption of caffeinated and decaffeinated instant coffee beverages on oxidative stress induced by strenuous exercise in rats. Plant Foods Hum Nutr. 2012;67:82–87. doi: 10.1007/s11130-011-0267-8. [DOI] [PubMed] [Google Scholar]
- 29.Acheson K, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jequier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr. 1980;33:989–997. doi: 10.1093/ajcn/33.5.989. [DOI] [PubMed] [Google Scholar]
- 30.Oh-ishi S, Heinecke J, Ookawara T, et al. Role of lipid and lipoprotein oxidation. In: Radák Z, editor. Free Radicals in Exercise and Aging. Champaign, IL: Human Kinetics; 2000. pp. 211–258. [Google Scholar]
- 31.Bloomer RJ, Kabir MM, Marshall KE, Canale RE, Farney TM. Postprandial oxidative stress in response to dextrose and lipid meals of differing size. Lipids Health Dis. 2010;9:79. doi: 10.1186/1476-511X-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloomer R, Trepanowski J, Kabir M, Alleman R, Dessoulavy M. Impact of short-term dietary modification on postprandial oxidative stress. Nutr J. 2012;11:16. doi: 10.1186/1475-2891-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloomer RJ, Ferebee DE, Fisher-Wellman KH, Quindry JC, Schilling BK. Postprandial oxidative stress: influence of sex and exercise training status. Med Sci Sports Exerc. 2009;41:2111–2119. doi: 10.1249/MSS.0b013e3181a9e832. [DOI] [PubMed] [Google Scholar]
- 34.Bloomer RJ, Fisher-Wellman KH. Lower postprandial oxidative stress in women compared with men. Gend Med. 2010;7:340–349. doi: 10.1016/j.genm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 35.del Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem. 2002;50:3698–703. doi: 10.1021/jf011702q. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Kitts DD. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res Int. 2011;44:2418–2424. [Google Scholar]
- 37.Fisher-Wellman KH, Bloomer RJ. Exacerbated postprandial oxidative stress induced by the acute intake of a lipid meal compared to isoenergetically administered carbohydrate, protein, and mixed meals in young, healthy men. J Am Coll Nutr. 2010;29:373–381. doi: 10.1080/07315724.2010.10719854. [DOI] [PubMed] [Google Scholar]
- 38.Ledikwe JH, Blanck HM, Kettel Khan L, et al. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83:1362–1368. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- 39.Neri S, Signorelli SS, Torrisi B, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Ther. 2005;27:1764–1773. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Neri S, Calvagno S, Mauceri B, et al. Effects of antioxidants on postprandial oxidative stress and endothelial dysfunction in subjects with impaired glucose tolerance and type 2 diabetes. Eur J Nutr. 2010;49:409–416. doi: 10.1007/s00394-010-0099-6. [DOI] [PubMed] [Google Scholar]
- 41.Ghanim H, Sia CL, Upadhyay M, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Covas MI, de la Torre K, Farre-Albaladejo M, et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic Biol Med. 2006;40:608–616. doi: 10.1016/j.freeradbiomed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Natella F, Belelli F, Gentili V, Ursini F, Scaccini C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J Agric Food Chem. 2002;50:7720–7725. doi: 10.1021/jf020346o. [DOI] [PubMed] [Google Scholar]
- 44.Lee I, Kim H, Bae J. Endothelial dysfunction: its relationship with acute hyperglycaemia and hyperlipidemia. Int J Clin Pract Suppl. 2002;(129):59–64. [PubMed] [Google Scholar]
- 45.De Gottardi A, Berzigotti A, Seijo S, et al. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am J Clin Nutr. 2012;96:584–590. doi: 10.3945/ajcn.112.040469. [DOI] [PubMed] [Google Scholar]