Abstract
Moderate acute malnutrition (MAM) affects 11% of children <5 y old worldwide and increases their risk for morbidity and mortality. It is assumed that successful treatment of MAM reduces these risks. A total of 1967 children aged 6–59 mo successfully treated for MAM in rural Malawi following randomized treatment with corn-soy blend plus milk and oil (CSB++), soy ready-to-use supplementary food (RUSF), or soy/whey RUSF were followed for 12 mo. The initial supplementary food was given until the child reached a weight-for-height Z-score (WHZ) >−2. The median duration of feeding was 2 wk, with a maximum of 12 wk. The hypothesis tested was that children treated with either RUSF would be more likely to remain well-nourished than those treated with CSB++. The primary outcome, remaining well-nourished, was defined as mid-upper arm circumference ≥12.5 cm or WHZ ≥−2 for the entire duration of follow-up. During the 12-mo follow-up period, only 1230 (63%) children remained well-nourished, 334 (17%) relapsed to MAM, 190 (10%) developed severe acute malnutrition, 74 (4%) died, and 139 (7%) were lost to follow-up. Children who were treated with soy/whey RUSF were more likely to remain well-nourished (67%) than those treated with CSB++ (62%) or soy RUSF (59%) (P = 0.01). A seasonal pattern of food insecurity and adverse clinical outcomes was observed. This study demonstrates that children successfully treated for MAM with soy/whey RUSF are more likely to remain well-nourished; however, all children successfully treated for MAM remain vulnerable.
Introduction
Acute malnutrition is implicated in 35% of the 7.5 million deaths of children <5 y in developing countries each year (1). Moderate acute malnutrition (MAM)10, defined as having a weight-for-height Z-score (WHZ) <−2 and ≥−3 without edema, affects 11% of children worldwide under the age of 5 y (1). Children with MAM are not only at 3 times greater risk of death than well-nourished children but also face greater risk of morbidity from infectious diseases and delayed physical and cognitive development (1, 2).
Children with MAM are generally treated as outpatients with supplementary foods, such as peanut-based, ready-to-use supplementary food (RUSF) or fortified blended flours, such as corn-soy blend and corn-soy blend plus milk and oil (CSB++) (3, 4). RUSF has been associated with higher recovery rates than corn-soy blend in children with MAM (5, 6); however, CSB++ and RUSF have been shown to be equally effective (7).
Systematically collected data on the outcomes of children who recover from MAM, particularly with respect to growth, mortality, and risk of further episodes of malnutrition, have not to our knowledge been reported in the medical/nutrition literature. In this study, we compared children successfully treated for MAM with CSB++ or 1 of 2 RUSF products to test the hypothesis that clinical outcomes 1 y after recovery from MAM will be better in children treated with RUSF.
Participants and Methods
Study population.
Children aged 6–59 mo who recovered from MAM at 1 of 14 rural malnutrition clinics in southern Malawi while participating in a randomized controlled trial that compared the clinical efficacy of CSB++, soy RUSF, and soy/whey RUSF in treating MAM were eligible for the study (7). Successful recovery was defined as reaching WHZ >−2 without developing bipedal edema within ≤6 biweekly follow-up visits. Children with known or suspected HIV or tuberculosis were included in the study; however, children with other chronic debilitating illnesses, such as cerebral palsy or congenital malformations, were excluded.
Participants came primarily from families of subsistence farmers. Maize, the staple crop, is harvested from household-level gardens in April, after a single rainy season. Thus, the incidence of acute malnutrition peaks before harvest (December–April). The prevalence of HIV infection in children is estimated to be 1.7% (8). Among Malawian children <5 y, 48% are stunted (9).
This study was approved by the University of Malawi College of Medicine Research and Ethics Committee and the Washington University in St. Louis Human Research Protection Office.
Study design.
This was a 12-mo, prospective, observational study comparing the clinical outcomes of children previously treated for and recovered from MAM. The study was carried out following the completion of a randomized clinical trial for MAM where children were treated to the goal of WHZ >−2 with CSB++, soy RUSF, or soy/whey RUSF. Caretakers were asked to return to the clinic with the child at 3, 6, and 12 mo after recovery and at any time they believed that the child might benefit from evaluation. At every visit, anthropometric and clinical status was assessed. Possible clinical outcomes at 12 mo were: well-nourished, defined as WHZ >−2 or mid-upper arm circumference (MUAC) ≥12.5 cm at every visit for 1 y; relapsed, defined as WHZ ≤−2 and MUAC <12.5 cm at any time during the year; developed severe acute malnutrition (SAM), defined as having a WHZ <−3 or bipedal edema at any time during the year; defaulted, defined as having been followed-up for <48 wk; or died. Children who relapsed or developed SAM were appropriately treated until recovery.
The primary outcome was remaining well-nourished. Secondary outcomes were development of SAM and death. A child was considered to have an adverse outcome if he/ she developed MAM or SAM or died. Identification of risk factors for death and the development of SAM were assessed through regression modeling. The sample size of 1967 eligible children was large enough to detect a difference in the absolute improvement of the primary outcome of at least 5 percentage points with either RUSF formulation, with a 5% α-error and 90% power, assuming that 90% of children remained well-nourished with CSB++.
Participation.
Once it was confirmed that caretakers gave informed consent, demographic information was collected and household food insecurity status was assessed using a validated, 9-item Household Food Insecurity Access Scale (HFIAS) (10, 11). Trained nutrition researchers and senior pediatric research nurses then evaluated children for acute malnutrition by measuring anthropometry and assessing for edema. Standard methodologies for anthropometric measurements were used: weight was measured using an electronic scale to the nearest 5 g; length was measured in triplicate to the nearest 0.2 cm using a rigid length board; and MUAC was measured with a standard insertion tape to the nearest 0.2 cm. After extensive training by one of the physicians supervising the study, field nutrition researchers evaluated participants for edematous malnutrition (kwashiorkor) by assessing for bilateral pitting edema.
At each visit, the child’s weight, length, and MUAC were measured and edema was reassessed and caretakers were interviewed regarding the child’s appetite, infectious symptoms, HFIAS, and antibiotic use during the prior 2 wk. Nutrition and general health counseling was provided to the caretakers.
Children whose WHZ was found to be <−2 and ≥−3 at any visit were given focused nutritional counseling and supplemental rations that provided ∼75 kcal · kg−1 · d−1 (314 kJ · kg−1 · d−1) and treated to recovery. Children found to have SAM at any visit were treated with ready-to-use therapeutic food as outpatients (12) or transferred to inpatient care, as clinically appropriate in each case. HIV testing was encouraged if the mother was HIV positive, if the child remained malnourished despite 4 wk of therapy, or if the child was suspected to have HIV infection by clinical assessment. Children who missed scheduled visits were sought by village health workers at their homes and encouraged to return for follow-up.
Initial treatment of MAM.
Prior to enrollment, all children were treated with 1 of 3 supplementary foods (Supplemental Table 1). CSB++ is a blended flour with 8% milk powder and is less energy dense but has more protein than both RUSF products. CSB++ costs US$1.10/kg or US$0.16/average daily ration. Soy RUSF contains no animal source ingredients and costs US$2.13/kg or US$0.22/average daily ration (1 sachet weighing 92 g). Soy/whey RUSF contains 2 g of protein from animal sources and costs US$3.59/kg or US$0.38/average daily ration. The median duration of feeding was 2 wk with a maximum of 12 wk. Children who did not recover after 12 wk were offered inpatient or outpatient treatment for SAM.
Data analyses.
Anthropometric indices were computed from the WHO’s 2006 Child Growth Standards (13), calculated using Anthro or AnthroPlus (WHO). These programs correct for the use of length instead of standing height in children >2 y old. Clinical outcomes, demographic and clinical characteristics, and anthropometric measurements were compared using chi-square tests for dichotomous variables and ANOVA for continuous variables. Intention–to-treat analysis was used. The Bonferroni test was used for post-test comparisons. All statistical assessments were performed with IBM SPSS Statistics 20. P values <0.05 were considered to be significant.
To determine risk factors for poor outcomes while controlling for confounding variables and differences in the enrollment characteristics of children treated with different supplementary foods, logistic regression models for remaining well-nourished, development of SAM, and death at 3 mo and 12 mo were created. The regression models were created using a stepwise backward method where the criteria for inclusion in the final model was P < 0.10 for the coefficient. Covariates initially included in the models were age, sex, whether the mother was alive, whether the father was alive, whether the mother was the primary caretaker of the child, whether the child’s father was present in the home, mother’s HIV status (if mother was alive), child’s HIV status, number of children in the household <5 y, type of food received during the initial MAM treatment, and, upon enrollment, caretaker’s report of fever, cough, diarrhea, vomiting, and appetite, child’s anthropometric status [MUAC, WHZ, and height-for-age Z-score (HAZ)], and HFIAS. Coefficients were ORs. Covariates with coefficients with a 95% CI that did not include 1 were considered significant.
To assess for the presence of seasonal variation in the occurrence of an adverse outcome, the number of children presenting for follow-up in each 4-mo period and the number of children who died, developed SAM, or developed MAM were tabulated. The data were plotted and tested by chi-square using a 2 × 3 table. A P value of <0.01 was considered to be reflective of a significant seasonal variation in these adverse outcomes.
Results
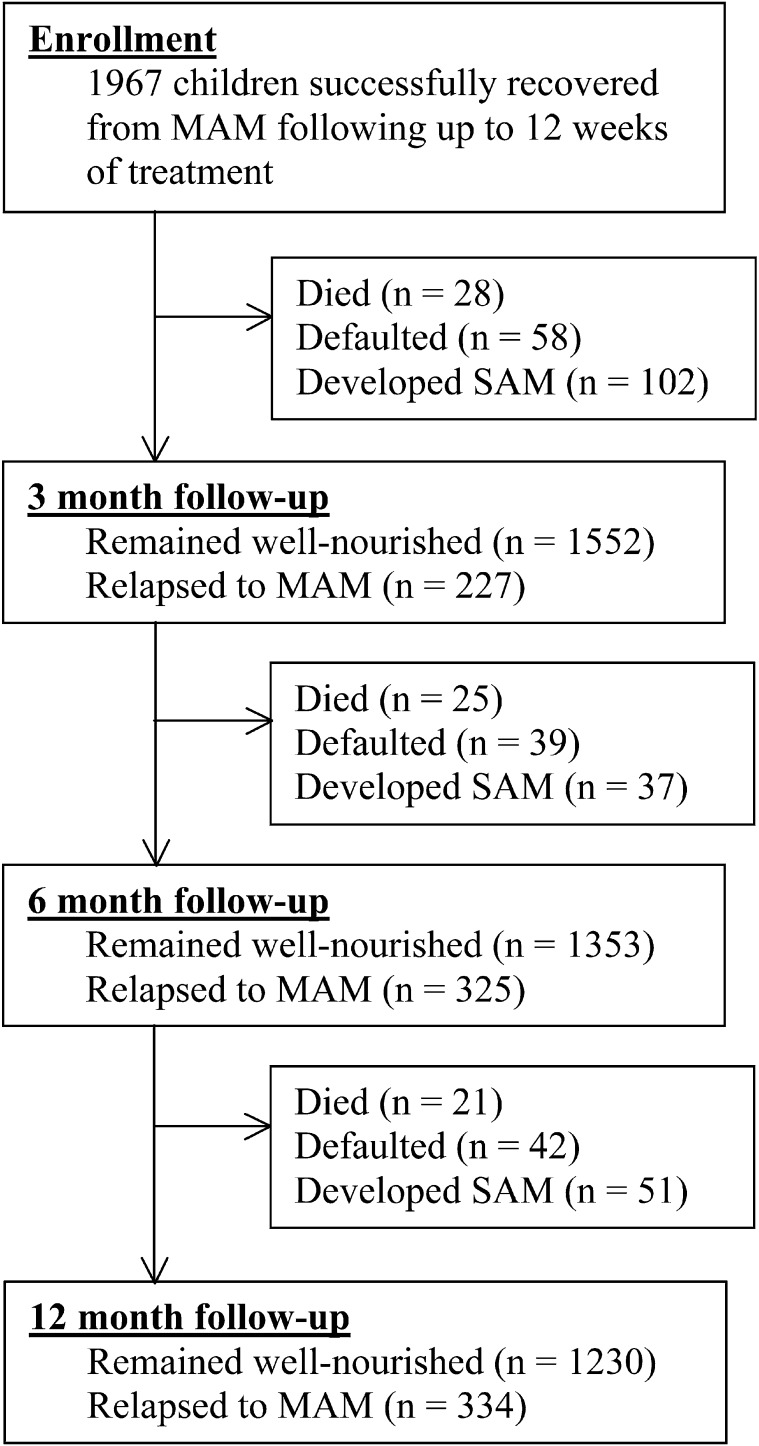
Between November 2009 and February 2011, 1967 children successfully recovered from MAM and were eligible for this study (Fig. 1;Table 1). Children who were treated with soy/whey RUSF had a higher MUAC than children who were treated with CSB++ or soy RUSF upon entry to this study (Table 1). A total of 139 (7%) children were lost to follow-up during the course of 1 y; 90 (65%) of these children were known to have moved away from the geographic proximity to the follow-up site. Enrollment characteristics of the children who defaulted were similar to those of children who completed the 1-y follow-up.
FIGURE 1.
Flow of participants throughout the 1-y follow-up study. MAM, moderate acute malnutrition; SAM, severe acute malnutrition.
TABLE 1.
Characteristics of children who successfully recovered from MAM and were enrolled in the 1-y follow-up study1
| CSB++ | Soy RUSF | Soy/whey RUSF | P value | |
| n | 653 | 647 | 667 | |
| Female, n (%) | 396 (61) | 396 (61) | 413 (62) | 0.89 |
| Age, mo | 21.0 ± 11.2 | 20.6 ± 10.9 | 20.6 ± 11.3 | 0.78 |
| MUAC, cm | 12.5 ± 0.9 | 12.5 ± 0.9 | 12.7 ± 0.9 | <0.001 |
| WHZ | −1.65 ± 0.47 | −1.63 ± 0.46 | −1.61 ± 0.49 | 0.24 |
| HAZ | −2.99 ± 1.29 | −2.96 ± 1.25 | −2.9 ± 1.21 | 0.54 |
| ≤−2, n (%) | 514 (79) | 512 (79) | 520 (78) | 0.84 |
| ≤−3, n (%) | 307 (47) | 314 (49) | 307 (46) | 0.66 |
| WAZ | −2.82 ± 0.82 | −2.79 ± 0.81 | −2.74 ± 0.8 | 0.30 |
| Twin, n (%) | 21 (3) | 50 (8) | 45 (7) | 0.001 |
| Primary caretaker is mother, n (%) | 625 (96) | 617 (95) | 637 (96) | 0.96 |
| Mother is alive, n (%) | 641 (98) | 637 (98) | 657 (99) | 0.87 |
| Father is alive, n (%) | 641 (98) | 634 (98) | 647 (97) | 0.32 |
| Father is in the home, n (%) | 499 (76) | 518 (80) | 506 (76) | 0.15 |
| Still breastfeeding, n (%) | 412 (63) | 420 (65) | 427 (64) | 0.75 |
| Child known to be HIV+, n (%) | 17 (3) | 11 (2) | 15 (2) | 0.60 |
| Mother known to be HIV+, n (%) | 82 (13) | 58 (9) | 55 (8) | 0.02 |
| Children in the home | 1.6 ± 0.7 | 1.6 ± 0.7 | 1.5 ± 0.7 | 0.15 |
| HFIAS score2 | 6.3 ± 5.4 | 6.1 ± 5.1 | 6.2 ± 5.3 | 0.64 |
Values are means ± SD or n (%). CSB++, corn-soy blend plus milk and oil; HAZ, height-for-age Z-score; HFIAS, Household Food Insecurity Access Scale; MAM, moderate acute malnutrition; MUAC, mid-upper arm circumference; RUSF, ready-to-use supplementary food; SAM, severe acute malnutrition; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
HFIAS: a higher score indicates more food insecurity, maximum 27.
A total of 1552 (79%) children remained well-nourished after 3 mo and 1353 (69%) were well-nourished after 6 mo. Among the 74 (4%) children who died, 28 (38%) died within 3 mo of enrollment and another 25 (34%) died between the 3- and 6-mo follow-up visits. Of the 53 caretakers that reported a cause of death, fever, diarrhea, and malaria accounted for 42 (79%) of these cases and SAM was mentioned in only 3 (6%) of the deaths.
Children who were treated with soy/whey RUSF for their initial episode of MAM were more likely to remain well-nourished (67%) than those treated with CSB++ (62%) or soy RUSF (59%) (P = 0.01) (Table 2). This comparison was not controlled for the difference in MUAC seen at enrollment. Logistic regression modeling was used to identify baseline characteristics that were associated with children who remained well-nourished, developed SAM, or died during the follow-up period (Table 3). The model identified lower MUAC and having a father who died as characteristics associated with death by 3 mo; additionally, HAZ and known HIV infection were also associated with death by 12 mo. Regression modeling did not identify an association between receiving soy/whey RUSF and remaining well-nourished.
TABLE 2.
Nutritional and anthropometric outcomes of children recovered from MAM 12 mo after enrollment1
| CSB++ | Soy RUSF | Soy/whey RUSF | P value | |
| n | 653 | 647 | 667 | |
| Maintained normal nutritional status,2 n (%) | 402 (62) | 382 (59) | 446 (67) | 0.01 |
| Relapsed to MAM,3 n (%) | 110 (17) | 117 (18) | 107 (16) | |
| Developed SAM during the year, n (%) | 63 (10) | 75 (12) | 52 (8) | 0.07 |
| Severe wasting, WHZ <−3, n (%) | 35 (5) | 28 (4) | 20 (3) | |
| Kwashiorkor, n (%) | 28 (4) | 47 (7) | 32 (5) | |
| Died, n (%) | 32 (5) | 19 (3) | 23 (3) | 0.17 |
| Did not return for 1-y follow-up, n (%) | 46 (7) | 54 (8) | 39 (6) | 0.25 |
| WHZ | −1.0 ± 0.9 | −1.0 ± 0.9 | −1.0 ± 0.8 | 0.87 |
| HAZ | −3.1 ± 1.2 | −3.1 ± 1.2 | −3.0 ± 1.2 | 0.10 |
| MUAC, cm | 13.6 ± 1.0 | 13.6 ± 1.1 | 13.7 ± 1.0 | 0.55 |
Values are means ± SD or n (%). CSB++, corn-soy blend plus milk and oil; HAZ, height-for-age Z-score; MAM, moderate acute malnutrition; MUAC, mid-upper arm circumference; RUSF, ready-to-use supplementary food; SAM, severe acute malnutrition; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
Defined as WHZ ≥−2 or MUAC ≥12.5 cm.
Defined as WHZ <−2, but ≥−3 and MUAC <12.5 cm.
TABLE 3.
Logistic regression model of factors associated with clinical outcomes in children successfully recovered from MAM1
| Independent variable | OR [95% CI] | P value |
| Success2 at 3 mo | ||
| Female child | 1.64 [1.27, 2.11] | <0.001 |
| Reported to have good appetite on enrollment | 2.91 [1.02, 8.29] | 0.045 |
| WHZ at enrollment | 2.67 [2.07, 3.45] | <0.001 |
| HFIAS score | 1.03 [1.01, 1.05] | 0.019 |
| Caretaker is mother | 3.06 [1.54, 6.11] | 0.001 |
| Mother alive | 3.11 [1.80, 5.37] | <0.001 |
| Child without HIV infection | 1.96 [1.21, 3.19] | 0.006 |
| Success at 12 mo | ||
| Female child | 2.35 [1.88, 2.94] | <0.001 |
| WHZ at enrollment | 3.90 [3.09, 4.91] | <0.001 |
| HAZ at enrollment | 1.08 [1.01, 1.16] | 0.046 |
| HFIAS score | 1.02 [1.01, 1.04] | 0.008 |
| Mother alive | 3.11 [1.80, 5.37] | <0.001 |
| Child without HIV infection | 2.60 [1.62, 4.18] | <0.001 |
| Developed SAM by 3 mo | ||
| MUAC at enrollment | 0.71 [0.65, 0.77] | <0.001 |
| WHZ at enrollment | 0.25 [0.16, 0.40] | <0.001 |
| Child without HIV infection | 0.39 [0.18, 0.84] | 0.002 |
| Developed SAM by 12 mo | ||
| Female gender | 0.58 [0.40, 0.84] | 0.004 |
| MUAC at enrollment | 0.82 [0.76, 0.89] | <0.001 |
| WHZ at enrollment | 0.41 [0.29, 0.59] | <0.001 |
| HAZ at enrollment | 0.87 [0.78, 0.98] | 0.026 |
| Child without HIV infection | 0.37 [0.20, 0.70] | 0.002 |
| Age (in months) | 0.97 [0.95, 0.98] | 0.001 |
| Died by 3 mo | ||
| MUAC at enrollment | 0.66 [0.62, 0.72] | <0.001 |
| Having a father who died | 8.19 [2.29, 29.4] | 0.001 |
| Died by 12 mo | ||
| MUAC at enrollment | 0.78 [0.72, 0.85] | 0.004 |
| HAZ at enrollment | 0.83 [0.71, 0.98] | 0.032 |
| Having a father who died | 3.21 [1.20, 8.59] | 0.020 |
| Child without HIV infection | 0.43 [0.19, 0.99] | 0.046 |
Logistic regression with stepwise backward exclusion of independent variables. Cox and Snell 2 for successful recovery: R2 = 0.30 for 3 mo, R2 = 0.10 for 12 mo; for development of SAM, R2 = 0.63 for 3 mo, R2 = 0.55 for 12 mo; for death, R2 = 0.71 for 3 mo, R2 = 0.66 for 12 mo. HAZ, height-for-age Z-score; HFIAS, Household Food Insecurity Access Scale; MAM, moderate acute malnutrition; MUAC, mid-upper arm circumference; WHZ, weight-for-height Z-score.
Success is defined as surviving with WHZ ≥−2 or MUAC ≥12.5 cm without developing edema.
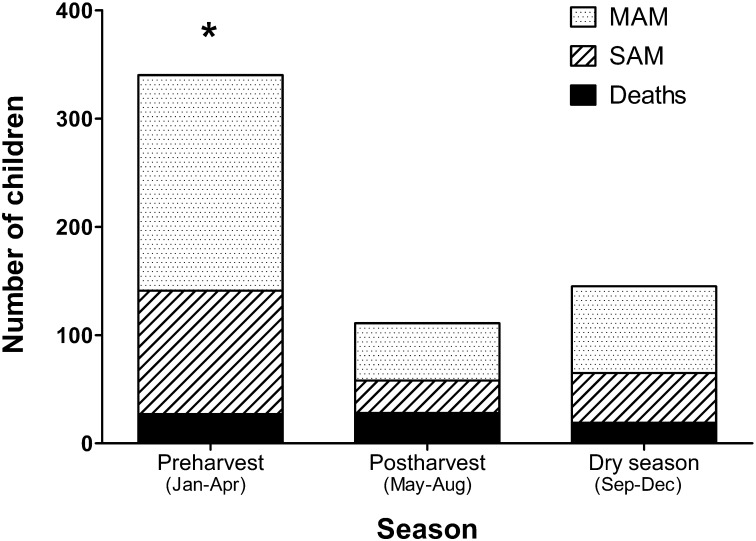
Repeat episodes of MAM and SAM were more frequently observed during the more food-insecure months of January through April (chi-square = 108 df = 3 degrees; P < 0.0001) (Fig. 2). A similar pattern of seasonal food insecurity was observed by HFIAS (data not shown).
FIGURE 2.
Numbers of children recovered from MAM that had adverse outcomes by season. Adverse outcomes included developing MAM or SAM and death. *Indicates adverse outcomes were higher during the food-insecure months of January to April compared with the rest of the year (chi-square, P < 0.0001). MAM, moderate acute malnutrition; SAM, severe acute malnutrition.
Discussion
In this 1-y observational study, only 63% of children who recovered from MAM remained well-nourished, while 4% died. Although simple comparison of this primary outcome on the basis of the type of supplementary food administered indicates that soy/whey RUSF is superior to soy RUSF and CSB++, logistic regression modeling to control for differences in MUAC, HAZ, and WHZ between the groups did not identify soy/whey RUSF as an independent predictor for a child remaining well-nourished.
The limitations of this study are that the study population was rural southern African children living in a climate with a distinct rainy season that provides adequate water for a single harvest annually; thus, the results should be extrapolated with caution to other populations with different patterns of rainfall and habitual diets. Measurement of stature was accurate to only the nearest 2 mm; however, changes in stature were assessed over a relatively long period of time, 3–12 mo, during which a child might be expected to grow 12 cm. The number of children lost to follow-up was 7%, modest considering that follow-up for each child took place over a 12-mo period. The initial treatment for MAM in the study population was conducted in the context of a randomized trial that provided specialized fortified foods and we speculate that outcomes after 12 mo would be worse in a programmatic setting.
In this study, children who relapsed to MAM were defined as those with a MUAC <12.5 cm and WHZ <−2, whereas in operational practice either of the criteria would be used to identify MAM. Use of WHZ criteria to define relapse to MAM is often problematic in operational practice, because upon recovery, children may grow in stature without concurrent weight gain sufficient to maintain WHZ. These children appear to relapse using WHZ criteria, when in fact their continued linear growth indicates that they may not be malnourished. Use of MUAC obviates this categorization artifact in the identification of MAM. To apply more stringent criteria in an effort to identify children who experienced a genuine deterioration in nutritional status, we used both MUAC and WHZ to define MAM in this study.
Supplementary feeding with soy/whey RUSF is associated with greater growth in MUAC in children with MAM, which confers a clinical benefit that extends for 12 mo thereafter. Although the rate of recovery was similar between the 3 foods after initial treatment (7), soy/whey RUSF achieved a slightly more durable recovery that resulted in maintaining normal nutritional status over the subsequent year (67% for soy/whey RUSF vs. 62% for CSB++ and 59% for soy RUSF; P = 0.01). We speculate that the most cost-effective approach to achieving greater MUAC may well be treatment with a less costly food, such as soy RUSF or CSB++, for a longer duration than increased use of soy/whey RUSF; however, a future study would need to be carried out to confirm this.
Continuing supplementary feeding for MAM longer than the time required to reach WHZ >−2 may increase the fraction of children that remain well-nourished over the subsequent year, which is certainly warranted given the poor outcomes for children that cease to receive supplementary food as soon as they obtain a WHZ >−2. Internationally, there is currently diversity in the WHZ discharge criteria that are used for MAM, ranging from WHZ >−2 to WHZ >−1, based on expert opinion. Evidence to suggest the optimal duration of feeding for a child with MAM in terms of long-term outcomes is lacking. The challenge that increasing the WHZ discharge criterion presents operationally is that WHZ may not continue to increase as nutritional status improves. Thus, supplementary feeding programs will find that many children will not qualify for discharge on the basis of WHZ, even though their linear growth is occurring at a normal rate. The use of MUAC along with WHZ to define MAM and recovery from MAM may solve this operational challenge.
The high prevalence of poor outcomes among children recovering from MAM suggests that although anthropometric recovery was achieved, children’s physiologic and immunologic status was not restored to normal. Among all Malawian children aged 1–5 y, the mortality rate is expected to be 1% annually (8), yet 4% of the children participating in this study died. Perhaps further interventions should be offered to these children during this period of vulnerability. Potential interventions include bed nets to prevent malaria, the most common infection, a supplementary food for a limited duration that would provide all of the micronutrients needed in the diet plus 100 kcal, HIV testing and treatment, deworming, and a review and update of the immunization status for each child. Regular monthly follow-up might be helpful as well, particularly during times of food insecurity or for those children that are not cared for by their mothers. Children having recovered from MAM should be considered at high risk and the subject of interventions to reduce this risk in further trials.
Although HIV infection was not implicated in most of the poor outcomes, it was strongly associated with SAM and death. This suggests that there is a benefit to linking HIV testing programs with targeted feeding programs in HIV endemic regions, with the goals being appropriate provision of ART and cotrimoxazole prophylaxis.
The incidence of adverse clinical outcomes followed a seasonal pattern of food shortage that is commonly recognized in Malawi, with a greater proportion of poor outcomes occurring during the “hungry season.” Correspondingly, a similar pattern of seasonal food insecurity was observed by HFIAS. Among children with poor outcomes, 60% of these occurred during the “hungry season,” suggesting that interventions to improve longer term outcomes for MAM in Malawi could be targeted in this season.
Future research should explore the optimal duration of treatment for MAM, what is the most appropriate discharge criteria for targeted MAM treatment programs, what types of physiologic and/or immunologic deficiencies persist after anthropometric MAM has resolved, and testing interventions to improve outcomes in this vulnerable population.
Supplementary Material
Acknowledgments
The authors thank Eleanor Chipofya, Rosemary Godwa, Lydia Kamena, Jean Mbawa, Nester Mwase, Horris Chikwiri, Jackson Makwinja, and Vegas Riscado for their dedicated service to the children of Malawi. I.T., K.M., M.D., and M.J.M. designed research; C.Y.C., I.T., R.J.W., C.T., and M.J.M. conducted research; C.Y.C. and M.J.M. first analyzed data and wrote paper; M.D., I.T., and R.J.W. contributed subsequent analyses; and M.J.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CSB++, corn-soy blend plus milk and oil; HAZ, height-for-age Z-score; HFIAS, Household Food Insecurity Access Scale; MAM, moderate acute malnutrition; MUAC, mid-upper arm circumference; RUSF, ready-to-use supplementary food; SAM, severe acute malnutrition; WHZ, weight-for-height Z-score.
Literature Cited
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 2.Pelletier DL, Low JW, Johnson FC, Msukwa LA. Child anthropometry and mortality in Malawi: testing for effect modification by age and length of follow-up and confounding by socioeconomic factors. J Nutr. 1994;124:S2082–105 [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Colorado C, Mason F, Shoham J. Measuring the effectiveness of Supplementary Feeding Programmes in emergencies. London: Humanitarian Practice Network; 2008 [Google Scholar]
- 4.de Pee S, Bloem MW. Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6- to 23-month old children and for treating moderate malnutrition among 6- to 59-month-old children. Food Nutr Bull. 2009;30:S434–63 [DOI] [PubMed] [Google Scholar]
- 5.Matilsky DK, Maleta K, Castleman T, Manary MJ. Supplementary feeding with fortified spreads results in higher recovery rates than with corn/ soy blend in moderately wasted children. J Nutr. 2009;139:773–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nackers F, Broillet F, Oumarou D, Djibo A, Gaboulaud V, Guerin PJ, Rusch B, Grais RF, Captier V. Effectiveness of ready-to-use therapeutic food compared to a corn/soy-blend-based pre-mix for the treatment of childhood moderate acute malnutrition in Niger. J Trop Pediatr. 2010;56:407–13 [DOI] [PubMed] [Google Scholar]
- 7.LaGrone LN, Trehan I, Meuli GJ, Wang RJ, Thakwalakwa C, Maleta K, Manary MJ. A novel fortified blended flour, corn-soy blend “plus-plus,” is not inferior to lipid-based ready-to-use supplementary foods for the treatment of moderate acute malnutrition in Malawian children. Am J Clin Nutr. 2012;95:212–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNICEF State of the world's children 2012. New York: UNICEF; 2012 [Google Scholar]
- 9.WHO World health statistics. Geneva: WHO; 2012 [Google Scholar]
- 10.Knueppel D, Demment M, Kaiser L. Validation of the Household Food Insecurity Access Scale in rural Tanzania. Public Health Nutr. 2010;13:360–7 [DOI] [PubMed] [Google Scholar]
- 11.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide. Washington, DC: USAID; 2007 [Google Scholar]
- 12. WHO, World Food Programme, United Nation System Standing Committee on Nutrition, UNICEF. Community management of severe acute malnutrition. Geneva: WHO; 2007.
- 13.WHO WHO child growth standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: WHO; 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.