Abstract
Monoacyglycerol acyltransferases (MGATs) and diacylglycerol acyltransferases (DGATs) catalyze two consecutive steps of enzyme reactions in the synthesis of triacylglycerols (TAGs). The metabolic complexity of TAG synthesis is reflected by the presence of multiple isoforms of MGAT and DGAT enzymes that differ in catalytic properties, subcellular localization, tissue distribution, and physiological functions. MGAT and DGAT enzymes play fundamental roles in the metabolism of monoacylglycerol (MAG), diacylglycerol (DAG), and triacylglycerol (TAG) that are involved in many aspects of physiological functions, such as intestinal fat absorption, lipoprotein assembly, adipose tissue formation, signal transduction, satiety, and lactation. The recent progress in the phenotypic characterization of mice deficient in MGAT and DGAT enzymes and the development of chemical inhibitors have revealed important roles of these enzymes in the regulation of energy homeostasis and insulin sensitivity. Consequently, selective inhibition of MGAT or DGAT enzymes by synthetic compounds may provide novel treatment for obesity and its related metabolic complications.
Keywords: monoacylglycerol acyltransferase, diacylglycerol acyltransferase, obesity
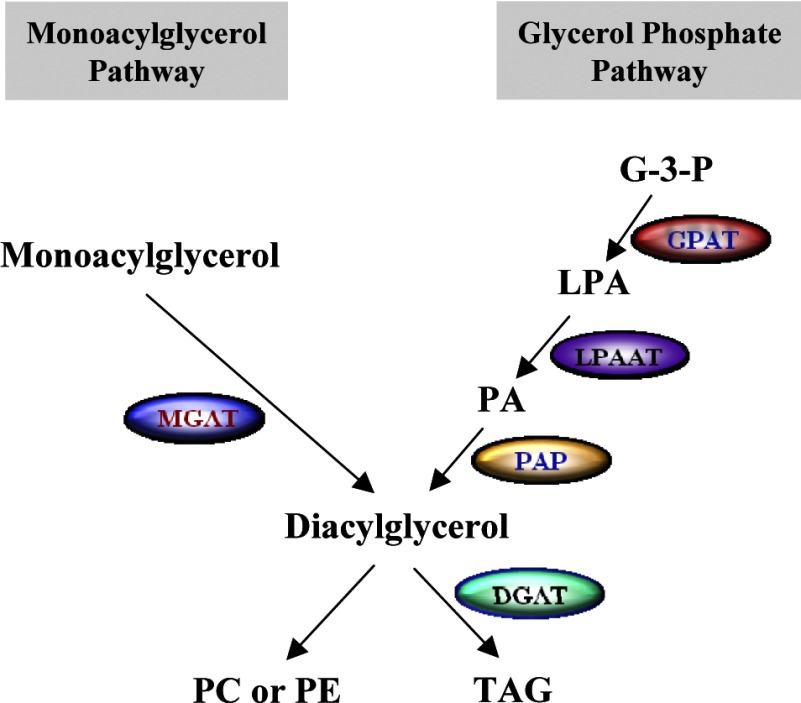
in mammals, the synthesis of triacylglycerol (TAG) serves critical functions in multiple physiological processes, including intestinal dietary fat absorption, intracellular storage of surplus energy, lactation, attenuation of lipotoxicity, lipid transportation, and signal transduction (26, 42, 61, 69). There are two major biochemical pathways for TAG synthesis (Fig. 1). The monoacylglycerol (MAG) pathway begins with the acylation of MAG with a fatty acyl-CoA by monoacylglycerol acyltransferase (MGAT). This pathway plays a predominant role in the enterocytes after feeding, where large amounts of 2-MAG and fatty acids (FA) are released from the digestion of dietary lipids (57). The MAG pathway is also active in adipose tissue (58), likely playing a role in storing excess energy in the form of TAG. A second pathway to TAG synthesis is the glycerol 3-phosphate (G-3-P) pathway, a de novo pathway in most tissues, including the small intestine. The G-3-P pathway begins with the acylation of G-3-P with a fatty acyl-CoA, producing lysophosphatidic acid, followed by further acylation and dephosphorylation to yield diacylglycerol (DAG) (5, 26). These two pathways share the final step in converting DAG to TAG, a reaction catalyzed by diacylglycerol acyltransferase (DGAT) (13, 26).
Fig. 1.
The 2 metabolic pathways involved in the synthesis of triacylglycerol (TAG). The monoacylglycerol (MAG) pathway, also known as the remodeling pathway, begins with the acylation of MAG with fatty acyl-CoA catalyzed by monoacylglycerol acyltransferase (MGAT). This pathway dominates in the small intestine, a tissue primarily responsible for dietary fat absorption. The glycerol 3-phosphate (G-3-P) pathway is a de novo pathway involved in TAG synthesis in most tissues. The G-3-P pathway begins with the acylation of G-3-P by glycerol-3-phosphate acyltransferase (GPAT) with fatty acyl-CoA, producing lysophosphatidic acid (LPA), followed sequentially by further acylation by LPA acyltransferase (LPAAT) and dephosphorylation by phosphatidic acid (PA) phosphorylase (PAP) to yield diacylgycerol (DAG). The 2 pathways share the final step in converting DAG into TAG, which is catalyzed by diacylglycerol acyltransferase (DGAT). DAG is also used as a substrate for the synthesis of phosphatidic choline (PC) and phosphatidic ethanolamine (PE).
In the past few years, great strides have been made in the identification and characterization of the enzymes of these two pathways. As a result of rapid progress in genomics, bioinformatics, and transgenic mouse models, much has been learned about the underlying mechanisms that regulate TAG synthesis. The use of bioinformatics bypasses the difficulties in the purification of these enzymes from primary tissues because most of the enzymes are intrinsic membrane proteins. For example, the identification of DGAT1 was achieved by its sequence homology with acyl-CoA:cholesterol acyltransferase 1 (ACAT1) (13, 15), and the recent purification and cloning of DGAT2 from the oleaginous fungus Mortierella remmaniana (40) sparked the identification of members of the DGAT2 gene family, including DGAT2 and three MGAT isoforms (MGAT1, MGAT2, and MGAT3) (11, 14, 22, 78, 79).
The importance of TAG synthesis is exemplified by severe insulin resistance in patients with lipodystrophy, a genetic condition characterized by defective TAG synthesis and storage in adipose tissues (1, 49). Whereas excess TAG accumulation in adipose leads to obesity, ectopic storage of TAG in nonadipose tissues such as liver and skeletal muscle is associated with insulin resistance (45). Recent progress in the identification and characterization of the MGAT and DGAT enzymes along with the phenotypic characterizations of mice with altered expression of these genes have provided important insights into their dynamic roles in the regulation of energy homeostasis and other physiological functions.
Catalytic Properties of MGATs and DGATs in the Synthesis of TAG
MGATs.
MGAT catalyzes the first step in TAG synthesis involved in dietary absorption by enterocytes. Three isoforms of MGAT enzymes, known as MGAT1, MGAT2, and MGAT3, have been identified so far. All three MGAT isoforms possess strong MGAT enzyme activity and are localized in the endoplasmic reticulum (ER) (11, 14, 22, 78, 79). However, they differ in tissue expression patterns and in catalytic properties. The MGAT1 mRNA has been detected mainly in stomach, kidney, and adipose tissue, whereas MGAT2 and MGAT3 exhibit highest expression in the small intestine (11, 22, 78, 79). Among the MGAT isoforms, MGAT3 possesses some unique features. The MGAT3 gene is found only in higher mammals and humans but not in rodents. Although named after its enzyme activity, MGAT3 shares higher sequence homology with DGAT2 than with the other MGAT isoforms. In addition, MGAT3 demonstrates significantly higher DGAT activity than MGAT1 and MGAT2 in the order MGAT3 > MGAT1 > MGAT2 when either MAG or DAG is used as substrate, suggesting that MGAT3 also functions as a TAG synthase (9). The DGAT activity of MGAT3 is relative to the concentration of MAG substrate. When low concentrations of 2-MAG are used as a substrate, the major enzyme product of MGAT3 is TAG (21). In contrast, DAG is the major enzymatic product when high concentrations of 2-MAG are used as a substrate (21). It can be envisaged that MGATs and DGAT2 enzymes may be evolved from a common ancestral gene. This notion is supported by their similar subcellullar localization pattern (9) and the colocalization of DGAT2 gene with MGAT2 gene on the same region of human chromosome 11. Although MGAT3 enzyme exhibits strong DGAT activity, its catalytic properties are quite different from those of DGAT1. Whereas MGAT3 is very sensitive to treatment with 1% 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonate, DGAT1 activity is stimulated by its application, indicating that they possess different catalytic mechanisms. Interestingly, MGAT3 activity is equally sensitive to detergent inactivation when either MAG or DAG is used as a substrate, suggesting that the DGAT and MGAT activities of the MGAT enzymes are inseparable (9).
DGATs.
DGAT catalyzes the final step in mammalian TAG synthesis that merges the MGAT and G-3-P pathways (Fig. 1) (26). In mammals, there are two isoforms of DGAT enzymes, DGAT1 and DGAT2. DGAT1 is a member of the mammalian ACAT gene family (13, 53), whereas DGAT2 belongs to a new family of acyltransferases that includes the three MGAT enzymes. In addition to DAG, both DGAT1 and DGAT2 also recognize MAG as a substrate in the synthesis of TAG (21, 75). Although both DGAT1 and DGAT2 enzymes catalyze the same reactions in TAG synthesis with DAG or MAG and acyl-CoA as substrates, they are functionally distinguished by their differences in catalytic properties (9, 21), subcellular localization (64), physiological regulation (50), and phenotypic consequences when rendered deficient in mice (61, 63). When MAG is used as a substrate, the major enzymatic product catalyzed by DGAT2 is TAG. In contrast, the enzymatic products catalyzed by DGAT1 depend on the concentration of MAG, but the TAG/MAG product and substrate relationship does not obey the classic Michaelis-Menten kinetics. At low concentrations of MAG, the major acylation product by DGAT1 is TAG. However, the DGAT activity of DGAT1 is dramatically inhibited when high concentration of MAG is used as a substrate, resulting in an increased production of DAG (21). Overexpression of DGAT1 results in the accumulation of small lipid droplets around the cell periphery, whereas overexpression of DGAT2 leads to increases in large cytosolic lipid droplets (63). This difference may be related to the previous reports on two proposed types of DGAT activities in liver microsomes, an overt activity that regulates the cytosolic TAG pools and another that is latent and plays a role in TAG secretion (55, 71). This is supported by studies in which inactivation of the yeast Dgat1 gene resulted in the reduction of DGAT activity on lipid particles, but without significant effect on DGAT activity in the ER (62). Upon the treatment of oleate, DGAT2, but not DGAT1 and MGAT2, can also be detected in mitochondria-associated membranes and lipid droplets in addition to ER (64). Furthermore, when DGAT1 is overexpressed in mouse liver via an adenoviral vector, the mice showed increased VLDL secretion and gonadal fat mass, whereas hepatic-specific DGAT2 overexpression increased liver TAG content but not VLDL secretion (74). DGAT1 has also been shown to recognize wax alcohol and retinol as acyl acceptors in potential acyl-CoA:wax alcohol acyltransferase and acyl CoA:retinol acyltransferase reactions, implicating a role of DGAT1 in the synthesis of wax esters and retinyl esters, respectively (54, 77).
Insights into the Physiological Function of MGAT and DGAT Enzymes
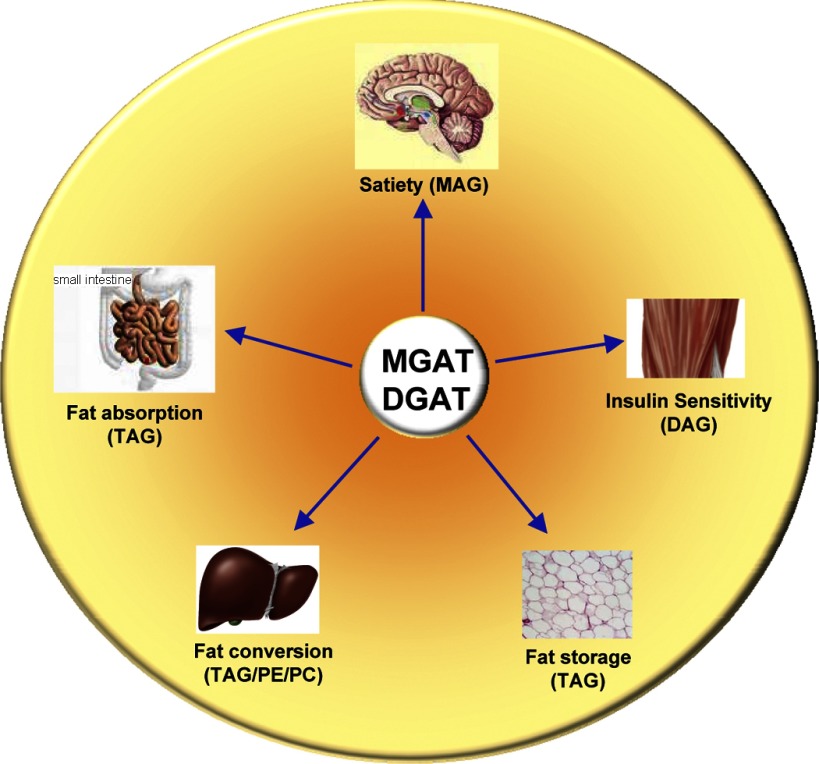
In addition to the synthesis of TAG, the MGAT and DGAT enzymes also modulate intracellular levels of MAG and DAG, two important signaling molecules. DAG is an activator for protein kinase C (PKC), which regulates insulin sensitivity in the liver and skeletal muscles (43). 2-Arachidonoylglycerol (2-AG) in the brain is a natural ligand for endocannabinoid receptors, which regulate various physiological events, including appetite (27, 48, 65). Consequently, the MGAT and DGAT families of enzymes are implicated in the regulation of various physiological functions, such as dietary fat absorption, lipid metabolism, fat storage, insulin sensitivity, satiety, and energy homeostasis (Fig. 2).
Fig. 2.
MGAT and DGAT enzymes are implicated in multiple pathways that regulate energy homeostasis. MGAT and DGAT enzymes play important roles in energy metabolism by regulating satiety in the brain (mediated by MAG), dietary fat absorption in the gut (in the form of TAG), phospholipid synthesis and VLDL secretion in the liver (in the form of DAG and TAG), fat storage in adipocytes (in the form of TAG), and insulin sensitivity in skeletal muscle (mediated by DAG).
Regulation of dietary fat absorption.
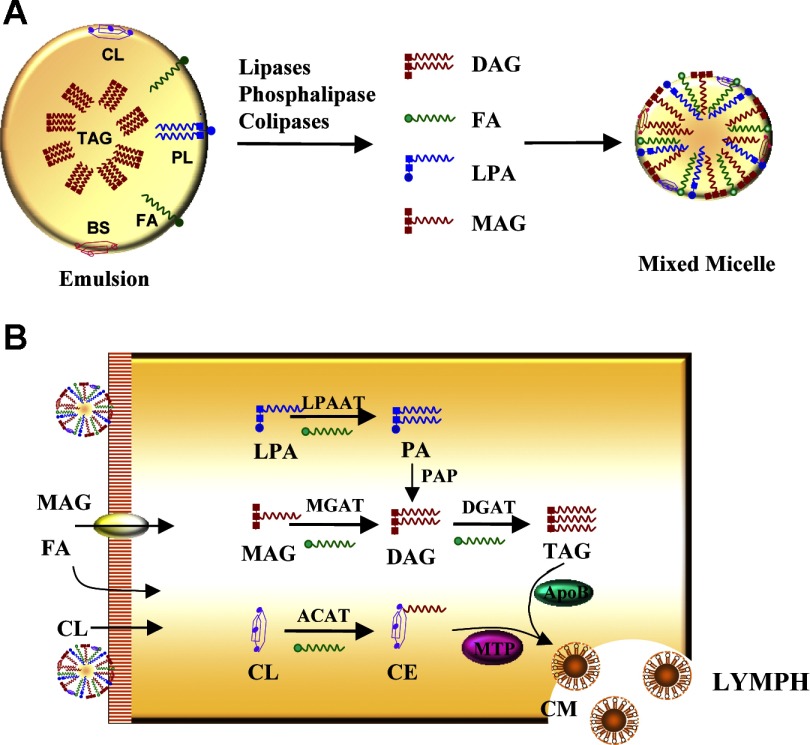
In mammals, the small intestine plays a major role in fat absorption (70). Dietary fat in the form of TAG is emulsified to fine lipid droplets in stomach, and further enzymatically digested to MAG and FA, mainly by pancreatic lipase and its cofactor. Facilitated by bile salts, MAG and FA are taken up by enterocytes (57), where they are resynthesized into TAG, packaged into chylomicrons, and secreted into circulation. As shown in Fig. 3, there are two main biochemical pathways for TAG synthesis in the enterocytes, the MGAT pathway and the G-3-P pathway. The relative activity of each pathway is determined by the abundance of 2-MAG and free FA. Under normal lipid absorption conditions, the MGAT pathway contributes to ∼80% of TAG incorporated into chylomicrons in the intestine (57, 70).
Fig. 3.
The process of dietary lipid digestion and absorption. A: the digestion of dietary lipids begins in the stomach, where lipids are subjected to partial digestion by gastric lipase, forming large fat globules with a hydrophobic TAG core surrounded by polar molecules, including phospholipids (PL), free cholesterol (CL), fatty acids (FA), and ionizing proteins. The digestive processes are completed in the intestinal lumen, where large emulsions of fat globules are mixed with bile salts (BS) and pancreatic juice containing lipid digestive enzymes to form an aqueous suspension of small fatty droplets with maximized exposure to the pancreatic lipases for lipid hydrolysis. MAG, LPA, DAG, and free FA that are released from lipid and phospholipid hydrolysis join BS, CL, and fat-soluble vitamins to form mixed micelles for dietary fat absorption at the brush border of the enterocytes. B: after entering the enterocyte, MAG, LPA, and CL have to be reacylated before they can be absorbed. MAG is sequentially acylated by MGAT and DGAT enzymes to form TAG. LPA is acylated by LPAAT to PA followed by dephosphorylation by PAP to yield DAG. Dietary cholesterol (CL) is acylated by acyl-CoA:cholesterol acyltransferase (ACAT) to cholesteryl esters (CE). Facilitated by microsomal triglyceride transfer protein (MTP), TAG joins CE and apolipoprotein B (apoB) to form chylomicrons that are secreted to the lymph for circulation.
In support of a key regulatory role of MGATs in dietary fat absorption, all three genes encoding the recently identified MGAT enzyme (MGAT1, MGAT2, and MGAT3) demonstrate predominant expression in the gastrointestinal tract (8, 11, 22, 78, 79). MGAT2 and MGAT3 are expressed mainly in small intestine, whereas MGAT1 is most abundantly expressed in the stomach. Although high mRNA levels of both MGAT2 and its short isoforms are present in human liver, very little MGAT enzyme activity was identified in that tissue, possibly reflecting a posttranscriptional regulation of the gene (44). The predominant role of MGATs in dietary fat absorption is also supported by the expression pattern of MGAT2, which mirrors that of dietary fat absorption (10), with the highest expression in the midgut. Consumption of Western diet enriched with animal fat is believed to be a contributing factor for the ongoing obesity epidemic (60). Diabetes, obesity, and lactation have been shown to induce liver MGAT enzyme activity in rodents (8, 46, 52) that is correlated with increased dietary fat absorption. Consequently, MGAT2 mRNA expression is significantly upregulated in sections of small intestine of mice fed a high-fat diet and lactation, supporting a predominant role of MGAT enzymes in dietary fat absorption (10, 24). Last, mice with targeted inactivation of MGAT2 gene exhibited a significant delay in dietary fat absorption, although MGAT2 knockout mice absorbed normal quantities of fat (76).
In contrast to the apparent restricted expression of the MGAT enzymes in the gastrointestinal tract, both DGAT1 and DGAT2 are widely expressed in a variety of tissues (13, 14), reflecting their important roles in both the remodeling and de novo pathways of TAG synthesis. DGAT1 and DGAT2 are also abundantly expressed in the small intestine, suggesting an important role in dietary absorption. In support of this notion, mice with targeted deletion of DGAT1 exhibit a delay in circulating postprandial hypertriglyceridemia and a reduction in chylomicron production (7). The involvement of DGAT1 in dietary fat absorption was further supported by the development of a DGAT1-specific enzyme inhibitor, XP620, which significantly decreased apolipoprotein B (apoB) secretion in Caco-2 cells, TAG and DAG synthesis in primary enterocytes, and dietary fat absorption in rats (21).
Genetic dissection of MGATs and DGATs in regulating energy homeostasis.
As reflected by their tissue distribution pattern and activities, the MGAT enzymes are likely to regulate energy homeostasis via their role in dietary fat absorption. The impact of the MGAT then may be very significant if a Western diet enriched with animal fat is the major source of food intake. This notion is supported by the recent phenotypic characterization of MGAT2-knockout mice, which exhibited a reduction of metabolic efficiency and increased thermogenic energy expenditure when fed a high-fat diet. MGAT2-knockout mice were protected from developing obesity, fatty liver, hyperlipidemia, and glucose intolerance under the challenge of chronic high-fat feeding (76).
DGAT1 polymorphisms have been linked with a certain type of human obesity (47). In plants, a single amino acid polymorphism of the DGAT1 gene is linked with oil content in maize (83). Likewise, a single nucleotide polymorphism of DGAT1 is associated with milk fat content in cattle (3). DGAT1 protein levels increased sharply following differentiation of 3T3-L1 into mature adipocytes. Overexpression of DGAT1 and DGAT2 results in a significant increase in TAG synthesis in mature adipocytes (63, 81). Conversely, mice with DGAT1 deficiency demonstrated a reduction in adiposity and resistance to high-fat diet-induced obesity (61), which is accompanied by increased energy expenditure and hyperlocomotive activity (17). This metabolic phenotype is likely caused by changes in adipocyte lipid metabolism resulting from DGAT1 deficiency. Wild-type mice with adipose transplanted from the DGAT1-deficient mice demonstrated physiological features that are similar to the DGAT1-knockout mice, including resistance to diet-induced obesity and an improvement in insulin sensitivity (16).
Regulation of fat storage by DGAT2.
In contrast to the phenotype of DGAT1 deficiency, targeted inactivation of DGAT2 enzyme resulted in lethal neonatal lipopenia and skin barrier abnormalities (63). DGAT2 plays a much more critical role in TAG synthesis in various tissues, as evidenced by the near absence of TAG in liver and in white adipose tissue and a >70% reduction in plasma levels of TAG, free FA, and glucose in the DGAT2-knockout mice. The lethal phenotype was not compensated by DGAT1, further suggesting the different roles played by these two enzymes in endogenous TAG synthesis (63). Interestingly, the content of linoleic acid (18:2) in free FA of liver and plasma was greatly reduced in DGAT2-null mice, suggesting a key role of DGAT2 in selective retention of essential FA that are vital for maintaining normal skin function. Although the heterozygous DGAT2-knockout mice appear indistinguishable from the wild-type controls, further analysis is needed to investigate whether they are more resistant to diet-induced obesity. The difference in the phenotype between the DGAT1- and DGAT2-knockout mice supports previous speculation that there are two different DGAT enzymes responsible for storage and mobilization, with separate pools of TAG for storage and oxidation (29).
Tissue-specific effects of DGAT enzymes in insulin sensitivity.
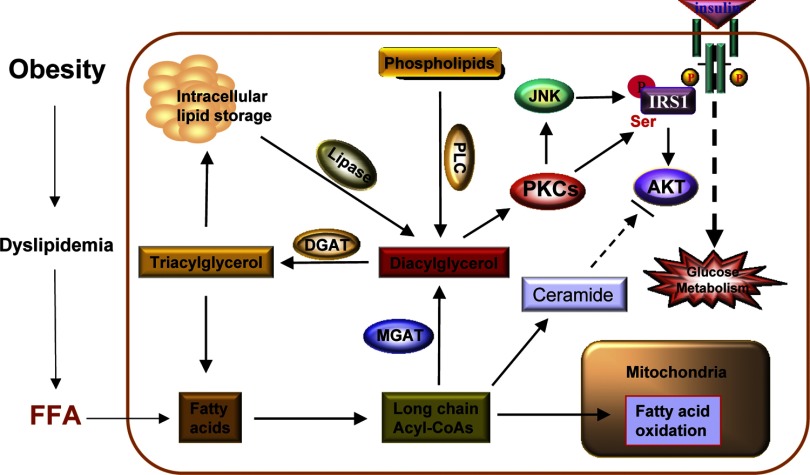
Increased TAG storage in nonadipose tissues is associated with increased level of insulin resistance, but the underlying mechanism is poorly understood. The DGAT-knockout mouse, however, has provided novel insights into the regulatory role of DGATs in regulating insulin sensitivity. DAG is both a substrate for DGAT enzymes as well as an activator for PKCs. Overactivation of DAG-responsive PKC isoforms in muscle, including PKCβ, -α, -δ, -ε, and -θ, is linked to muscle insulin resistance (4, 31, 37–39, 59, 80). Since DAG is both a product of MGAT enzymes and a substrate for DGAT enzymes, it is expected that mice deficient in MGAT will improve insulin sensitivity, whereas DGAT deficiency will cause insulin resistance as outlined by a hypothetic model in Fig. 4.
Fig. 4.
A hypothetical model for the regulation of insulin sensitivity by the MGAT and DGAT enzymes. Obesity is associated with increased free fatty acid (FFA) influx to skeletal muscle, resulting in increased levels of intramyocellular TAG and other intracellular lipotoxic FA derivatives, such as DAG and ceramide. FFA enters muscles cells, where it is converted to long-chain fatty acyl-CoAs that are used for either the synthesis of DAG, ceramide, or β-oxidation in the mitochondria. DAG can also be generated from hydrolysis of triglyceride or phospholipids catalyzed by phospholipase C (PLC). DAG and ceramide are known to cause hyperactivation of multiple isoforms of protein kinase Cs (PKCs) and c-Jun NH2-terminal kinase (JNK), which phosphorylate serine residues on insulin receptor substrate-1 (IRS-1), leading to insulin resistance. Accordingly, inactivation of MGAT or overexpresion of DGAT in skeletal muscle is envisaged to improve insulin sensitivity by preventing the intracellular accumulation of DAG.
In support of a regulatory role of DGAT1 in insulin sensitivity, transgenic overexpression of DGAT1 in type 1 skeletal muscle, slow-twitch muscle, protected mice from high-fat diet-induced insulin resistance. In contrast, DGAT1 deficiency diminishes insulin sensitivity and renders the muscles completely vulnerable to FA challenges, suggesting a muscle-specific effect of DGAT1 in regulating insulin sensitivity (43). Consistent with the model outlined in Fig. 4, the transgenic expression not only increased intramuscular TAG levels but also decreased myocellular DAG and ceramide levels, leading to an improvement in muscle and whole body insulin sensitivity. The improved insulin sensitivity in the transgenic mice was associated with an attenuated fat-induced activation of DAG-responsive PKCs and the stress mediator JNK1 (2, 80). Ceramide is a signaling molecule that inhibits Akt phosphorylation. Elevated ceramide in insulin-responsive tissues causes insulin resistance (66). Transgenic overexpression of DGAT1 in the skeletal muscle stimulates Akt phosphorylation and plasma membrane translocation of GLUT4 as well as reduction of serine phosphorylation of the insulin receptor substrate-1 (43). Moreover, overexpression of DGAT1 in rodent skeletal muscle was sufficient to recreate the athlete's paradox of increased intramyocellular fat stores and improved insulin sensitivity. The dissociation of increased intramyocellular TAG from insulin resistance depicted in Fig. 4 is also supported by the phenotype of adipose triglyceride lipase (ATGL)-knockout mice. ATGL is required for the initial conversion of TAG to DAG during lipolysis. ATGL-knockout mice were shown to exhibit a greater glucose tolerance in skeletal muscle despite a marked accumulation of intramyocellular TAG (34). The transgenic effect of DGAT1 on insulin sensitivity in skeletal muscle appears to contradict the phenotype of whole body DGAT1-knockout mice that exhibit an improvement in insulin sensitivity and resistance to high-fat diet-induced obesity (17, 19, 61). This discrepancy underscores the critical tissue-specific effect of DGAT enzymes on metabolism. The importance of DGAT1 in maintaining insulin sensitivity is also highlighted by transplantation of fat from Dgat1−/− mice to wild-type mice, leading to improved insulin sensitivity (18).
In contrast to the transgenic effect of DGAT1 in insulin sensitivity in skeletal muscles, transgenic overexpression of DGAT2 in glycolytic (type II) muscle causes an increased level of TAG content, ceramides, and unsaturated long-chain fatty acyl-CoAs in young adult mice (41). This lipid accumulation was accompanied by impaired insulin signaling and insulin-mediated glucose uptake in glycolytic muscle and impaired whole body glucose and insulin tolerance (41). The sharp contrast of phenotypes in insulin sensitivity for mice overexpressing DGAT1 and DGAT2 in the muscle could be because of three reasons. 1) The DGAT1 transgenics express the transgene in both oxidative (type I, slow-twitch, red) and glycolytic (type II, fast-twitching, white) fibers, whereas DGAT2 transgenics only express DGAT2 in glycolytic fiber. 2) DGAT1 has broader substrate specificity, and with an apparent high Km, than DGAT2. It has been speculated that DGAT1 might be more closely involved with the esterification of exogenous FA, whereas DGAT2 might be more involved with the esterification of FA synthesized de novo (41). 3) DGAT1 and DGAT2 are localized in different subcellular locations. Therefore, their cellular implications of dynamic metabolic flux of DAG, fatty acyl-CoA, could be drastically different (64).
The regulatory roles of DGAT enzymes in insulin sensitivity in other tissues are further complicated by conflicting results from phenotypic characterization reported in several studies. In one study, transgenic overexpression of DGAT1 in the white adipose tissue of C57/Bl6 mice rendered the mice more obese without significant effect on insulin sensitivity (20), which is consistent with the metabolic consequences of transgenic expression in skeletal muscles. In contrast, adipose-specific overexpression of Dgat1 in FVB mice led to diet-induced insulin resistance and fatty liver without affecting muscle TAG content or the onset of diet-induced obesity. Adipocytes from Dgat1 transgenic mice displayed increased basal and isoproterenol-stimulated lipolysis rates, leading to the redistribution of fat from adipose tissue to the liver. Consequently, Dgat1 transgenic mice are both insulin and leptin resistant with markedly elevated plasma free FA levels. The phenotypic discrepancy of these studies could be due to different genetic backgrounds; sFVB has been shown to be more resistant to diet-induced obesity (36), whereas C57/Bl6 is more sensitive.
In contrast to the adipogenic expression of DGAT1, transgenic overexpression of DGAT2 in mouse liver led to hepatic steatosis, with increased amounts of TAG, DAG, ceramides, and unsaturated long-chain fatty acyl-CoAs in the liver (51). However, the transgenic mice exhibited no insulin resistance or impairment of insulin signaling in the liver. Likewise, DGAT1 overexpression in the liver also failed to induce glucose or insulin intolerance (51). Of interest, suppression of DGAT2, but not DGAT1, by antisense oligo treatment reverses diet-induced hepatic insulin resistance concurrent with reduction of DAG and TAG but not long-chain acyl-CoAs (23). These results further suggest different functions of the two DGAT enzymes in regulating insulin sensitivity in the liver.
Effects on satiety.
MGAT catalyzes the synthesis of DAG using acyl-CoA and MAG as substrates. In addition to being a substrate for MGAT enzymes, MAG, especially 2-AG, also functions as an endogenous ligand for the endocannabinoid receptors (65). 2-AG is a unique molecular species of MAG that was initially isolated from rat brain and gut. 2-AG has also been identified from adipocytes where MGAT is expressed (30). It binds to the cannabinoid receptors (CB1 and CB2) and exhibits a variety of cannabimimetic activities in vitro and in vivo, including stimulation of appetite (27, 32, 48). Consequently, antagonists of CB1 receptor have recently been demonstrated with antiobesity effects both in animal models of obesity and in obese humans (28, 35). However, the metabolic pathways involved in the synthesis of MAG in brain and gut are not well characterized. In support of a potential role of DGAT in regulating appetite, Dgat1-knockout mice exhibit hyperphagia, particularly when they are exposed to cold (17), but the underlying mechanism has not been investigated. It will be interesting to study whether the increased appetite in DGAT1-knockout mice is associated with an elevated level of MAG in the central nervous system and gut.
MGATs and DGATs as Drug Targets for Obesity and the Related Metabolic Complications
Obesity is characterized by excessive accumulation of TAG in adipose tissue. As discussed in the previous sections, the MGAT and DGAT enzymes are implicated in various steps in energy homeostasis from dietary fat absorption and fat storage to regulation of energy expenditure. Therefore, inhibiting these enzymes may offer pharmacological strategies for antiobesity treatment (60). Consumption of a Western diet rich in animal fat is considered to be one of the major factors contributing to the ongoing obesity epidemic. In industrialized countries, dietary fat intake provides >40% of the caloric content of daily food consumption (25). Inhibitors of dietary fat absorption can be used to treat obesity, as exemplified by the development of pancreatic lipase inhibitors for the treatment of obesity. As one of the only two drugs currently marketed for obesity (56), Orlistat, also known as Xenical, is a potent and specific inhibitor of both gastric and pancreatic lipases (6, 12, 33, 72) used in the clinic for the treatment of obesity. The drug acts nonsystemically and reduces fat absorption by ∼30%. However, Orlistat is not widely used by obese patients due to its major gastrointestinal side effects related to malabsorbed TAG, such as abdominal pain, urgency to defecate, increased flatus, steatorrhea, and diarrhea. Therefore, the MGAT and DGAT enzymes present an alternative drug target for dietary fat absorption due to their major role in fat absorption (8). Furthermore, the three MGAT enzymes are expressed predominantly in gastrointestinal tract, which could possibly alleviate potential side effects of chemical inhibitors in other tissues. Discharge of MAG and free FA as an unabsorbed energy source in the stool may avoid the unwanted gastrointestinal side effects caused by Orlistat. This strategy is supported by the recent successful development of Slentrol, a microsomal triglyceride transfer protein (MTP) inhibitor developed specifically for the treatment of canine obesity (73). Slentrol causes significant weight loss in canines by inhibiting dietary fat absorption and reducing food intake. Like MTP inhibitors, inhibition of MGAT enzymes may reduce food intake, since high concentration of MAG and free FA is expected to stimulate the secretion of norexigenic peptides, such as cholecystokinin, peptide YY, and glucagon-like peptide-1, that mimic the effects observed in gastrointestinal bypass patients (67). One major concern for a potential MGAT inhibitor is the theoretical excessive accumulation of FA and MAG in enterocytes, which could be lipotoxic. However, this has not been a concern with MTP inhibitors, at least in canines, likely due to the high turnover rate of the gastrointestinal lining (68). Detailed phenotypic characterization on MGAT-knockout mice is needed to better understand the pharmaceutical potentials of MGAT inhibitors for the treatment of obesity and its related metabolic complications.
Multiple patents have been filed on DGAT inhibitors for the treatment of obesity and its related metabolic complications. For example, the DGAT1 inhibitor XP620 has recently been shown to reduce apoB secretion in Caco-2 cells, TAG and DAG synthesis in primary enterocytes, and dietary fat absorption in mice (21). Recently, a highly potent DGAT1 inhibitor was shown to cause weight loss, reduction in liver TAG, and depletion of serum TAG following a lipid challenge in a dose-dependent manner, recapturing the major phenotype of DGAT1-knockout mice (82). However, it remains unclear whether a pan-inhibitor for both DGAT enzymes is more beneficial than isoform-specific inhibitors. Although inhibition of DGAT2 in the liver would likely offer the advantages of the prevention of steatosis and/or dyslipidemia, inhibition of DGAT2 in skeletal muscle and skin could exacerbate insulin resistance associated with obesity (43). On the other hand, it could be argued that an agent that inhibits both DGAT activities might be more desirable to achieve maximal effects on inhibition of TAG synthesis in all tissues. Practically, development of isoform-specific inhibitors may be beneficial due to the tissue- and intracellular compartment-specific distribution of DGAT1 and DGAT2 and the lack of sequence homology between these two isoforms. When targeting DGAT1 and/or DGAT2 in obesity treatments, one must also consider the potential side effects in skin, lactation, and teratogenic consequences as predicted from the pathophysiological conditions associated with DGAT1 and DGAT2 deficiency (61, 63). Whether these pharmacological approaches would succeed awaits definitive answers from clinical trials of DGAT1 and MGAT inhibitors that may be delivered soon by several pharmaceuticals.
Future Perspectives
Recent advances in the identification and characterization of MGAT and DGAT enzymes have laid a solid foundation for the understanding of their functional importance in energy metabolism. Generation and phenotypic characterization of transgenic and knockout mouse models of the DGAT enzymes has shed important light on the dynamic functional roles of these enzymes in regulating TAG synthesis and their related physiological functions, such as insulin signaling, energy homeostasis, and lactation. Studies on the MGAT enzymes lag behind those of the DGAT enzymes, partly due to the fact that they have only recently been identified and characterized. Although both MGAT and DGAT enzymes are involved in TAG synthesis, they likely differ significantly in their physiological functions as suggested by their differences in tissue expression patterns and substrate preferences. The MGAT enzymes are expected to play an important role in regulating the energy homeostasis in the gastrointestinal tract, in dietary fat absorption, and within the gastrointestinal-pancreas-brain axis in the regulation of food intake. In contrast, the DGATs are involved in regulating both intestinal and de novo TAG synthesis in liver and adipose tissues. Since 2-AG is a regulator of appetite by activating the CB1 receptor, the potential functional role of the MGAT enzymes in regulating appetite in the central nervous system deserves to be further explored. The recent generation of MGAT2-knockout mice will greatly facilitate such studies. The question remains as to why mammals possess multiple isoforms of the MGAT enzymes and how they differ in metabolic and physiological functions. Finally, although much has been learned from murine MGAT and DGAT transgenic and knockout models, precaution must be taken in making generalizations to humans. For example, MGAT3 is found only in higher mammals but not in rodents. Much anticipated are clinical trials utilizing DGAT and MGAT inhibitors in treating obesity and its related metabolic complications.
GRANTS
This research project was partly sponsored by the American Diabetes Association (7-07-RA-148; to Y. Shi) and by the National Institute of Diabetes and Digestive and Kidney Diseases (1-R01-DK-076685).
Acknowledgments
We thank Dr. Charles H. Lang for critically reading the manuscript.
REFERENCES
- 1.Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab 14: 214–221, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Aguirre V, Uchida T, Yenush L, Davis R, White M. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo A, Amills M, Urrutia B, Domenech A, Sastre Y, Badaoui B, Jordana J. Identification of a single nucleotide polymorphism at intron 16 of the caprine acyl-coenzyme A: diacylglycerol acyltransferase 1 (DGAT1) gene. J Dairy Res 74: 47–51, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Avignon A, Yamada K, Zhou X, Spencer B, Cardona O, Saba-Siddique S, Galloway L, Standaert M, Farese R. Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes 45: 1396–1404, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem 49: 459–487, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Bitou N, Ninomiya M, Tsujita T, Okuda H. Screening of lipase inhibitors from marine algae. Lipids 34: 441–445, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV Jr. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem 277: 25474–25479, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cao J, Burn P, Shi Y. Properties of the mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J Biol Chem 278: 25657–25663, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Cheng L, Shi Y. Catalytic properties of MGAT3, a putative triacylgycerol synthase. J Lipid Res 48: 583–591, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cao J, Hawkins E, Brozinick J, Liu X, Zhang H, Burn P, Shi Y. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J Biol Chem 279: 18878–18886, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Lockwood J, Burn P, Shi Y. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J Biol Chem 278: 13860–13866, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Carrière F, Renou C, Ransac S, Lopez V, De Caro J, Ferrato F, De Caro A, Fleury A, Sanwald-Ducray P, Lengsfeld H, Beglinger C, Hadvary P, Verger R, Laugier R. Inhibition of gastrointestinal lipolysis by Orlistat during digestion of test meals in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 281: G16–G28, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 95: 13018–13023, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 276: 38870–38876, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Chang CC, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 268: 20747–20755, 1993 [PubMed] [Google Scholar]
- 16.Chen HC, Jensen DR, Myers HM, Eckel RH, Farese RV Jr. Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 111: 1715–1722, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HC, Ladha Z, Smith SJ, Farese RV Jr. Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab 284: E213–E218, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Chen HC, Rao M, Sajan MP, Standaert M, Kanoh Y, Miura A, Farese RV Jr, and Farese RV. Role of adipocyte-derived factors in enhancing insulin signaling in skeletal muscle and white adipose tissue of mice lacking Acyl CoA:diacylglycerol acyltransferase 1. Diabetes 53: 1445–1451, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV Jr. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 109: 1049–1055, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV Jr. Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes 51: 3189–3195, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Cheng D, Iqbal J, Devenny J, Chu CH, Chen L, Dong J, Seethala R, Keim WJ, Azzara AV, Lawrence RM, Pelleymounter MA, Hussain MM. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J Biol Chem 283: 29802–29811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng D, Nelson TC, Chen J, Walker SG, Wardwell-Swanson J, Meegalla R, Taub R, Billheimer JT, Ramaker M, Feder JN. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J Biol Chem 278: 13611–13614, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282: 22678–22688, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Chon S, Zhou Y, Dixon J, Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem 282: 33346–33357, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Clandinin MT, Cheema S, Field CJ, Garg ML, Venkatraman J, Clandinin TR. Dietary fat: exogenous determination of membrane structure and cell function. FASEB J 5: 2761–2769, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43: 134–176, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol 20, Suppl 1: 110–115, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez JR, Allison DB. Rimonabant Sanofi-Synthélabo. Curr Opin Investig Drugs 5: 430–435, 2004 [PubMed] [Google Scholar]
- 29.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta 1483: 37–57, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Gonthier MP, Hoareau L, Festy F, Matias I, Valenti M, Bès-Houtmann S, Rouch C, Robert-Da Silva C, Chesne S, Lefebvre d'Hellencourt C, Césari M, Di Marzo V, Roche R. Identification of endocannabinoids and related compounds in human fat cells. Obesity (Silver Spring) 15: 837–845, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Griffin M, Marcucci M, Cline G, Bell K, Barucci N, Lee D, Goodyear L, Kraegen E, White M, Shulman G. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Guijarro A, Osei-Hyiaman D, Harvey-White J, Kunos G, Suzuki S, Nadtochiy S, Brookes PS, Meguid MM. Sustained weight loss after Roux-en-Y gastric bypass is characterized by down regulation of endocannabinoids and mitochondrial function. Ann Surg 247: 779–790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J 256: 357–361, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner E, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Halford JC. Obesity drugs in clinical development. Curr Opin Investig Drugs 7: 312–318, 2006 [PubMed] [Google Scholar]
- 36.Hu C, Qing K, Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res 12: 1264–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Itani S, Ruderman N, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Itani S, Zhou Q, Pories W, MacDonald K, Dohm G. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes 49: 1353–1358, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Fillmore J, Sunshine M, Albrecht B, Higashimori T, Kim D, Liu Z, Soos T, Cline G, O'Brien W, Littman D, Shulman G. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823–827, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ. DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J Biol Chem 276: 38862–38869, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Farese RV Jr. Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Endocrinol Metab 293: E1772–E1781, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Listenberger L, Han X, Lewis S, Cases S, Farese RJ, Ory D, Schaffer J. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100: 3077–3082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockwood JF, Cao J, Burn P, Shi Y. Human intestinal monoacylglycerol acyltransferase: differential features in tissue expression and activity. Am J Physiol Endocrinol Metab 285: E927–E937, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Lowell B, Shulman G. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Luan Y, Hirashima T, Man ZW, Wang MW, Kawano K, Sumida T. Pathogenesis of obesity by food restriction in OLETF rats—increased intestinal monoacylglycerol acyltransferase activities may be a crucial factor. Diab Res Clin Pract 57: 75–82, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Ludwig EH, Mahley RW, Palaoglu E, Ozbayrakci S, Balestra ME, Borecki IB, Innerarity TL, Farese RV Jr. DGAT1 promoter polymorphism associated with alterations in body mass index, high density lipoprotein levels and blood pressure in Turkish women. Clin Genet 62: 68–73, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Mol Cell Endocrinol 286: S66–S78, 2008 [DOI] [PubMed] [Google Scholar]
- 49.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258: 766–770, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Meegalla R, Billheimer J, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun 298: 317–323, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Hevener AL, Farese RV Jr. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Mostafa N, Bhat BG, Coleman RA. Increased hepatic monoacylglycerol acyltransferase activity in streptozotocin-induced diabetes: characterization and comparison with activities from adult and neonatal rat liver. Biochim Biophys Acta 1169: 189–195, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Oelkers P, Behari A, Cromley D, Billheimer J, Sturley S. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J Biol Chem 273: 26765–26771, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Orland MD, Anwar K, Cromley D, Chu CH, Chen L, Billheimer JT, Hussain MM, Cheng D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: diacylglycerol acyltransferase 1. Biochim Biophys Acta 1737: 76–82, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Owen MR, Corstorphine CC, Zammit VA. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem J 323: 17–21, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes 27: 1437–1446, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Phan CT, Tso P. Intestinal lipid absorption and transport. Front Biosci 6: D299–D319, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Polheim D, David JS, Schultz FM, Wylie MB, Johnston JM. Regulation of triglyceride biosynthesis in adipose and intestinal tissue. J Lipid Res 14: 415–421, 1973 [PubMed] [Google Scholar]
- 59.Schmitz-Peiffer C, Browne C, Oakes N, Watkinson A, Chisholm D, Kraegen E, Biden T. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes 46: 169–178, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Shi Y, Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov 3: 695–710, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87–90, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Sorger D, Daum G. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J Bacteriol 184: 519–524, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV Jr. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 279: 11767–11776, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Stone SS, Levin MC, Zhou P, Han J, Walther TC, Farese RV Jr. The endoplasmic reticulum enzyme, DGAT2, is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. In press. [DOI] [PMC free article] [PubMed]
- 65.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res 45: 405–446, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Summers S, Garza L, Zhou H, Birnbaum M. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18: 5457–5464, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki S, Ramos E, Goncalves C, Chen C, Meguid M. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 138: 283–290, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Thomson AB, Cheeseman CI, Keelan M, Fedorak R, Clandinin MT. Crypt cell production rate, enterocyte turnover time and appearance of transport along the jejunal villus of the rat. Biochim Biophys Acta 1191: 197–204, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Toker A. The biology and biochemistry of diacylglycerol signalling. Meeting on molecular advances in diacylglycerol signalling. EMBO Rep 6: 310–314, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tso P, Crissinger K. Digestion and absorption of lipids. In: Biochemical and Physiological Aspects of Human Nutrition, edited by Stipanuk MH. Philadelphia, PA: W. B. Saunders, 2000, chapt. 7, p. 125–141.
- 71.Waterman IJ, Zammit VA. Activities of overt and latent diacylglycerol acyltransferases (DGATs I and II) in liver microsomes of ob/ob mice. Int J Obes Relat Metab Disord 26: 742–743, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Weibel EK, Hadvary P, Hochuli E, Kupfer E, Lengsfeld H. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity. J Antibiot (Tokyo) 40: 1081–1085, 1987 [DOI] [PubMed] [Google Scholar]
- 73.Wren J, Gossellin J, Sunderland S. Dirlotapide: a review of its properties and role in the management of obesity in dogs. J Vet Pharmacol Ther 30: 11–16, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J Biol Chem 280: 21506–21514, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Yen CL, Monetti M, Burri BJ, Farese RV Jr. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res 46: 1502–1511, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Yen C, Cheong J, Moriwaki J, Zhou P, Wong J, Farese RV Jr. Reduced metabolic efficiency in response to dietary fat in mice lacking the intestinal enzyme MGAT2. Molecular mechanisms involved in the nutrient control of cellular function. FESAB Summer Research Conferences, Carefree, AZ, July 20–25, 2008
- 77.Yen C, Monetti M, Burri B, Farese RJ. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res 46: 1502–1511, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Yen CL, Farese RV Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem 278: 18532–18537, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Yen CL, Stone SJ, Cases S, Zhou P, Farese RV Jr. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc Natl Acad Sci USA 99: 8512–8517, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu C, Chen Y, Cline G, Zhang D, Zong H, Wang Y, Bergeron R, Kim J, Cushman S, Cooney G, Atcheson B, White M, Kraegen E, Shulman G. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Yu YH, Zhang YY, Oelkers P, Sturley SL, Rader DJ, Ginsberg HN. Posttranscriptional control of the expression and function of diacylglycerol acyltransferase-1 in mouse adipocytes. J Biol Chem 277: 50876–50884, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Zhao G, Souers A, Voorbach M, Falls H, Droz B, Brodjian S, Lau Y, Iyengar R, Gao J, Judd A, Wagaw S, Ravn M, Engstrom K, Lynch J, Mulhern M, Freeman J, Dayton B, Wang X, Grihalde N, Fry D, Beno D, Marsh K, Su Z, Diaz G, Collins C, Sham H, Reilly R, Brune M, Kym P. Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J Med Chem 51: 380–383, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Zheng P, Allen W, Roesler K, Williams M, Zhang S, Li J, Glassman K, Ranch J, Nubel D, Solawetz W, Bhattramakki D, Llaca V, Deschamps S, Zhong G, Tarczynski M, Shen B. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat Genet 40: 367–372, 2008 [DOI] [PubMed] [Google Scholar]