Abstract
MDCO-216, a complex of dimeric recombinant apoA-IMilano (apoA-IM) and palmitoyl-oleoyl-phosphatidylcholine (POPC), was administered to cynomolgus monkeys at 30, 100, and 300 mg/kg every other day for a total of 21 infusions, and effects on lipids, (apo)lipoproteins, and ex-vivo cholesterol efflux capacity were monitored. After 7 or 20 infusions, free cholesterol (FC) and phospholipids (PL) were strongly increased, and HDL-cholesterol (HDL-C), apoA-I, and apoA-II were strongly decreased. We then measured short-term effects on apoA-IM, lipids, and (apo)lipoproteins after the first or the last infusion. After the first infusion, PL and FC went up in the HDL region and also in the LDL and VLDL regions. ApoE shifted from HDL to LDL and VLDL regions, while ApoA-IM remained located in the HDL region. On day 41, ApoE levels were 8-fold higher than on day 1, and FC, PL, and apoE resided mostly in LDL and VLDL regions. Drug infusion quickly decreased the endogenous cholesterol esterification rate. ABCA1-mediated cholesterol efflux on day 41 was markedly increased, whereas scavenger receptor type B1 (SRB1) and ABCG1-mediated effluxes were only weakly increased. Strong increase of FC is due to sustained stimulation of ABCA1-mediated efflux, and drop in HDL and formation of large apoE-rich particles are due to lack of LCAT activation.
Keywords: palmitoyl-oleoyl-phosphatidylcholine, ABCA1-mediated cholesterol efflux
Epidemiological studies in the past showed an inverse correlation between plasma concentrations of HDL and risk of coronary heart disease (1–3). The protective effects of HDL are assumed to be related to its ability to promote the efflux of cholesterol from cholesterol-laden foam cells in the atherosclerotic plaque and to mediate the transport of this cholesterol to the liver for catabolism or excretion (4–6).
ApoA-I, the major structural protein of HDL, is an essential player in the process of reverse cholesterol transport (RCT): it serves as a cofactor for LCAT and as a ligand and activator of ABCA1, one of the key membrane-associated cholesterol efflux carriers enabling HDL to remove cholesterol from macrophages.
ApoA-I is a 243-amino acid amphipathic protein, in which distinctive domains and amino acids determine lipid binding and particle size (7), LCAT activation (8), and binding to cholesterol efflux carriers (9). Several mutations have been described to occur in human subjects/families that affect apoA-I functionality, one of which is known as the apoA-IMilano (apoA-IM) mutation. This mutation, first described in 1980 (10), consists of an Arg→Cys substitution at position 173 of apoA-I (11, 12). The presence of the cysteine residue permits dimerization of apoA-IM, and in apoA-IM carriers, a sizeable proportion of the apoA-IM protein occurs as a homodimer (11, 13–15). In carriers of this mutation, all of whom are heterozygous, the mutation is associated with a decrease of HDL-cholesterol (HDL-C) accompanied by a loss of the larger HDL2 subpopulation (13, 14). Strikingly, however, carriers of the mutation seem to be protected against atherosclerotic diseases (10), suggesting that their HDL particles can fully exert their atheroprotective functions in spite of their lower plasma concentration.
Since the discovery of this apoA-I variant, considerable efforts have been made to establish and develop apoA-IM as antiatherosclerotic treatment modality. Indeed, administration of the predecessor compound ETC-216 was shown to reduce atherosclerotic plaque size and volume in mice and rabbit models (16–20) and in patients with acute coronary syndromes (21). However, no studies have been reported describing the effects of repeated administration of this product candidate on lipid and lipoprotein levels, either in animals or in man.
The Medicines Company has continued development of apoA-IM as a therapeutic agent to regress atherosclerotic plaque burden by using a genetically modified expression organism and an improved process to manufacture dimeric apoA-IM (22), with the drug product now named MDCO-216. As part of a comprehensive six-week toxicology study of MDCO-216 in cynomolgus monkeys, we investigated the effects of repeated administration of MDCO-216 on lipid and lipoprotein levels and on cholesterol efflux capacity. The drug was found to cause strong and sustained increases in free cholesterol (FC) and phospholipid (PL) levels, which were highly correlated with ABCA1-mediated cholesterol efflux capacity of serum ex vivo. The drug also caused marked decreases of HDL-C, apoA-I, and apoA-II; dramatic shifts of lipids and of apoE from the HDL toward the LDL and VLDL region; and a strongly reduced rate of endogenous cholesterol esterification, indicating LCAT inhibition by MDCO-216.
MATERIALS AND METHODS
MDCO-216 is a complex of highly purified dimeric recombinant apoA-IM and palmitoyl-oleoyl-phosphatidylcholine (POPC). The production of the recombinant protein in E. coli and its purification has been described (22). Complexation with POPC was performed using a high-pressure homogenization procedure. The final product contained 13 mg/ml protein, 14 mg/ml POPC, 1.3 mg/ml di-sodium hydrogen phosphate heptahydrate, 0.178 mg/ml sodium dihydrogen phosphate dehydrate, 62 mg/ml sucrose, and 8.2 mg/ml mannitol. It had an osmolarity of 287 mOsmol/kg (pH of 7.5), and it was characterized by gel permeation chromatography showing that 89% of protein and phospholipid was recovered in a single peak of apparent molecular weight of around 150 kDa (supplementary Fig. I). Nondenaturing gradient-gel electrophoresis revealed that the majority of the drug product displayed an apparent diameter of 8 nm, and no free apoA-IM was observed (supplementary Fig. II).
Animals and treatment
The study was part of a pivotal toxicology study performed by Covance, Harrogate, UK, and carried out in conformity with the Public Health Service Standards for Humane Care and Use of Laboratory Animals and approved by the Covance Institutional Ethical Review Committee.
Monkeys were housed in pens that conform to the Code of Practice for the Housing and Care of Animals Used in Scientific Procedures (Home Office, London, 1989). Wood shavings (Beta Bedding, Datesand Ltd., Manchester, UK) and corncob (IPS Product Supplies Ltd., London, UK) were provided as bedding. Animals of the same group and sex were housed in the same pen. Animals of each group/sex were not able to interact with the other treatment groups/sex. Each animal was offered approximately 100 g of SQC Mazuri Primate Diet (Special Diets Services Ltd., Witham, UK). Each batch of diet was analyzed for specific constituents and contaminants. In addition, the animals were given daily supplements of a Bonio biscuit (Spillers), a diluted fruit drink, and one of the following: fresh fruit, fresh vegetables, forage mix, peanuts, raisins, or sunflower seeds that did not require analyses as a form of environmental and dietary enrichment. The supplements were supplied by UK human food suppliers. Mains water was provided ad libitum via water bottles. The water was periodically analyzed for specific contaminants.
Male and female cynomolgus monkeys were divided into four treatment groups and received MDCO-216 by intravenous infusion during 1 h every two days for a period of six weeks as follows: Group 1, 0 (vehicle saline) (N = 5 per sex); Group 2, 30 mg/kg/occasion (N = 3 per sex); Group 3, 100 mg/kg/occasion (N = 3 per sex); and Group 4, 300 mg/kg/occasion (N = 5 per sex).
Each animal received a total of 21 doses over the treatment period. To prepare plasma, blood samples were taken in EDTA tubes from all animals at baseline six days prior to the first infusion (week 1), on day 15 (week 3), and on day 41 (week 6). The samples on days 15 and 41 were taken about 1 h prior to the drug infusion on that day (i.e., 46 h after the 7th and 20th infusions, respectively). Two monkeys/sex of groups 1 and 4 were also sampled after a four-week, treatment-free recovery phase (day 71, week 10). In addition, serial blood samples were taken on days 1 and 41 at 5 min, 1 h, 2 h, 4 h, 8 h, 24 h, and 48 h, which were let to coagulate to prepare serum.
Analytical methods
In the plasma samples, total cholesterol (TC), FC, triglyceride (TG), PL, HDL-C, and LDL-cholesterol (LDL-C) were measured on a Cobas-Bio analyzer using Roche/Hitachi kits (HDL-C Plus third generation and LDL-C Plus second generation). CE was calculated as the difference between TC and FC. Monkey apoA-I, apoA-II, and apoB were determined using immune-nephelometric methods employing commercially available antibodies. Test kits were from Thermo Scientific for apoA-I (Ref. 981702) and apoB (Ref. 981703) and from Rolf Greiner BioChemica for apoA-II (Cat. No. KAI-003). In an in-vitro spiking experiment, addition of pure MDCO-216 to monkey plasma was found to artificially increase the measured amount of monkey apoA-I in a concentration-dependent manner, although there was no interference by MDCO-216 with the apoA-II and apoB measurements. The plasma values for monkey apoA-I were corrected accordingly, taking into account the apoA-IM concentrations in the 48 h serum samples on day 41 (see below). Since these kits use human apoproteins as references, the values reported here may deviate from true absolute values, but the effects of MDCO-216 administration shown in the Results section can be assumed to reliably reflect relative changes.
In the serial serum samples taken on days 1 and 41, levels of ApoA-IM were determined with a selective ELISA employing the mouse monoclonal antibody 17F3 from Mabtech (Nacka, Sweden), which does not recognize monkey wild-type apoA-I. Levels of TC, FC, PL, and TG in these serum samples were measured by standard enzymatic methods. In pooled sera of the 100 mg/kg dose group, lipoproteins were separated by fast-protein liquid chromatography (FPLC) on a Superose 6B column, and lipids in the elution fractions were assessed by standard enzymatic techniques. In addition; apoprotein composition of the lipoprotein fractions was evaluated by 4–20% SDS-PAGE and immunodetection with antibodies against apoB (Calbiochem), apoE (Calbiochem), apoA-I (Calbiochem), apoA-IMilano, and albumin (Rockland). Two PAGE slab gels were used for analysis of the relevant FPLC fractions, with nine fractions per slab gel. These two gels were run, transferred, and blotted together; films with the same exposure times are shown.
Total serum apoE was evaluated by SDS-PAGE, and immunodetection of blots of these gels was carried out with an antihuman apoE antibody (Calbiochem). A standard curve of human plasma of known apoE concentration was run in the same assay to calculate plasma apoE content.
Cholesterol efflux and endogenous cholesterol esterification measurements.
Cholesterol efflux capacities of serum samples taken at day 41 were determined as described in detail elsewhere (23–25). In brief, global and ABCA1-mediated cholesterol efflux were measured using J774 mouse macrophage cells in the presence and absence of cAMP. SRB1-mediated efflux was measured using Fu5AH rat hepatoma cells, and ABCG1-mediated efflux was measured using ABCG1-transfected BHK cells in the presence and absence of mifepristone. For all assays, cells were preincubated with 3H-cholesterol and ACAT inhibitor Sandoz 58-035 (but not preloaded with mass cholesterol) overnight. Cells were then incubated overnight in 0.2% BSA [Fu5AH (no additive), J774 (with or without cpt-cAMP), BHK (with or without mifepristone)]. After washing, the cells were incubated for 4 h with the serum samples added at 2% (v/v). [3H]cholesterol released to monkey serum after incubation with cells for 4 h was measured by liquid scintillation counting. Cholesterol efflux is expressed as the radiolabel released as a percentage of [3H]cholesterol within cells before addition of serum. All efflux values were corrected by subtracting the small amount of radioactive cholesterol released from cells incubated with serum-free medium. The cholesterol efflux from J774 cells preincubated with cAMP comprises effluxes mediated by ABCA1, SRB1, ABCG1, passive diffusion, or other unknown carriers (24) and is denoted herein as global efflux, while the efflux in the absence of cAMP is denoted as basal efflux. ABCA1-dependent efflux from J774 cells was calculated as difference between global and basal efflux. The ABCG1-mediated efflux from BHK cells was determined as the difference between the efflux in the presence and absence of mifepristone (25). SRB1-mediated efflux was measured as the total efflux from Fu5AH rat hepatoma cells.
The esterification of cholesterol within endogenous lipoproteins (cholesterol esterification rate, CER) was determined on pooled plasma samples as previously described (26).
Statistical analyses
For each monkey and measured parameter, the delta (week X – baseline at week 1) were calculated, with X being 3, 6, or 10 weeks. ANOVA was performed on these delta values, with treatment dose as independent experimental variable, followed by Dunnett's post-hoc test to see whether the delta in each drug-treated group (30, 100, or 300 mg/kg/occasion) differed significantly (P < 0.05) from the vehicle-treated group.
Pearson correlation coefficients were calculated using the Pearson feature in Excel. Values greater than 0.349 are significant at P < 0.05, and values greater than 0.449 are significant at P < 0.01 for 30 degrees of freedom (32 monkeys).
RESULTS
Changes in plasma lipid, lipoprotein, and apolipoprotein levels before, during, and after six weeks of administration of MDCO-216
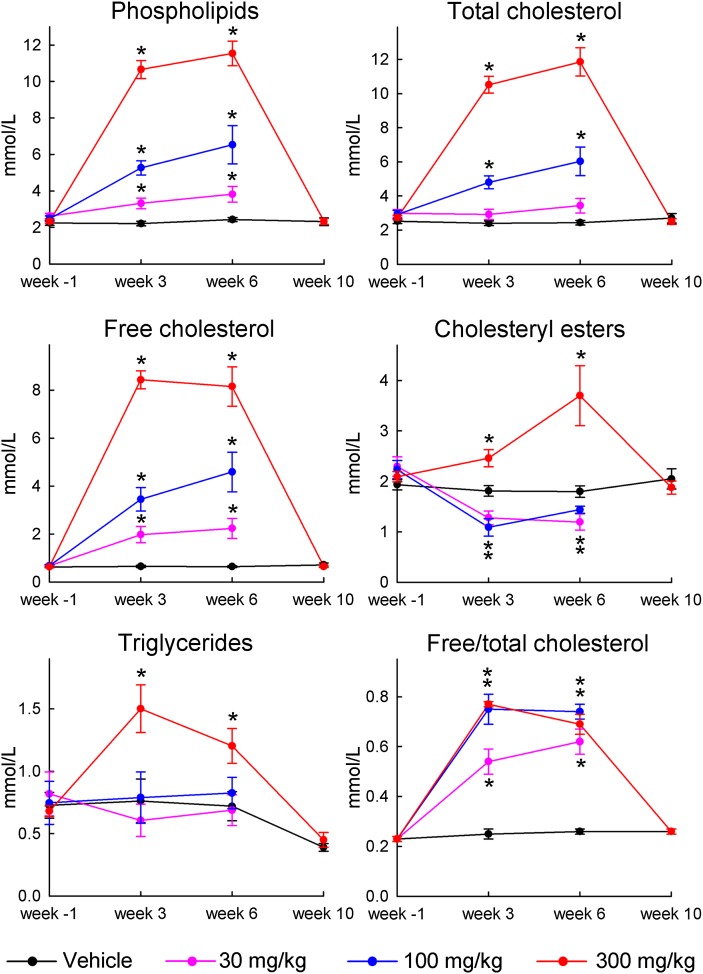
As shown in Fig. 1, compared with values at baseline (week 1), pronounced and dose-dependent increases of plasma levels of PL, TC, and FC were observed after the 7th (week 3) and 20th (week 6) administration of MDCO-216. The increases in PL and TC were significant already for the lowest dose. The drug caused a significant drop in the level of CE at the 30 and 100 mg/kg doses but a significant increase at the 300 mg/kg dose, while the level of TG was significantly increased at the 300 mg/kg doses but not affected by the 30 and 100 mg/kg doses. The FC/TC ratio increased from 0.24 at baseline to 0.77 at the dose of 100 mg/kg during the six weeks of treatment.
Fig. 1.
Plasma levels of PL, TC, FC, CE, and TG, and the FC/TC ratio, before, during, and after 6 weeks of repetitive administration of MDCO-216 to cynomolgus monkeys. The values for week 3 or week 6 of drug administration refer to plasma samples taken 1 h prior to the infusion occurring on day 15 or 41, respectively. Since effects on lipids and apoproteins were of similar size in males and females, values for both genders were pooled for calculation of averages and SEM. Week 1; plasma sample taken 7 days before start of first infusion. Week 10: plasma sample taken 70 days after the first infusion and 28 days after last infusion. Data are averages ± SEM of n = 10 (5 males and 5 females) for monkeys receiving vehicle (0) and 300 mg/kg, and n = 6 (3 males and 3 females) for monkeys receiving 30 mg/kg and 100 mg/kg, except on week 10 where data are averages ± SEM for n = 4 (2 males and 2 females). For each monkey and measured parameter, the delta (week X − baseline at week 1) were calculated, with X being 3, 6, or 10 weeks. ANOVA was performed on these delta-values, with treatment dose as independent experimental variable, followed by Dunnett's post-hoc test to see whether deltas in each of the drug-treated groups (30, 100, or 300 mg/kg/occasion) differed significantly (P < 0.05) from the vehicle-treated group. Dots marked with an asterisk indicate significant differences between the drug-treated and vehicle-treated groups (P < 0.05).
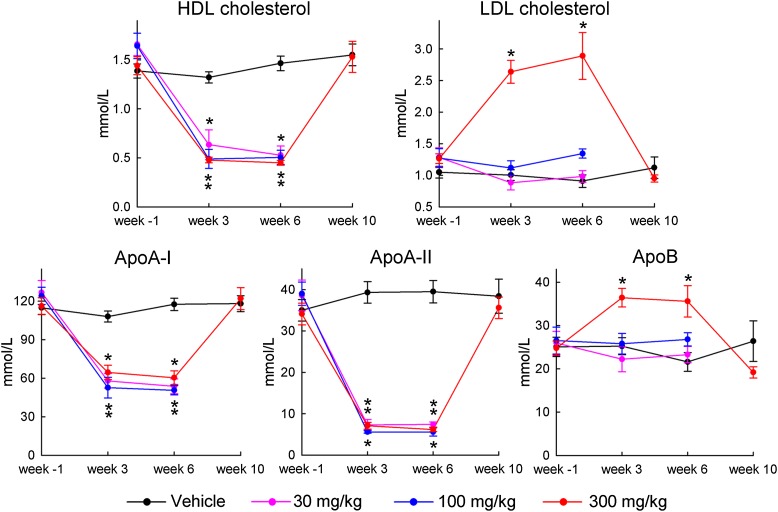
As shown in Fig. 2, levels of HDL-C, apoA-I, and apoA-II were strongly decreased at all doses, with the strongest effect on apoA-II. In contrast, LDL-C and apoB were not affected by the 30 and 100 mg/kg doses and only moderately increased at the 300 mg/kg dose, in line with the effects seen for TG.
Fig. 2.
Plasma levels of HDL-C, LDL-C, apoA-I, apoA-II, and apoB in the plasma samples described in legend of Fig. 1. Dots marked with an asterisk indicate significant differences between the drug-treated and vehicle-treated groups (P < 0.05).
Four monkeys in the vehicle and 300 mg/kg dose groups were also assessed on day 71 (week 10), four weeks after the last infusion. As shown in Figs. 1 and 2, all lipid, lipoprotein, and apolipoprotein levels in the MDCO-216-treated monkeys had returned to pretreatment or vehicle values at that time.
Serum levels of apoA-IM, lipids, and (apo)lipoproteins in sera before and after administration of MDCO-216 on day 1
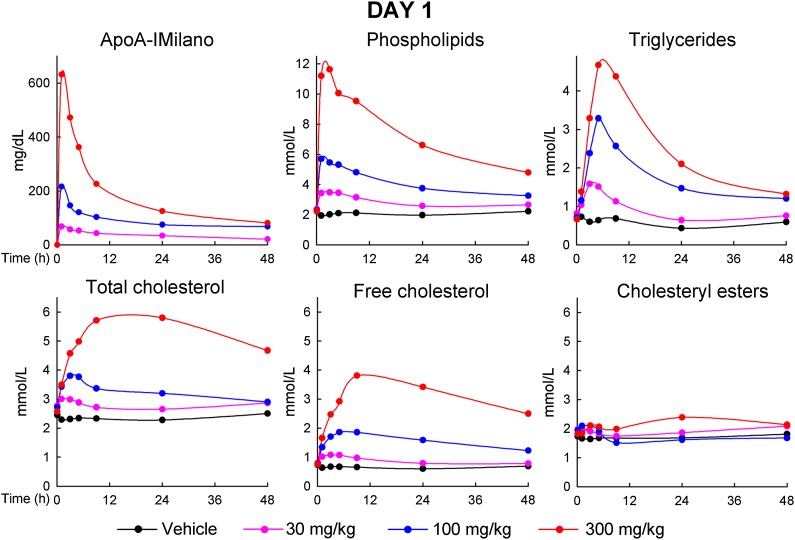
As shown in Fig. 3, the serum level of apoA-IM peaked immediately at the first time point (5 min) after infusion and thereafter declined at a rapid rate, with 50% of the peak level reached between 4 and 8 h after infusion, followed by a second phase of slower clearance. The PL level also reached peak at 5 min or 1 h after infusion but did not decrease as rapidly as apoA-IM. At 46 h after infusion, PL was still above pretreatment level. TG, TC, and FC levels also rose rapidly after infusion, with TG reaching peak at 4 h after infusion, and TC and FC at 8 h. Rates of decrease of these levels were similar or even lower than seen for PL, with baseline levels not yet reached at 46 h.
Fig. 3.
Serum levels of apoA-IM, PL, TG, TC, FC, and CE just prior to and at various time points after the first infusion of MDCO-216 on day 1. Data points are averages for n = 10 (5 males and 5 females) for monkeys receiving vehicle (0) and 300 mg/kg, and n = 6 (3 males and 3 females) for monkeys receiving 30 mg/kg and 100 mg/kg.
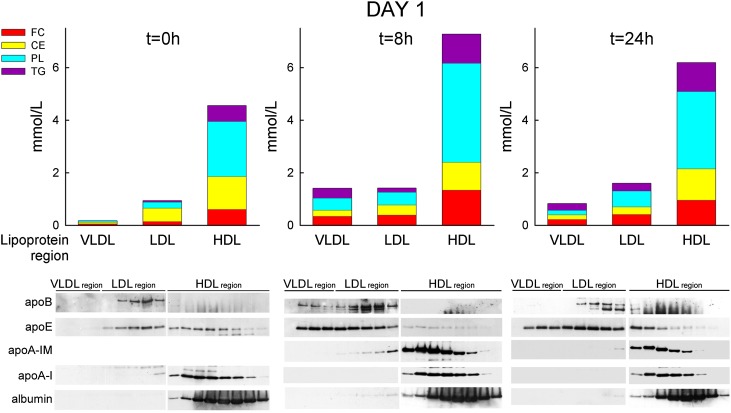
To establish the lipoproteins in which these lipid changes occurred, serum samples of the 100 mg/kg dose group were pooled and subjected to FPLC separation. As shown in Fig. 4, at baseline, the majority of all lipids were found in the HDL fractions, with only small amounts in the LDL and VLDL regions. At 8 h or 24 h after infusion, PL, FC, and TG increased in HDL but relatively more markedly also in LDL and VLDL.
Fig. 4.
FPLC separation of pooled sera of male monkeys prior to and at various time points after the first infusion of 100 mg/kg of MDCO-216 on day 1. Bars graphs represent the serum concentrations of the various lipids in the VLDL, LDL, or HDL region, calculated as (amount of lipid X in LP-area) × (concentration of lipid X in whole serum) / (total amount of lipid X recovered in all FPLC fractions). Bottom panels are Western blots of PAGE gels showing various apoproteins in the FPLC fractions. Two PAGE slab gels were used for analysis of the relevant FPLC fractions, with nine fractions per slab-gel. These two gels were run, transferred, and blotted together; films with the same exposure times are shown.
Fig. 4 also shows that apoA-I wild-type was found only in the HDL region before and after infusion; apoB at baseline was detected in the LDL region only but also in the VLDL region at 8 h after infusion. At 8 h and 24 h after infusion, apoA-IM was detected only in the HDL region. Apo-E at baseline occurred in both the HDL and LDL regions but moved out of the HDL region into the LDL and VLDL regions in the 8 h and 24 h serum samples. Serum albumin occurred only in the post-HDL fractions at baseline and stayed there after infusion.
Serum levels of apoA-IM, lipids, and (apo)lipoproteins in sera before and after administration of MDCO-216 on day 41
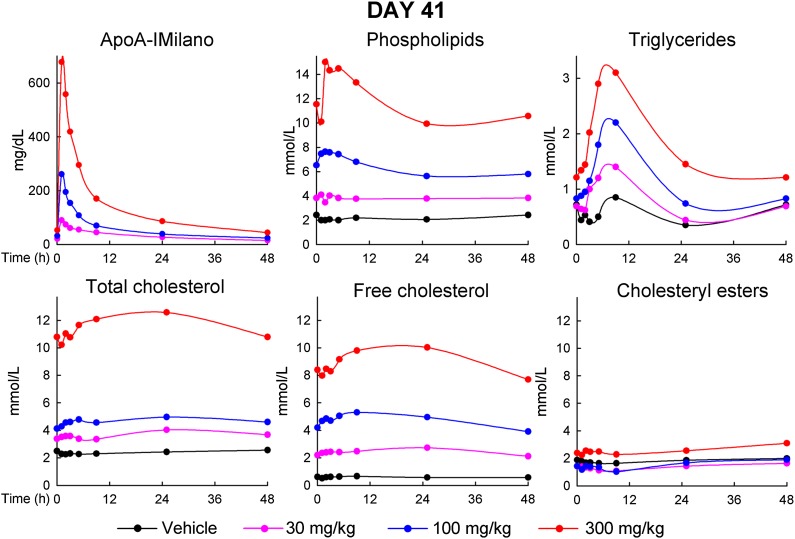
As shown in Fig. 5, the serum level of apoA-IM showed a very similar kinetic profile after infusion on day 41 compared with day 1. Just prior to infusion, serum lipids were very similar to those in the plasma samples taken on day 41 at 1 h prior to infusion (values shown for week 6 in Fig. 1). After infusion, lipids showed similar changes as seen on day 1, with a rapid increase of PL (peak reached at 5 min or 1 h after infusion), a slower increase of TG (peak reached at 4 h), an even slower increase in FC, and no or little effect on CE. At 46 h, lipids had returned to the preinfusion values.
Fig. 5.
Serum levels of apoA-IM, PL, TG, TC, FC, and CE just prior to and at various time points after the 21st infusion of MDCO-216 on day 41. Data points are averages for n = 10 (5 males and 5 females) for monkeys receiving vehicle (0) and 300 mg/kg, and n = 6 (3 males and 3 females) for monkeys receiving 30 mg/kg and 100 mg/kg.
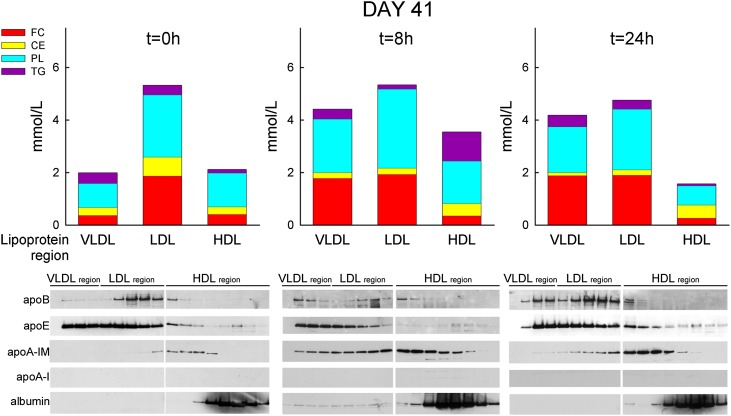
Again, serum samples of the 100 mg/kg dose group were pooled and subjected to FPLC separation. As shown in Fig. 6, just prior to infusion, the majority of PL, TG, and TC was found in the LDL and VLDL regions. At 8 h after infusion, PL and FC increased strongly in both the VLDL and the HDL regions, with little change in the LDL region. At 24 h, PL and FC decreased in the HDL region compared with the predose distribution and remained high in the VLDL region.
Fig. 6.
FPLC separation of pooled sera of male monkeys prior to and at various time points after infusion of 100 mg/kg of MDCO-216 on day 41. Bars graphs represent the serum concentrations of the various lipids in the VLDL, LDL, or HDL region, calculated as (amount of lipid X in LP-area) × (concentration of lipid X in whole serum) / (total amount of lipid X recovered in all FPLC fractions). Bottom panels are western blot of PAGE gels showing various apoproteins in the FPLC fractions. Two PAGE slab gels were used for analysis of the relevant FPLC fractions, with nine fractions per slab-gel. These two gels were run, transferred, and blotted together; films with the same exposure times are shown.
Fig. 6 also shows that apoA-IM occurred in the HDL region in both the predose and 24 h time points, but it showed up in the LDL region at 8 h. In contrast, apoB was invariably detected in the LDL region only. ApoA-I wild-type was not detected in any of these FPLC fractions. Both at baseline and at 24 h after infusion, apo-E was found to reside mainly in the VLDL and LDL regions.
Increase in surface lipids in VLDL and LDL and in serum apoE
Table 1 shows that the administration of MDCO-216 led to pronounced increases in the ratio of surface lipid (PL plus FC) over core lipids (TG and CE) in the particles of the size of LDL and VLDL, both on day 1 and in particular at 8 and 24 h after infusion on day 41.
TABLE 1.
Ratio of surface lipids (PL+FC) to core lipids (TG+CE) in lipoproteins isolated by FPLC
| Day 1 (T = 0 h) | Day 1 (T = 24 h) | Day 41 (T = 0 h) | Day 41 (T = 8 h) | Day 41 (T = 24 h) | Reference Cyno'sa | |
| VLDL region | — | 0.95 | 1.80 | 6.40 | 3.89 | 0.53 |
| LDL region | 0.65 | 1.77 | 3.85 | 12.3 | 4.32 | 0.66 |
| HDL region | 1.46 | 1.69 | 4.04 | 1.25 | 1.54 | 1.96 |
T, time.
Calculated from Ref. 61.
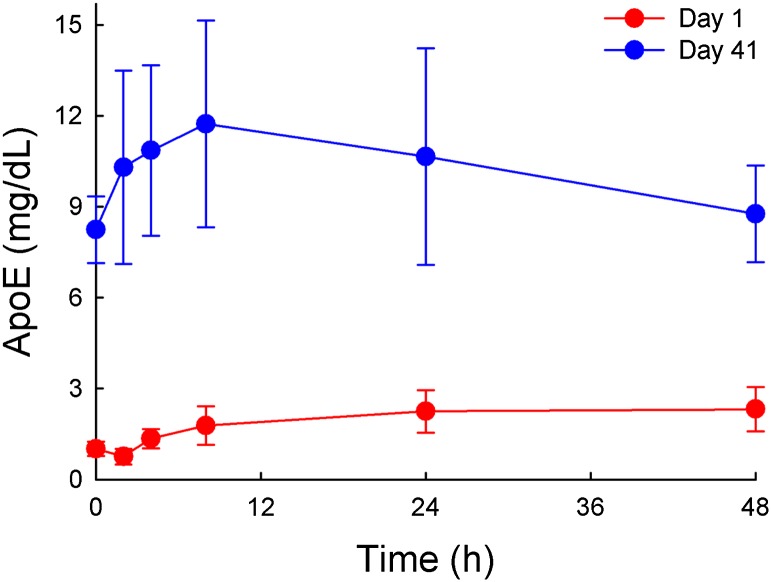
After observing the strong increase of the polar surface lipids PL and FC in the LDL and VLDL regions and the enrichment of these particles with apoE, we measured the total serum level of apoE in the monkeys before and after infusion of 100 mg/kg MDCO-216. As shown in Fig. 7, at baseline the apoE was 8-fold higher on day 41 than on day 1. On day 41, the infusion caused a transient further increase in apoE, with a similar time course as seen for FC.
Fig. 7.
Apo-E levels in sera of female monkeys prior to and at various time points after the infusion of 100 mg/kg of MDCO-216 on day 1 or day 41.
Changes in endogenous cholesterol esterification
The relative lack of effect on CE and the increase in the FC/TC ratio (Fig. 1) suggested that the LCAT reaction in these monkeys was only minimally stimulated by MDCO-216. More direct evidence of lack of CE formation was obtained by measuring the endogenous CER in sera incubated ex vivo. As shown in Table 2, CER was decreased noticeably at 8 h and 24 h after the first infusion of 30 mg/kg or 300 mg/kg of MDCO-216 compared with the preinfusion value, and it was zero prior to and at 8 h and 24 h after infusion on day 41.
TABLE 2.
Cholesterol esterification rates in sera of monkeys prior to and at 8 or 24 h after infusion of 30 or 300 mg/kg MDCO-216 on day 1 or day 41
| Day 1 | Day 41 | ||||
| Dose (mg/kg) | Time (h) | FC (mM) | CER (nmol/ml/h) | FC (mM) | CER (nmol/ml/h) |
| 30 | 0 | 0.80 | 39.3 | 2.20 | 0 |
| 30 | 8 | 0.96 | 20.6 | 2.65 | 0 |
| 30 | 24 | 0.78 | 16.6 | 2.71 | 0 |
| 300 | 0 | 0.75 | 43.3 | 9.83 | 0 |
| 300 | 8 | 3.67 | 29.7 | 10.0 | 0 |
| 300 | 24 | 3.18 | 4.7 | 11.1 | 0 |
Cholesterol efflux from cultured cells incubated with MDCO-216 or with serum from MDCO-216-treated monkeys
We first wanted to ascertain that MDCO-216 behaved like a pre-beta-like particle with respect to inducing cholesterol efflux in cultured cells expressing the ABCA1 and SRB1 cholesterol efflux carriers. As shown in supplementary Fig. III, MDCO-216 by itself at a low concentration (14 μg/ml) caused a vigorous global cholesterol efflux from J774 cells, of which about 60% was ABCA1-mediated. Efflux from Fu5AH cells (SRB1 mediated) was relatively small. At a 7-fold higher drug concentration (100 μg/ml), global efflux from J774 cells was 2-fold higher compared with the lower concentration, most of this increase due to an 3-fold increase in basal efflux, whereas the ABCA1-mediated efflux was only 60% higher than with the lower concentration. A similar strong increase (about 4-fold) was seen for the SRB1-mediated efflux from Fu5AH cells compared with the lower concentration.
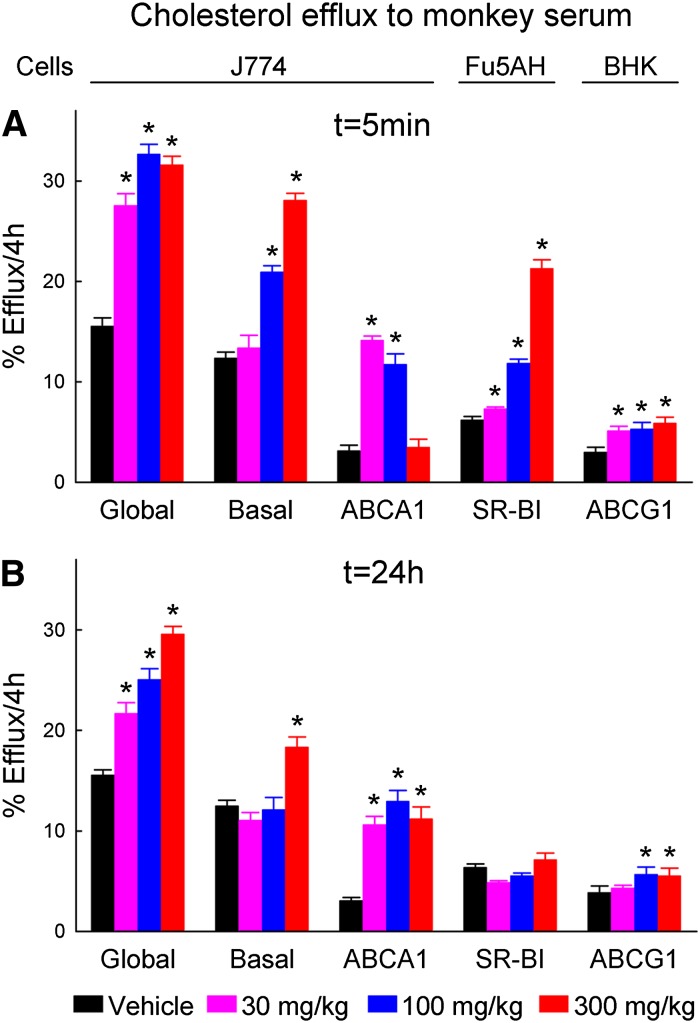
Cholesterol efflux by sera of MDCO-216-treated monkeys was determined in sera taken at 5 min and 24 h of day 41. When taken at 5 min after infusion (Fig. 8A), the sera of monkeys treated with 30 mg/kg showed marked increases in the global and ABCA1-mediated effluxes in J774 cells, but the infusion had only small effects on the basal efflux from these cells or on the SRB1-mediated efflux from Fu5AH cells. The ABCA1-mediated efflux was also strongly elevated at the 100 mg/kg dose but surprisingly did not differ from the vehicle control at the 300 mg/kg dose. In contrast, the SRB1-mediated efflux from Fu5AH cells was strongly increased by the middle and high doses. The sera also significantly but weakly stimulated the ABCG1-mediated efflux from BHK cells at all doses. In these 5 min sera, the global and basal effluxes from J774 cells and the SRB1-mediated efflux from Fu5AH cells strongly correlated with the corresponding serum PL and FC levels, whereas the ABCG1-mediated efflux showed a weaker correlation, and the ABCA1-mediated efflux in these sera did not correlate at all with these lipids (Table 3, sample A).
Fig. 8.
Cholesterol efflux measured in sera taken at (A) 5 min or (B) 24 h after infusion on day 41, as function of administered dose of MDCO-216. Global, basal, and ABCA1-mediated effluxes were measured in J774 cells, SRB1-mediated efflux in Fu5AH cells, and ABCG1-mediated efflux in BHK cells as described in Materials and Methods. Concentration of serum in the efflux assays was 2% by volume. Data for male and female monkeys were pooled. Bars represent averages ± SEM for n = 10 (vehicle and 300 mg/kg doses) or n = 6 (30 and 100 mg/kg doses) monkeys. Effluxes marked with an asterisk indicate significant differences between the drug-treated and vehicle-treated groups (ANOVA followed by Dunnet's test).
TABLE 3.
Correlations between the various cholesterol effluxes and levels of FC and serum PL
| Sample A (5 min) | |||||
| Global | ABCA1 | Basal | SRB1 | ABCG1 | |
| FC | 0.92** | 0.17 | 0.86** | 0.83** | 0.67** |
| PL | 0.84** | −0.06 | 0.93** | 0.91** | 0.62** |
| Sample B (24 h) | |||||
| Global | ABCA1 | Basal | SRB1 | ABCG1 | |
| FC | 0.91** | 0.64** | 0.70** | 0.36* | 0.51** |
| PL | 0.93** | 0.67** | 0.70** | 0.39* | 0.49** |
Serum samples were taken 5 min (A) or 24 h (B) after the last infusion on day 41, for all 32 monkeys studied. *P < 0.05 for 30 degrees of freedom; **P < 0.01 for 30 degrees of freedom. Correlations are Pearson correlation coefficients.
When taken at 24 h after infusion, the sera of monkeys treated with all doses of MDCO-216 caused marked increases in the global and ABCA1-mediated effluxes in J774 cells, while the basal efflux from these cells was only stimulated by serum from the high-dose group (Fig. 8B). These sera had only weak stimulatory effects on ABCG1-mediated efflux from BHK cells, with significance reached only for the middle and high doses, and SRB1-mediated efflux from Fu5AH cells was not affected by any dose. In these sera, the global, ABCA-mediated, and basal effluxes from J774 cells strongly correlated with the corresponding serum PL and FC levels, while the SRB1- and ABCG1-mediated effluxes showed weaker correlations with these lipids (Table 3, sample B).
DISCUSSION
This study was performed as part of a pivotal toxicology study, assessing the safety and tolerability of six weeks of repeated administration of MDCO-216 to cynomolgus monkeys. The primary goal of this study was to establish no-adverse-effect levels as guidance for human studies. The product was well tolerated at all doses without any adverse effects on a broad array of anatomical, histological, and clinical-chemical parameters (The Medicines Company, unpublished data). The study was performed in normolipidemic monkeys fed a standard diet. It offered the opportunity to assess the short- and long-term effects of repeat administrations of MDCO-216 on plasma lipid and lipoprotein levels, and the effect of treatment on the ex-vivo serum cholesterol efflux capacity via currently known efflux mechanisms. The opportunity had additional relevance since little information has been published on the effects of exogenous apoA-1M administration on lipids and lipoproteins in animals fed a normal diet; previous animal studies were focused on the effect of apoA-IM administration on lipid levels and atherosclerosis in cholesterol-fed animals (16–20).
Changes in lipids and apolipoprotein levels
Repeated administration of MDCO-216 every second day caused marked and dose-dependent increases in TC, FC, and PL and strong decreases in HDL-C, apoA-I, and apoA-II. Moderate increases of TG, LDL-C, and apoB were observed only at the highest dose. CE levels decreased at the two lower doses but increased at the highest dose. The changes on day 15 (after 7 administrations) were similar to those on day 41 (after 20 administrations), indicating that steady-state lipid levels were reached after the 7th administration, except for CE levels in which the increase was more prominent at week 6 than week 3. In the monkeys receiving the highest dose (300 mg/kg/occasion), all lipid and apolipoprotein values returned to pretreatment levels four weeks after the last dose. No gender differences were observed.
The increase in PL is likely due to administration of the POPC in the MDCO-216 drug product, although a stimulation of PL efflux via ABCA1 may also contribute. The increase in FC is considered the primary pharmacodynamic response to the drug, and in view of the high correlation of FC levels with total and ABCA1-mediated cholesterol effluxes (Table 3), it can reasonably be ascribed to the strong cholesterol efflux capacity of MDCO-216 mainly via ABCA1. Strikingly, there was no commensurate increase in CE, and consequently, the FC/TC ratio increased very strongly. This indicates that LCAT activity was either inhibited or not activated by apoA-IM, which was confirmed by ex-vivo measurement of the CER (Table 2). This is not surprising since partial LCAT deficiency was reported earlier for carriers of the apoA-IM mutation (26, 27). Furthermore, in sera of mice doubly transgenic for both apoA-IM and human apoA-II, LCAT activity was decreased and the serum FC/TC ratio strongly elevated compared with mice doubly transgenic for human wild-type apoA-I and human apoA-II, which was ascribed to a lower endogenous CER in the former animals (28). In addition, Calabresi et al. found that discoidal HDL particles containing apoA-IM dimer and POPC were clearly less potent in activating LCAT compared with similar-sized particles with wild-type apoA-I (29). The particles tested in that study were very similar to those used in the present study, lending further support that MDCO-216 indeed leads to a decreased LCAT activity.
Very notable were the observed decreases in steady-state levels of HDL-C, apoA-I, and apoA-II that are described here for the first time. The decrease observed in our study is in line with the fact that, in humans carrying the apoA-IM mutation, HDL-C levels are about 2-fold lower than normal (27). In human volunteers receiving a single dose of 50–100 mg/kg ETC-216, the predecessor compound of MDCO-216, an increase was seen at these doses when HDL-C was quantified by FPLC (30). Note, however, that the increases reported for ETC-216 were limited to sera obtained at 30 min or 2 h after drug administration. At later time points in the same study, HDL-C measured by both the enzymatic method and FPLC showed a marked decrease at doses of 50 mg/kg or higher (C. Bisgaier, unpublished data).
The mechanism for the drop in HDL is unclear at this time. Carriers of the apoA-IM mutation (who are all heterozygous) are known to have very low HDL-C levels (10, 14). In a careful metabolic study in carriers of the apoA-IM mutation, the lower HDL level was ascribed to rapid clearance of HDL carrying apoA-IM as monomer (31), whereas the residence time of HDL containing apoA-IM as dimer was much longer than normal HDL containing wild-type apoA-I. A conversion of the MDCO-216 into HDL particles with monomeric apoA-IM followed by rapid clearance of the latter would then offer an explanation for the prominent drop. So far, no evidence for such a conversion in our study is at hand. Alternatively, the drop in apoA-I seen in our study may be due to feedback suppression of the endogenous apoA-I synthesis by the administered A-IM protein. Transgenic mice expressing the gene of human apoA-I had high serum levels of human apoA-I and strongly decreased serum levels of their own mouse apoA-I due to a posttranscriptional suppression of mouse apoA-I synthesis in the liver (32). Finally, the drop in HDL may be a consequence of the lack of LCAT activation, which is in line with the observed decrease in endogenous CER (Table 2). Subjects with familial LCAT deficiency are known to have very low levels of HDL-C and apoA-I (33).
MDCO-216 at the highest dose caused increases in TG, CE, and apoB levels. This is in line with the observation that human carriers of apoA-IM also have increased levels of TG (26), and similar increases in TG were seen in mice doubly transgenic for wild-type human apoA-I and human apoA-II (34). Taken together, these data indicate that MDCO-216 on days 15 and 41 at the highest dose caused an increase in plasma VLDL concentration still visible 46 h after the previous infusion. This may be the result of an increased delivery of cholesterol and phospholipids to the liver, described in other animal models to lead to increased VLDL production (35, 36), or alternatively, of decreased VLDL clearance as observed after administration of high doses of reconstituted HDL to apoE knockout mice (37) or apoE3-Leiden mice (38).
Short-term effects on lipids and (apo)lipoproteins on days 1 and 41
It is clear from Fig. 3 that MDCO-216 had rapid, immediate effects on all measured serum lipid levels except CE. In this short-term time frame, peaks were reached first for PL, then for TG, and lastly for FC. Noticeably, 46 h after the first infusion, just prior to the second administration, PL, TG (for the highest dose only), and FC levels did not quite return to preinfusion levels. This explains why on day 15 after 6 infusions or on day 41 after 20 infusions, plasma levels of these lipids at 46 h after the previous infusion were (much) higher than at baseline. In the two days after each infusion, new amounts of lipids entered the circulation on top of levels that were still elevated above baseline after the previous infusion. Apparently, it took six administrations to reach a new steady state in which the amounts of lipids leaving the plasma compartment were approximately equal to the amounts entering that compartment during the 47 h after drug infusion.
Looking at the distribution of lipids among lipoprotein particles separated by FPLC, we found that the increases in PL and FC in serum after the first and the last infusion showed up in the VLDL and LDL regions in addition to the HDL region. This was paralleled by a shift of apoE away from the HDL region into the LDL and VLDL regions. Lipoprotein lipids on day 41 (Fig. 6) already showed much lower HDL lipids prior to infusion compared to those on day 1 (Fig. 4), in line with the clear drop of HDL-C in plasma (Fig. 2). In addition, dramatic increases of PL and FC in the VLDL and LDL regions were observed in the day 41 sera compared with the day 1 sera, and they were not accompanied by proportional increases in the “core lipids” TG and CE. We suggest that infusion of MDCO-216 leads to formation of membranous particles containing only PL and FC as lipids, which have sizes in the range of LDL or even VLDL. The absence of albumin in the particles eluting in these regions suggests that these new particles are not composed of plasma-filled vesicles, as has been described for Lp-X (39). Furthermore, the strong increase in total serum apoE level on day 41 compared with day 1, and the enrichment of apoE in the VLDL and LDL regions, suggests that these new membranous disks are carrying apoE. Further work is needed to test these hypotheses.
The appearance of FC and PL in the VLDL and LDL regions has been observed before by others infusing reconstituted human HDL with wild-type apoA-I in mice (40); it was attributed to lack of activation of mouse LCAT by human apoA-I. Likewise, a shift of eluted FC and PL to VLDL and LDL regions upon FPLC separation was seen after administration of apoA-I mimetic peptides in mice (41), attributed to displacement of apoA-I from HDL thus disabling the LCAT-activating capacity of apoA-I. Also, administration of high doses ETC-216 to rabbits led to an increased appearance of FC in the VLDL region upon FPLC separation (18). In the latter study, ETC-216 was shown to clearly deplete lipids from fatty streaks, demonstrating that these high levels of FC in the VLDL region were not detrimental with respect to atherosclerosis regression. Finally, also in the earlier human volunteer study, the lipids in the VLDL and LDL regions were markedly increased after single administration of ETC-216 in doses of 50 mg/kg or higher (C. Bisgaier, unpublished data).
A stimulatory effect of apoA-I and reconstituted HDL on apoE production by various cell types has been well described for cultured macrophages (42, 43), hepatocytes (44), and adipocytes (45) by a mechanism that is unrelated to binding of apoA-I to ABCA1 (43). We propose that the infusion of MDCO-216 has likewise led to enhanced production and secretion of apoE in the monkeys. Other researchers (46, 47) have shown that apoE is able to cause efflux of FC and PL from macrophages, thus producing apoE-containing nascent HDL in an ABCA1-dependent and independent manner. It will be of interest to determine to what extent the increase in FC levels observed in our study actually is caused by ABCA1-mediated efflux or by stimulation of this apoE-rich nascent HDL production.
Effects of MDCO-216 on ex-vivo cholesterol effluxes
A recent careful study (48) reported the effects of several defined sizes of reconstituted HDL (wild-type apoA-I-POPC) particles on the cholesterol efflux via ABCA1, ABCG1, and SRB1 transporters. Only the particles with the smaller diameters (7.8 and 9.6 nm) promoted the ABCA1-mediated efflux, reaching a plateau at 20 μg/ml (as protein). In contrast, the larger-sized (10.8 to 17 nm) particles showed a concentration-dependent increase in efflux via SRB1 but not ABCA1. Conversely, the small (7.8 nm) particles did not promote SRB1-mediated efflux. Our findings with pure MDCO-216 (supplementary Figs. II and III) are in good agreement with this, as the majority of the product has a size range of 7.8 nm, and at low concentration (14 μg/ml protein), the product mainly promotes ABCA1-mediated efflux. The product contains a small amount of particles in the range of 10.8 nm or larger, which is in line with a marked increase of SRB1-mediated efflux only when a high concentration of the drug product (100 μg/ml protein) is used.
Known pathways of cholesterol efflux were markedly induced by sera from drug-treated animals (Fig. 8). Sera taken 24 h after the infusion showed marked increases of global and ABCA1-mediated efflux at all MDCO-216 doses, whereas the efflux from J774 cells via mechanisms other than ABCA1 was only stimulated by sera at the highest dose, and SRB1-mediated efflux from Fu5AH cells was not stimulated at any dose. Stimulation of ABCG1-mediated efflux from BHK cells was small and only significant for the serum from monkeys treated with middle and high doses. In contrast, sera taken 5 min after infusion stimulated ABCA1 efflux for the monkeys receiving the low dose but enhanced the SRB1-mediated efflux for the monkeys treated with the high dose. This pattern (stimulation of SRB1-mediated efflux only in the early serum samples and only with sera from the mid- and high-dose monkeys) can be explained by the high drug product concentration at 5 min after infusion of these high doses. As shown in Fig. 5, in the monkeys receiving 300 mg/kg, the mean serum level of apoA-IM at this time point was 6.8 mg/ml, corresponding to 136 μg/ml in the efflux assay (containing 2% serum by volume), which is above the concentration in which the pure drug led to a marked stimulation of SRB1- mediated efflux (supplementary Fig. III). At the 24 h time point, the mean serum level of apoA-IM in these monkeys was decreased to 0.9 mg/ml, corresponding to18 μg/ml in the efflux assay. At this level, pure MDCO-216 had little effect on SRB1-mediated efflux (supplementary Fig. III).
Recently, Favari et al. reported that serum of apoA-IM carriers caused stronger activation of ABCA1-mediated efflux than serum of matching control subjects (49). The authors identified a unique 8 nm-sized HDL in the serum of these carriers occurring in addition to the normal pre-β-1HDL; the sum of concentrations of these two particles correlated with the ABCA1-mediated efflux in the serum of these patients. They also found that reconstituted HDL containing one molecule of dimeric apoA-IM was as equally potent as similarly sized (8 nm) particles containing two molecules of wild-type apoA-I in stimulating ABCA1-mediated efflux from J774 macrophages. In agreement with that, Weibel et al. (50) found no intrinsic difference in cholesterol efflux capacities between apoA-IM and wild-type apoA-I in reconstituted HDL particles or in HDL isolated from wild-type or apoA-IM-expressing mice.
ABCA1 primarily interacts with lipid-free or lipid-poor apoA-I, such as pre-β-1HDL present in low concentrations in serum (48). SRB1-mediated cholesterol efflux is stimulated by discoidal pre-β-HDL particles, except the smallest 7.8 nm size (51), as well as by spherical α HDL (52, 53) and by liposomes. ABCG1 mediates cholesterol efflux mainly to spherical HDL particles with α-mobility (25). Animal studies using selective knockout models have shown that ABCA1 and ABCG1 act in a sequential fashion in the process of macrophage RCT in vivo, whereas SRB1 does not seem to play a significant role (53–58). In our study, the ABCG1-mediated efflux was not or only to a small extent increased in the sera of the drug-treated monkeys. This suggests that in these monkeys infused MDCO-216 particles do not mature in the circulation, in line with the lack of LCAT activation by MDCO-216 discussed above.
The observation that serum of monkeys on day 41 at 24 h after the last infusion, in spite of the high concentration of FC and low level of apoA-IM, was still able to strongly promote global and ABCA1-mediated efflux suggests that pre-β particles remain present and active in the circulation (and the lymphatic/interstitial fluid). Noticeably, the ex-vivo ABCA1-mediated and basal effluxes from J774 cells induced by these sera were highly correlated with the levels of FC and PL. This suggests that MDCO-216 is continuously picking up FC and PL from cells and tissues and regenerated by unloading these lipids to the large-sized particles in the circulation, thus reflecting a “shuttle and synergism” scenario previously shown to operate in a cell culture model (59). In line with this, other researchers recently showed the importance of non-HDL lipoproteins in the overall efflux capacity of human plasma after infusion of reconstituted HDL (60).
Overall conclusions
Administration of MDCO-216 in cynomolgus monkeys leads to marked and sustained increases of PL and FC and to decreases of HDL-C, apoA-I, and apoA-II. This change in lipid and lipoprotein profile sustained over a six-week period was well tolerated and did not lead to any obvious adverse effects. Although administration of exogenous apoA-IM clearly differs from endogenous HDL biogenesis in carriers of the apoA-IM mutation, the dynamic constellation described here would promote a sustained cholesterol efflux from macrophages that is mainly ABCA1 mediated. Since this transporter is a major player in macrophage RCT, MDCO-216 may be expected to have potent antiatherosclerotic activity, as was indeed observed in the past in several animal models and in acute coronary syndrome patients. The fact that the ABCA1-mediated efflux was already maximally stimulated at the lowest dose suggests that even lower doses may already have clinically relevant effects.
Limitations
These findings pertain to monkeys receiving multiple, high doses of MDCO-216 with short treatment intervals of 48 h. Ex-vivo effluxes do not necessarily reflect cholesterol effluxes in vivo. We did not measure effluxes in sera taken at day 1 in which the PL- and FC-rich particles were not yet prominently present. The formation of the large PL- and FC-rich apoE-containing particles and their potential contribution to cholesterol effluxes remains to be confirmed in human studies.
Supplementary Material
Acknowledgments
The authors thank Alice Ossoli and Ilaria Bellusci for excellent technical support, and Drs. Cesare Sirtori and Charles Bisgaier for valuable contributions in drafting the manuscript.
Footnotes
Abbreviations:
- apoA-IM
- apoA-IMilano
- CE
- cholesteryl ester
- CER
- cholesterol esterification rate
- FC
- free cholesterol
- FPLC
- fast-protein liquid chromatography
- HDL-C
- HDL-cholesterol
- LDL-C
- LDL-cholesterol
- PL
- phospholipid
- POPC
- palmitoyl-oleoyl-phosphatidylcholine
- RCT
- reverse cholesterol transport
- SRB1
- scavenger receptor type B1
- TC
- total cholesterol
- TG
- triglyceride
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures and three tables.
REFERENCES
- 1.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 2.Rifkind B. M. 1990. High-density lipoprotein cholesterol and coronary artery disease: survey of the evidence. Am. J. Cardiol. 66: 3A–6A. [DOI] [PubMed] [Google Scholar]
- 3.Wilson P. W. F. 1990. High-density lipoprotein, low density lipoprotein and coronary artery disease. Am. J. Cardiol. 66: 7A–10A. [DOI] [PubMed] [Google Scholar]
- 4.Glomset J. A. 1968. The plasma lecithin:cholesterol acyltransferase reaction. J. Lipid Res. 9: 155–167. [PubMed] [Google Scholar]
- 5.Tall A. R. 1990. Plasma high density lipoproteins: metabolism and relationship to atherogenesis. J. Clin. Invest. 86: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acton S., Rigotti A., Landschulz K. T., Xu S., Hobbs H. H., Krieger M. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 271: 518–520. [DOI] [PubMed] [Google Scholar]
- 7.Palgunachari M. N., Mishra V. K., Lund-Katz S., Phillips M. C., Adeyeye S. O., Alluri S., Anantharamaiah G. M., Segrest J. P. 1996. Only the two ends of eight tandem amphipathic helical domains of human apoA-I have significant lipid affinity: implications for HDL assembly. Arterioscler. Thromb. Vasc. Biol. 16: 328–338. [DOI] [PubMed] [Google Scholar]
- 8.Banka C. L., Bonnet D. J., Black A. S., Smith R. S., Curtiss L. K. 1991. Localization of an apolipoprotein A-I epitope critical for activation of lecithin-cholesterol acyltransferase. J. Biol. Chem. 266: 23886–23892. [PubMed] [Google Scholar]
- 9.Rothblat G. H., Mahlberg F. H., Johnson W. J., Phillips M. C. 1992. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J. Lipid Res. 33: 1091–1097. [PubMed] [Google Scholar]
- 10.Sirtori C. R., Calabresi L., Franceschini G., Baldassarre D., Amato M., Johansson J., Salvetti M., Monteduro C., Zulli R., Muiesan M. L., et al. 2001. Cardiovascular status of carriers of the apolipoprotein a-i(milano) mutant: The Limone sul Garda study. Circulation. 103: 1949–1954. [DOI] [PubMed] [Google Scholar]
- 11.Weisgraber K. H., Bersot T. P., Mahley R. W., Franceschini G., Sirtori C. R. 1980. A-IMilano apoprotein: isolation and characterization of a cysteine-containing variant of the A-I apoprotein from human high density lipoproteins. J. Clin. Invest. 66: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisgraber K. H., Rall S. C., Jr, Bersot T. P., Mahley R. W., Franceschini G., Sirtori C. R. 1983. Apolipoprotein A-IMilano: detection of normal apoA-I in affected subjects and evidence for a cysteine for arginine substitution in the variant A-I. J. Biol. Chem. 258: 2508–2513. [PubMed] [Google Scholar]
- 13.Franceschini G., Frosi T. G., Manzoni C., Gianfranceschi G., Sirtori C. R. 1982. High density lipoprotein-3 heterogeneity in subjects with the apoA-IMilano variant. J. Biol. Chem. 257: 9926–9930. [PubMed] [Google Scholar]
- 14.Franceschini G., Calabresi L., Tosi C., Gianfranceschi G., Sirtori C. R., Nichols A. V. 1990. Apolipoprotein A-IMilano: disulfide-linked dimers increase high density lipoprotein stability and hinder particle interconversion in carrier plasma. J. Biol. Chem. 265: 12224–12231. [PubMed] [Google Scholar]
- 15.Calabresi L., Vecchio G., Longhi R., Gianazza E., Palm G., Wadensten H., Hammarstrom A., Olsson A., Karlstrom A., Sejlitz T., et al. 1994. Molecular characterization of native and recombinant apolipoprotein A-IMilano dimers: the introduction of an interchain disulfide bridge remarkably alters the physicochemical properties of apolipoprotein A-I. J. Biol. Chem. 269: 32168–32174. [PubMed] [Google Scholar]
- 16.Ameli S., Hultgardh-Nilsson A., Cercek B., Shah P. K., Forrester J. S., Ageland H., Nilsson J. 1994. Recombinant apolipoprotein A-I Milano reduces intimal thickening after balloon injury in hypercholesterolemic rabbits. Circulation. 90: 1935–1941. [DOI] [PubMed] [Google Scholar]
- 17.Shah P. K., Nilsson J., Kaul S., Fishbein M. C., Ageland H., Hamsten A., Johansson J., Karpe F., Cercek B. 1998. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 97: 780–785. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa G., Monteggia E., Marchesi M., Lorenzon P., Laucello M., Lorusso V., Di Mario C., Karvouni E., Newton R. S., Bisgaier C. L., et al. 2002. Recombionant apolipoprotein A-IMilano infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ. Res. 90: 974–980. [DOI] [PubMed] [Google Scholar]
- 19.Parolini C., Marchesi M., Lorenzon P., Castano M., Balconi E., Miragoli L., Chaabane L., Morisetti A., Lorusso V., Martin B. J., et al. 2008. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 51: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 20.Ibanez B., Vilahur G., Cimmino G., Speidl W. S., Pinero A., Choi B. G., Zafar M. U., Santos-Gallego C. G., Krause B., Badimon L., et al. 2008. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J. Am. Coll. Cardiol. 51: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 21.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes. A randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 22.Caparon M. H., Rust K. J., Hunter A. K., McLaughlin J. K., Thomas K. E., Herberg J. T., Shell R. E., Lanter P. B., Bishop B. F., Dufield R. L., et al. 2010. Integrated solution to purification challenges in the manufacture of a soluble recombinant protein in E. coli. Biotechnol. Bioeng. 105: 239–249. [DOI] [PubMed] [Google Scholar]
- 23.Mweva S., Paul J. L., Cambillau M., Goudouneche D., Beaune P., Simon A., Fournier N. 2006. Comparison of different cellular models measuring in vitro the whole human serum cholesterol efflux capacity. Eur. J. Clin. Invest. 36: 552–559. [DOI] [PubMed] [Google Scholar]
- 24.de la Llera-Moya M., Drazul-Schrader D., Asztalos B. F., Cuchel M., Rader D. J., Rothblat G. H. 2010. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sankaranarayanan S., Oram J. F., Asztalos B. F., Vaughan A. M., Lund-Katz S., Adorni M. P., Phillips M. C., Rothblat G. H. 2009. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 50: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschini G., Baio M., Calabresi L., Sirtori C. R., Cheung M. C. 1990. Apolipoprotein AIMilano. Partial lecithin:cholesterol acyltransferase deficiency due to low levels of a functional enzyme. Biochim. Biophys. Acta. 1043: 1–6. [DOI] [PubMed] [Google Scholar]
- 27.Franceschini G., Sirtori C. R., Bosisio E., Gualandri V., Orsini G. B., Mogavero A. M., Capurso A. 1985. Relationship of the phenotypic expression of the A-IMilano apoprotein with plasma lipid and lipoprotein patterns. Atherosclerosis. 58: 159–174. [DOI] [PubMed] [Google Scholar]
- 28.Bielicki J. K., Forte T. M., McCall M., Stoltzfus L. J., Chiesa G., Sirtori C. R., Franceschini G., Rubin E. M. 1997. High density lipoprotein particle size restriction in apolipoprotein A-I(Milano) transgenic mice. J. Lipid Res. 38: 2314–2321. [PubMed] [Google Scholar]
- 29.Calabresi L., Franceschini G., Burkybile A., Jonas A. 1997. Activation of lecithin cholesterol acyltransferase by a disulfide-linked apolipoprotein A-I dimer. Biochem. Biophys. Res. Commun. 232: 345–349. [DOI] [PubMed] [Google Scholar]
- 30.Cole T. G., Nowatzke W. L., Bisgaier C. L., Krause B. 2002. Method-dependent changes in “HDL-cholesterol” with recombinant apolipoprotein A-I(Milano) infusion in healthy volunteers. Clin. Chem. 48: 680–681. [PubMed] [Google Scholar]
- 31.Roma P., Gregg R. E., Meng M. S., Ronan R., Zech L. A., Franceschini G., Sirtori C. R., Brewer H. B., Jr 1993. In vivo metabolism of a mutant form of apolipoprotein A-I, apoA-IMilano, associated with familial hypoalphalipoproteinemia. J. Clin. Invest. 91: 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin E. M., Ishida B. Y., Clift S., Krauss R. M. 1991. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc. Natl. Acad. Sci. USA. 88: 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuivenhoven J. A., Pritchard H., Hill J., Frohlich J., Assmann G., Kastelein J. 1997. The molecular pathology of lecithin:cholesterol acyltransferase (LCAT) deficiency syndromes. J. Lipid Res. 38: 191–205. [PubMed] [Google Scholar]
- 34.Chiesa G., Stoltzfus L. J., Michelangoli S., Bielicki J. K., Santi M., Forte T. M., Sirtori C. R., Franceschini G., Rubin E. M. 1998. Elevated triglycerides and low HDL cholesterol in transgenic mice expressing human apoA-I(Milano). Atherosclerosis. 136: 139–146. [DOI] [PubMed] [Google Scholar]
- 35.Stone B. G., Schreiber D., Alleman L. D., Ho C. Y. 1987. Hepatic metabolism and secretion of a cholesterol-enriched lipoprotein fraction. J. Lipid Res. 28: 162–172. [PubMed] [Google Scholar]
- 36.Wiersma H., Nijstad N., Gautier T., Iqbal J., Kuipers F., Hussain M. M., Tietge U. 2010. Scavenger receptor B1 facilitates hepatic very low density lipoprotein production in mice. J. Lipid Res. 51: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah P. K., Yano J., Reyes O., Chyu K. Y., Kaul S., Bisgaier C. L., Drake S., Cercek B. 2001. High-dose recombinant apolipoprotein A-I(Milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mixce. Potential applications for acute plaque stabilization. Circulation. 103: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Berbee J. F. P., Stroes E. S., Smit J. W. A., Havekes L. M., Romijn J. A., Rensen P. C. N. 2011. CETP expression reverses the reconstituted HDL-induced increase in VLDL. J. Lipid Res. 52: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patsch J. R., Aune K. C., Gotto A. M., Morrisett J. D. 1977. Isolation, chemical characterization and biophysical properties of three different abnormal lipoproteins: LP-X1, Lp-X2 and LP-X3. J. Biol. Chem. 252: 2113–2120. [PubMed] [Google Scholar]
- 40.Chen Z., O'Neill E., Meurer R. D., Gagen K., Luell S., Wang S-P., Ichetovkin M., Franz-Wattley B., Eveland S., Strack A. M., et al. 2012. Reconstituted HDL elicits marked changes in plasma lipids following single-dose injection in C57Bl/6 Mice. J. Cardiovasc. Pharmacol. Ther. 17: 315–323. [DOI] [PubMed] [Google Scholar]
- 41.Carballo-Jane E., Chen Z., O'Neill E., Wang J., Burton C., Chang C. H., Chen X., Eeveland S., Frantz-Wattley B., Gagen K., et al. 2010. ApoA-I mimetic peptides promote pre-β HDL formation in vivo causing remodeling of HDL and triglyceride accumulation at higher dose. Bioorg. Med. Chem. 18: 8669–8678. [DOI] [PubMed] [Google Scholar]
- 42.Bielicki J. K., McCall M. R., Forte T. M. 1999. Apolipoprotein A-I promotes cholesterol release and apolipoprotein E recruitment from THP-1 macrophage-like foam cells. J. Lipid Res. 40: 85–92. [PubMed] [Google Scholar]
- 43.Kockx M., Rye K. A., Gaus K., Quinn C. M., Wright J., Sloane T., Sviridov D., Fu Y., Sullivan D., Burnett J. R., et al. 2004. Apolipoprotein A-I stimulated apolipoprotein E secretion from human macrophages is independent of cholesterol efllux. J. Biol. Chem. 279: 25966–25977. [DOI] [PubMed] [Google Scholar]
- 44.Farkas M. H., Swift L. L., Hasty A. H., Linton M. F., Fazio S. 2003. The recycling of apolipoprotein E in primary cultures of mouse hepatocytes. Evidence for a physiologic connection to high density lipoprotein metabolism. J. Biol. Chem. 278: 9412–9417. [DOI] [PubMed] [Google Scholar]
- 45.Bencharif K., Hoareau L., Murumalla R. K., Tarnus E., Tallet F., Glerc R. G., Gardes C., Cesari M., Roche R. 2010. Effect of apoA-I on cholesterol release and apoE secretion in human mature adipocytes. Lipids Health Dis. 9: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Z. H., Fitzgerald M. L., Mazzone T. 2006. Distinct cellular loci for the ABCA1-dependent and ABCA1-independent lipid efflux mediated by endogenous apolipoprtein E expression. Arterioscler. Thromb. Vasc. Biol. 26: 157–162. [DOI] [PubMed] [Google Scholar]
- 47.Zannis V. I., Koukos G., Drosatos K., Vezeridis A., Zanni E. E., Kypreos K. E., Chroni A. 2008. Discrete roles of apoA-I and apoE in the biogenesis of HDL species: lessons learned from gene transfer studies in different mouse models. Ann. Med. 40: 14–28. [DOI] [PubMed] [Google Scholar]
- 48.Favari E., Calabresi L., Adorni M. P., Jessup W., Simonelli S., Franceschini G., Bernini F. 2009. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 48: 11067–11074. [DOI] [PubMed] [Google Scholar]
- 49.Favari E., Gomaraschi M., Zanotti I., Bernini F., Lee-Rueckert M., Kovanen P. T., Sirtori C. R., Franceschini G., Calabresi L. 2007. A unique protease-sensitive high-density lipoprotein particle containing the apolipoprotein A-I(Milano) dimer effectively promotes ATP-binding cassette A1-mediated cell cholesterol efflux. J. Biol. Chem. 282: 5125–5132. [DOI] [PubMed] [Google Scholar]
- 50.Weibel G. L., Alexander E. T., Joshi M. R., Rader D. J., Lund-Katz S., Phillips M. C., Rothblat G. H. 2007. Wild-type apoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler. Thromb. Vasc. Biol. 27: 2022–2029. [DOI] [PubMed] [Google Scholar]
- 51.Liadaki K. N., Liu T., Xu S., Ishida B. Y., Duchateaux P. N., Krieger J. P., Kane J., Krieger M., Zannis V. I. 2000. Binding of high density lipoprotein (HDL) and discoidal reconstituted HDL to the HDL receptor scavenger receptor class B type I. Effect of lipid association and apo-A-I mutations on receptor binding. J. Biol. Chem. 275: 21262–21271. [DOI] [PubMed] [Google Scholar]
- 52.Liu T., Krieger M., Kan H. Y., Zannis V. I. 2002. The effects of mutations in helices 4 and 6 of apoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein is required for efficient lipid transport. J. Biol. Chem. 277: 21576–21584. [DOI] [PubMed] [Google Scholar]
- 53.Wang X., Collins H. L., Ranalletta M., Fuki I. V., Billheimer J. T., Rothblat G. H., Tall A. R., Rader D. J. 2007. Macrophage ABCA1 and ABCG1, but not SR-B1, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117: 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuchel M., Lund-Katz, M. de la Llera-Moya, J. S. Millar, D. Chang, I. Fuki, G. H. Rothblat, M. C. Phillips, and D. J. Rader S. 2010. Pathways by which reconstituted high-density lipoprotein mobilizes free cholesterol from whole body and from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Out R., Hoekstra M., Hildebrand R. B., Kruit J. K., Meurs I., Li Z., Kuipers F., Van Berkel T. J., Van Eck M. 2006. Macrophage ABC-G1 deletion disrupts lipid homeostasis in lung alveolar macrophages and moderately influences atherosclerotic lesions development in LDL-receptor deficient mice. Arterioscler. Thromb. Vasc. Biol. 26: 2295–2300. [DOI] [PubMed] [Google Scholar]
- 56.Out R., Hoekstra M., Habets K., Meurs I., de Waard V., Hildebrand R. B., Wang Y., Chimini G., Kuiper J., Van Berkel T. J., et al. 2008. Combined deletion of macrophage ABC-A1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 28: 258–264. [DOI] [PubMed] [Google Scholar]
- 57.Vaughan A. M., Oram J. F. 2006. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 47: 2433–2443. [DOI] [PubMed] [Google Scholar]
- 58.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. 2007. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigueza W. V., Williams K. J., Rothblat G. H., Phillips M. C. 1997. Remodeling and shuttling. Mechanisms for the synergistic effects between different acceptor particlles in the mobilization of cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 17: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoang A., Drew B. G., Low H., Remaley A. T., Nestel P., Kindwell B. A., Sviridov D. 2012. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. Eur. Heart J. 33: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagdade J. D., Wagner J. D., Rudel L. L., Clarkson T. B. 1995. Accelerated cholesteryl ester transfer and altered lipoprotein composition in diabetic cynomolgus monkeys. J. Lipid Res. 36: 759–766. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.