Abstract
People vary widely in how much they discount delayed rewards, yet little is known about the sources of these differences. Here we demonstrate that neural activity in ventromedial prefrontal cortex (VMPFC) and ventral striatum (VS) when human subjects are asked to merely think about the future—specifically, to judge the subjective length of future time intervals—predicts delay discounting. High discounters showed lower activity for longer time delays, while low discounters showed the opposite pattern. Our results demonstrate that the correlation between VMPFC and VS activity and discounting occurs even in the absence of choices about future rewards, and does not depend on a person explicitly evaluating future outcomes or judging their self-relevance. This suggests a link between discounting and basic processes involved in thinking about the future, such as temporal perception. Our results also suggest that reducing impatience requires not suppression of VMPFC and VS activity altogether, but rather modulation of how these regions respond to the present versus the future.
Introduction
People differ in their willingness to defer immediate gratification to pursue long-term goals. These individual differences are important, because a tendency to delay gratification is associated with better educational outcomes in children and beneficial health behaviors in adults (Mischel et al., 1988; Bickel and Marsch, 2001; Duckworth and Seligman, 2005; Chabris et al., 2008). However, we know little about the source of these differences. This article links individual differences in delay discounting to brain activity while individuals are merely thinking about future durations, in the absence of any explicit tradeoffs between immediate and delayed gratification.
Delay discounting tasks provide a measure of preference for immediate versus delayed rewards. The extent of preference for immediate rewards is captured by the discount rate, which expresses how much the subjective value of a delayed reward declines as a function of delay. Neuroimaging studies of discounting have found that BOLD activity in ventral striatum (VS) and ventromedial prefrontal cortex (VMPFC) scales with the subjective value of the options being considered (Kable and Glimcher, 2007, 2010; Ballard and Knutson, 2009; Peters and Büchel, 2009; Pine et al., 2009). This implies that individual differences in discounting are linked to differences in the neural sensitivities of VS and VMPFC. In high discounters, there is much greater activity in these regions for immediate compared with equally sized delayed rewards, while this difference is smaller in low discounters. Contextual manipulations that shift discount rates within an individual may involve the same mechanism, affecting BOLD activity within VS and VMPFC (Peters and Büchel, 2010a).
More recent work has suggested a link between discounting and VS or VMPFC activity in other task contexts. Hariri et al. (2006) have shown that higher discounters exhibit a greater VS response to unpredicted rewards. Others have shown that higher discounters exhibit a greater difference in BOLD activity between judgments of oneself in the present compared with the future (Ersner-Hershfield et al., 2009; Mitchell et al., 2011).
A more basic question is whether brain activity when individuals are merely prompted to think about the future can predict discount rates. Is there a relationship between VS and VMPFC activity and discount rates even when individuals are not explicitly evaluating present or future outcomes or judging their self-relevance? Behavioral studies have recently demonstrated the role of time perception in intertemporal choice (Kim and Zauberman, 2009; Zauberman et al., 2009), but, to date, there are no neuroimaging data on this relationship. Here we show that the delay sensitivity of VS and VMPFC BOLD responses when individuals make simple value-free judgments about the subjective length of future time intervals predicts behavioral discount rates measured 10 d later. This suggests that the association between neural activity and discounting arises from a basic process involved in thinking about the future, such as judging temporal distance. Additionally, our results suggest that reducing impatience requires not suppressing VS and VMPFC activity altogether, but rather modulating how these regions respond to the present versus the future.
Materials and Methods
Subjects.
Forty participants (16 males and 24 females; 88% were right handed) were recruited from the University of Pennsylvania and surrounding community. Participants had a mean age of 21.75 years (SD, 3.27 years). All participants were compensated for their time on both of 2 testing days and received an additional monetary payment based on their decisions in the discounting task. All participants provided consent in accordance with the procedures of the Institutional Review Board of the University of Pennsylvania.
Tasks.
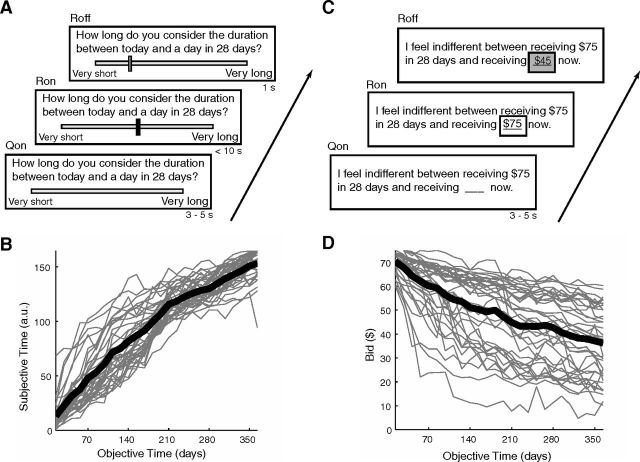
All participants completed two sessions, separated by an average of 10 d (SD, 5 d; range, 4–21 d). Participants were told that there were two sessions involving different tasks, but they were not told the details of each task until that session began. In the first session, participants completed the temporal judgment task (Fig. 1A). In this task, participants were asked to judge the subjective duration of a future delay interval on a visual analog scale. This scale was bounded by the labels “very short” and “very long” (Zauberman et al., 2009). Participants were asked questions of the form, “How long do you consider the duration between today and a day in 28 d?” In each scan, participants were asked about 26 different delay durations, uniformly distributed between 14 and 364 d. Participants were informed beforehand about the entire range of delays they would be judging. Participants entered their judgment by moving the response bar along the scale. The response bar always appeared in the middle of the scale. Participants used two buttons to move the bar to the right and left, and a third to submit their response. Ratings were recorded in arbitrary screen units, ranging from 1 to 165. All participants completed four scans of this task.
Figure 1.
Temporal judgment and temporal discounting tasks. A, The sequence of events during a trial in the temporal judgment task is shown. Participants viewed each question for 3–5 s then were given 10 s to respond. Participants moved a black bar (which always appeared first in the center) along a scale bounded by the labels “very short” and “very long” to indicate their perceived duration of each delay. When a final response was submitted, the bar turned red and remained on screen for 1 s. B, Plot of subjective time ratings against objective time durations. Gray lines are individual subjects, and the black line is the mean. C, The sequence of events during a trial in the temporal discounting task is shown. Participants viewed each question for 3–5 s and were then given 10 s to respond. Bids for each trial always began at $75, and participants were able to increase and decrease the monetary amount. After pressing a button to submit their response, the text turned red and remained on the screen for 1 s. D, Plots of bids against objective durations. Gray lines are individual subjects' bids, and the black line is the mean.
In the second session, participants completed the temporal discounting task (Fig. 1C). In this task, participants bid on delayed monetary rewards. They provided the amount of money received immediately that they felt was equivalent to receiving a larger amount after a specified delay. Participants were asked questions of the form, “I feel indifferent between receiving $75 in 28 d and receiving $? now.” From trial to trial, the $75 value of the delayed amount remained constant, and only the delay varied. In each of four scans, participants were asked about the same 26 delays used in the temporal judgments task (i.e., between 14 and 364 d). Again, participants were informed beforehand about the range of possible delays. The response amount always began at $75 now. Participants entered their bid by using two buttons to increase or decrease this amount and a third button to submit their response. The behavioral data from one scan of one subject in this task was lost due to experimenter error.
The timing of the two tasks was similar. The intertrial interval was variable, between 0.5 and 13.5 s. In the “question period,” participants saw the delay they were judging or the delayed amount they were evaluating but could not yet enter their response. This period lasted from 3 to 5 s. This period terminated with the appearance of the response bar or of “$75 now” in the respective tasks. The “response period” began at this point and lasted until the participant submitted their response. The response period timed-out after 10 s in both tasks, and the current location of the response bar or the immediate amount was taken as the participant's response.
Participants knew that if they did not press a button to submit their response, the value that the cursor was on when the 10 s response period ended would be taken as their response. Participants also knew that there was no penalty for not pressing the submit button. Because of this, several participants adopted a strategy of never pressing the submit button. Overall, participants did not submit a response within 10 s on 12% of trials in the temporal judgment task, and on 22% of trials in the temporal discounting task. However, these “timed-out” trials appeared to be clearly strategic rather than indicative of an inability to complete the task. Indeed, the discount rates estimated using all trials and those excluding timed-out trials were very highly correlated (r(36) = 0.998). We therefore kept all timed-out trials in our analyses.
Only a very small minority of timed-out trials were the result of participants not responding on the trial at all. Across both scans, only five participants had any instance of not moving the cursor along the response scale at all; each of these participants made no response on only 1 of a total of 104 trials.
Payments.
In addition to a flat $15 per hour fee for their participation, subjects were paid an additional amount according to their decisions in the time discounting task, using the mechanism of Becker et al. (1964). First, participants rolled a die to randomly select one of the trials. The delayed option on that trial (e.g., $75 in 28 d) and the participant's bid for that option (e.g., $70 now) were determined. The participant then rolled a die a second time to generate a random “counteroffer.” The generation of counteroffers was such that they would be uniformly distributed between $0 and $75. If the participant's bid was greater than the counteroffer, they received $75 at the delay specified. If the participant's bid was below the counteroffer, they received the counteroffer amount immediately. The payment procedure provides incentive for the participant to bid their true valuation of the delayed option. All payments were made using prepaid debit cards, as described previously (Kable and Glimcher, 2007, 2010). These cards have the advantage of making receipt of the delayed payment easy and reliable, minimizing the effects of greater uncertainty about and effort needed for the future reward.
Behavioral data analysis.
Behavioral data were analyzed in MATLAB (Mathworks). In the time discounting task, we characterized the relationship between the objective delay (OT) and the participant's evaluation of $75 received after that delay (BID). We fit this relationship with the following function: BID = 75/(1 + k × OT; Mazur, 1987). Because discount rates are not normally distributed, statistics are performed on the log-transform of the discount parameter k.
For one subject, this function could not be fit because the subject always bid $75. This subject is excluded from all analyses and figures, with one exception. This subject is included in additional analyses that use an alternative method of estimating discount rates, the area under the curve (AUC) method (Myerson et al., 2001). We report the correlation between brain activity and discount rates estimated with both the hyperbolic k and AUC measures, and observe similar results with both sets of estimates.
To reduce the influence of possible accidental responses in the time discounting task, we excluded all trials for any delay where the range of the subject's responses spanned more than half the range of the response scale (range greater than $37.50). We excluded a total of 12 delay bins in a total of five subjects (maximum of 4 delay bins per subject, ∼1% of the data). Our reported results do not differ when these trials are included.
MRI image acquisition.
In both sessions, functional and anatomical images were collected using a 3 T Siemens Trio scanner equipped with an eight-channel head coil. T2*-weighted functional images were collected using an EPI sequence (TR = 3 s; TE = 30 ms; 45 axial slices, 3 × 3 × 3 mm; 64 × 64 matrix). Each scan consisted of 150–152 images. All participants completed four scans in each session. High-resolution T1-weighted anatomical images were collected using an MPRAGE sequence (TI = 1100 ms; 160 axial slices, 0.9375 × 0.9375 × 1.000 mm; 192 × 256 matrix).
Imaging data analysis.
Functional images were analyzed using VoxBo, incorporating tools from SPM2. Functional images were first sinc interpolated in time to adjust for staggered slice acquisition, corrected for head motion by realigning all volumes to the first volume of the scanning session using six-parameter rigid-body transformations, and detrended and high-pass filtered (cutoff of 3 cycles/scan, or 0.0066 Hz) to remove low-frequency drift in the fMRI signal. Images were coregistered with each subject's high-resolution anatomical scan and normalized into MNI space. Normalized data were then spatially smoothed (kernel FWHM = 9 mm) and thresholded to remove voxels outside of the brain.
Single-subject analyses were performed using the general linear model as implemented in VoxBo. Estimation was by ordinary least squares.
Only the neuroimaging data from the temporal judgments task is discussed here. The neuroimaging data from the temporal discounting task will be addressed in a separate report, which we have previously presented in abstract form (Kable et al., 2011). That report focuses on the similarities between activation during the bidding task that we used here and the choice task used in previous studies (Kable and Glimcher, 2007, 2010).
The first model for the temporal judgments task included 15 covariates of interest. These covariates modeled activity at the following three different time points in the trial: (1) the time at which a trial began and participants saw the delay to be judged (Qon); (2) the time at which the response bar appeared and the participant could begin entering the response (Ron); and (3) the time at which the participant submitted the response (Roff). The first 13 regressors divided the Qon periods into equally sized bins based on the objective delay being judged. Since there was a small number of trials for each delay (four trials), we combined consecutive rank-ordered delays (i.e., the four trials from the shortest delay with the four trials from the second shortest delay, and so on) to obtain 13 delay bins from the 26 unique objective delays presented. The last two regressors modeled all of the Ron periods and all of the Roff periods. All of these covariates were constructed by convolving a delta function at the time of each event (i.e., only the first 100 ms of each event is modeled) with an empirically estimated hemodynamic response function. This hemodynamic response function is distributed with the VoxBo software package and was empirically estimated by Aguirre et al. (1998) from motor cortex responses during a motor performance task.
The second model for the temporal judgments task included six covariates of interest: three covariates modeling activity at Qon, Ron, and Roff; and three additional parametric modulator covariates, one at each of these three time points in the trial. All six regressors modeled the first 100 ms of each event. The first three covariates were constructed by convolving a delta function at the time of each event with an empirically estimated hemodynamic response function (Aguirre et al., 1998). These first three covariates fit the mean activity at a given point in a trial, across all trials. The parametric modulator was the OT being judged on that trial. These values were all mean centered and then convolved with the hemodynamic response, so the parametric modulator covariates fit the deviations from mean activity that were correlated with the objective delay across trials.
Region of interest analysis.
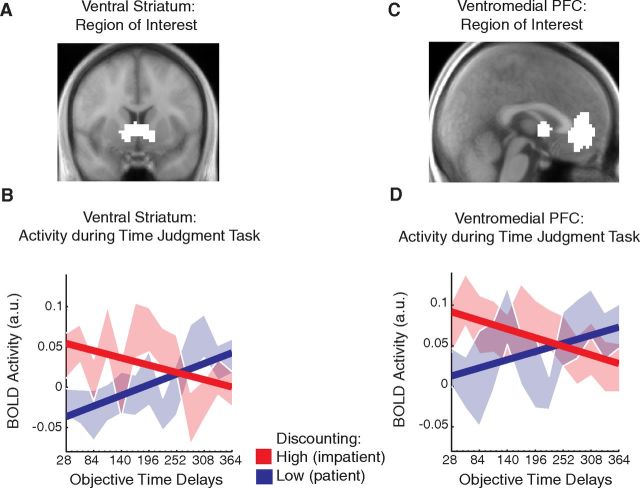
We used regions of interest (ROIs) from Bartra et al. (2013). This is a quantitative meta-analysis of studies that report value-related neural signals during decision making. The regions of interest used here were defined from an analysis of 27 studies reporting subjective value effects (i.e., increased BOLD signal for increasingly valuable rewards) at the time when subjects were evaluating the available choice options. Regions of interest in bilateral ventral striatum and ventromedial prefrontal cortex defined by this contrast are available for download (http://www.sas.upenn.edu/∼mcguirej/meta-analysis.html). Slight differences in the downloadable masks and the masks used here are due to the downsampling process from 2 to 3 mm voxel sizes. The resulting regions are bilateral ventral striatum (300 voxels at 3 × 3 × 3 mm, centered on MNI coordinates −6, 8, and −4 on the left, and 6, 10, and −8 on the right) and ventromedial prefrontal cortex (609 voxels at 3 × 3 × 3 mm, centered on MNI coordinates −2, 40, and −8). The regions of interest used are shown in Figure 2.
Figure 2.
BOLD activity during the temporal judgment task differentiates high and low discounters. A, C, Region of interest encompassing ventral striatum (A) and ventromedial prefrontal cortex (C), identified from previous meta-analysis (Bartra et al., 2013). B, D, BOLD activity in the ventral striatum (B) and ventromedial prefrontal cortex (D) ROIs at question onset during the temporal judgments task is plotted based on the length of delay being judged on that trial (26 delay lengths represented in 13 bins). Participants are divided at the median into high (n = 20) and low (n = 19) discounters based on their behavior during the temporal discounting task. Average BOLD activity at each delay is plotted for the high (red) and low (blue) discounting groups. Light colors are SE ranges.
Results
On each trial of the temporal judgment task, participants indicated their perceived duration of a future delay on a visual analog scale (Fig. 1A). Delays ranged from 14 to 364 d. In the temporal discounting task, participants indicated how much they valued a delayed gain of $75 (Fig. 1C). The delays were identical to those used in the temporal judgment task. For each subject, we fit the relationship between objective time and the person's valuation with the following function: BID = 75/(1 + k × OT), to estimate each subject's k value.
Our analyses concentrated on the link between neural responses in the temporal judgment task and delay discount rates as measured in the temporal discounting task 10 d later. Previous studies of intertemporal choice have identified correlates of subjective value during decision making in ventral striatum and ventromedial prefrontal cortex (Kable and Glimcher, 2007, 2010; Peters and Büchel, 2009, 2010b). We defined regions of interest in the ventral striatum and ventromedial prefrontal cortex based on a quantitative meta-analysis that examined subjective value correlates during decision making (Bartra et al., 2013).
We used two different approaches to examine the link between neural sensitivity to delay in the time judgment task and behavior on the time discounting task. In both cases, we looked at the neural response in the temporal judgment task at the time when the participant first saw the future duration to be judged on that trial, before they could enter their response (Qon). First, we split participants at the median k value into two groups of high (n = 20) and low (n = 19) discounters. In both ROIs, we estimated the BOLD activity in the two groups at Qon as a function of the delay being judged (delays were divided into 13 bins). High discounters showed a higher response in both ventral striatum and ventromedial prefrontal cortex to short delay periods that decreased as delay lengths increased (Fig. 2). Low discounters showed the opposite pattern, with responses increasing from short delays to long delays (Fig. 2). The estimated slopes of individual subjects in the high and low discounting groups are significantly different (VS, p = 0.0026, two-tailed t test; VMPFC, p = 0.037, two-tailed t test).
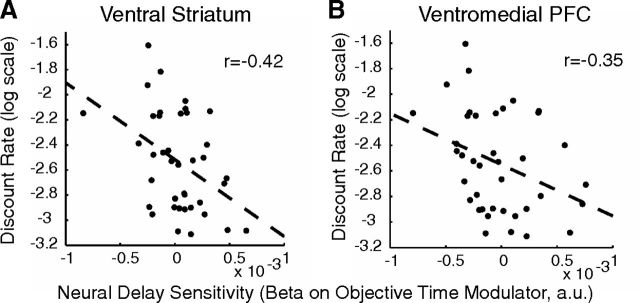
Our second approach looked at this same effect in a continuous rather than categorical manner. For each subject, we estimated the neural sensitivity to delay in both regions in the temporal judgment task. We modeled the objective delay as a parametric modulator in the temporal judgment task and took the β-coefficient on this parameter as our estimate of neural sensitivity. This provides a measure of how activity in these regions changes as a function of delay in the temporal judgment task; for example, whether activity increases or decreases as the delay judged gets longer, and how strongly it does so, in each subject. We then related this neural sensitivity to delay (from the temporal judgment task) to individual discount rates (from the behavioral measures in the time discounting task). Delay discount rates were negatively correlated with neural delay sensitivities. There was a significant linear relationship between discount rates and neural response to objective duration during the temporal judgment task in both regions (ventral striatum: r(37) = −0.42, p = 0.0076; ventromedial prefrontal cortex: r(37) = −0.35, p = 0.0294; see Figure 3). Using the AUC estimation of discount rates, which allows us to include all participants, this relationship is just as strong (ventral striatum: r(38) = 0.44, p = 0.0049; ventromedial prefrontal cortex: r(38) = 0.34, p = 0.0314; note that larger AUC corresponds to less discounting). Higher discounters showed an increasingly negative relationship between BOLD activity and objective durations, while lower discounters had an increasingly positive relationship.
Figure 3.
Individual neural delay sensitivities during temporal judgment predict delay discount rates. Individual discount rates (log scale) calculated from behavior on the temporal judgment task are plotted against individual neural delay sensitivity, or the fit of a parametric objective time modulator to neural activity in the temporal judgments task. A, B, This is plotted for activity in ventral striatum (A) and ventromedial prefrontal cortex (B). Dotted line is the linear trendline.
These data show that neural delay sensitivity can account for ∼15% of the variance in discount rates. Given recent concerns regarding the interpretation of effect sizes in imaging experiments (Vul et al., 2009), we hasten to add that the effect sizes we estimated here are unbiased and not artificially inflated, because we carefully identified valuation regions a priori, based on separate data entirely. Indeed, estimating the same brain–behavior relationship in a circular manner, by using only those voxels in ventromedial prefrontal cortex and ventral striatum that show a significant across-subjects correlation, results in inflated effect size estimates (ventral striatum: r(37) = −0.51, p = 0.0008; ventromedial prefrontal cortex: r(37) = −0.51, p = 0.0009).
Discussion
We scanned 40 people while they made judgments about the perceived length of future time durations and found that brain activity during this outcome-free task was predictive of how the same individuals discounted future monetary rewards. We identified regions in ventromedial prefrontal cortex and ventral striatum where activity consistently scaled with subjective value in previous fMRI studies of decision making, and measured the delay sensitivity of these regions during the temporal judgment task. This neural sensitivity to time delays was negatively correlated with discount rates measured 10 d later. Steep discounters tended to have larger neural responses when judging short delays than when judging long delays, while shallow discounters tended to have the opposite pattern. Our results show that prompting someone to think about the future, in a fairly minimal manner with no mention of outcomes, elicits brain activity that predicts discount rates. These results suggest that individual differences in the propensity to delay gratification derive from differences in basic cognitive and neural processes engaged when one thinks about the future, rather than, or in addition to, an individual's drive toward immediate rewards and/or their ability to control that drive.
Our study builds on previous work in several ways. First, we identified regions involved in valuation a priori. This is critical to the claim that temporal judgments elicit activity in the same regions as decision making, since there is heterogeneity within valuation regions, and in general nearby anatomical regions can be implicated in different cognitive processes (Kable, 2011; Poldrack, 2011). Second, we measured the sensitivity of these regions to parametrically varying delays. This allowed us to determine that activity differences between individuals are not solely due to the response of these regions to the immediate present, but instead involve how activity in these regions varies as people consider intervals that extend farther into the future. Third, our temporal judgment task did not involve explicit choices, evaluation of rewards or future scenarios, or judgments of self-relevance. The simplicity of our task allows us to show that even very basic judgments about the future are sufficient to elicit brain activity in valuation regions that predict discount rates.
Our findings leave open the exact nature of the cognitive processes that mediate this link. The neural processes of temporal judgment and reward valuation could be directly or indirectly linked, in several ways. One possibility is that judging prospective future durations itself activates ventromedial prefrontal cortex and ventral striatum, and that the activity we observe does not explicitly or implicitly reflect valuations. Under this account, neural activity is predictive because the perception of future durations predicts discount rates. Alternatively, since the waiting duration is a necessary input for evaluating delayed rewards, judgments of future durations may be sufficient to elicit value-related neural activity, as an epiphenomenon. Another possibility is that evaluative processes are critically involved in the judgment of prospective future durations. People might directly use their evaluation of future reward as a cue for judging the length of a future duration. Or, they might imagine a specific future event when judging duration. Cues in the simulated future event might be used to judge temporal distance, and the same simulated event might also be automatically evaluated.
Of course, these possibilities are not mutually exclusive, and the implicated cognitive processes are highly intertwined. Temporal estimates are an important factor in the evaluation of delayed rewards, and a major reason for simulating different possible futures is to evaluate whether these are desirable or not (Gilbert and Wilson, 2007). More than one of these cognitive processes might drive activation in ventromedial prefrontal cortex and ventral striatum, and these different processes may be instances of a more general function performed by these brain areas. Future experiments might use techniques that can separate neural signals that overlap spatially (e.g., adaptation or multivoxel pattern analysis) to tease apart the component processes in this network.
Regardless of the details of the link, our results demonstrate that outcome-free temporal judgments and temporal discounting of rewards share neural processes, as would be predicted by theories that attribute some features of discounting and impulsivity more generally to properties of temporal perception (Wittmann and Paulus, 2008; Wittmann et al., 2011). Some of our previous work has focused on how the curvature of time perception can affect the shape of the discount function (Zauberman et al., 2009). We did not observe a similar relationship in these data, perhaps because of the smaller sample size or larger time lag between the two tasks. In contrast, these data call attention to another link we have explored recently, between the magnitude of a temporal perception and the extent of discounting (Kim and Zauberman, 2009, 2013). Given that the absolute perceived magnitude is difficult to measure behaviorally, because it can be confounded with scale use, the individual differences in neural delay sensitivities discovered here might provide a useful tool in further exploring this relationship.
Previous work has shown that discount rates vary widely across individuals, and that these differences are relatively stable across time (Ohmura et al., 2006; Kirby, 2009; Senecal et al., 2012). Despite this intraindividual reliability, there are surprisingly few reliable predictors of discount rates. Perhaps the strongest is cognitive ability, which is reliably associated with discount rates with an effect size of approximately r = −0.20 (Shamosh and Gray, 2008; Burks et al., 2009). The relationship between discount rates and self-reported personality traits is usually smaller than this. In this context, the 15% of the variance in discount rates that can be accounted for by neural delay sensitivities is a sizable and important effect. These data show that, avoiding the previously described bias from circular estimates (Vul et al., 2009), the correlations between BOLD activity and individual differences in behavior can be comparable to or even outperform those of standard psychometric variables.
Our results also have implications for how to enhance patient, future-oriented behavior. Much work has concentrated on the hypothesis that lateral prefrontal cortex promotes patient choices, and that (correspondingly) such choices require a greater degree of cognitive control (McClure et al., 2004; Hare et al., 2009; Figner et al., 2010; Luo et al., 2012). Some have additionally proposed that lateral prefrontal cortex acts in opposition to ventromedial prefrontal and ventral striatal regions, and that suppressing activity in these two regions promotes patient behavior. In contrast, our results show that patient individuals show a different pattern of activity in ventromedial prefrontal cortex and ventral striatum, rather than reduced activity in these regions altogether. Shallower discounters showed greater activity in ventromedial prefrontal cortex and ventral striatum when considering durations lasting farther into the future. This suggests that patient behavior might be promoted by enhancing activity in ventromedial prefrontal cortex and ventral striatum in response to future outcomes, relative to that for immediate outcomes. This proposal is consistent with several recent studies showing that medial prefrontal BOLD activity predicts the effectiveness of messages promoting future-oriented behavior (Falk et al., 2010; Chua et al., 2011). Given that ventromedial prefrontal cortex has been linked to a “prospective brain network” that simulates the future (Buckner and Carroll, 2007; Gilbert and Wilson, 2007; Schacter et al., 2007) and to predicting the subjective value of different possible actions during decision making (Kable and Glimcher, 2009; Rangel and Hare, 2010), these neural findings may suggest novel psychological interventions to promote the delay of gratification.
Footnotes
We thank Li Jiang for assistance in data collection; Kourosh Zakeri for assistance in data analysis; and Jim Bettman, Wes Hutchinson, Ifat Levy, and Joe McGuire for comments on previous versions.
References
- Aguirre GK, Zarahn E, D'esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker GM, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behav Sci. 1964;9:226–232. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burks SV, Carpenter JP, Goette L, Rustichini A. Cognitive skills affect economic preferences, strategic behavior, and job attachment. Proc Natl Acad Sci U S A. 2009;106:7745–7750. doi: 10.1073/pnas.0812360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris CF, Laibson D, Morris CL, Schuldt JP, Taubinsky D. Individual laboratory-measured discount rates predict field behavior. J Risk Uncertain. 2008;37:237–269. doi: 10.1007/s11166-008-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Seligman ME. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Ersner-Hershfield H, Wimmer GE, Knutson B. Saving for the future self: neural measures of future self-continuity predict temporal discounting. Soc Cogn Affect Neurosci. 2009;4:85–92. doi: 10.1093/scan/nsn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. J Neurosci. 2010;30:8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW. The cognitive neuroscience toolkit for the neuroeconomist: a functional overview. J Neurosci Psychol Econ. 2011;4:63–84. doi: 10.1037/a0023555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. J Neurophysiol. 2010;103:2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Kim BK, Senecal N, Zauberman G. Heterogeneity in the neural substrates of time perception and time discounting. Paper presented at 2nd Interdisciplinary Symposium in Decision Neuroscience; September; Philadelphia, PA. 2011. [Google Scholar]
- Kim BK, Zauberman G. Perception of anticipatory time in temporal discounting. J Neurosci Psychol Econ. 2009;2:91–101. doi: 10.1037/a0017686. [DOI] [Google Scholar]
- Kim BK, Zauberman G. Can Victoria's Secret change the future? A subjective time perception account of sexual-cue effects on impatience. J Exp Psychol Gen. 2013;142:328–335. doi: 10.1037/a0028954. [DOI] [PubMed] [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychon Bull Rev. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Pollini D, Giragosian L, Monterosso JR. Moderators of the association between brain activation and farsighted choice. Neuroimage. 2012;59:1469–1477. doi: 10.1016/j.neuroimage.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Nevin JE, Rachlin H, editors. Quantitative analysis of behavior, Vol 5: the effects of delay and intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 1988;54:687–696. doi: 10.1037/0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Schirmer J, Ames DL, Gilbert DT. Medial prefrontal cortex predicts intertemporal choice. J Cogn Neurosci. 2011;23:857–866. doi: 10.1162/jocn.2010.21479. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Exp Clin Psychopharmacol. 2006;14:318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29:15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010a;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010b;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ. Encoding of marginal utility across time in the human brain. J Neurosci. 2009;29:9575–9581. doi: 10.1523/JNEUROSCI.1126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Senecal N, Wang T, Thompson E, Kable JW. Normative arguments from experts and peers reduce delay discounting. Judgm Decis Mak. 2012;7:568–589. [PMC free article] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. Delay discounting and intelligence: a meta-analysis. Intelligence. 2008;36:289–305. doi: 10.1016/j.intell.2007.09.004. [DOI] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Simmons AN, Flagan T, Lane SD, Wackermann J, Paulus MP. Neural substrates of time perception and impulsivity. Brain Res. 2011;1406:43–58. doi: 10.1016/j.brainres.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman G, Kim BK, Malkoc SA, Bettman JR. Discounting time and time discounting: subjective time perception and intertemporal preferences. J Mark Res. 2009;46:543–556. doi: 10.1509/jmkr.46.4.543. [DOI] [Google Scholar]