Abstract
Background
Application of distractive forces to small bowel induces intestinal growth, or enterogenesis. This emerging area of research may provide treatment for short bowel syndrome (SBS). Glucagon-like peptide 2(GLP-2) has also been reported to induce small bowel growth after bowel resection. We hypothesized that exogenous GLP-2 will result in enhanced distraction-induced enterogenesis.
Methods
Distraction-induced model was performed in 10-week-old C57B6 mice using osmotic forces with high molecular weight polyethylene glycol (PEG-stretch). Four groups were studied: Control group (PEG−/GLP-2−); PEG-stretch (PEG+/GLP-2−); GLP-2 control (PEG−/GLP-2+); and GLP-2 stretch (PEG+/GLP-2+). GLP-2 was given via subcutaneous osmotic pump over the 5 days of experiment. Morphology was measured by histomicrography. Epithelial cell (EC) proliferation was measured with PCNA immunofluorescent staining. Total intestinal growth and blood vessel volume was assessed with Micro CT volumetry. VEGF, FGF1 and 2, and PDGF were measured by RT-PCR.
Results
EC proliferation increased significantly in all groups compared to Controls, but was greatest in the GLP-2 stretch group. Diameter and length significantly increased in the PEG stretch and GLP-2 stretch groups. Moreover, there was statistically greater diameter, crypt depth and EC proliferation in the GLP-2 stretch vs. PEG stretch groups. GLP-2 stretch vessel volume was greater than all other groups and was significantly increased compared to controls. The relative expression of PDGF increased significantly in the PEG stretch group vs. the control group.
Conclusions
GLP-2 had an additive effect on EC proliferation, tissue growth, histomorphology and vascularization. The combination of enterogenesis and GLP-2 may yield an improved approach to treat SBS.
Keywords: GLP-2, distraction-induced growth, enterogenesis, vascularity, Microfil, Polyethylene glycol (PEG), cell proliferation
Introduction
Short bowel syndrome (SBS), caused by loss of small intestinal length, has an incidence of 3–5 per 100,000 births per year(1) and is associated with a reduced quality of life (2). Patients are dependent on parental nutrition(3) and have a high risk of severe complications which include, but is not limited to, sepsis and cholestatic liver disease(4). Outcomes of SBS patients have been linked to remaining small bowel length after operation (3). To overcome this, surgical procedures have been developed to increase bowel length (5–7). Success of these procedures have been limited, however, by surgical complications and reliance on dilated segments of bowel(8). An alternative treatment for SBS, small bowel transplantation, has limited success as well, with graft failure approaching 60% at 5 years(9).
Recently, application of distractive forces to the small bowel has been shown to induce significant growth of the intestine, or enterogenesis. This emerging area may provide a novel treatment for SBS(10–12). To address a better understanding of how distraction leads to intestinal growth, we developed a murine model of enterogenesis. This model utilizes high molecular weight polyethylene glycol (PEG) to create osmotic pressure within a 3cm isolated segment of bowel (13). We have termed this distractive-force model “PEG-stretch”. Previous work using the PEG-stretch model in our laboratory has shown significant increases in crypt depth, villus height, and epithelial cell (EC) proliferation(13).
Glucagon-like peptide 2(GLP-2) has been reported as another method to induce small bowel growth after bowel resection(14). Recent studies describe GLP-2’s action to be associated with increased EC proliferation, crypt fission and an increase in villus height (15). Other reports on the administration of exogenous GLP-2 have shown the growth factor to increase wet weights of rodent jejunum and ileum, enhance crypt cell proliferation, reduce enterocyte apoptosis, and increase thickness of the epithelial mucosa(16–18). GLP-2 analogs (Teduglutide, NPS Pharmaceuticals, Bedminster, NJ) have been the subject of clinical trials that have shown safety and efficacy in the promotion of intestinal growth in SBS patients(19, 20). GLP-2 has subsequently been demonstrated to increase blood flow to the superior mesenteric artery(21–23) and portal vein(24). Increased blood flow is necessary during growth of the small bowel; however, the mechanism by which GLP-2 affects blood flow is unknown. In fact, the interaction between enterogenesis and vascularity is poorly understood. The purpose of this study is to examine if GLP-2 would enhance intestinal growth during the process of enterogenesis. We hypothesized that GLP-2 would increase the enterogenetic potential of our murine PEG stretch model. We further hypothesized that this enhanced growth would be associated with an increase in intestinal vascularity. We investigated the effects of systemic GLP-2 on growth characteristics in stretched segments of small bowel including a detailed analysis of vascularity.
Material and Methods
Experimental Animals
All animal experiments were conducted with approval from the University Committee on the Use and Care of Animals (protocol number 07703/03986). Specific pathogen-free 10 week C57BL/6 male mice, with body weight over 22.0g (Jackson Bar Harbor, MA) were maintained in a 12-h night rhythm at 23°C and a relative humidity of 40–60%. Animals were fed standard rodent diet (LabDiet 5001Rodent Diet, PMI Nutrition International, LLC, Brentwood, MO) ad libitum. Forty eight hours before surgery, the diet was changed to micro-stabilized rodent liquid diet (TestDiet, Richmond, IN).
Surgical method
Details of the operation and study plan are shown in Figure 1. The day before the short bowel operation, an ALZET osmotic pump (#1007D) was prepared with 100 μl GLP-2 analog (25). Isoflurane anesthesia was induced and maintained by inhalational administration. A 2cm midline incision was made and the ligament of Treitz was identified. A well vascularized proximal-small bowel segment (containing at least 2 of the mesenteric arteries), at least 5 cm distal to the ligament of Treitz, was isolated on its mesenteric pedicle. Length and diameter were measured and the distal end of the isolated segment was ligated with 6-0 silk sutures. In the PEG stretch groups, polyethylene glycol (350 μl PEG: 3350KDa) was gently injected into the isolated segment, through a silastic laboratory tubing (ID: 0.64mm, OD: 1.19mm). The catheter was gradually removed and the end ligated using 6-0 silk suture. The isolated segment was returned to the abdomen. In controls, the segment was sealed, but not injected. This decision was based on previous work in which a saline injection group was found to produce no intestinal distension in a similar model as the saline was rapidly absorbed (13). Intestinal continuity was then reestablished by end-to-end anastomosis with interrupted sutures (9-0 nylon). Normal saline (1.5 ml) was injected to the peritoneal cavity before closing. Peritoneum and skin were closed using 4-0 polyglactin sutures. The mouse was turned in prone position and a 1cm incision was made in the flank. For the GLP-2 treated groups, the previously prepared osmotic pump containing GLP-2 was placed in the subcutaneous space and back incision was closed using 4-0 polyglactin sutures.
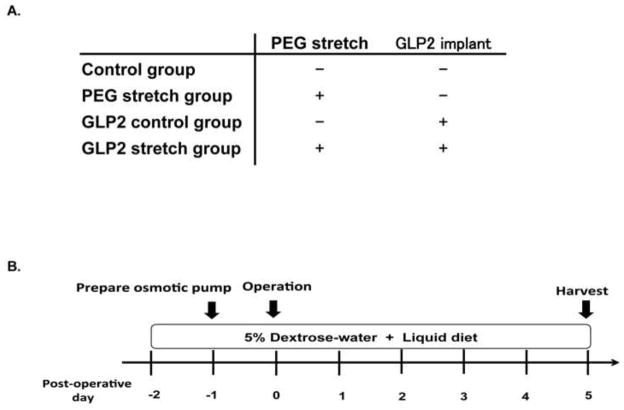
Figure 1.
A. Study groupsWe studied 4 groups of mice: Control group, PEG stretch group, GLP-2 control group, GLP-2 stretch group. Control mice did not receive PEG, and did not receive an implanted osmotic pump. The PEG stretch group had PEG instilled into the isolated lumen, but without implantation of an osmotic pump containing GLP-2. The GLP-2 control group received an osmotic pump with GLP-2, but did not have PEG implanted into the isolated segment. The GLP-2 stretch group had PEG instilled into the isolated segment lumen, and had an osmotic pump with GLP-2 implanted. A total of 9 mice were studied in each group; and 3 mice from each group were utilized for Microfil® vascularity analysis.
B. Study flow
Mice were provided 5% dextrose water and transition from regular chow to a liquid diet 2 days before the operation. Osmotic pump was prepared to contain GLP-2 on preoperative day 1, and stored at 37°C. Mice were euthanized 5 days after the operation.
Collection of tissue
Mice were allowed dextrose 5% in water for the first twelve hours following the procedure. For the remainder of the experimental period, the mice were maintained on a micro-stabilized rodent liquid diet. After 5 days, the mice were euthanized. Morphological changes (length and diameter) were recorded from the distended intestinal segments at the time of harvest and compared to the segment in the same animal at the time of operation. Two 0.5 cm portions of the segments were excised and placed into either 10% formaldehyde or embedded in an optimum cutting temperature (OCT) compound (Sakura Finetek Europe B.V., Netherland). Formalin-preserved sections were preserved in paraffin, sectioned transversely (5 μm), and stained with hematoxylin and eosin. OCT preserved sections were snap-frozen in liquid nitrogen and later used for immunofluorescence staining (see PCNA below). The remaining segment was immediately processed for mucosal cell isolation, and RT-PCR as described previously(26). Whole isolated segments were also used for vascularity evaluation.
Realtime PCR (RT-PCR)
Scraped intestinal mucosal tissue was added into RNeasy micro kit (Qiagen, Hilden, Germany) and homogenized. cDNA was purified and processed as previously described (26). All primers for selected gene sequences were selected by Primer-BLAST software http://blast.ncbi.nlm.nih.gov/Blast.cgi. Vascular endothelial growth factor(VEGF), fibroblast growth factor (FGF)1 and 2, platelet-derived growth factor(PDGF)were examined as vascular markers. Sodium-dependent glucose co-transporter 1(SGLT1), Glucose transporter 2 (GLUT2) and Glucose transporter 5 (GLUT5) were investigated as a measures of intestinal function. Real-time PCR (RT-PCR) was performed using a Rotor-Gene 6000 (Qiagen). GLP-2 treatment in vivo studies altered the expression the expression of glyceraldehyde-3-phosphate dehydrogenase (p<0.05), β-actin (p<0.01) and ribosomal protein L 13a (p<0.01) but not 18S RNA. Therefore, 18S RNA was used as an internal control for all quantitative analyses of mRNA expression(27, 28).
Epithelial cell proliferation (PCNA staining)
Cell proliferation was evaluated with immunofluorescence staining using PCNA antibody (Cell signaling, Danvers, MA), which was applied overnight (dilution- 1:1000). Slides were incubated with secondary antibody for 2-hrs and counterstained with DAPI for detection of nuclei. Images were visualized on a Nikon Eclipse Ti (Nikon Instruments Inc, Tokyo, Japan) under 20X magnification. All slides were counted in 15 crypts per slide. Cell proliferation rate was recorded as the number of PCNA positive crypt cells per the total number of crypt cells, as previously described(29).
Intestinal vascularity analysis
Microfil® has recently been used described to evaluate vascularity in small intestine after lengthening in a pig model (30). In the present study, the abdominal and chest cavities were exposed after euthanasia. The right atrium was cut for blood drainage, and a 26-gauge needle was used to puncture the left ventricle. Heparinized normal saline (10cc) was injected until drainage fluid was clear. The Microfil® was prepared in a 5:4 ratio of MV-diluent to MV-122 yellow compound according to the manufacturer’s manual. The mixture was catalyzed with 5% (by volume) of MV Curing Agent and injected into the systemic vascular system after saline clearance. After a 24 h incubation in 4°C, the tissue was taken for CT scanning (University of Michigan’s Center for Molecular Imaging for MicroCT). GE Healthcare’s Microviewer software was next utilized to create a 3D polygonal isosurface (3D structure) based on a series of 2-dimensional CT images. Using discrepancies in Hounsfield units, the percentage of vessels within the generated 3D polygon was reported based on CT volumetry. With this methodology, we obtained total intestinal volume, vascularity volume and the ratio of vascularity volume to total intestinal volume.
Statistics
Data are expressed as mean ± SEM. For comparison of two groups, unpaired T-tests were used. Comparisons between GLP-2 stretch group and GLP-2 control group, PEG stretch group and Control group were performed using the unpaired T-tests. For multiple group comparisons, ANOVA was performed with a post hoc Tukey Multiple Comparison analysis. Differences between groups were tested using pairwise comparisons within the model. P values less than 0.05 were considered statistically significant.
Results
Gross tissue analysis and morphometrics
The GLP-2 control was unchanged in terms of gross bowel length and diameter compared to the control group; this was somewhat unexpected given previous literature which showed that GLP-2 could drive intestinal growth in non-surgically manipulated bowel (31). On gross inspection, PEG stretch resulted in significant growth of the isolated segment in both length and diameter compare to the control group (Figure 2, p<0.05); and this was consistent with our previous study (13). Interestingly, with the addition of GLP-2 to the PEG stretch model (GLP-2 stretch) there was yet another significant increase in intestinal diameter compared to the PEG stretch group (p<0.05). However, there was no additional increase in bowel length with the addition of GLP-2.
Figure 2. Gross morphologic changes in the isolated segment.
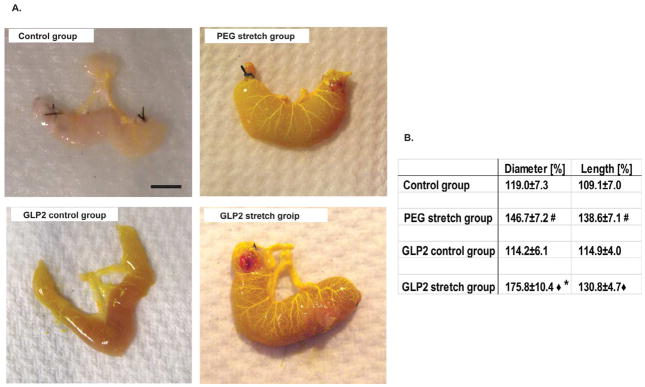
A. Representative images showing overall size and the vascularity of the isolated segments at the time of harvesting. The yellow color Microfil® compound replaced the blood in vessels. Scale bar is 0.5mm.
B. Table showing the summation of macroscopic changes in the isolated segment by PEG-mediated distractive forces and GLP-2. Changes are shown by the percent change from the starting measurements. The isolated segments of the PEG stretch group were expanded significantly in both percentage change of diameter and length from pre-injection of PEG, and was also significantly greater than that of the control group (#p<0.05). Similarly, there were statistically significant increases for the GLP-2 stretch group compared to GLP-2 control group (◆p<0.05). Interestingly, the GLP-2 stretch group had a significantly increased diameter compared to the PEG stretch group (*p<0.05).
This pattern held true with regard to the histomorphologic evaluation. The GLP-2 control was unchanged from the control group with regards to villus height and crypt depth (Table 1, villus height p= 0.53, crypt depth p=0.52). However, the PEG stretch and GLP-2 stretch groups both showed a significant increase in villus height and crypt depth compared to the control group (p<0.05). Further, the GLP-2 stretch group exhibited a significant increase in crypt depth compared to the PEG stretch alone (p<0.05). There was also a trend towards increased villus height in the GLP-2 stretch vs. PEG stretch; however, this failed to achieve statistical difference.
Table 1.
| Villus height [μm] | Crypt depth [μm] | |
|---|---|---|
| Control group | 279.1±15.8 | 85.3±1.8 |
| PEG stretch group | 350.1±32.1 * | 105.1±2.1 * |
| GLP2 control group | 293.6±6.3 | 90.1±7.0 |
| GLP2 stretch group | 380.4±18.8# | 118.8±4.2 #◆ |
Results are expressed as mean ± SEM
An unpaired two-tailed T-test was used to compare expression between groups, p<0.05
p<0.05 for compared to Control group
p<0.05 for compared to GLP2 control group
p<0.05 for compared to PEG stretch group
Effect of GLP-2 on epithelial cell proliferation in the enterogenesis model
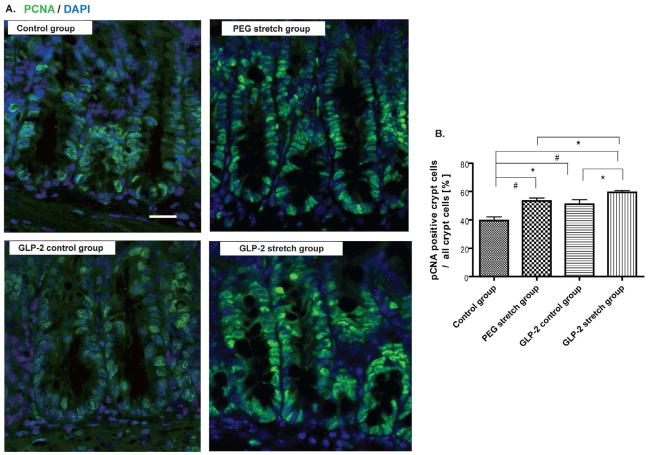
To insure that sufficient exogenous GLP-2 was delivered to our mice, a preliminary set of experiments were performed with Alzet® osmotic pumps containing the differing concentrations of GLP-2. Once optimized we demonstrated that plasma levels (measured by radioimmunoassays, kindly performed by the J. Holst laboratory) of GLP-2 rose from a fasting baseline of 32.1±9.2 picoM to 657.0±169.0 picoM in the exogenously delivered group. Intestinal EC proliferation was assessed with PCNA immunofluorescent staining (Figure 3). Both distraction groups demonstrated a significantly increased rate of EC proliferation compared to the control group (p<0.01). EC proliferation was also significantly increased in the GLP-2 control compared to the Control group (p<0.05); and this was consistent with previously reported literature(15). The GLP-2 stretch group showed significantly greater EC proliferation compared to the PEG stretch group; and the GLP-2 stretch group had the greatest rate of EC proliferation of all groups studied (p<0.05). These results demonstrate the additive effect of GLP-2 action on EC proliferation in the distraction-mediated intestinal growth model.
Figure 3. GLP-2 and the distractive force stimulated cell proliferation.
A. Immunofluorescence staining was performed for evaluation of epithelial cell proliferation in each group using PCNA staining. Scale bar is 50 μm.
B. Epithelial cell proliferation is shown as the ratio of PCNA positive crypt cells to all crypt cells, and was used as an epithelial cell proliferation marker.
PEG stretch group increased EC proliferation compared to the Control group (#p<0.01). GLP-2 stretch group also up-regulated EC proliferation compared to the GLP-2 control group (*p<0.05) and the control group (#p<0.01). The GLP-2 control group showed a higher EC proliferation compared to Control group (*p<0.05). The GLP-2 stretch group showed significantly higher EC proliferation compared to the PEG stretch group (*p<0.05). Values are means ± SEM.
GLP-2 and vascularization of the isolated segment
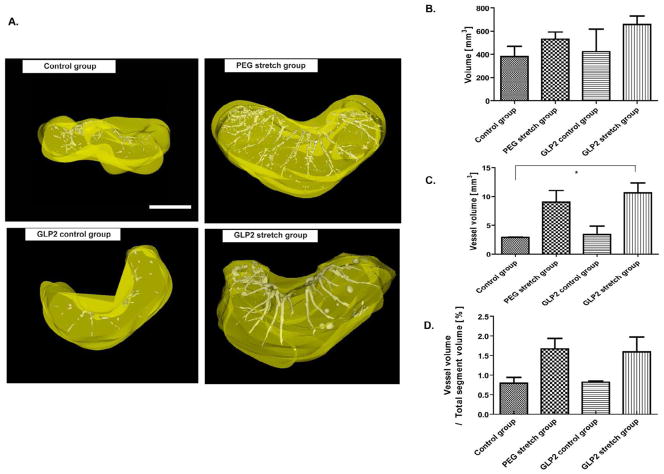
Vascularity of the intestinal wall was investigated after enterogenesis with Microfil® vascular analysis. The 3D reconstruction data was similar to that of our gross inspection in that the GLP-2 stretch group had the largest total intestinal volume (Figure 4A and 4B). As well, the GLP-2 stretch group had the largest vessel volume in the isolated segments after induction of enterogenesis (Figure 4C); and this was the only group to achieve statistical significance compared to the Control group (p<0.05). Although the PEG stretch group also showed increased vessel volume compared to the control group, there was no statistically significant difference between the GLP-2 stretch group versus the PEG stretch group, nor between the Control group versus GLP-2 control group. To further analyze vascularization, a vessel volume ratio (vessel volume / total isolated segment volume) was calculated (Figure 4D). This showed an increase in the vessel volume ratio in the GLP-2 stretch groups compared to the GLP-2 control group, but the difference was not statistically significant (p=0.21). Interestingly, both the GLP-2 stretch group and PEG stretch group showed an increase in the vessel volume ratio compared to the other study groups, but there was no significant difference between the these two groups (PEG stretch vs. the control group, p=0.09, GLP-2 stretch vs. GLP-2 control group, p=0.21).
Figure 4. Analysis of vascularization.
A. Representative images of the 3 dimension reconstructions of the vascularization of isolated segments from each study group as reconstructed using Microview software. The white colored area indicates the vessel structure. Likewise yellow color area indicates intestinal volume. Scale bar is 0.5mm.
B. Summary of the changes in intestinal volume in each study group. Although there was no significant change, a trend of increased volume was observed in the PEG stretch and GLP-2 stretch groups, with the largest volume in the GLP-2 stretch group.
C. Mean vessel volume, the GLP-2 stretch group led to a statistically significant increase in vessel volume compared to the Control group (*p<0.05).
D. The ratio of vessel volume to total intestinal segment size is shown. Not a marked increase in this ratio for both the PEG stretch and GLP-2 stretch groups. (p=0.87)
Values are means ± SEM.
GLP-2 did not alter vascular-related regulatory genes
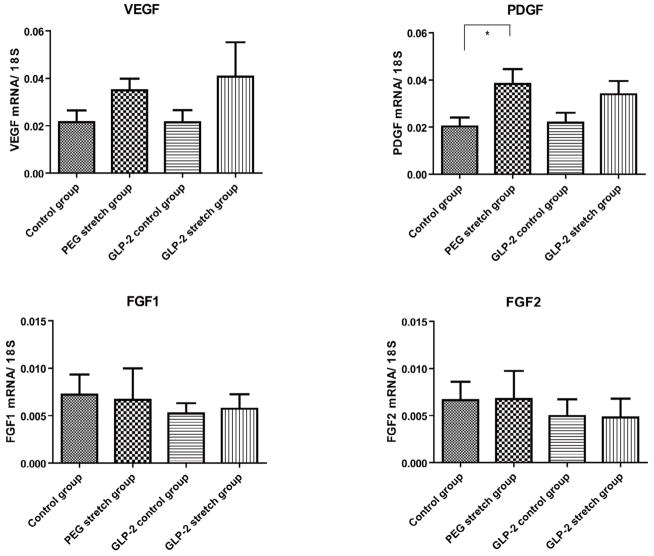
We next examined VEGF, FGF1, FGF2 and PDGF mRNA expression as they are all potentially responsible for driving the increased vascularity observed in this model (Figure 5). In comparing all groups, only PDGF expression was statistically increased in the PEG stretch group compared to the Control group (p<0.05). This, as well as VEGF trended towards an increase in the GLP-2 stretch group, but failed to reach statistical significance. Although other genes were increased in the PEG stretch group compared to the Control group, none of these achieved statistical significance (VEGF; p=0.20, FGF1; p=0.18, FGF2; p=0.55). Quite interestingly, despite the GLP-2 group having increased vascularization to at least the level of the PEG stretch group, the GLP-2 stretch group had the lowest levels of FGF1 and FGF2.
Figure 5. Vascularity associated gene expression.
Mucosally-derived mRNA expression of several vascularization-associated control genes were studied with RT-PCR and expressed as a ratio to 18S RNA. Note that the abundance of each of these factors rose in the PEG stretch group. Interestingly, the addition of GLP-2 actually led to a reduction of these values for FGF1 and FGF2. The only significant change was the expression of PDGF between the PEG stretch and Control groups (p<0.05). Other changes were not statistically different between these two groups (VEGF; p=0.20, FGF1; p=0.18, FGF2; p=0.55). Values are means ± SEM.
Effect of GLP-2 on intestinal function during enterogenesis
Relative expression of SGLT1, GLUT2 and GLUT5 mRNA was measured to evaluate intestinal function in the setting of enterogenesis (Table 2). SGLT1 expression was statistically increased in the GLP-2 stretch group compared to the PEG stretch group or GLP-2 groups (p<0.05). This suggested that the addition of GLP-2 and mechanical stretching had an additive effect that was not achieved with either of these approaches by themselves. GLUT2 expression trended towards an increase in both stretch groups, but failed to reach statistical significance when compared to the control group. (Control group vs. PEG stretch group, p=0.19, GLP-2 control group vs. GLP-2 stretch group, p=0.14).
Table 2.
| SGLT1 | GLUT2 | GLUT5 | |
|---|---|---|---|
| Control group | 10.7±3.8 | 2.4±0.7 | 2.7±1.4 |
| PEG stretch group | 8.8±2.7 | 4.1±0.8 | 3.2±0.9 |
| GLP2 control group | 9.5±2.0 | 1.7±0.3 | 0.9±0.2 |
| GLP2 stretch group | 26.3±8.5◆ | 3.5±1.0 | 2.5±1.0 |
Fold changes of target genes are relative to 18S RNA abundance; all data are × 103
Results are expressed as mean ± SEM
An unpaired two-tailed T-test was used to compare expression between groups, p<0.05
p<0.05 for compared to PEG stretch group
Discussion
This study demonstrates that exogenous GLP-2 administration has an additive effect on intestinal growth in a murine PEG stretch model. This was shown grossly with significantly increased diameter in isolated intestinal segments in the GLP-2 stretch group compared to the PEG stretch group. This was also seen on a cellular level with increased EC proliferation with the addition of GLP-2 into this enterogenesis model. In addition, the crypt depth was statistically elongated in the GLP-2 stretch group compared to the PEG stretch group. Both the PEG stretch and the GLP-2 stretch group had a marked increase in intestinal volume compared to the control group, and this was mirrored by an increase in vessel volume in these groups. This may suggest this larger volume of tissue requires increased vascularity to accommodate nutrient and oxygen demands of an increased intestinal mass. The vessel volume ratio was similar between PEG stretch and GLP-2 stretch group, but overall vessel volume was greater in the GLP-2 stretch group; again, suggesting that GLP-2 potentially drove greater vascularization in this model.
GLP-2 positively affects the multiple functions in the gastrointestinal intestinal tract (32–34). GLP-2 has been shown to stimulate intestinal growth through crypt fission and reduced EC apoptosis resulting in an expansion of mucosal surface area(35). Our results also showed that GLP-2 led to increased EC proliferation with and without the application of distractive forces. However, GLP-2 mediated EC proliferation was enhanced by the application of distractive forces. Moreover, the addition of GLP-2 stimulated a further increase in crypt depth, but not villus height. This suggests that the addition of GLP-2 led to augmentation of the crypt architecture via increased EC proliferation rate; and resulted in further morphological growth than the distraction-mediated intestinal growth alone. On the other hand, while our results showed that GLP-2 significantly increased EC proliferation, there was no difference in the villus height and crypt depth when GLP-2 was used alone. GLP-2’s effect on morphology could only be appreciated after the addition of distractive forces. It is possible that longer degrees of distraction and GLP-2 administration may have led to a greater augmentation of growth with GLP-2 in this distraction model. This may also be attributed to the fact that our model differs from small bowel resection models used in previous publications in both surgical procedure and experimental timeframe. For example, Garrison et al demonstrated that a 7-day administration of GLP-2 increased villus height and crypt depth, but only villus height with 4- days of administration. Hence, GLP-2 may affect cellular proliferation at early stages, which may lead to greater morphological changes in the isolated intestinal segments without enterogenesis at a later time point. However the development of EC proliferation and intestinal histomorphological growth may be altered with enterogenesis at early stages.
GLP-2 has been shown to stimulate intestinal blood flow(22) and increase portal blood flow(24) through a nitric oxide-dependent pathway(36). GLP-2 has also been shown to raise jejunal microcirculatory flow in SBS patients with an enterostomy(37). While flow has been examined, actual growth of intestinal vascularity has not been examined. Microfil contrast agent was used to assess vessel volume and overall vessel density. PEG stretch led to an increase in vessel volume. The addition of GLP-2 to the PEG stretch model led to a further increase in vessel volume that reached statistical significance compared to the control group. Admittedly, it is difficult to determine if GLP-2 stimulated vessel growth to allow for increased tissue growth, or if the increased proliferation seen in the GLP-2 group stimulated an increase in neovascularization. The mechanism of the increased growth remains unclear, as the relative expression of VEGF and PDGF only trended towards an increased level with the addition of GLP-2.
Intestinal function was also enhanced with the addition of GLP-2 to the PEG stretch model. This is evident through the increased expression of SGLT1 in the GLP-2 stretch group compared to the PEG stretch group. This matches previous studies where GLP-2 was shown to increase SGLT1 expression in short bowel resection model (38) and in tissue-engineered neointestine (39). Therefore, GLP-2 appears to contribute to increases of SGLT1 expression in these models of intestinal growth, regardless of the approach.
This study did have several limitations. We demonstrated that GLP-2 led to increased intestinal growth and vascularity in the enterogenesis model; however, mRNA expressions of vascular related genes were not significantly different. It is possible that statistical significance could be reached with larger number of mice. Secondly, the abdominal cavity in mice allows only for 5 days of expansion. Longer periods of distractive forces could perhaps clarify some unanswered questions; however, the small abdominal cavity volume actually led to a compartment syndrome with further degrees of enterogenesis. These could possibly be carried out in a larger animal model, such as a rat with its given increased abdominal capacity. Further, we did not investigate blood flow in this study. The technique of Microfil® vascularity analysis requires euthanasia and prevents other analyses to be performed on that animal. Thus real-time flow analysis could not be performed at the same time on the same animal. Consequently, tissue samples were collected from different mice from which the mice were examined by Microfil® imaging. However, the demonstration of increases in vessel growth actually demonstrated a new and novel action of enterogenesis and GLP-2.
In this study, we show that exogenous GLP-2 accelerates intestinal epithelial cell proliferation, histomorphology and over all tissue growth when given in addition to enterogenesis driven by distractive forces. This may potentially be due to GLP-2’s effect on vascularity. This data implies that the addition of GLP-2 to the novel approach of distraction-mediated enterogenesis may increase the potential for bowel growth. This suggestion should be confirmed in future studies including large animals with the combination of different enterogenesis procedures with and without the addition of GLP-2. Potentially, the use of distractive forces applied to remaining intestine of patients with SBS, along with GLP-2, may yield an improved approach to treat this very challenging clinical disorder.
Acknowledgments
Grant Support: Hartwell Foundation, Biomedical Research Award and FDA Pediatric Device Consortia Grant 2-P50-FD-003787-03
We thank Amanda Fair, Kevin Heist, Ben Hoff and Pele Browner for their excellent technical assistance.
Footnotes
Disclosures: The authors have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeLegge M, Alsolaiman MM, Barbour E, Bassas S, Siddiqi MF, et al. Short bowel syndrome: parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci. 2007;52:876–892. doi: 10.1007/s10620-006-9416-6. [DOI] [PubMed] [Google Scholar]
- 2.Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, et al. Natural History of Pediatric Intestinal Failure: Initial Report from the Pediatric Intestinal Failure Consortium. The Journal of pediatrics. 2012 doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer AU, Neaga A, West B, Safran J, Brown P, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–409. doi: 10.1097/01.sla.0000179647.24046.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyasaka EA, Okawada M, Utter B, Mustafa-Maria H, Luntz J, et al. Application of distractive forces to the small intestine: defining safe limits. J Surg Res. 2010;163:169–175. doi: 10.1016/j.jss.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi A. Intestinal loop lengthening--a technique for increasing small intestinal length. Journal of pediatric surgery. 1980;15:145–151. doi: 10.1016/s0022-3468(80)80005-4. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Soper RT. Isolated bowel segment (model 1): creation by myoenteropexy. Journal of pediatric surgery. 1990;25:512–513. doi: 10.1016/0022-3468(90)90562-n. [DOI] [PubMed] [Google Scholar]
- 7.Kim HB, Lee PW, Garza J, Duggan C, Fauza D, et al. Serial transverse enteroplasty for short bowel syndrome: a case report. Journal of pediatric surgery. 2003;38:881–885. doi: 10.1016/s0022-3468(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 8.Sudan D, Thompson J, Botha J, Grant W, Antonson D, et al. Comparison of intestinal lengthening procedures for patients with short bowel syndrome. Annals of surgery. 2007;246:593–601. doi: 10.1097/SLA.0b013e318155aa0c. discussion 601–594. [DOI] [PubMed] [Google Scholar]
- 9.Lao OB, Healey PJ, Perkins JD, Horslen S, Reyes JD, et al. Outcomes in children after intestinal transplant. Pediatrics. 2010;125:e550–558. doi: 10.1542/peds.2009-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga H, Sun X, Yang H, Nose K, Somara S, et al. Distraction-induced intestinal enterogenesis: preservation of intestinal function and lengthening after reimplantation into normal jejunum. Ann Surg. 2012;255:302–310. doi: 10.1097/SLA.0b013e318233097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Puapong DP, Wu BM, Atkinson JB, Dunn JC. Enterogenesis by mechanical lengthening: morphology and function of the lengthened small intestine. J Pediatr Surg. 2004;39:1823–1827. doi: 10.1016/j.jpedsurg.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Puapong DP, Wu BM, Lam MM, Atkinson JB, Dunn JC. Distension enterogenesis: increasing the size and function of small intestine. J Pediatr Surg. 2006;41:763–767. doi: 10.1016/j.jpedsurg.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Okawada M, Mustafa Maria H, Teitelbaum DH. Distraction Induced Enterogenesis: A Unique Mouse Model Using Polyethylene Glycol. Journal of Surgical Research. 2011;170:41–47. doi: 10.1016/j.jss.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brubaker PL, Crivici A, Izzo A, Ehrlich P, Tsai CH, et al. Circulating and tissue forms of the intestinal growth factor, glucagon-like peptide-2. Endocrinology. 1997;138:4837–4843. doi: 10.1210/endo.138.11.5482. [DOI] [PubMed] [Google Scholar]
- 15.Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301:28. doi: 10.1152/ajpgi.00039.2011. [DOI] [PubMed] [Google Scholar]
- 16.Drucker DJ, Deforest L, Brubaker PL. Intestinal response to growth factors administered alone or in combination with human [Gly2]glucagon-like peptide 2. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1997;273:G1252–G1262. doi: 10.1152/ajpgi.1997.273.6.G1252. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CH, Hill M, Drucker DJ. Biological determinants of intestinotrophic properties of GLP-2 in vivo. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1997;272:G662–G668. doi: 10.1152/ajpgi.1997.272.3.G662. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CH. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. The American journal of physiology. 1997;273:E77–84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- 19.Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin GRPB, Sigalet DL. Gut hormones, and short bowel syndrome: The enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation. World J Gastroenterol. 2006;26:4117–4129. doi: 10.3748/wjg.v12.i26.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bremholm L, Hornum M, Henriksen BM, Larsen S, Holst JJ. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol. 2009;44:314–319. doi: 10.1080/00365520802538195. [DOI] [PubMed] [Google Scholar]
- 22.Bremholm L, Hornum M, Andersen UB, Holst JJ. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept. 2010;159:67–71. doi: 10.1016/j.regpep.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Stephens J, Stoll B, Cottrell J, Chang X, Helmrath M, et al. Glucagon-like peptide-2 acutely increases proximal small intestinal blood flow in TPN-fed neonatal piglets. Am J Physiol Regul Integr Comp Physiol. 2006;290:15. doi: 10.1152/ajpregu.00588.2005. [DOI] [PubMed] [Google Scholar]
- 24.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Holst JJ, Teitelbaum DH. Total parenteral nutrition (TPN)-associated atrophy is associated with loss of intestinal epithelial cell (EC) migration: modulation of action by epidermal growth factor (EGF) and glucagon-like peptide-2 (GLP-2) Gastroenterology. 2011;140:S-170–S-171. [Google Scholar]
- 26.Spencer A, Yang H, Haxhija E, Wildhaber B, Greenson J, et al. Reduced Severity of a Mouse Colitis Model with Angiotensin Converting Enzyme Inhibition. Digestive Diseases and SciencesSpringer Netherlands. 2007:1060–1070. doi: 10.1007/s10620-006-9124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvey S, Thompson EW, Matthaei K, Lea RA, Irving MG, et al. Beta-actin--an unsuitable internal control for RT-PCR. Mol Cell Probes. 2001;15:307–311. doi: 10.1006/mcpr.2001.0376. [DOI] [PubMed] [Google Scholar]
- 28.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131:589–605. doi: 10.1053/j.gastro.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Teitelbaum DH. Epidermal growth factor/TNF-alpha transactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2012;302:10. doi: 10.1152/ajpgi.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralls MW, Sueyoshi R, Herman RS, Utter B, Czarnocki I, et al. Mesenteric neovascularization with distraction-induced intestinal growth: enterogenesis. Pediatr Surg Int. 2012;16:16. doi: 10.1007/s00383-012-3204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkman AS, Murali SG, Hitt S, Solverson PM, Holst JJ, et al. Enteral nutrients potentiate glucagon-like peptide-2 action and reduce dependence on parenteral nutrition in a rat model of human intestinal failure. Am J Physiol Gastrointest Liver Physiol. 2012;303:28. doi: 10.1152/ajpgi.00184.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, et al. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol. 2007;293:29. doi: 10.1152/ajpgi.00530.2006. [DOI] [PubMed] [Google Scholar]
- 34.Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol. 1997;273:R1965–1971. doi: 10.1152/ajpregu.1997.273.6.R1965. [DOI] [PubMed] [Google Scholar]
- 35.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–147. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 37.Hoyerup P, Hellstrom PM, Schmidt PT, Brandt CF, Askov-Hansen C, et al. Glucagon-like peptide-2 stimulates mucosal microcirculation measured by laser Doppler flowmetry in end-jejunostomy short bowel syndrome patients. Regul Pept. 2012;16:00243–00241. doi: 10.1016/j.regpep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:12. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 39.Ramsanahie A, Duxbury MS, Grikscheit TC, Perez A, Rhoads DB, et al. Effect of GLP-2 on mucosal morphology and SGLT1 expression in tissue-engineered neointestine. Am J Physiol Gastrointest Liver Physiol. 2003;285:14. doi: 10.1152/ajpgi.00374.2002. [DOI] [PubMed] [Google Scholar]