Abstract
Background/aim
The pathophysiological basis of relapse and recrudescence in type 1 autoimmune hepatitis (AIH) is poorly understood. This study examined factors associated with biochemical relapse in type 1 AIH, and specifically addressed whether psychological stress was associated with a relapsing-remitting disease course.
Methods
A case–control study design was used to analyse 33 patients with AIH followed at the Yale Liver Clinic during a 4-year period. Twenty-two patients with 46 episodes of relapse or recrudescence (cases) and 11 controls in long-term remission were identified during this period. Clinical variables were collected to establish factors associated with relapse. All patients were administered the Social Readjustment Rating Scale (SRRS), a psychological stress questionnaire consisting of 43 weighted life events. Stress is judged to be low when the score is 0–150; mild, 151–200; moderate, 201–300 and major > 300.
Results
AST, ALT, prednisone dose and SRRS score were all significantly different between cases and controls. The mean SRRS score for cases with relapse/recrudescence was 239 vs 152 for the control group, P = 0.048 and remained significant on ancova analysis which accounted for covariables, P = 0.05. Cases also identified additional stressors not represented in the SRRS at a significantly higher rate than controls. Conversely, the controls spontaneously identified coping strategies that may have allowed them to manage stress more effectively.
Conclusion
Psychological stress is a significant factor that is associated with relapse in type 1 autoimmune hepatitis. Management of AIH may benefit from strategies to reduce stress and promote psychological well being.
Keywords: autoimmune hepatitis, psychological stress, relapse
Type 1 autoimmune hepatitis (AIH) is a chronic hepatitis of unknown aetiology that is marked by the presence of circulating auto-antibodies, hypergammaglobulinaemia, and liver biopsy showing plasma cell infiltration, inter-face hepatitis and piece-meal necrosis (1). Its course is marked by responsiveness to immunosuppressive medications with episodes of remission and relapse (2). Sixty-five percent of patients enter remission within 18 months and 80% achieve remission within 3 years (3, 4). Even with achievement of remission, studies indicate that 50–87% of patients will relapse within 1 year after stopping medication (3-5). In one study > 80% had one or more relapses, despite treatment from 7 to 43 years (5). It remains unclear what factors account for the high frequency of relapse in AIH, although both short treatment duration and failure to normalize disease activity have been suggested (6, 7).
To date, no study has examined pathogenic mechanisms which might lead to relapse, although psychological stress, particularly life events perceived as stressful, have been cited anecdotally by physicians and patients to worsen disease activity (5). Psychological stress activates the hypothalamic-pituitary axis and the sympathetic nervous system and ultimately leads to immune dysregulation (8) marked by increased levels of circulating pro-inflammatory cytokines (9, 10). In autoimmune diseases, augmentation of a pro-inflammatory response could have deleterious effects in tissues that are particularly susceptible to immune stimulation.
In this study we hypothesize that life events equated with psychological stress might be associated with relapse or recrudescence of disease activity, and seek to determine factors associated with a relapsing-remitting course. The Social Readjustment Rating Scale (SRRS), a well-recognized life events scaling instrument, was used to measure psychological stress (11). Our findings suggest that psychological stress is associated with relapse in type 1 autoimmune hepatitis.
Methods
Study population
This study was approved by the Yale University Human Investigation Committee (IRB) and informed consent was obtained from all subjects. Thirty-three adult patients who were followed at the Yale Liver Clinic from January 2004 through December 2007 (4-year study period) met criteria for definite or probable type 1 autoimmune hepatitis as defined by the International Autoimmune Hepatitis Group (12) and consented to participate in this study. Fifty-four percent of cases and 46% of controls met the criteria for definite type 1 AIH, and there was no significant difference between groups (P = 0.63). Type 2 AIH was excluded by antibody testing and all patients had a liver biopsy that was consistent with AIH. Most patients had been evaluated at presentation and follow-up by one of us (J. L. B.). Patients were excluded if they could not read or speak English, had decompensated cirrhosis (defined as ascites, encephalopathy, variceal haemorrhage), hepatocellular carcinoma or post-liver transplantation.
Cases consisted of patients who were either in remission or were incomplete responders, and subsequently experienced relapse or recrudescence of disease activity. Remission was defined as disappearance of clinical symptoms and complete normalization of aminotransferases on or off maintenance dose immunosuppression (prednisone 2.5–10 mg/day +/− azathioprine 50 mg/day). An incomplete response was defined as aminotransferases within 10 IU/ml of the upper limits of normal (ULN) on immunosuppression therapy. Relapse was defined in two ways once immunosuppression has been tapered off: (i) An increase in the AST or ALT ≥ 2 × ULN or (ii) an increase in AST and/or ALT ≥ 2 × the prior level of AST or ALT on routine labs checked Q1–3 months depending on disease activity or stability. Recrudescence was defined as relapse on maintenance or suppression therapy. The controls consisted of patients followed for at least 2 years, currently in remission on or off maintenance therapy, and who had no evidence of biochemical relapse or recrudescence for the entire duration of the 4-year study period. A subset of these patients experienced a sustained remission, defined as normal AST/ALT off all immunosuppression with no subsequent relapse in long-term follow-up. Patients could meet criteria for only one group during the study period. A subset of patients did not meet criteria for either group, and had higher levels of aminotranferases despite aggressive treatment efforts.
The medical record was reviewed on a retrospective or prospective basis from January 2004 to December 2007, and all events that constituted a relapse or recrudescence during that period were recorded. Charts were reviewed retrospectively for cases of relapse or recrudescence that occurred between January 2004 and December 2005 and these events were recorded. Thereafter, all cases of relapse or recrudescence were captured prospectively until December 2007.
The normal range for the upper limits of AST and ALT varied from lab to lab and ranged from 30 to 65 IU/ml; therefore, relapses and recrudescences were individually determined for each patient based on his or her lab’s reference range. Furthermore, to standardize AST and ALT levels between laboratories with disparate ranges, a ratio of AST/(upper limits of AST) and ALT/(upper limits of ALT) was calculated and recorded to effectively compare cases and controls. Demographical and outcomes data were collected, including age, sex, duration of disease, presence of cirrhosis, presence of other autoimmune diseases, total number of relapses/recrudescences during the study period, prerelapse or entry AST/ALT, prerelapse or entry prednisone dose, change in AST and ALT pre- and post-relapse (delta AST and ALT). For patients with multiple relapses, all treatment-specific variables (AST, ALT, prednisone dose, delta AST/ALT) were averaged so that multiple relapsing events in one patient would not count as separate cases and create a measurement bias, which could otherwise magnify differences between cases and controls.
Treatment regimen and definitions of response
Our treatment regimen, definitions of response to therapy, protocol for follow-up and biochemical and medication monitoring are described in detail elsewhere (5). Patients are usually maintained on prednisone 10 mg/day and azathioprine 50 mg/day until liver function are within normal range, and then slowly tapered. Recrudescence leads to an increase in either the dose of prednisone by 5–10 mg/day or azathioprine to 75 mg/day. The steroid taper is restarted once aminotransferases return to normal again. Once liver function has normalized, follow-up clinical visits and tests of liver function are routinely obtained at 3-month intervals in the absence of clinical symptoms. A liver biopsy is not typically performed before withdrawal of immunosuppression. After two to three relapses, maintenance doses of medication are usually continued indefinitely.
Measurement of psychological stress
Stressful life events were measured using the SRRS, the most widely known and internationally used life events scaling instrument (11) in clinical research. The SRRS consists of 43 major and minor life events that are scored based on the magnitude of the event. A cumulative score is derived by summing each of the individual events.
The SRRS was administered to cases on a prospective basis right after the date that abnormal labs were obtained, and subjects were asked to select events that occurred within the 1 year before relapse or recrudescence; in each case the questionnaire was administered within 1 month of the date of relapse. For cases contacted retrospectively, subjects were asked to recall events that occurred 1 year before the date of their recorded relapse on chart review. Controls were also asked to identify events that occurred within the past 1 year. Seventy percent of the subjects received the questionnaire in the mail and 30% received it during their clinic visit. All subjects returned the questionnaire via the mail. Five patients declined to participate in this study. Five patients did not meet strict criteria for case or control groups. These 10 patients represent ‘non-participants’ and are not included in this study’s 33 participants who completed the study consent and questionnaires required for participation.
A cumulative stress score was derived for each relapse/recrudescence in the case group and for the control group: 0–150 is defined as low stress, 151–200 is mild stress, 201–300 is moderate stress and > 300 is major stress. For cases with multiple relapses, the SRRS scores were averaged to obtain one SRRS score per case to minimize magnifying differences between groups.
Patients also recorded if there were other stressful life events not addressed in the questionnaire, and described coping mechanisms.
Data analysis
Pearson’s χ2 analyses were conducted to assess if relationships exist between categorical variables based on group (case vs. control). The Mann–Whitney U-test was used to compare differences in the means of continuous variables. Data are presented as the mean with 95% confidence intervals, median, standard deviation, minimum and maximum values. The Fisher’s exact test was used in the analysis of contingency tables with small sample size. Because the variables in this study had been constructed a priori and then systematically assessed in our study population, an unadjusted P value of ≤ 0.05 was used to determine statistical significance.
An analysis of covariance (ancova) was conducted to assess if differences exist in the SRRS by group (case vs. control) after controlling for age, gender, duration, cirrhosis, psychiatric history, other autoimmune disease.
A power calculation was also performed prior to the start of this study. Being that this study requires an ancova with an independent variable that has two levels (case vs. control), approximately 26 participants would be needed for each group summing to a total of 52 participants. Having an α-value set at 0.05, 52 participants will yield a power of 0.80 with a large effect size.
The constant comparative method (13-15) of qualitative data analysis was used to develop and implement consistent coding of data collected from the written content submitted by subjects. Key themes were summarized which (i) determined other stressors that were not identified on the SRRS, (ii) determined whether specific coping strategies were used to manage or alleviate stress.
Results
Factors associated with relapse/recrudescence vs. remission
The case–control study consisted of 33 adult patients between the ages of 20 and 79 years with definite or probable type 1 AIH. A power analysis before this study indicated that 26 patients in the case group and 26 in the control group (total 52 patients) would be needed to demonstrate a large effect. Twenty-two patients experienced one or more relapse or recrudescence during the study period, totaling 46 events that met criteria (mean 2.09 relapses or recrudescences per patient; range 1–5 events per patient); seven events (15%) constituted relapse and 39 events (85%) constituted recrudescence. Of the 46 recorded events, 35 were captured retrospectively (76%) while 11 were captured prospectively (24%). Thirteen out of the 22 cases (59%) had greater than one relapse/recrudescence and nine had only one relapse (39%). Sixteen out of these 22 cases (73%) had at least one retrospective event. Twelve cases had only retrospective events (55%), and six cases had only prospective events recorded (27%). The mean post-relapse/recrudescence ASTand ALT levels were 3.49 and 3.35 times higher than their ULN, which is a 255 and 259% difference between pre- and post-relapse (and recrudescence) levels respectively. Seventy-three percent of controls were off all immunosuppression. Five patients in the control group (45% of control; 15% of the entire case–control study) achieved sustained remission.
Tables 1, 2a, and 2b show the patient and treatment specific variables that were analysed to determine factors associated with relapse. Higher ASTand ALT level, higher prednisone dose, and higher SRRS score (stress levels) were all significantly associated with relapse. The remaining factors in the analysis proved to be insignificant, albeit, longer duration of disease in the control group trended towards significance.
Table 1.
Pearson’s χ2 analyses on group by sex, cirrhosis, prior psychiatric history, other autoimmune diseases
| Case n = 22 | Control n =11 | χ 2 | df | P value | |
|---|---|---|---|---|---|
| Sex (% female) | 19 (86%) | 9(82%) | 0.118 | 1 | 0.731 |
| Cirrhosis (%)* | 9 (41%) | 6(55%) | 0.550 | 1 | 0.458 |
| Prior Psychiatric history (%) | 2 (9%) | 3 (27%) | 1.886 | 1 | 0.170 |
| Other autoimmune diseases (%)† | 5 (23%) | 4 (36%) | 0.688 | 1 | 0.407 |
Cirrhosis has been defined either on liver biopsy specimen, positive liver–spleen scan, radiographic imaging or varices on endoscopy.
Other autoimmune diseases that were diagnosed in our study population include Graves disease (1), Ulcerative colitis (1), Crohn’s disease (1), Hashimoto’s thryoiditis (4), celiac sprue (1), asthma (1), DM type 1 (1), lipodystrophy (1). Two patients had multiple autoimmune diseases.
Table 2a.
Independent samples Mann–Whitney U-tests on age, duration, mean prednisone dose, mean AST/ULN ratio, mean AST/ULN ratio, and social re-adjustment rating scale by group
| Case n=22 |
Control n= 11 |
U | Significance | |
|---|---|---|---|---|
| Age (years) | 47.09 | 51.45 | 102.5 | 0.479 |
| Duration (years) | 9.73 | 15.91 | 81.5 | 0.133 |
| Mean prednisone (mg)f | 5.62 | 0.91 | 33.5 | 0.001 |
| Mean AST*/† | 0.94 | 0.66 | 41.0 | 0.002 |
| Mean ALT*/† | 0.76 | 0.44 | 36.0 | 0.001 |
| SRRS score† | 239.31 | 152.55 | 69.5 | 0.048 |
ASTand ALT were converted to ratio of ASTor ALT to the upper limits of normal for reference lab to create standard levels between patients.
For each individual case with multiple relapses, an average prednisone dose, AST, ALT and SRRS score was calculated to prevent double counting of cases with multiple relapses.
Table 2b.
Descriptive statistics for Mann–Whitney U-tests
| Variable | Group | Statistics | ||
|---|---|---|---|---|
| Age | Case | Mean | 47.09 | |
| 95% CI for mean | Lower bound | 40.38 | ||
| Upper bound | 53.80 | |||
| Median | 50 | |||
| Standard deviation | 15.13 | |||
| Minimum | 20 | |||
| Maximum | 79 | |||
| Control | Mean | 51.45 | ||
| 95% CI for mean | Lower bound | 42.82 | ||
| Upper bound | 60.09 | |||
| Median | 53 | |||
| Standard deviation | 12.86 | |||
| Minimum | 34 | |||
| Maximum | 77 | |||
| Duration | Case | Mean | 9.73 | |
| 95% CI for mean | Lower bound | 6.12 | ||
| Upper bound | 13.33 | |||
| Median | 7.5 | |||
| Standard deviation | 8.13 | |||
| Minimum | 1 | |||
| Maximum | 30 | |||
| Control | Mean | 15.91 | ||
| 95% CI for mean | Lower bound | 8.00 | ||
| Upper bound | 23.82 | |||
| Median | 10 | |||
| Standard deviation | 11.78 | |||
| Minimum | 3 | |||
| Maximum | 38 | |||
| Prednisone (mg) |
Case | Mean | 5.62 | |
| 95% CI for mean | Lower bound | 3.96 | ||
| Upper bound | 7.29 | |||
| Median | 5 | |||
| Standard deviation | 3.75 | |||
| Minimum | 0 | |||
| Maximum | 13.75 | |||
| Control | Mean | 0.91 | ||
| 95% CI for mean | Lower bound | −0.22 | ||
| Upper bound | 2.04 | |||
| Median | 0 | |||
| Standard deviation | 1.69 | |||
| Minimum | 0 | |||
| Maximum | 5 | |||
| AST/ULN ratio |
Case | Mean | 0.94 | |
| 95% CI for mean | Lower bound | 0.84 | ||
| Upper bound | 1.03 | |||
| Median | 0.97 | |||
| Standard deviation | 0.21 | |||
| Minimum | 0.52 | |||
| Maximum | 1.46 | |||
| Control | Mean | 0.66 | ||
| 95% CI for mean | Lower bound | 0.53 | ||
| Upper bound | 0.79 | |||
| Median | 0.72 | |||
| Standard deviation | 0.20 | |||
| Minimum | 0.29 | |||
| Maximum | 0.97 | |||
| ALT/ULN ratio |
Case | Mean | 0.76 | |
| 95% CI for mean | Lower bound | 0.66 | ||
| Upper bound | 0.85 | |||
| Median | 0.71 | |||
| Standard deviation | 0.22 | |||
| Minimum | 0.41 | |||
| Maximum | 1.23 | |||
| Control | Mean | 0.44 | ||
| 95% CI for mean | Lower bound | 0.31 | ||
| Upper bound | 0.58 | |||
| Median | 0.45 | |||
| Standard deviation | 0.20 | |||
| Minimum | 0.18 | |||
| Maximum | 0.81 | |||
| SRRS | Case | Mean | 239.31 | |
| 95% CI for mean | Lower bound | 192.21 | ||
| Upper bound | 286.42 | |||
| Median | 232 | |||
| Standard deviation | 106.23 | |||
| Minimum | 56 | |||
| Maximum | 430 | |||
| Control | Mean | 152.55 | ||
| 95% CI for mean | Lower bound | 66.71 | ||
| Upper bound | 238.38 | |||
| Median | 130 | |||
| Standard deviation | 127.77 | |||
| Minimum | 0 | |||
| Maximum | 426 |
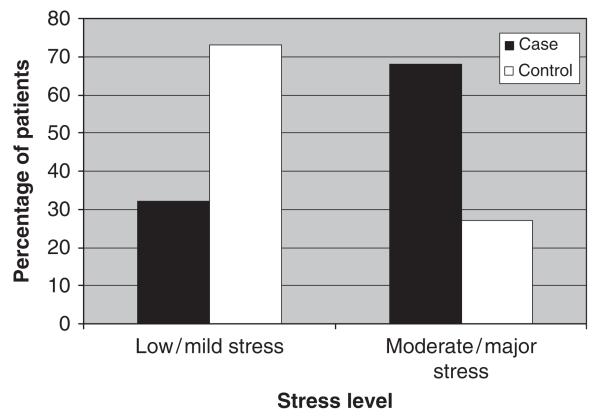
The average SRRS score for all cases with > 1 relapse/recrudescence was 239.31 (192–286; 95% confidence interval; SD 106) and the average SRRS score for all controls was 152.55 (66–238; 95% confidence interval; SD 126), P value of 0.048. The mean stress level for the case group is classified as ‘moderate/borderline major’ and the control group’s level of stress is ‘mild/borderline low’. A larger proportion of participants in the case group tended to have major/moderate stress levels compared to low/mild; and a larger proportion of those participants with major/moderate stress tended to be in the case group compared with the control (Fig. 1); while this trended towards significance, the Fisher exact test on the small sample analysis indicated an insignificant result (P = 0.061) (Table 4).
Fig. 1.
Moderate/major stress levels were seen in more patients who relapsed or recrudesced (cases) than controls. The Fisher exact test value is 0.061. Cross tabs are presented in Table 4.
Table 4.
Cross tabs between stress levels (low/mild vs. moderate/ major) and group (case vs. control)
| Stress level | Case | Control |
|---|---|---|
| Low/mild | 7 | 8 |
| Major/moderate | 15 | 3 |
P = 0.061.
An analysis of covariance (ancova) assessed if the group differences on SRRS (case vs. control) persisted after controlling for age, gender, duration of disease, presence of compensated cirrhosis, psychiatric history and other autoimmune diseases (Table 3). These results remained significant F (1, 25) = 4.21, P = 0.05. Prednisone dose and AST/ALT were not included in the ancova analysis because the study criteria allowed for entry of cases with slightly higher aminotransferases and prednisone dose compared with controls (because these patients constitutes a more ‘difficult to manage’ group compared to controls), and would have created statistical bias against the SRRS stress score if included as a covariate. Attempts to only include cases with completely normal prerelapse AST/ALT on low dose or no prednisone who subsequently relapse would have significantly affected the power of this study.
Table 3.
ancova on social re-adjustment rating scale by group (case vs. control) after controlling for age, gender, duration, cirrhosis, psychiatric history and other autoimmune disease
| Source | F | Significance | Partial η2 | Power |
|---|---|---|---|---|
| Age | 0.30 | 0.586 | 0.01 | 0.08 |
| Gender | 0.15 | 0.702 | 0.01 | 0.07 |
| Duration | 0.17 | 0.687 | 0.01 | 0.07 |
| cirrhosis | 0.03 | 0.867 | 0.00 | 0.05 |
| Psychiatric History | 1.02 | 0.321 | 0.04 | 0.16 |
| Other Autoimmune Diseases | 1.51 | 0.230 | 0.06 | 0.22 |
| Group | 4.21 | 0.05 | 0.14 | 0.51 |
Qualitative analysis of patient quotations
Table 5 lists additional stressors not identified on the SRRS. Eleven additional categories or ‘themes’ were extrapolated from the patient’s description, with 13/22 (59%) of the cases and 5/11 (46%) of the control group identifying additional stressors (P = 0.5), albeit the difference was not significant. However, the case group identified 20 additional stressors (20 stressors/22 cases = 0.9/case), while the control group identified five additional stressors (five stressors/11 controls = 0.45/control), a difference that was significant between groups (P < 0.008).
Table 5.
The key themes characterizing other stressors experienced by the case group that were not identified in the social re-adjustment rating scale
| Key theme | Examples from coded data |
|---|---|
| (1) Physically demanding activities (2 cases/0 controls) |
|
| (2) Combat and wartime stresses (1 case/1 control) |
|
| (3) Increase in arguments or tension in relationship with children, boy/girlfriends, close friends, family members aside from spouse (5 cases/1 control) |
|
| (4) Stress relating to being a student (3 cases) |
|
| (5) Close friends becoming ill (0 case/1 control) |
|
| (6) Mental illness in a close family member (2 case/0 controls) |
|
| (7) Events that occur to one’s living space (3 cases/1 control) |
|
| (8) New role as a caretaker for children and/or other family members (2 cases/0 controls) |
|
| (9) Threat to self, living space, or personal property (1 case/0 controls) |
|
| (10) Child participation in sporting events (1 case/0 controls) |
|
| (11) Difficulty getting pregnant (0 cases/1 control) |
|
|
| |
| Total stressors | 20 stressors for 22 cases/5 stressors for 11 controls |
Both cases and controls spontaneously identified strategies for coping with stress listed in Table 6. Overall, 7/11 controls (64%) and 3/22 cases (14%) reported one or more coping strategies that helped to manage their disease (P = 0.002). The 11 patients in the control group identified 16 coping strategies (1.45 strategies/control) and 22 patients in the case group identified five coping strategies (0.23 strategies/case), a significant difference between groups (P < 0.0001).
Table 6.
The key themes characterizing coping strategies used by control subjects to manage stressful life events
| Coping strategy | Number of cases/controls |
|---|---|
| Support of family/close friends to dealing with major stresses | 1 case/4 controls |
| Perception of stability in life and lack of stressors (stable finances, good job, ‘happy life’) | 1 case/4 controls |
| Increased awareness of stress to promote early utilization of coping interventions | 0 case/1 control |
| Exercise and meditative medicine (yoga, massage, reiki) | 2 cases/2 controls |
| Healthy diet/lifestyle | 1 case/2 controls |
| Faith based support | 0 case/1 control |
| Having a positive outlook despite challenges and major stresses | 0 case/1 control |
| Focusing on other goals and achievements | 0 case/1control |
|
| |
| Total coping strategies | 5 strategies for 22 cases/16 strategies for 11 controls |
Discussion
This is the first study in patients with AIH that attempts to associate psychological stress with relapse or recrudescence. Although the cohort is not large, it is somewhat unique in that it was followed clinically by one physician/investigator over many years, thus providing uniform management as described in detail elsewhere (5). Our findings indicate that higher AST/ALT levels, higher prednisone dose, and increased psychological stress are important factors associated with exacerbations in disease activity. Patients with one or more relapses/recrudescences have significantly higher stress scores and number of stressful events compared to patients in prolonged remission. Significant ancova analysis with consideration of covariates further lends credence to this assertion. Subjects also identified additional stressors that were not in the SRRS, suggesting that stress levels were probably underestimated, particularly for the case group that already had identified significantly higher number of additional stressors compared to controls. Furthermore, there were a higher number of subjects in the control group who described positive coping strategies that were likely to have enhanced their ability to deal with stressful events and their disease, a finding that was also significant between the two groups of patients. These findings suggest that techniques to augment adaptive or positive coping mechanisms might be beneficial therapeutically.
Historically, clinical outcome studies in AIH have attempted to identify factors, which differentiate patients who achieve sustained remission from those who repeatedly relapse. One study suggests that longer duration of treatment may reduce the incidence of relapse [sustained remission was achieved because of drug cessation in 67% of patients treated for > 4 years, but only in 10% of patients treated for < 2 years (6)]; while others emphasize the need to normalize aminotransferase levels (5, 7), immunoglobulins and IgG (7) to ensure maintenance of remission. While earlier studies have emphasized the need to eliminate histological activity prior to drug withdrawal, a large percentage of patients with inactive liver biopsies will also relapse after withdrawal of therapy (7, 16).
Our results support prior studies, which showed that normalization of aminotransferases is a major factor in relapse prevention and the major goal in treatment. However the mean AST and ALT levels for both groups fell in the ‘normal range’ based on lab reference ranges, and so we were surprised that there was a small but highly statistically significant difference in aminotransferases levels between groups that otherwise were in the ‘normal range’. The case group had mean AST and ALT values that were below (but close to) the upper limits of the normal range, while controls had levels that were ~30% less and fell into the middle-of-normal and low range. This finding suggests that patients that frequently relapse have a more difficult time bringing aminotransferase levels into a completely normal range, and that the AST/ALT levels can fall to significantly lower levels within the normal range in patients in remission. This lower level of ‘activity’ may constitute a truly normal aminotransferase level not accurately reflected in lab reference ranges. Furthermore, our study shows that despite close attention to optimizing prednisone doses to control disease activity while minimizing toxicity, patients on higher doses of immunosuppression (cases) continue to relapse more frequently than patients on low dose or no immunosuppression (controls). This confirms that cases were treated with adequate immunosuppresion and were not ‘under’ treated, while controls were not overly suppressed to maintain normal aminotransferases. Therefore, factors other than suboptimal treatment are likely to account for difficulty with bringing aminotransferase levels into the low normal range. We can further speculate that psychological stress, higher aminotransferases, and higher doses of prednisone may be inter-related. Increased stress may promote disease activity, lead to borderline elevations in AST/ALT and/or relapse, thus subsequently leading to higher prednisone dose, which may itself lead to side effects including anxiety and stress. Future studies will be needed to assess these speculations.
The impetus for our study was based on anecdotal observations from our patients, many of whom have been observed over a period of > 3 decades, who have noted that significant stressful life events (death in the family, change or loss of job, difficulties with spouse or children, as examples) have often been associated with exacerbations in disease activity as assessed by aminotransferase levels (5).
The rationale for linking psychological stress with exacerbation of autoimmune hepatitis is rooted in the field of psychoneuroimmunology, which studies the complex interaction between the endocrine, nervous and immune systems in a stress response. Stress has been found to be a contributing factor in cardiovascular disease, some forms of cancer and viral diseases such as HIV (17). Acute psychological stress is postulated to activate the hypothalamic-pituitary axis and the sympathetic nervous system in a fight or flight response. This leads to increased leukocyte trafficking to protect against infection and increased peripheral levels of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α. Stress during final exams increased the peripheral production of IL-6, TNF-α, and interferon-γ in medical students (18) and higher levels of peripheral IL-6 production are described in caregivers for demented family members compared with controls (19). Psychological stress has also been studied in other autoimmune diseases including multiple sclerosis (MS) and ulcerative colitis (UC). In MS, psychological stress has been associated with increased recurrence rates and the development of white matter brain lesions (20-22). In UC, experimental induction of acute psychological stress led to significant increase in peripheral and rectal mucosa production of LPS-stimulated TNF-α and IL-6 compared with relaxed controls (23).
Despite an association between psychological stress and relapse/recrudescence in AIH, our study has some limitations that might challenge these conclusions. Firstly, despite a good correlation between high aminotransferases and high stress score (> 200; 68% of cases), and conversely, low aminotransferases and low stress score (< 150; 73% of controls), 32% of cases had a low stress score (< 150) and 27% of controls had a high stress score (> 200). Thus, additional mechanisms other than responsiveness to stressful events must be involved (Table 7). Second psychological stress is a ‘soft outcome’ that is difficult to measure, and surrogate instruments, such as the SRRS, must be relied upon to validate its presence or absence. Because there is no gold standard or ‘valid’ way for ascertaining psychological stress in patients, these instruments are only an approximation of true psychological stress. Indeed, although we used an instrument widely validated in clinical studies over many decades it does not account for key modifiers of stress including differences in stress perception and coping capabilities that can provide additional nuances in measuring stress (24). Thirdly, this study questionnaire was administered on a retrospective basis for some of the patients and on a prospective basis for other patients, which makes the test–retest reliability for capturing remote events more problematic; however, given that most patients usually remember major stressful events that occurred to them over the past several years, we assume that these major events have been accurately captured. Fourthly, our patients identified additional stressors in our qualitative analysis that were not listed in the questionnaire, which may have underestimated their stress score. Fifthly, this study could not exclude that the relapses themselves may contribute to feelings of increased stress and that subjects may have chosen to include this information in the calculation of their stress score. However, both the subjects and the control groups had the exact same disease (type 1 AIH), and therefore had an equal chance for allotting ‘personal injury/illness’ as a stressor. It should also be emphasized that the relapses consisted of only small biochemical changes in aminotransferases found on routine blood work so that symptoms were rarely associated with the relapse. Finally, AIH is a relatively uncommon disease with a small sample size, precluding our ability to reach power that would have led to a large statistical effect; nevertheless the result remained significant. Most studies in AIH are retrospective chart reviews and therefore can pool many more patients over decades to increase the study power. Our study’s use of directed questionnaires in a contemporary population over a relatively limited period of time made our study small but not insignificant. Because of the small sample, the present study proposes only a ‘proof of concept’ that will require a multicentre trial to validate the results. Indeed, given the limitations, which arise from a retrospective study design, it is important to await future studies in order to reach a more confident conclusion regarding the association between psychological stress and relapse.
Table 7.
Mechanisms which may account for patients with low aminotransferases and high stress, and conversely high aminotransferases and low stress
| High aminotransferases (case) | Low aminotransferases (control) | |
|---|---|---|
| High stress score (> 200) | High aminotransferases/high stress 15/22 = 68% |
Low aminotransferases/high stress 3/11 = 27% |
| Consistent with our hypothesis | Possible mechanisms: | |
|
||
| Low stress score (< 150) | High aminotransferases/low stress 7/22 = 32% |
Low aminotransferases/low stress 8/11 = 73% |
| Possible mechanisms: | Consistent with our hypothesis | |
|
In summary, our study suggests that psychological stress is a factor that is associated with relapse or recrudescence of disease activity in AIH. Because recurrent relapse is a predictor for development of cirrhosis and a decreased survival in this disease (25), strategies that focus on relapse prevention and enhance the rates of sustained remission, including stress reduction, should be considered mainstays of therapy for this disease.
Acknowledgements
This study was supported in part by the Yale Liver Center Clinical Translational Core Facility (USPH DK P30-34989) and National Institutes of Health Training Grant (# T32 – DK07356-28). We would also like to thank the faculty of the Robert Wood Johnson Clinical Scholars Program at the Yale University School of Medicine for assistance in developing this study.
References
- 1.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.Al-Chalabi T, Heneghan MA. Remission in autoimmune hepatitis: what is it, and can it ever be achieved? Am J Gastroenterol. 2007;102:1013–5. doi: 10.1111/j.1572-0241.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 3.Albert JC, Ammon HV, Summerskill WH. Clinical features and pronosis of severe chronic active liver disease (CALD) afer corticosteroid-induced remission. Gastroenterolgy. 1980;78:518–23. [PubMed] [Google Scholar]
- 4.Hegarty JE, Nouri Aria KT, Portmann B, et al. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology. 1983;3:685–9. doi: 10.1002/hep.1840030510. [DOI] [PubMed] [Google Scholar]
- 5.Seela S, Boyer JL. Autoimmune Hepatitis type I: safety and efficacy of prolonged medical therapy. Liver Int. 2005;25:734–9. doi: 10.1111/j.1478-3231.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanzler S, Gerken G, Lohr H, et al. Duration of immunosuppressive therapy in autoimmune hepatitis. J Hepatol. 2001;34:354–5. doi: 10.1016/s0168-8278(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 7.Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol. 2007;102:1005–12. doi: 10.1111/j.1572-0241.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 8.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–7. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 9.Sanders VM, Kohm AP. Sympathetic nervous system interaction with the immune system. Int Rev Neurobiol. 2002;52:17–41. doi: 10.1016/s0074-7742(02)52004-3. [DOI] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser JK, Loving TJ, Stowell JR, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–84. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 11.Holmes T, Rahe R. The social readjustment rating scale. J Psychosom Res. 1967;11:213–8. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez F, Berg PA, Bianchi FB, et al. International autoimmune hepatitis group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 13.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd edn. Sage Publications; Thousand Oaks, CA: 1994. [Google Scholar]
- 14.Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Sage Publications; London, UK: 1998. [Google Scholar]
- 15.Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Aldine Publishing Company; Chicago, IL: 1967. [Google Scholar]
- 16.Czaja AJ, Carpenter HA. Histological features associated with relapse after corticosteroid withdrawal in type 1 autoimmune hepatitis. Liver Int. 2003;23:116–23. doi: 10.1034/j.1600-0676.2003.00810.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–8. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 19.Schulz R, Belle SH, Czaja SJ, et al. Long-term care placement of dementia patients and caregiver health and well being. JAMA. 2004;292:961–7. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 20.Buljevac D, Hop WC, Reedeker W, et al. Self reported stressful life events and exacerbations in multiple sclerosis: prospective study. BMJ. 2003;327:646. doi: 10.1136/bmj.327.7416.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohr D, Goodkin D, Nelson S, et al. Moderating effects of coping on the relationship between stress and the development of new brain lesions in multiple sclerosis. Psychosom Med. 2002;64:803–9. doi: 10.1097/01.PSY.0000024238.11538.EC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr D, Hart S, Julian L, et al. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328:731. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131:410–9. doi: 10.1053/j.gastro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz M, Schaefer C, Hiroto D, et al. Life event questionnaires for measuring presumptive stress. Psychosom Med. 1977;39:413–31. doi: 10.1097/00006842-197711000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007;27:507–15. doi: 10.1111/j.1478-3231.2007.01444.x. [DOI] [PubMed] [Google Scholar]