Abstract
In previously published work, we have described heparin-binding synthetic peptides that preferentially recognize amyloid deposits in a mouse model of reactive systemic (AA) amyloidosis and can be imaged by using positron and single photon emission tomographic imaging. We wanted to extend these findings to the most common form of visceral amyloidosis, namely light chain (AL); however, there are no robust experimental animal models of AL amyloidosis. To further define the binding of the lead peptide, p5, to AL amyloid, we characterized the reactivity in vitro of p5 with in situ and patient-derived AL amyloid extracts which contain both hypersulfated heparan sulfate proteoglycans as well as amyloid fibrils. Histochemical staining demonstrated that the peptide specifically localized with tissue-associated AL amyloid deposits. Although we anticipated that p5 would undergo electrostatic interactions with the amyloid-associated glycosaminoglycans expressing heparin-like side chains, no significant correlation between peptide binding and glycosaminoglycan content within amyloid extracts was observed. In contrast, following heparinase I treatment, although overall binding was reduced, a positive correlation between peptide binding and amyloid fibril content became evident. This interaction was further confirmed using synthetic light chain fibrils that contain no carbohydrates. These data suggest that p5 can bind to both the sulfated glycosaminoglycans and protein fibril components of AL amyloid. Understanding these complex electrostatic interactions will aid in the optimization of synthetic peptides for use as amyloid imaging agents and potentially as therapeutics for the treatment of amyloid diseases.
Keywords: AL amyloid, peptide binding, fibrils, glycosaminoglycans, heparinase, peptide p5
Introduction
Amyloid is a complex pathological deposit principally composed of protein fibrils and hypersulfated heparan sulfate proteoglycans (HSPG) [1]. It is associated with numerous disorders including Alzheimer's disease (AD; Aβ amyloid), type 2 diabetes (T2DM; AIAPP amyloid), rheumatoid arthritis (reactive AA amyloid) and plasma cell dyscrasias (AL amyloid) [2]. The deposition of certain amyloid types, in modest amounts, can lead to regional cytotoxicity and organ dysfunction as in patients with AD or T2DM [3]. Alternatively, substantial AA and AL amyloid deposits can occur systemically in visceral organs (notably the liver, spleen, kidney and heart as well as major nerves and bone marrow) resulting in mechanical and architectural perturbation of the organ causing loss of function, morbidity and death [4]. For patients with systemic amyloidosis, it has become increasingly important to develop non-invasive molecular imaging techniques to monitor the whole organ/body burden of amyloid which aids in prognostication, patient stratification and monitoring response to therapy.
Numerous amyloid-reactive, and often pan-amyloid, reagents have been reported. Proteins and monoclonal antibodies (mAbs), including serum amyloid P component (SAP), bovine aprotinin, mAb 11-1F4, and camelid antibody B10, as well as the heparin-reactive peptide p5, have all been shown to associate with amyloid deposits or amyloid fibrils [4,5,6,7,8]. Many of them have also proven effective as imaging agents for the detection of various visceral amyloid deposits in patients [4,5,6,7] or in preclinical studies using murine models of systemic amyloid disease [4]. However, in the US, none are FDA-approved or routinely employed in the clinic. Certain of these amyloid-reactive biological molecules, including the antibody B10, aprotinin, and likely the mAb 11-1F4, bind amyloid essentially via electrostatic interactions [8,9]. The B10 mAb has been shown to bind synthetic amyloid fibrils in vitro[8]. This interaction is perturbed by the presence of 0.5 M NaCl in the reaction medium. The pan-amyloid reactive mAb 11-1F4 has been shown to cross-react with both κ and λ types of AL amyloid in vitro and in patients with the disease; however, this reactivity is completely lost when lysine side chains are modified by addition of succinimidyl adducts (Wall et al., unpublished observations).
The specific detection of amyloid deposits in AA mice, using radiolabeled p5, makes it an excellent candidate for imaging many types of amyloid in vivo since all contain heparan sulfate proteoglycans (HSPG; notably perlecan) with hypersulfated glycosaminoglycan (GAG) side chains [10]. The peptide has been assumed, due to its interaction with heparin, to bind the GAGs that are ubiquitously associated with all amyloid HSPG [11]. However, this has not been empirically determined in any system. In the present study, we have tested the hypothesis that p5 specifically binds the GAG associated with amyloid. To achieve this, we have employed a radioactive peptide-binding assay using a panel of tissue-derived human AL amyloid extracts containing varying amounts of fibril and GAG material, based on the interaction of specific dyes. In addition, we validated our findings by examining the reactivity of p5 with synthetic fibrils composed of light chain fragments.
Materials and Methods
AL extract preparation
The extracts were prepared using the water flotation method as described by Pras et al. [12] without modification. Amyloid material isolated in the water wash was collected and freeze dried.
Alcian blue measurements
The method used to determine the sulfated (acidic) glycosaminoglycan content of the AL amyloid extracts was that described by Karlsson and Björnsson [13].
Thioflavin T(ThT) analysis
Ten μL of 1 mg/mL amyloid extract, before or after heparinase I treatment, was added to each of three wells on a 96-well microplate. Thirty μL of phosphate buffered saline, pH 7.5 (PBS; containing 0.15 M NaCl unless 1 M NaCl was used), was added to each of the wells prior to the addition of 10 μL of 300 μM ThT (Sigma-Aldrich, St. Louis, MO). A set of 3 wells containing only PBS and ThT was used as a background control. The ThT fluorescence emission (490 nm, excitation at 450 nm) was measured using a fluorescence plate reader (Victor 1420 multilabel counter, Perkin Elmer, Wellesly, MA), and measurements for amyloid-containing wells were corrected by subtraction of mean background fluorescence.
Peptide histochemistry
The biotinylated p5 peptide was used for tissue amyloid staining as previously described by Wall et al. [4]. Biotinylated peptide was detected using peroxidase conjugated streptavidin coupled with HRPO and visualized with diamino-benzidine substrate.
Peptide radiolabeling
A 5 mL-volume Sephadex G-25 resin-packed column (PD10, GE Healthcare, Piscataway, NJ) was equilibrated with 10 mL of filtered (0.2 μm pore-sized) 0.1 % gelatin in PBS. Fifty μg of peptide p5 in 10-50 μL of water was added to 10 μL of NaPO4 buffer (pH 7.6) in a 1.5 mL microfuge tube. Ten μL of 125I (∼1 mCi; Perkin-Elmer) was added to the reaction mixture followed by 5 μL of Chloramine T (4 mg/mL). The reaction was quenched by addition of 10 μL of 4 mg/mL sodium metabisulfite. The peptide with blue dextran exclusion marker was applied to the G-25 column, and the first 10 fractions (∼250 μL each) were collected as the blue dextran eluted from the column. Peak fractions of radioactivity were pooled. Radiochemical purity was established by SDS/PAGE analyzed by a phosphor imager [14].
Peptide binding assay
Twenty-five μL of 1 mg/mL AL extract or synthetic recombinant λ6 Wil variable domain (rVλ6Wil) fibrils [15] were centrifuged in a 0.5 mL microfuge tube at 21,000 × g for 5 min. The supernatant was discarded and pellet resuspended in 200 μL of PBS with 0.05 % tween-20 (PBST). Ten μL of a 1:100 dilution of radioiodinated peptide p5 (∼ 100,000 counts per minute (CPM); ∼ 5 ng peptide) stock was added to the suspension. The mixture was rotated at RT for 1 h. Samples were then centrifuged at 15,000 × g for 10 min. Supernatants were collected in glass tubes, and pellets were resuspended in 200 μL PBST before another centrifugation at 15,000 × g for 10 min. Supernatants were removed and added to those previously collected. Pellets were transferred to glass tubes using a total of 400 μL of PBST. The radioactivity in each tube was measured using a Cobra II gamma counter (Perkin-Elmer) with a 1 min acquisition, and the percentage of 125I-p5 bound to pellet was determined as follows: Pellet CPM/(Pellet CPM + Supernatant CPM)× 100
To demonstrate the effect of increasing monovalent cation concentration on the peptide-amyloid interaction, the peptide binding assay was performed as above in solutions of 10 mM phosphate buffer (pH 7.6) containing 0.15 (PBS), 0.25, 0.5, or 1 M NaCl.
Heparinase I treatment
Approximately 250 μg (50 U) of lyophilized heparinase I (Sigma-Aldrich) was dissolved in 250 μL of 20 mM Tris-HCl, 50 mM NaCl, 4 mM CaCl2 and 0.01% BSA at pH 7.5 (Hep buffer). Amyloid extracts were suspended in 500 μL (1 mg/mL) of Hep buffer prior to the addition of 20 μL (4 U) of heparinase I. Samples were rotated at 25°C for 2 hours then centrifuged at 21,000 × g for 5 min. The supernatant was discarded and pellet resuspended in 500 μL of PBST. Samples were washed again by centrifugation and the supernatant discarded. The pellet was resuspended in the same volume of PBST and stored at -80°C.
Statistical methods
Pearson correlation and paired t-test statistical analyses were performed using SPSS Version 19 (Armonk, NY: IBM Corp) and Prism (Ver. 6.01, Graphpad Software Inc, San Diego, CA). Significance was set at p < 0.05.
Results
To demonstrate the reactivity of p5 with AL amyloid deposits in tissue sections, a biotinylated conjugate of the peptide was used as a histochemical detection agent. Specific localization of biotinyl-p5 with AL amyloid in various tissues was evidenced by the brown tissue staining (Fig. 1). Validation of amyloid-specific reactivity was performed by staining consecutive tissue sections with Congo red and examining red-green birefringence when viewed microscopically with cross-polarized illumination. Images in Figure 1 demonstrate that the peptide bound preferentially to the regions rich in Congo red-staining material (amyloid deposits) and not to normal tissue regions of the section.
Figure 1.
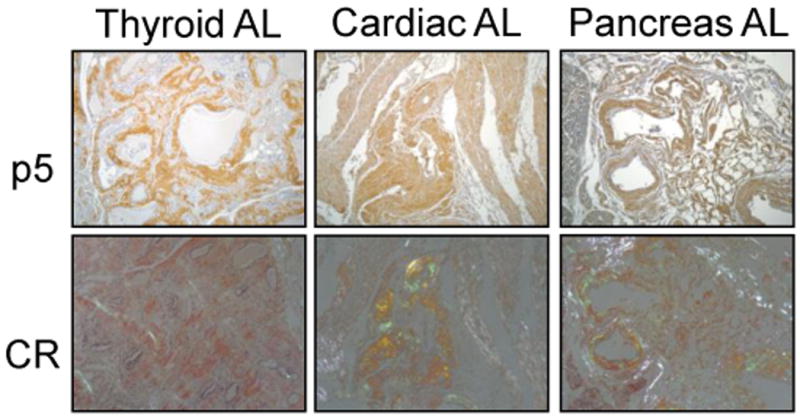
Histochemical analysis of p5 binding to AL amyloid deposits in tissue sections. Biotinylated p5 was added to AL-containing tissue sections (p5) and binding evidenced by brown diaminobenzidine staining. Co-localization with amyloid deposits was shown by staining consecutive tissue sections with Congo red (CR) and examining the presence red-green birefringence indicative of amyloid.
Since the peptide histochemistry revealed that p5 binds to AL amyloid in tissue sections, we further characterized this interaction by studying the peptide's ability to bind human ALκ and ALλ amyloid extracts. These extracts contain various amounts of both GAGs and fibrils [16]; thus, Alcian blue and ThT fluorescence assays were used to estimate the amounts of these components within each extract, respectively (Table 1). Because p5 binds heparin, we reasoned that amyloid binding would correlate significantly with the amount of sulfated GAG present in the extracts.
Table 1. Physical characteristics of AL amyloid extracts.
| Amyloid (organ)a | Amyloid type | Alcian blueb | Thtc |
|---|---|---|---|
| Cab (L) | ALκ4 | 0.152 | 16542 |
| Car (L) | ALλ | 0.217 | 16147 |
| Tal (L) | ALκ | 0.016 | 17311 |
| Hig (L) | ALκ1 | 0.177 | 23724 |
| Bal (S) | ALλ4 | 0.194 | 15079 |
| Shi (L) | ALλ2 | 0.155 | 41257 |
| Shi (S) | ALλ2 | 0.125 | 75745 |
| Art (S) | AH/ALIgGκ1 | 0.173 | 34622 |
| Tyl (S) | ALλ3 | 0.119 | 51179 |
L, liver and S, spleen.
Presence of sulfated GAGs based on Alcian blue absorbance at 450 nm (au)
Presence of amyloid fibrils measured by ThT fluorescence at 490 nm (em. = 450 nm).
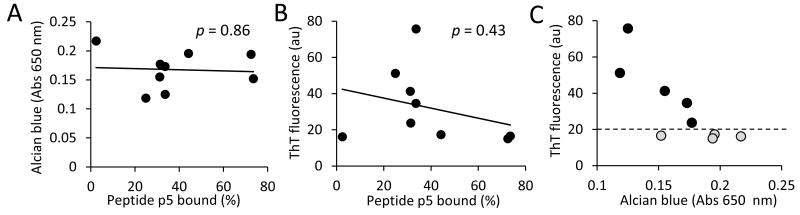
The radioiodinated peptide binding assay was used to quantify binding of p5. The amount of 125I-p5 that was bound to pellets (% of total) unexpectedly did not correlate with either the GAG content (Alcian blue measurement [Fig. 2A]) or the amyloid fibril mass (ThT fluorescence [Fig. 2B]). Notably, two discreet populations of AL extract were apparent based on the Alcian blue and ThT binding (Fig. 2C). The first was characterized by high amyloid fibril content (black points above the dashed line) and the second with increasing amounts of GAGs (gray points below the dashed line).
Figure 2.
Peptide p5 binding to AL amyloid extract does not correlate with GAG or fibril content. Correlation (Pearson) analyses between 125I-p5 binding GAG content measured by Alcian blue (A) or fibril content measured by ThT fluorescence emission (B). Correlation analysis of GAG and fibril content revealed 2 discreet populations (C). AL amyloid extracts with high fibril content (●) were further analyzed.
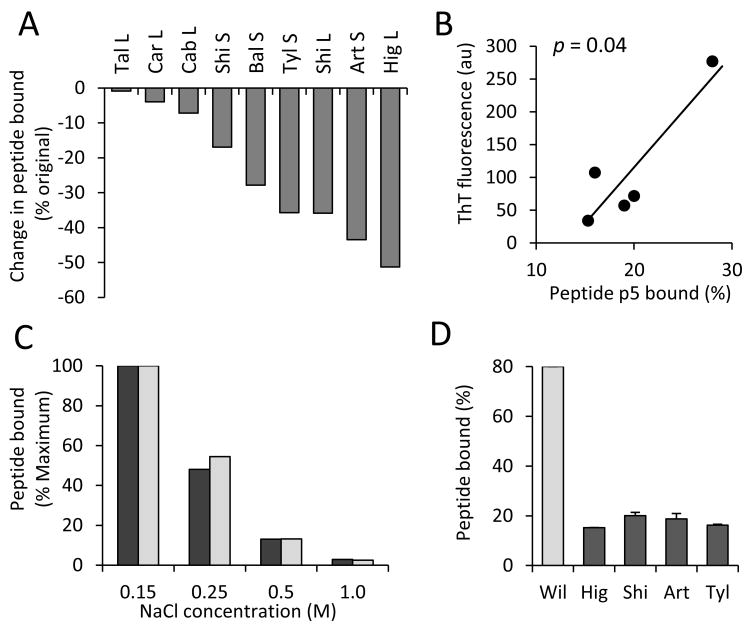
Given that the interaction of 125I-p5 with AL extracts was more complex than we anticipated (Fig. 1) and may involve both the fibrillar and GAG components of amyloid, we further characterized the binding of the peptide to amyloid after treatment with heparinase I to remove heparan sulfate GAGs. Aliquots of each of the treated extracts were used in an 125I-p5 binding assay. We posited that, since p5 was thought to bind GAGs, less peptide would bind to the pellets following heparinase treatment. Indeed, we observed a decrease in the peptide's reactivity with each of the AL extracts consistent with the removal of the GAGs (Fig. 3A) substantiating the hypothesis that p5 can bind this component of the amyloid.
Figure 3.
125I-p5 binds with heparinase-treated AL extracts and synthetic light chain fibrils. (A) Heparinase treatment resulted in a decrease of 125I-p5 binding to all extracts studied. (B) Using the subset of extracts, a significant correlation between 125I-p5 binding to heparinase-treated amyloid fibril content was observed (Pearson analysis). (C) Increasing NaCl concentration in the reaction solution decreased the binding of 125I-p5 to Bal (S) amyloid extract (pre- [black] and post- [gray] heparinase treatment). (D) 125I-p5 binding to synthetic rVλ6Wil fibrils (25 μg) was compared to AL amyloid extracts (25 μg) and shown to be 4-fold greater.
We further hypothesized that, due to the complexity of the p5-amyloid reactivity (Fig. 2), the peptide may also bind to the amyloid fibrils within AL extracts, which would result in a significant positive correlation between fibril content and bound 125I-p5 post heparinase treatment. Therefore, we examined the relationship between the post-heparinase fibril load and the percentage of 125I-p5 bound to each extract. ThT analysis revealed no significant loss of fibrils following enzymatic digestion. Surprisingly, there was no correlation when all 9 AL extracts were included in the analysis (data not shown). However, when the 5 extracts with high fibril content were used, there was a significant positive correlation (p = 0.04, Fig 3B) which supported our thesis that 125I-p5 can also bind to the fibril component of AL amyloid after heparinase treatment.
To examine whether p5 binding with AL amyloid extracts was governed by electrostatic interactions, the reactivity was assessed in solutions of increasing ionic strength. We first demonstrated, using both an untreated and a heparinase-treated sample of a representative AL extract (Bal (S)), that incubation in 1 M NaCl did not cause a loss of fibril content (data not shown). The binding of 125I-p5 with AL amyloid extracts was shown to depend almost entirely on electrostatic interactions that could be inhibited by incubating peptide with amyloid in the presence of increasing NaCl concentrations, up to 1 M (Fig. 3C).
To further explore our finding that the peptide likely binds AL amyloid fibrils, synthetic rVλ6Wil light chain fibrils were prepared and tested for their ability to bind 125I-p5. In the ex vivo binding assay, 80% of radiolabeled p5 was observed bound to synthetic rVλ6Wil fibrils, which was 4-fold greater than that seen for an equivalent weight of AL amyloid extract (Fig. 3D).
Discussion
There is a critical need to develop amyloid-reactive molecules that might be used for the treatment and non-invasive detection of whole body amyloid burden by using molecular imaging techniques. Several biological reagents that bind amyloid, including mAbs, serum proteins and peptides have been described for this purpose [4,5,6,7]. We have previously described a synthetic, heparin-reactive peptide, designated p5, which binds numerous types of amyloid and, when radiolabeled, can be used for the specific visualization of reactive (AA) amyloidosis in mice [4]. In man, light chain (AL) amyloidosis is the most common form of visceral amyloid disease, but there are no natural or experimental animal models that effectively recapitulate the pathology. The biochemical forces governing the interactions of p5 with amyloid components are poorly understood and difficult to ascertain in vivo. Therefore, the interactions of p5 with AL must be evaluated in vitro.
Currently, there are no routine methods available in the US for detecting the whole body burden of amyloid in patients. Therefore, p5 is being developed to achieve this end. The reactivity of the peptide with human AL amyloid, in tissue sections or as patient-derived amyloid extracts, was assessed to further characterize the peptide-amyloid interaction. AL amyloid is a complex, heterogeneous matrix composed of highly sulfated HSPG, fibrils and other accessory proteins [17]. Amyloid-associated HSPG differs from the sulfated GAGs that are found throughout healthy tissues; due to its greater degree of sulfation, it is structurally and electrochemically similar to heparin [11]. Our hypothesis was that p5, due to its affinity for heparin, may exploit this characteristic thus providing a novel agent for the detection of amyloid deposits [4,11,18]. Our data show that while GAGs play a role in p5 binding, the overall interaction of the peptide with amyloid deposits is more complex, involving association with amyloid fibrils themselves.
Other amyloid-binding reagents, such as the camelid antibody B10, recognize electrostatic motifs that are present on the surface of synthetic amyloid-like fibrils composed of Aβ and other amyloidogenic proteins [8]. This was evidenced by the fact that introduction of mutations into the B10 mAb, which altered the net charge of the reagent, resulted in a disruption of the binding to fibrils. Additionally, chemical modification of the negatively charged carboxyl groups on synthetic fibrils similarly hindered the antibody's ability to react [8]. Given that amyloid contains heparin-like HSPG presenting a linear array of negatively charged sulfate groups [19], we anticipated that the binding of p5 to the GAG moiety would, like mAb B10, involve an electrostatic interaction.
Reactivity of p5 with patient-derived samples varied in intensity which likely reflects the heterogeneous nature of the AL amyloid and differences in the HSPG and amyloid fibril content. This was evidenced by the variability in the uptake of GAG and fibril-reactive dyes as well as the differing degrees of p5 reactivity. We were unable to demonstrate a significant linear correlation between p5 binding and the amount of GAGs or fibrils in the AL extracts, which implied that the reactivity of the peptide with the amyloid matrix may be more complex and involve both targets. Following heparinase treatment, however, a positive correlation between the fibril content and p5 binding was observed using a subset of extracts with high amyloid fibril load. Furthermore, we demonstrated that, similar to the B10 mAb, p5 was able to bind synthetic light chain-derived fibrils and that the association (with AL amyloid) is dependent on electrostatic interactions. Since amyloid fibrils, like heparin, project linear, repeating, electronegative amino acidic side chains, it is reasonable to suggest that p5 interacts with these regularly-spaced moieties.
The interaction of p5 with human-derived AL amyloid extracts is complex. Both heparinase-sensitive GAGs and amyloid fibrils are involved. A more complete understanding of this system may provide a paradigm for how p5, and similar peptides, interact with the various amyloid components.
Highlights.
Polybasic peptide p5 binds human light chain amyloid extracts.
The binding of p5 with amyloid involves both glycosaminoglycans and fibrils.
Heparinase treatment led to a correlation between p5 binding and fibril content.
p5 binding to AL amyloid requires electrostatic interactions.
Acknowledgments
We wish to thank Craig Wooliver for the histochemical stains. Amyloid extracts were kindly supplied by Dr. Alan Solomon and isolated by Ms. Teresa Williams. This work was supported by Award Number R01DK079984 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rocken C, Shakespeare A. Pathology, diagnosis and pathogenesis of AA amyloidosis. Virchows Arch. 2002;440:111–122. doi: 10.1007/s00428-001-0582-9. [DOI] [PubMed] [Google Scholar]
- 2.Westermark P. Aspects on human amyloid forms and their fibril polypeptides. FEBS J. 2005;272:5942–5949. doi: 10.1111/j.1742-4658.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 3.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 4.Wall JS, Richey T, Stuckey A, Donnell R, Macy S, Martin EB, Williams A, Higuchi K, Kennel SJ. In vivo molecular imaging of peripheral amyloidosis using heparin-binding peptides. Proc Natl Acad Sci U S A. 2011;108:E586–594. doi: 10.1073/pnas.1103247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins PN, Pepys MB. Imaging amyloidosis with radiolabelled SAP. Eur J Nucl Med. 1995;22:595–599. doi: 10.1007/BF01254559. [DOI] [PubMed] [Google Scholar]
- 6.Schaadt BK, Hendel HW, Gimsing P, Jonsson V, Pedersen H, Hesse B. 99mTc-aprotinin scintigraphy in amyloidosis. J Nucl Med. 2003;44:177–183. [PubMed] [Google Scholar]
- 7.Wall JS, Kennel SJ, Stuckey AC, Long MJ, Townsend DW, Smith GT, Wells KJ, Fu Y, Stabin MG, Weiss DT, Solomon A. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116:2241–2244. doi: 10.1182/blood-2010-03-273797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haupt C, Morgado I, Kumar ST, Parthier C, Bereza M, Hortschansky P, Stubbs MT, Horn U, Fandrich M. Amyloid fibril recognition with the conformational B10 antibody fragment depends on electrostatic interactions. J Mol Biol. 2011;405:341–348. doi: 10.1016/j.jmb.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso I, Pereira PJ, Damas AM, Saraiva MJ. Aprotinin binding to amyloid fibrils. Eur J Biochem. 2000;267:2307–2311. doi: 10.1046/j.1432-1327.2000.01237.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevens FJ, Kisilevsky R. Immunoglobulin light chains, glycosaminoglycans, and amyloid. Cell Mol Life Sci. 2000;57:441–449. doi: 10.1007/PL00000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall JS, Richey T, Macy S, Heidel E, Wooliver C, Kennel SJ. A novel method for quantifying peripheral tissue amyloid load by using the radiolabeled amyloidophilic peptide, p5. Amyloid. 2013;20:21–26. doi: 10.3109/13506129.2012.757216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin EC. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968;47:924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson M, Bjornsson S. Quantitation of proteoglycans in biological fluids using Alcian blue. Methods Mol Biol. 2001;171:159–173. doi: 10.1385/1-59259-209-0:159. [DOI] [PubMed] [Google Scholar]
- 14.Wall JS, Paulus MJ, Gleason S, Gregor J, Solomon A, Kennel SJ. Micro-imaging of amyloid in mice. Methods Enzymol. 2006;412:161–182. doi: 10.1016/S0076-6879(06)12011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wall J, Schell M, Murphy C, Hrncic R, Stevens FJ, Solomon A. Thermodynamic instability of human lambda 6 light chains: correlation with fibrillogenicity. Biochemistry. 1999;38:14101–14108. doi: 10.1021/bi991131j. [DOI] [PubMed] [Google Scholar]
- 16.Wall JS, Kennel SJ, Williams A, Richey T, Stuckey A, Huang Y, Macy S, Donnell R, Barbour R, Seubert P, Schenk D. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PLoS One. 2012;7:e52686. doi: 10.1371/journal.pone.0052686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta. 2005;1753:11–22. doi: 10.1016/j.bbapap.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Wall JS, Richey T, Williams A, Stuckey A, Osborne D, Martin E, Kennel SJ. Comparative analysis of peptide p5 and serum amyloid P component for imaging AA amyloid in mice using dual-isotope SPECT. Mol Imaging Biol. 2012;14:402–407. doi: 10.1007/s11307-011-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindahl B, Lindahl U. Amyloid-specific heparan sulfate from human liver and spleen. J Biol Chem. 1997;272:26091–26094. doi: 10.1074/jbc.272.42.26091. [DOI] [PubMed] [Google Scholar]