Abstract
Chronic exposure of skeletal muscle to saturated fatty acids, such as palmitate (C16:0), enhances proinflammatory IKK-NFκB signaling by a mechanism involving the MAP kinase (Raf-MEK-ERK) pathway. Raf activation can be induced by its dissociation from the Raf-kinase inhibitor protein (RKIP) by diacylglycerol (DAG)-sensitive protein kinase C (PKC). However, whether these molecules mediate the proinflammatory action of palmitate, an important precursor for DAG synthesis, is currently unknown. Here, involvement of DAG-sensitive PKCs, RKIP, and the structurally related monounsaturated fatty acid palmitoleate (C16:1) on proinflammatory signaling are investigated. Palmitate, but not palmitoleate, induced phosphorylation/activation of the MEK-ERK-IKK axis and proinflammatory cytokine (IL-6, CINC-1) expression. Palmitate increased intramyocellular DAG and invoked PKC-dependent RKIPSer153 phosphorylation, resulting in RKIP-Raf1 dissociation and MEK-ERK signaling. These responses were mimicked by PMA, a DAG mimetic and PKC activator. However, while pharmacological inhibition of PKC suppressed PMA-induced activation of MEK-ERK-IKK signaling, activation by palmitate was upheld, suggesting that DAG-sensitive PKC and RKIP were dispensable for palmitate's proinflammatory action. Strikingly, the proinflammatory effect of palmitate was potently repressed by palmitoleate. This repression was not due to reduced palmitate uptake but linked to increased neutral lipid storage and enhanced cellular oxidative capacity brought about by palmitoleate's ability to restrain palmitate-induced mitochondrial dysfunction.
Keywords: saturated fatty acid, monounsaturated fatty acid, diacylglycerol, nuclear factor kappa-B, interleukin-6, cytokine
Chronic, low-grade inflammation is a characteristic feature of obesity (1) that involves enhanced activation of proinflammatory signaling in tissues, such as skeletal muscle, and is associated with increased expression and secretion of cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor α (TNFα) (2, 3). An important factor contributing to the enhanced inflammatory drive in skeletal muscle is an increase in the circulating concentration of fatty acids, in particular saturated fatty acids (SFA), such as palmitate, which are notably elevated during obesity (4). Increases in SFAs are thought to be of significance given that numerous studies have demonstrated that these possess a greater pathogenic potential than mono- or polyunsaturated fatty acids (MUFA and PUFA) (5–8), which, in contrast, not only confer beneficial metabolic effects (9) but also possess the capacity to counter some of the deleterious effects associated with SFA oversupply (6, 10). Indeed, palmitoleate, a MUFA, released from white adipose tissue has been coined a “lipokine” based on its ability to promote systemic metabolic homeostasis (11). How fatty acids exert their differential effects upon signaling pathways that impact upon cellular metabolism and inflammation remains unclear, but such effects may be attributable, in part, to the observation that SFAs are less well oxidized than MUFAs and PUFAs (12, 13). Consequently, sustained exposure of muscle cells to elevated SFAs will result in greater intramyocellular conversion to fatty-acid-derived metabolites, such as diacylglycerol (DAG) and ceramide, whose accumulation is known to dysregulate proximal signaling events downstream of receptor tyrosine kinases (14–17) and induce expression of proinflammatory genes that are regulated in a nuclear factor kappa-B (NFκB)-dependent manner (2, 18, 19).
There has been considerable interest in delineating how palmitate or its lipid derivatives trigger inflammatory signaling in skeletal muscle. Toll-like receptors (TLR) of the innate immune system, in particular TLR2 and TLR4 (for which SFAs may be ligands), have been implicated in the activation of NFκB (20, 21), but there is also considerable evidence showing that members of the protein kinase C (PKC) (22, 23) and MAP kinase families (7, 18) can promote SFA-induced NFκB signaling independently of TLR involvement in skeletal muscle cells. We recently reported that palmitate induces activation of JNK, p38 MAPK, and MEK-ERK signaling but that only pharmacological inhibition of the MEK-ERK pathway attenuated SFA-induced NFκB signaling and IL-6 expression in L6 skeletal muscle cells (18). The activation of the classical MAP kinase (ERK) pathway by palmitate is unlikely to be initiated by TLRs in these muscle cells as it does not manifest for at least 6–8 h (18), whereas TLR-induced MAP kinase signaling is typically initiated within minutes of receptor activation in other tissues (24). The lag period of 6–8 h that we observe with respect to SFA-induced activation of the MEK-ERK-NFκB signaling axis in muscle cells may reflect the time required to accumulate palmitate-derived lipid intermediates to a threshold that promotes activation of molecules that subsequently stimulate the classical MAP kinase pathway. One family of molecules that may respond in this manner are the novel and conventional PKC isoforms, which can be activated by increases in intracellular DAG occurring in response to palmitate oversupply (25). PKCs have been linked to activation of MEK-ERK signaling via their ability to modulate the activity of the serine/threonine kinase Raf, which lies immediately upstream of MEK (26, 27). Modulation of Raf relies, in part, upon regulating its association with raf kinase inhibitory protein (RKIP), which when bound to Raf, suppresses Raf-mediated phosphorylation/activation of MEK (28, 29). RKIP is a physiological target for PKCs, and phosphorylation of RKIP on Ser153 induces its dissociation from Raf thereby enabling increased phosphorylation/activation of MEK and consequently that of ERK (30). However, it remains currently unknown whether DAG generated in response to palmitate overprovision helps drive MEK-ERK-NFκB signaling in response to palmitate in muscle cells, and if so, whether DAG-sensitive PKCs and RKIP are crucial regulators within this process and whether they are subject to counter-modulation by MUFAs, such as palmitoleate. Addressing these questions was the over-arching objective of the studies presented here.
MATERIALS AND METHODS
Materials
α-Minimal Essential Medium (α-MEM), fetal bovine serum (FBS), Trypsin/EDTA solution, and penicillin/streptomycin solution were purchased from Life Technologies (Paisley, UK). Other reagent-grade chemicals were purchased from Sigma-Aldrich (Poole, UK), including palmitate, palmitoleate, lauric acid, oleate, stearic acid, dimethyl sulphoxide (DMSO), etomoxir, and antibodies to β-actin and GAPDH. Fraction V fatty acid-free bovine serum albumin (BSA) and complete protein phosphatase inhibitor tablets were purchased from Boehringer-Roche Diagnostics (Basel, Switzerland). [3H]palmitate (0.5 mCi/ml) was from Perkin Elmer UK. Gö6976 and Gö6983 were from Tocris Bioscience (Bristol, UK), and phorbol 12-myristate 13-acetate (PMA) was from Ascent Scientific (Bristol, UK). Triglyceride assay kit was purchased from Sigma-Aldrich. Antibodies to IKKα/β phospho-Ser180/181, ERK1/2, ERK 1/2 phospho-Thr202/Tyr204, Raf-1 phospho-Ser338, MEK 1/2 phospho-Ser217/221, and PKCθ were purchased from Cell Signaling Technology (MA). Antibodies to IκBα and Raf-1 were from Santa Cruz Biotechnology (CA). Antibody to RKIP was from Upstate Biotechnology (VA) and to RKIP phospho-Ser153 was from Epitomics (CA). Anti-α1 Na/K-ATPase (α6F) antibody was obtained from the University of Iowa Hybridoma Bank. The anti-CD36 mAb clone 63 (anti-CD36-cl63; also known as clone CRF D2717) was from BD Biosciences (Oxford, UK).
Cell culture and fatty acid treatment
L6 muscle cells were maintained as myoblasts and differentiated into myotubes in α-MEM containing 2% (v/v) FBS and 1% (v/v) antibiotic/antimycotic solution (100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B) at 37°C with 5% CO2 as described previously (31). Myotubes were exposed to fatty acids (or vehicle) that were preconjugated to BSA for the times and at the concentrations indicated in the figure legends.
Cell lysis and isolation of total membranes
Following the incubations stated in the figure legends, cells were washed three times in ice-cold phosphate-buffered saline (PBS) and lysed using lysis buffer (50 mM TRIS at pH 7.4, 0.27 M sucrose, 1 mM Na-Orthovanodate at pH 10, 1 mM EDTA, 1 mM EGTA, 10 mM Na-β-glycerophosphate, 50 mM NaF, 5 mM Na-pyrophosphate, 1% (v/v) Triton X-100, 0.1% 2-mercaptoethanol, and protease inhibitors). Whole-cell lysates were centrifuged at 4,000 g at 4°C, snap-frozen in liquid nitrogen, and stored at −20°C. In some experiments, total-cell membranes were isolated as described previously (31).
Generation of recombinant adenovirus and transduction of L6 cells
The RKIP sequence was amplified from L6 cDNA and cloned into the vector pCR2.1-TOPO (Invitrogen). Site-directed mutagenesis was carried out using the QuickChange II kit (Stratagene) and was used to mutate serine 153 to valine. RKIP S153V was then subcloned into the shuttle vector pShuttleCMV. The resultant plasmid was linearized by restriction endonuclease PmeI and cotransformed with the adenoviral vector pAdEasy1 into the Escherichia coli strain BJ5183. Recombinants were selected for by kanamycin resistance and screened by endonuclease digestion with BamHI, PacI, and SpeI. The final recombinant adenoviral construct was linearized with the restriction endonuclease PacI and transfected into the packaging cell line Ad293 using Lipofectamine (Life Technologies). After seven days, cells were lysed by four freeze-thaw cycles, centrifuged to pellet cell debris, and then the supernatant containing virus was used to further propagate the recombinant adenovirus in Ad293 cells. Adenovirus was collected when approximately 70% of the cell monolayer showed cytopathic effect. Cell culture supernatant containing virus was collected and stored at −80°C. Viral titers were determined by plaque assay in Ad293 cells. L6 cells at day 4 of differentiation were transduced for 2 h with 86.8 × 106 pfu/ml of virus (either containing or lacking the RKIP S153V construct) and then incubated until fully differentiated as described previously (31).
Immunoblotting
Cell lysates or total membranes (30 μg protein) were diluted in Laemmli buffer and subjected to SDS-PAGE on 10% resolving gels and transferred onto immobilin-P membranes as described previously (31). Membranes were probed with primary antibodies against proteins of interest prior to incubation with the appropriate peroxidize-conjugated IgG antibodies. Proteins were detected using chemiluminesence involving exposure to autoradiographic film (31).
Immunoprecipitation
Coimmunoprecipitations of Raf-1 and RKIP were carried out in detergent-free, mild conditions as described previously (32). Protein-G sepharose beads were incubated with anti-RKIP antibody for 2 h at 4°C on an orbital platform shaker. L6 myotubes were lysed by sonication in PBS containing protease inhibitors, and 500 μg of the resulting lysate was incubated with the beads for a further 2 h at 4°C. The immunoprecipitates were washed four times in ice-cold PBS and then boiled for 5 min in Laemmli buffer.
RNA extraction and PCR
Total RNA extraction and quantitative real-time PCR were carried out as previously described (18). The sequences for the primers used were as follows: IL-6 forward, 5′-GACTGATGTTGTTGACAGCCA-′3; IL-6 reverse, 5′-ATGCTTAGGCATAACGCACTAGGTT-3′ CINC-1 forward, 5′-ACCCGCTCGCTTCTCTGTGC-3′ CINC-1 reverse, 5′-CAGCGCAGCTCATTGGCGAC-3′ UCP2 forward, 5′-CCTACAAGACCATTGCACGAGAG-3′ UCP2 reverse, 5′- GGCAAGGGAGGTCGTCTGTC-3′ GAPDH forward, 5-TGGAAAGCTGTGGCGTGAT-3; and GAPDH reverse 5-GCTTCACCACCTTCTTGAT-3.
Analysis of DAG and ceramide content
DAGs and ceramides were extracted by the use of an extraction mixture composed of isopropanol:water:ethyl acetate (35:5:60; v/v/v). Quantitative measurement was made using an Agilent 6460 triple quadrupole mass spectrometer. Diacylglycerols and ceramides were analyzed using positive ion electrospray ionization (ESI) source with multiple reaction monitoring (MRM). The chromatographic separation was performed using an Agilent 1290 Infinity Ultra Performance Liquid Chromatography (UPLC) as recently described (33). The analytical column was a reverse-phase Zorbax SB-C8 column 2.1 × 150 mm, 1.8 μm (Agilent). Chromatographic separation was conducted in binary gradient using 2 mM ammonium formate, 0.15% formic acid in methanol as Solvent A, and 1.5 mM ammonium formate, 0.1% formic acid in water as Solvent B at the flow rate of 0.4 ml/min. 1,3-dipentadecanoyl-rac-glycerol was used as an internal standard.
Measurement of intramyocellular triglyceride, fatty acid uptake, and whole-cell oxygen consumption
Intramyocellular triglyceride accumulation was assayed using an enzyme (lipase)-coupled colorimetric assay kit (MAK040, Sigma-Aldrich) as per manufacturer's instructions. Palmitate uptake in L6 myotubes was investigated as described previously (8). Oxygen consumption rate (OCR) was assayed by plating out L6 myoblasts onto 24-well plates at a density of 6,000 cells/well and allowing them to differentiate into myotubes over a seven-day period with media changes every two days. For chronic fatty acid treatments, myotubes were treated for 14 h with palmitate and/or palmitoleate in 2% BSA α-MEM and then transferred into Krebs-Henseleit buffer (111 mM NaCl, 4.7 mM KCl, 2 mM Mg2SO4, 1.2 mM Na2HPO4, 2.5 mM glucose, 0.5 mM carnitine) containing 2% (w/v) BSA and the relevant fatty acids. After 1 h, the OCR was measured continuously over a period of 2 h using a Seahorse Extracellular Flux (XF) Analyzer.
Statistical analyses
For multiple comparisons, statistical analysis was performed using one-way ANOVA (ANOVA) followed by a Bonferroni posttest. For individual comparisons, statistical analysis was performed using a t-test. Data analysis was performed using GraphPad Prism software and considered statistically significant at P < 0.05.
RESULTS
Effects of palmitate and MUFAs on ERK, IKKα/β phosphorylation, and IκBα abundance
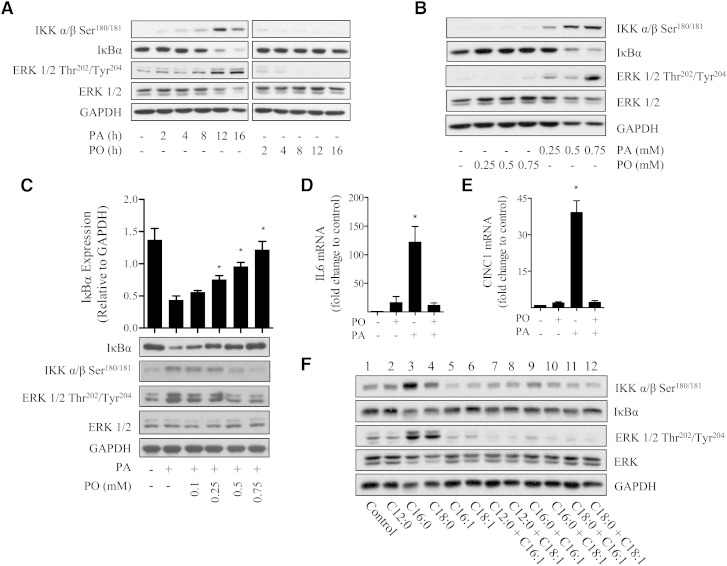
To explore the mechanisms underpinning the effect of increased SFA availability upon proinflammatory signaling, we initially monitored the phosphorylation/activation status of IKKα/β and the cellular abundance of its downstream target IκBα, which serves as a readout of NFκB activation. In line with our recent work (18), palmitate (C16:0) induced a dose- and time-dependent increase in IKK phosphorylation/activation that was associated with a corresponding loss in IκBα. These responses were maximal after 16 h incubation with 0.75 mM palmitate (Fig. 1A, B). In contrast, palmitoleate (a MUFA, C16:1) did not exert any detectable effect upon IKKα/β phosphorylation or upon IκBα abundance over the same dose and time range. We have previously demonstrated that the increase in NFκB signaling in response to palmitate is dependent upon the upstream activation of ERK (18). Consistent with these observations, palmitate but not palmitoleate enhanced ERK phosphorylation (Fig. 1).
Fig. 1.
Effects of palmitate, palmitoleate, and select MUFAs on proinflammatory signaling in L6 myotubes. (A, B) L6 myotubes (A) were treated with fatty acid-free BSA (vehicle control) or BSA conjugated to PA (0.75 mM) or PO (0.75 mM) for times indicated. Muscle cells (B) were incubated for 16 h with the fatty acids at the indicated concentrations. Cells were lysed and lysates subjected to immunoblot analysis using antibodies against IKKα/β Ser180/181, IκBα, and ERK 1/2 Thr202/Tyr204. (C) L6 myotubes were incubated in the absence or presence of 0.75 mM palmitate and/or palmitoleate at concentrations indicated for 16 h prior to lysis and immunoblotting for ERK 1/2 Thr202/Tyr204, IKKα/β Ser180/181, IκBα, and GAPDH. The cellular abundance of IκBα was quantified and expressed relative to GAPDH, which was used a gel loading control. (D, E) Alternatively, following treatment in the absence or presence of 0.75 mM palmitate and/or 0.75 mM palmitoleate, RNA was extracted from L6 myotubes and (D) IL-6 and (E) CINC-1 mRNA were determined by real time qPCR. Bars represent mean ± SEM from three separate experiments, and asterisks signify a significant difference from the untreated control values (P < 0.05). (F) Effect of different fatty acids upon proinflammatory signaling was assessed. L6 myotubes were treated for 16 h with 0.75 mM lauric acid (C12:0), 0.75 mM palmitate (C16:0), or 0.1 mM stearic acid (C18:0) alone or in combination with 0.75 mM palmitoleate (C16:1) or 0.75 mM oleate (C18:1). Lysates were immunoblotted to assess the phosphorylation status of IKKα/β and ERK 1/2 and total protein abundance of IκBα. Equal gel loading was ascertained by immunoblotting with an antibody against total ERK 1/2 and GAPDH. The blots are representative of three separate experiments.
Because palmitoleate did not elicit any increase in NFκB signaling, we subsequently assessed whether it could affect the proinflammatory drive induced by palmitate. Fig. 1C shows that palmitoleate antagonized the palmitate-induced activation/phosphorylation of ERK and IKKα/β and restrained IκBα loss in a dose-dependent manner. Subsequent real-time quantitative PCR analysis of two NFκB-regulated muscle genes, IL-6 and CINC-1 (the rat homolog of IL-8) (34, 35), revealed that while palmitate induced expression of IL-6 and CINC-1 by ∼120-fold and 40-fold, respectively (Fig. 1D, E), this was fully repressed in cells simultaneously incubated with equivalent concentrations (0.75 mM) of both palmitate and palmitoleate.
Although palmitoleate is a rather minor circulating MUFA, the data presented in Fig. 1A–E highlight the contrasting effects of introducing a single double-bond into an otherwise very prevalent C16 SFA. We therefore compared the extent to which fatty acyl chain length and saturation were important with respect to inducing their pro- or anti-inflammatory effects. L6 muscle cells were chronically incubated with laurate (C12:0, SFA), palmitate (C16:0), stearate (C18:0, SFA), palmitoleate (C16:1), or oleate (C18:1, MUFA) either alone or with SFA and MUFA in the combinations shown in Fig. 1F. Our data indicate that, unlike long chain (C16:0 and C18:0) SFAs, laurate does not induce phosphorylation/activation of ERK or IKKα/β, and it does not promote loss of IκBα (Fig. 1F). In line with much of the palmitoleate data presented in Fig. 1, muscle cells did not exhibit an increase in proinflammatory signaling when chronically exposed to oleate, a very prevalent circulating MUFA. Furthermore, like palmitoleate, oleate was able to attenuate the proinflammatory effects of both palmitate and stearate based on the reduced phosphorylation of ERK and IKKα/β and retention of IκBα (Fig. 1F, compare lanes 10 and 12 with lanes 3 and 4).
Palmitate induces RKIP phosphorylation and its dissociation from Raf1
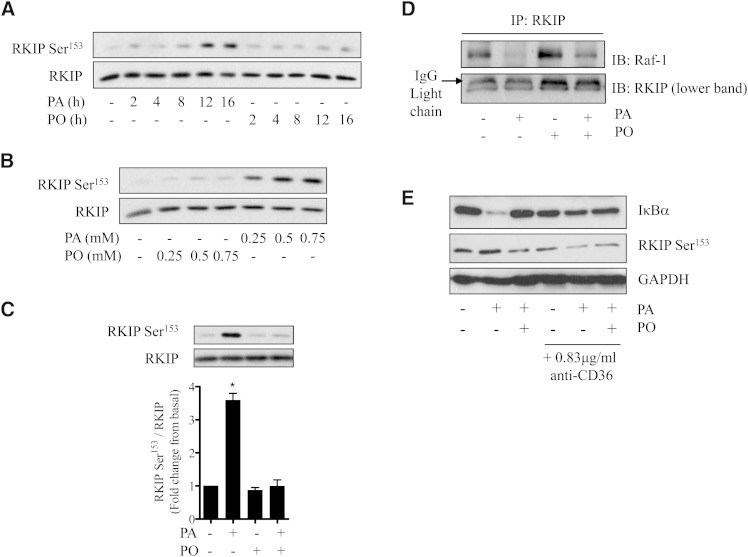
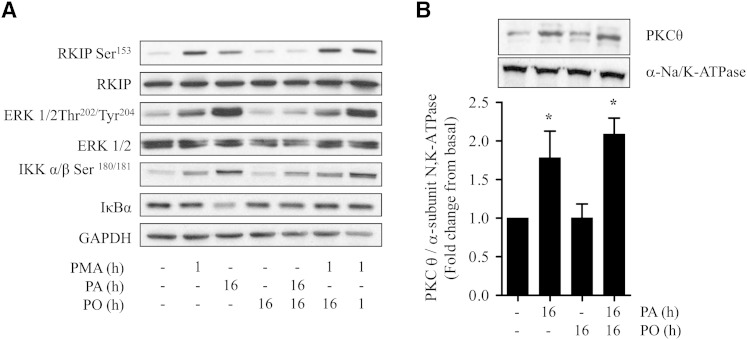
In an attempt to understand the mechanisms contributing to the differential effect that SFAs and MUFAs have upon the ERK-IKKα/β-NFκB signaling axis, we focused on regulation of molecules upstream of ERK. We have shown that intramyocellular accumulation of DAG is a significant feature associated with incubation of muscle cells with palmitate under conditions that result in proinflammatory NFκB signaling (14, 18). RKIP is an inhibitor of Raf1 activity and phosphorylation of RKIP on Ser153 by DAG-sensitive PKC promotes its dissociation from Raf1 and consequently alleviates RKIP-mediated inhibition of Raf1 (28, 29). Fig. 2A, B show that RKIP Ser153 phosphorylation is elevated in L6 muscle cells by palmitate in a time- and dose-dependent manner similar to that observed for ERK and IKKα/β (Fig. 1). This response was not observed in muscle cells treated with palmitoleate, but strikingly, the MUFA was able to fully repress RKIP phosphorylation on Ser153 when presented to cells at the same time as palmitate (Fig. 2C). Immunoprecipitation of RKIP from muscle cells that had not been exposed to fatty acids led to coimmunoprecipitation of Raf1 (Fig. 2D). However, significant depletion of Raf1 in RKIP immunoprecipitates from muscle cells that had been chronically incubated with palmitate was observed. Such depletion was not observed when muscle cells were incubated with palmitoleate, which was able to also repress loss of Raf1 in RKIP immunoprecipitates induced by palmitate (Fig. 2D).
Fig. 2.
Effects of palmitate and palmitoleate on RKIP phosphorylation and its association with Raf-1 in L6 muscle cells. L6 myotubes were (A) treated with PA and PO (each at 0.75 mM) for the times indicated, (B) incubated with fatty acids for 16 h at the stated concentrations, or (C, D) incubated for 16 h individually or in combination (each at 0.75 mM). Lysates were immunoblotted for RKIP Ser153 and total RKIP (A–C) or used for immunoprecipitating RKIP (D) and coprecipitation analysis of Raf-1. (E) L6 myotubes were treated in the absence or presence of PA and PO (each at 0.75 mM) and anti-CD36 (0.83 μg/ml) as indicated for 16 h. Cells were lysed, and lysates were immunoblotted for IκBα, RKIP Ser153, and GAPDH as indicated. Bars in (C) represent mean ± SEM from three separate experiments and asterisks signify a significant difference from the untreated control values (P < 0.05).
It is conceivable that the observed effects that palmitate has upon RKIP and IκBα shown in Figs. 1 and 2 may be initiated by signaling cascades that start at the cell membrane and do not require palmitate to enter the cell. To test this possibility, we investigated the effects of an antibody that inhibits palmitate uptake via the CD36 fatty acid transporter (36). Fig. 2E shows that while palmitate induces phosphorylation of RKIP on Ser153 and promotes loss of IκBα in L6 myotubes, both events were noticeably reduced in muscle cells that had been preincubated with anti-CD36 at concentrations previously reported to inhibit CD36-mediated fatty acid uptake (36). Antibody treatment did not modify palmitoleate action, consistent with the idea that the ability of this MUFA to repress the proinflammatory effect of palmitate was contingent on cellular fatty acid uptake.
Do DAG and DAG-sensitive PKCs play a role in palmitate-induced proinflammatory signaling?
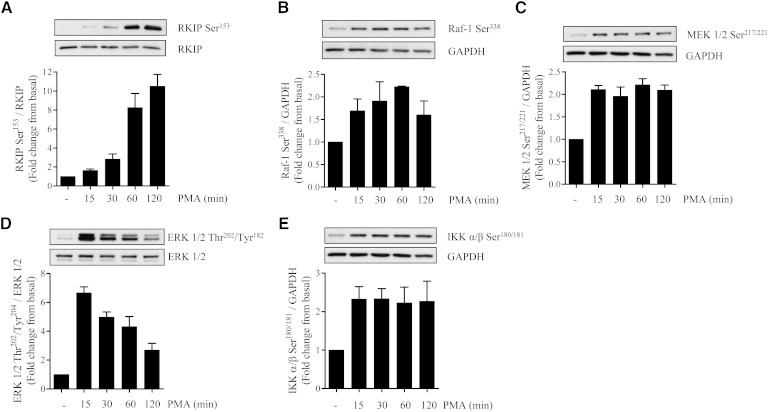
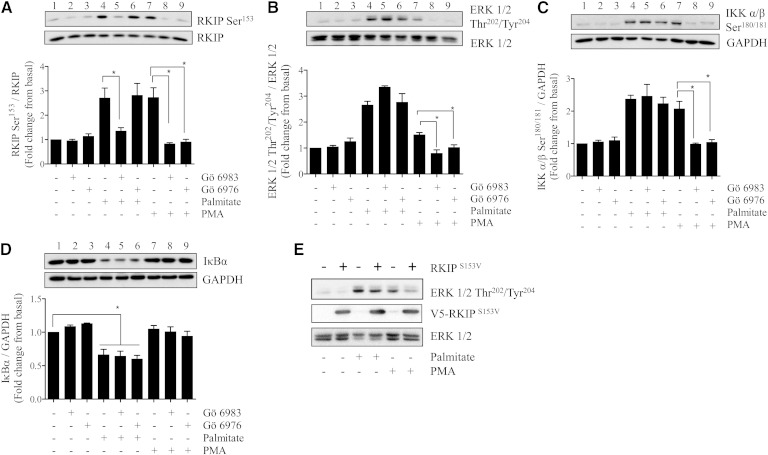
We hypothesized that if an increase in intramyocellular DAG and activation of DAG-sensitive PKCs were an important driver of palmitate-induced proinflammatory signaling, then it ought to be emulated by phorbol 12-myristate 13-acetate (PMA), a DAG mimetic. Fig. 3 shows that acute incubation of L6 myotubes with PMA for periods up to 2 h led to a rapid induction of RKIP Ser153 phosphorylation, which was associated with increased phosphorylation of Raf1 (on Ser338, a site supporting Raf1 activation (37)) and downstream activation of MEK, ERK, and IKKα/β. It is worth highlighting that, in separate experiments, combined exposure of muscle cells to palmitate and PMA did not induce any additive enhancement in RKIP Ser153 phosphorylation, suggesting that both stimuli are likely to act via a common mechanism (i.e., PKC activation) to promote RKIP phosphorylation (supplementary Fig. I-A). To assess the contribution of PKC to these signaling events, we subsequently tested the effect of two PKC inhibitors; Gö6983 (which inhibits both novel and conventional PKCs) and Gö6976 (which selectively targets conventional PKCs) (38). Fig. 4A shows that phosphorylation of RKIP in response to palmitate was suppressed by Gö6983 (compare lanes 4 and 5) but not by Gö6976 (compare lanes 4 and 6). However, despite the observed sensitivity of RKIP phosphorylation toward Gö6983, the inhibitor did not antagonize the fatty acid-induced phosphorylation of ERK or IKKα/β or curtail the loss of IκBα (Fig. 4B–D, compare lanes 4 and 5 in each panel). In contrast, both Gö6983 and Gö6976 were effective in negating the effects of PMA upon RKIP, ERK, and IKKα/β (Fig. 4A–D, compare lanes 8 and 9 with lane 7 in each panel). It should be stressed that PMA did not induce any appreciable proteosomal loss of IκBα in these experiments given that, unlike palmitate (which was present for 16 h), muscle cells were only exposed to PMA for 30 min in these studies, which is likely to be an insufficient period of time to observe depletion of this protein. The notion that RKIP may serve as an important intermediate in PKC-directed activation of the MEK-ERK pathway in response to PMA but that it is dispensable in response to palmitate is further supported by our finding that expression of dominant interfering RKIPSer153Val mutant (28) suppressed phosphorylation of ERK in response to PMA but not to palmitate (Fig. 4E).
Fig. 3.
PMA, a DAG mimetic and novel/conventional PKC activator, induces phosphorylation of RKIP and of the ERK-IKK signaling axis. L6 mytotubes were treated with 100 nM PMA for the times indicated. Cells were lysed and subsequently immunoblotted to assess the phosphorylation status of (A) RKIP, (B) Raf-1, (C) MEK 1/2, (D) ERK 1/2, and (E) IKKα/β. Phospho signals were either normalized to the unphosphorylated native protein or to GAPDH. Values shown are the mean ± SEM from three separate experiments.
Fig. 4.
Effects of Gö6983 and Gö6976, two PKC inhibitors, upon PMA and palmitate-induced phosphorylation of RKIP Ser153, ERK1/2 and IKK and IκBα abundance. Myotubes were exposed to PMA (100 nM, 30 min) and palmitate (0.75 mM, 16 h) in the absence or presence of either 1 μM Gö6983 or 1 μM Gö6976. At the end of these treatments, cells were lysed and lysates were immunoblotted to assess the phosphorylation status of (A) RKIP, (B) ERK 1/2, (C) IKKα/β, and (D) the total protein abundance of IκBα. Immunoblot signals were normalized to the abundance of the native protein of interest or GAPDH. Bars represent mean ± SEM from three separate experiments, and asterisks signify significant difference between the indicated bar values (P < 0.05). (E) L6 muscle cells (at day 4 of culture) were transduced with adenovirus containing or lacking (control) a V5-tagged RKIPS153V mutant and allowed to differentiate in culture until day 7. Infected L6 myotubes were subsequently incubated with either PMA (100 nM, 2 h) or with palmitate (0.75 mM, 16 h) prior to being lysed. Lysates were immunoblotted with antibodies to phospho-ERK1/2, native ERK1/2, or V5. Blots are representative from two separate experimental repeats.
Effects of palmitoleate upon proinflammatory signaling and PKC activation
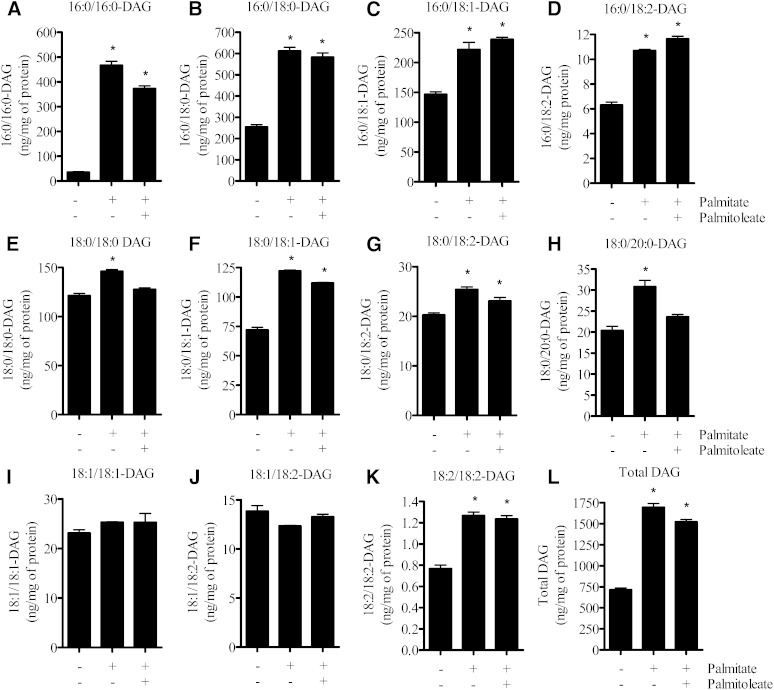
The findings presented in Fig. 4 imply that although palmitate induces activation of novel PKCs in L6 myotubes and these can mediate phosphorylation of RKIP, this signaling event appears not to be a crucial requirement for activation of the ERK-IKK proinflammatory pathway. To validate this proposition further, we compared the effect of palmitoleate on proinflammatory signaling induced by palmitate and PMA. Fig. 5A shows that while palmitoleate antagonizes palmitate-induced phosphorylation of RKIP, ERK, and IKKα/β, phosphorylation of these proteins in response to PMA was not suppressed by the MUFA. This latter observation suggests that the ability of palmitoleate to antagonize the proinflammatory effect of palmitate was unlikely to be the result of a targeted reduction in the activation of DAG-sensitive PKCs. This view is supported by the finding that PKCθ, a DAG-sensitive novel PKC that participates as an effector of fatty acid-induced signaling (39), was activated in response to palmitate as judged on the basis of its increased membrane association. However, unlike activation of the ERK-IKKα/β signaling axis, the SFA-induced activation of PKCθ could not be repressed by palmitoleate (Fig. 5B). In line with these findings, quantitative analysis of the fatty acid composition of different intramyocellular DAG species revealed that cell incubation with palmitate induced significant increases in DAG lipids with both saturated and unsaturated fatty acyl chains (Fig. 6A–K). Our analysis revealed that, with the exception of DAG lipids composed of fatty acyl chains with either two saturated C18:0 fatty acids (Fig. 6E) or with one C18:0 and one C20:0 fatty acid (Fig. 6H), the accumulation of other DAG species could not be suppressed by palmitoleate provision (Fig. 6).
Fig. 5.
Palmitoleate does not prevent PMA-induced activation of the RKIP/ERK signaling axis and does not block PKCθ activation in palmitate-treated cells. L6 myotubes were incubated with fatty acid-free BSA (vehicle) alone or with BSA conjugated for the times indicated with PA (0.75 mM), PO (0.75 mM), or PMA (100 nM) alone or in combination. Cells were harvested, and (A) total cell lysates were immunoblotted using antibodies against the proteins indicated or (B) total membranes were immunoblotted with antibodies against PKCθ and the α-subunit of the N,K-ATPase, a plasma membrane marker protein. The blots are representative of three separate experiments, and bars represent mean ± SEM from 3 separate experiments. Asterisks signify a significant difference from the control values (P < 0.05).
Fig. 6.
Effects of palmitate and palmitoleate upon intramyocellular abundance of different DAG species. L6 myotubes were incubated with palmitate (0.75 mM) and/or palmitoleate (0.75 mM) for 16 h. Following incubation, muscle cells were harvested in ice-cold PBS, and lipids were extracted to assess DAG content as described in Methods. Bars represent mean ± SEM from three separate experiments, and asterisks signify a significant difference from the untreated control values (P < 0.05).
Fatty acid uptake and the effect of palmitate and palmitoleate on cellular oxygen consumption, mitochondrial integrity, and cellular lipid deposition
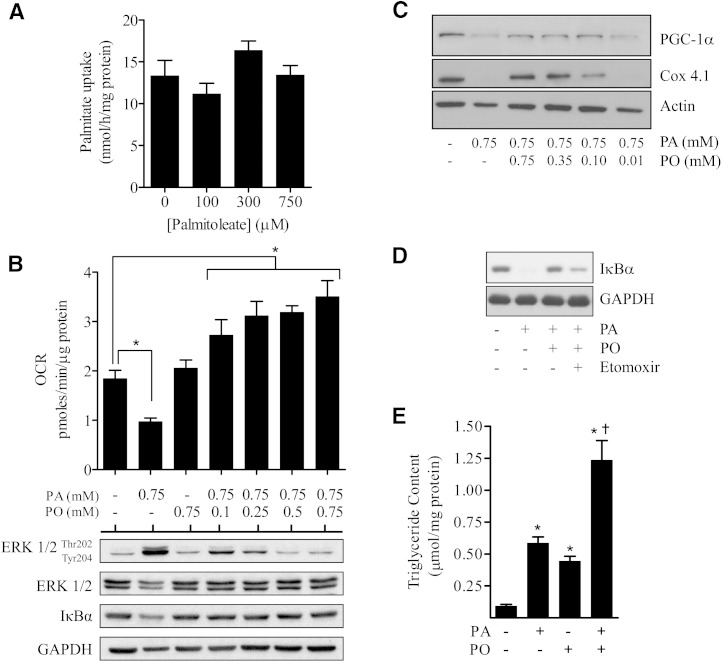
It is plausible that the ability of palmitoleate to suppress the proinflammatory potential of palmitate may be linked to a competitive reduction in palmitate uptake. However, Fig. 7A shows that palmitoleate had no significant impact upon palmitate uptake. In light of this finding, we postulated that an important factor contributing to the observed proinflammatory action of palmitate in myotubes may be the efficiency with which this SFA is oxidized when present for sustained periods at high concentrations. Analysis of the cellular OCR, which serves as an index of mitochondrial respiration, revealed that sustained (16 h) exposure of muscle cells to 0.75 mM palmitate reduced OCR by ∼50% (Fig. 7B). The reduced respiratory rate is associated with a marked impairment in mitochondrial integrity/function as reflected by a profound reduction in the abundance of Cox 4.1 (a subunit of cytochrome C-oxidase that functions within the mitochondrial respiratory chain) and cellular expression of PGC1α, which plays a major role in regulating mitochondrial biogenesis (Fig. 7C). It is noteworthy that such changes were not observed in response to acute or chronic PMA treatment, thus potentially excluding upstream effector molecules, such as PKC and RKIP, as contributors to mitochondrial dysfunction (supplementary Fig. I-B). Exposing muscle cells to palmitoleate had no detrimental effect on OCR (Fig. 7B), and intriguingly, when coincubated with palmitate, the MUFA induced a dose-dependent increase in OCR that was greater than that in cells treated with palmitoleate alone (Fig. 7B). Strikingly, under these circumstances, coprovision of palmitoleate antagonized the palmitate-induced loss in both Cox4.1 and PGC1α (Fig. 7C), which should serve to maintain mitochondrial integrity and function and thereby facilitate more efficient respiratory use of both palmitate and palmitoleate as fuels.
Fig. 7.
Fatty acid uptake and effects of palmitate and palmitoleate on oxygen consumption, mitochondrial protein expression, and accumulation of neutral lipids. (A) L6 myotubes were incubated in the absence and presence of palmitoleate (PO) at the indicated concentrations and uptake of [3H]palmitate determined as described in Methods. (B) L6 skeletal muscle cells were treated with the stated concentrations of fatty acid for 16 h, at which point cells were either used for analysis of oxygen consumption rate (OCR) which was assayed using Seahorse Bioscience technology as described in Methods, or L6 skeletal muscle cells lysed and lysates immunoblotted using antibodies against phospho or native proteins indicated. (C) L6 myotubes were treated with fatty acids at concentrations indicated as in (B) and lysates immunoblotted using antibodies against PGC1α and Cox 4.1. (D) Myotubes were incubated with fatty acids (0.75 mM) as indicated in the absence or presence of etomoxir (100 μM) prior to lysis and immunoblotting using antibodies against IκBα and GAPDH. (E) L6 muscle cells were treated with 0.75 mM PA and/or PO for 12 h prior to assaying TAG content as indicated in Methods. Bars represent mean ± SEM from three separate experiments. Asterisks signify a significant difference from the untreated control bar (P < 0.05), and the cross signifies a significant difference from the single fatty acid treatments alone (P < 0.05).
For correlative analysis, we also monitored the effect of titrating increasing concentrations of palmitoleate on palmitate-induced activation of ERK1/2 and upon loss of IκBα, and we found that palmitoleate concentrations as low as 0.1 mM can antagonize the effects of palmitate on these molecules (Fig. 7B). These observations imply that modest MUFA provision confers beneficial effects not only upon cellular respiration but also on proteins implicated in proinflammatory signaling and that enhanced fuel oxidation may help offset some of the lipotoxic effects associated with SFA oversupply. Indeed, the finding that inhibiting mitochondrial fatty acid uptake using etomoxir blunted palmitoleate's ability to fully antagonize loss of IκBα in response to palmitate underscores the importance of sustaining mitochondrial function (Fig. 7D).
In addition to promoting cellular respiration and maintaining mitochondrial function, palmitoleate provision may induce metabolic benefits by facilitating increased storage of fatty acids as neutral lipid. To assess this, we quantified intramyocellular triglyceride content and found that while treatment with palmitate or palmitoleate alone induced a significant increase in neutral lipid, it was enhanced in a greater than additive manner when myotubes were coincubated with both fatty acids together (Fig. 7E).
DISCUSSION
Studies in rodent models of obesity indicate that chronic, low-grade inflammation is a characteristic feature of the obese state that may, in part, be a consequence of a sustained increase in the circulating concentration of SFAs (e.g., palmitate) (40, 41), which can induce a potent inflammatory response in numerous tissues, including skeletal muscle. By contrast, MUFAs do not elicit this response and, intriguingly, are able to counter the proinflammatory effect of SFAs by as yet poorly understood mechanisms. Delineating how MUFAs modulate SFA-induced inflammatory tone in skeletal muscle in vivo is a major challenge given that, in addition to direct effects that SFAs may have upon muscle, tissue responses may be confounded by other systemic consequences of obesity. These include, for example, changes in adipokine levels (TNFα, IL-6, and IL-1β) (42), interorgan nutrient flow, and activation of both resident and infiltrating macrophages (43) that could affect diverse signaling pathways within muscle. Thus, in an attempt to gain a clearer understanding of how MUFAs may antagonize palmitate-induced proinflammatory signaling in muscle, this study utilized an established cultured rat skeletal muscle cell line (L6) that exhibits a robust SFA-induced proinflammatory response (18).
We recently reported that chronic exposure of L6 skeletal muscle cells to palmitate induces proinflammatory NFκB signaling in a manner dependent upon the upstream activation of the MEK-ERK signaling axis (18). Given that a rise in intramyocellular DAG is a major outcome associated with sustained oversupply of palmitate, we postulated that DAG-sensitive PKCs may feature prominently in the activation of the Raf-MEK-ERK pathway, a supposition based on evidence in the literature showing that PKCs can promote activation of this pathway in a number of different cell types (26, 28, 44). One mechanism linking activated PKCs to the MAP kinase cascade involves modulation of RKIP, a molecule whose association with Raf1 helps maintain the kinase in a repressed state (28). PKC-mediated phosphorylation of RKIP on Ser153 results in its dissociation from Raf1, thus facilitating activation of MEK-ERK signaling. Consistent with such a mechanism, we show that cell treatment with palmitate or the DAG mimetic PMA results in phosphorylation of RKIPSer153 and subsequent activation of Raf1, MEK, ERK, and IKK. Intriguingly, phosphorylation of RKIP induced in response to palmitate and PMA displays differential sensitivity to Gö6983 and Gö6976, the two PKC inhibitors used in our study. Both inhibitors suppress the PMA-induced phosphorylation of RKIP, but only Gö6983 inhibited phosphorylation of RKIP in response to palmitate. Since Gö6983 inhibits both novel and conventional PKC isoforms and Gö6976 is considered more selective against conventional PKC isoforms (45, 46), our findings imply that palmitate-induced phosphorylation of RKIP is mediated by novel PKCs, whereas PMA invokes a broad-spectrum response involving both novel and conventional PKC isoforms. Surprisingly, our findings indicate that the PKC-mediated phosphorylation of RKIP triggered by palmitate is dispensable with respect to the downstream activation of ERK and IKK, unlike the response to PMA. This proposition is based on two separate lines of evidence. First, while PKC inhibitors suppress RKIPSer153 phosphorylation in response to both PMA and palmitate, the downstream activation of ERK was only halted in response to PMA. Second, stable overexpression of a phosphorylation-resistant dominant negative RKIP mutant (RKIPSer153Val) only suppresses PMA and not palmitate-induced ERK activation. Collectively, these observations imply that while the Raf-MEK-ERK signaling axis can be stimulated in a PKC- and RKIP-dependent manner by PMA and palmitate, the stimulation of this pathway in response to palmitate is not exclusively mediated via the PKC-RKIP-Raf link. Our observations thus implicate a separate, as yet undefined, palmitate-derived signal that feeds into the MEK-ERK cascade at a point beyond RKIP/Raf that helps sustain palmitate's proinflammatory signaling potential.
Previous studies have demonstrated that oleate (a C18:1 MUFA) reduces intramyocellular DAG accumulated in response to palmitate oversupply (6). In such studies, DAG content was determined by assaying incorporation of radiolabeled fatty acids into lipid extracts that were resolved by thin layer chromatography, which does not allow quantification of different DAG species. In contrast, the present study utilized an ESI mass spectrometry-based approach that allows profiling and quantification of multiple DAG species. Our analysis focused on the most abundant DAG species and revealed, somewhat surprisingly, that while chronic exposure of L6 myotubes to palmitate induced a greater than 2-fold increase in total DAG content, cosupplementation of palmitoleate did not significantly reduce total DAG abundance. Intriguingly, however, the MUFA did promote a selective reduction in the palmitate-induced increase of C18:0/C18:0 DAG and C18:0/C20:0 DAG. Since DAG-sensitive PKCs mediate phosphorylation of RKIPSer153 and palmitoleate blocks SFA-induced phosphorylation of this site, it is tempting to speculate that increases in these two DAG species may specifically participate in palmitate-induced regulation of RKIP. If so, given that abundance of numerous other DAG species in response to palmitate was not altered by palmitoleate, our results may indicate that activation of novel PKCs (induced by increases in C18:0/C18:0 DAG and C18:0/C20:0 DAG) and phosphorylation of RKIP may occur in specific cell compartments. We cannot exclude the possibility that other MUFAs, such as oleate, may exert a broader suppressive effect on SFA-induced accumulation of different DAG lipids that may potentially help explain the discrepancy between our findings and those reported in the literature (6). In any event, our data indicate that the ability of palmitoleate to inhibit the proinflammatory potential of palmitate in muscle cells is independent of any suppressive effect the MUFA has upon DAG-mediated RKIP modulation.
Increased provision of SFAs is also known to promote intramyocellular accumulation of ceramide and induce endoplasmic reticulum (ER) stress, both of which have been implicated in the cellular proinflammatory response (47, 48). However, while we find that sustained exposure of L6 myotubes to palmitate elevates total cell ceramides (attributable to specific increases in numerous long-chain ceramide species), this increase, unlike the increase in NFκB-mediated proinflammatory signaling, is not antagonized by palmitoleate (supplementary Fig. II). This finding is consistent with our previous work showing that sustained (16 h), low-grade exposure to 10 μM C2 ceramide or short (2 h) exposure to 100 μM C2 ceramide fails to increase proinflammatory signaling in muscle cells (18). In line with work of other groups (48), we also find that palmitate induces expression of ER stress markers ATF3 and CHOP. Expression of these stress genes was also markedly enhanced in response to thapsigargin, a known ER stress-inducing agent. Intriguingly, however, while palmitoleate repressed palmitate-induced expression of ATF3 and CHOP, the MUFA failed to avert expression of these ER stress markers in response to thapsigargin (supplementary Fig. III). Moreover, cotreatment of muscle cells with palmitate and two separate ER stress inhibitors (4-phenylbutyric acid and taurourssodeoxycholic acid) at concentrations previously shown to prevent ER stress in L6 myotubes (49) failed to suppress palmitate-induced IκBα loss or IL-6 gene expression (supplementary Fig. IV). These observations collectively suggest that, while ER stress is a feature associated with palmitate overprovision, it is unlikely to be a major driver of palmitate's proinflammatory potential in muscle cells.
While numerous studies have demonstrated that increased fatty acid availability results in elevated intramyocellular lipid deposition and that this correlates with loss of skeletal muscle insulin sensitivity (50–53), there is also evidence that suggests that intramuscular accumulation of neutral lipid (triacylglycerol, TAG) need not be lipotoxic or insulin desensitizing (54–56). Such observations have invariably been reported in skeletal muscle of chronically exercised individuals who remain markedly insulin-sensitive despite having high intramyocellular triglycerides (i.e., the so called “athletes paradox”) and most likely reflects an adaptive state in which skeletal muscle of such individuals has become more efficient in “turning over” stored lipid (57). Partitioning of fatty acids into storage or oxidative pathways is, however, a highly variable process. Compared with MUFAs, SFAs have been shown to be less well oxidized (58) and more poorly incorporated into TAG (56), but intriguingly, when copresented with MUFAs, their esterification into TAG (6, 59) as well as their mitochondrial oxidation has been shown to be enhanced (6). We found that muscle cells incubated with palmitate exhibited a lower OCR than those exposed to palmitoleate alone but that cellular respiration and TAG accumulation was augmented in the presence of both fatty acids together. The reduced oxidative capacity associated with oversupply of SFA is likely to be a consequence of mitochondrial dysfunction given that i) we observed a reduction in Cox4.1 and PGC1α (two proteins with important but distinct roles in mitochondrial biology) and ii) recent studies show that palmitate causes mitochondrial damage/fragmentation in muscle cells by inducing a greater inflammatory drive, insulin resistance, and apoptosis (60, 61). In contrast, promoting oxidation and/or storage of SFAs by coprovision of MUFAs reduces the proapoptotic drive of palmitate (58), and as we and others (6) found, it also suppresses NFκB-mediated proinflammatory signaling.
In summary, the current study excludes any direct involvement of DAG-sensitive PKCs and RKIP in the palmitate-induced activation of the MEK-ERK-IKK proinflammatory signaling axis in L6 myotubes. Our results suggest that it is highly likely that the heightened proinflammatory response triggered by palmitate oversupply is primarily a manifestation of not being able to effectively oxidize and/or channel the fatty acid for storage as neutral triglyceride. These findings add to the growing body of evidence showing that the presence of MUFA stimulates metabolic handling of palmitate and that this helps subvert some of the lipotoxic (i.e., proinflammatory) effects associated with palmitate overload. Precisely how MUFAs achieve this protective effect remains unclear, but recent work in SRD-13A cells (a CHO-derived cell line) has identified the presence of Ubxd8, a protein capable of sensing availability of unsaturated fatty acids and regulating triglyceride synthesis (62). Ubxd8 is an ubiquitin-like (UBX) domain containing protein involved in ER-associated degradation of Insig-1, a protein involved in ER membrane retention of sterol regulatory element binding protein-1 (SREBP-1). SREBP-1 functions as a transcription factor regulating expression of numerous genes involved in lipid homeostasis. Under conditions of low fatty acid availability when synthesis of TAG would be low, Ubxd8 promotes degradation of Insig-1, thus enabling SREBP1 to be transported to the golgi where it is cleaved and then routed to the nucleus to activate genes promoting fatty acid synthesis. However, the presence of unsaturated fatty acids leads to oligomerization and inactivation of Ubxd8, thereby preserving Insig1 and promoting ER retention of SREBP1. Under such circumstances, expression of genes supporting fatty acid synthesis would be suppressed in favor of a greater shift toward TAG and lipid droplet formation (62). Intriguingly, SFAs do not bind to or promote oligomerization of Ubxd8 and are very inefficient in repressing Ubxd8, but their incorporation into TAG is likely to be enhanced when presented to cells alongside unsaturated fatty acids. It is currently unknown whether Ubxd8 or closely related homologs are expressed in skeletal muscle and whether they function in a similar manner to that reported in SRD-13A cells, but it is conceivable that pharmacological modulation of such a sensor may induce dynamic changes in fatty acid metabolism that could help combat lipid-induced metabolic disorders.
Supplementary Material
Footnotes
Abbreviations:
- CINC-1
- cytokine-induced neutrophil chemoattractant-1
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- IκB
- inhibitor of NFκB
- IKK
- inhibitor of NFκB kinase
- IL-6
- interleukin 6
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen/ERK-activated protein kinase
- NFκB
- nuclear factor kappa-B
- OCR
- oxygen consumption rate
- PA
- palmitate
- PKB
- protein kinase B
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- PO
- palmitoleate
- RKIP
- raf-kinase inhibitor protein
- TLR
- Toll-like receptor
- TNFα
- tumor necrosis factor α
This work was supported by a Biotechnology and Biological Sciences Research Council (BBSRC)/Industrial (AstraZeneca) CASE Quota Award, by BBSRC, and by Diabetes UK.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of seven figures.
REFERENCES
- 1.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 2.Boden G. 2006. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab. Rep. 6: 177–181. [DOI] [PubMed] [Google Scholar]
- 3.Green C. J., Pedersen M., Pedersen B. K., Scheele C. 2011. Elevated NF-kappaB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes. 60: 2810–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden G., Shulman G. I. 2002. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 32(Suppl. 3): 14–23. [DOI] [PubMed] [Google Scholar]
- 5.Lee J. S., Pinnamaneni S. K., Eo S. J., Cho I. H., Pyo J. H., Kim C. K., Sinclair A. J., Febbraio M. A., Watt M. J. 2006. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J. Appl. Physiol. 100: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 6.Coll T., Eyre E., Rodriguez-Calvo R., Palomer X., Sanchez R. M., Merlos M., Laguna J. C., Vazquez-Carrera M. 2008. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 283: 11107–11116. [DOI] [PubMed] [Google Scholar]
- 7.Kadotani A., Tsuchiya Y., Hatakeyama H., Katagiri H., Kanzaki M. 2009. Different impacts of saturated and unsaturated free fatty acids on COX-2 expression in C2C12 myotubes. Am. J. Physiol. Endocrinol. Metab. 297: E1291–E1303. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos N., Watson M., Sakamoto K., Hundal H. S. 2006. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem. J. 399: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vessby B., Unsitupa M., Hermansen K., Riccardi G., Rivellese A. A., Tapsell L. C., Nalsen C., Berglund L., Louheranta A., Rasmussen B. M., et al. 2001. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 44: 312–319. [DOI] [PubMed] [Google Scholar]
- 10.Peng G., Li L., Liu Y., Pu J., Zhang S., Yu J., Zhao J., Liu P. 2011. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology. 152: 2206–2218. [DOI] [PubMed] [Google Scholar]
- 11.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., Hotamisligil G. S. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyton J., Drury P. J., Crawford M. A. 1987. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 57: 383–393. [DOI] [PubMed] [Google Scholar]
- 13.Gaster M., Rustan A. C., Beck-Nielsen H. 2005. Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes. 54: 648–656. [DOI] [PubMed] [Google Scholar]
- 14.Powell D. J., Turban S., Gray A., Hajduch E., Hundal H. S. 2004. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem. J. 382: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., Summers S. A. 2003. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 278: 10297–10303. [DOI] [PubMed] [Google Scholar]
- 16.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277: 50230–50236. [DOI] [PubMed] [Google Scholar]
- 17.Powell D. J., Hajduch E., Kular G., Hundal H. S. 2003. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 23: 7794–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green C. J., Macrae K., Fogarty S., Hardie D. G., Sakamoto K., Hundal H. S. 2011. Counter-modulation of fatty acid-induced pro-inflammatory nuclear factor kappaB signaling in rat skeletal muscle cells by AMP-activated protein kinase. Biochem. J. 435: 463–474. [DOI] [PubMed] [Google Scholar]
- 19.Coll T., Alvarez-Guardia D., Barroso E., Gomez-Foix A. M., Palomer X., Laguna J. C., Vazquez-Carrera M. 2010. Activation of peroxisome proliferator-activated receptor-{delta} by GW501516 prevents fatty acid-induced nuclear factor-{kappa}B activation and insulin resistance in skeletal muscle cells. Endocrinology. 151: 1560–1569. [DOI] [PubMed] [Google Scholar]
- 20.Senn J. J. 2006. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J. Biol. Chem. 281: 26865–26875. [DOI] [PubMed] [Google Scholar]
- 21.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barma P., Bhattacharya S., Bhattacharya A., Kundu R., Dasgupta S., Biswas A., Bhattacharya S., Roy S. S., Bhattacharya S. 2009. Lipid induced overexpression of NF-kappaB in skeletal muscle cells is linked to insulin resistance. Biochim. Biophys. Acta. 1792: 190–200. [DOI] [PubMed] [Google Scholar]
- 23.Jove M., Planavila A., Sanchez R. M., Merlos M., Laguna J. C., Vazquez-Carrera M. 2006. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology. 147: 552–561. [DOI] [PubMed] [Google Scholar]
- 24.Hoareau L., Bencharif K., Rondeau P., Murumalla R., Ravanan P., Tallet F., Delarue P., Cesari M., Roche R., Festy F. 2010. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J. Inflamm. (Lond.). 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson M. L., Coghlan M., Hundal H. S. 2009. Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem. J. 417: 791–801. [DOI] [PubMed] [Google Scholar]
- 26.Kang D. W., Park M. H., Lee Y. J., Kim H. S., Kwon T. K., Park W. S., Min S. 2008. Phorbol ester up-regulates phospholipase D1 but not phospholipase D2 expression through a PKC/Ras/ERK/NFkappaB-dependent pathway and enhances matrix metalloproteinase-9 secretion in colon cancer cells. J. Biol. Chem. 283: 4094–4104. [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Wang Q., Hu W., Evers B. M. 2004. Regulation of phorbol ester-mediated TRAF1 induction in human colon cancer cells through a PKC/RAF/ERK/NF-kappaB-dependent pathway. Oncogene. 23: 1885–1895. [DOI] [PubMed] [Google Scholar]
- 28.Corbit K. C., Trakul N., Eves E. M., Diaz B., Marshall M., Rosner M. R. 2003. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J. Biol. Chem. 278: 13061–13068. [DOI] [PubMed] [Google Scholar]
- 29.Hagan S., Garcia R., Dhillon A., Kolch W. 2006. Raf kinase inhibitor protein regulation of raf and MAPK signaling. Methods Enzymol. 407: 248–259. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz K., Lohse M. J., Quitterer U. 2003. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 426: 574–579. [DOI] [PubMed] [Google Scholar]
- 31.Hajduch E., Alessi D. R., Hemmings B. A., Hundal H. S. 1998. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 47: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 32.Yeung K., Seitz T., Li S., Janosch P., McFerran B., Kaiser C., Fee F., Katsanakis K. D., Rose D. W., Mischak H., et al. 1999. Suppression of Raf-1 kinase activity and MAP kinase signaling by RKIP. Nature. 401: 173–177. [DOI] [PubMed] [Google Scholar]
- 33.Blachnio-Zabielska A. U., Persson X. M., Koutsari C., Zabielski P., Jensen M. D. 2012. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun. Mass Spectrom. 26: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein B., Yang M. X. 1995. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 15: 4971–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaishi K., Ohtsuka T., Tsuneyoshi S., Maehara N., Harada M., Yoshida H., Watanabe K., Tsurufuji S. 2000. Inhibition of the production of rat cytokine-induced neutrophil chemoattractant (CINC)-1, a member of the interleukin-8 family, by adenovirus-mediated overexpression of IkappaBalpha. J. Biochem. 127: 511–516. [DOI] [PubMed] [Google Scholar]
- 36.Angin Y., Steinbusch L. K., Simons P. J., Greulich S., Hoebers N. T., Douma K., van Zandvoort M. A., Coumans W. A., Wijnen W., Diamant M., et al. 2012. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem. J. 448: 43–53. [DOI] [PubMed] [Google Scholar]
- 37.Zang M., Gong J., Luo L., Zhou J., Xiang X., Huang W., Huang Q., Luo X., Olbrot M., Peng Y., et al. 2008. Characterization of Ser338 phosphorylation for Raf-1 activation. J. Biol. Chem. 283: 31429–31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kewalramani G., Fink L. N., Asadi F., Klip A. 2011. Palmitate-activated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase C theta and epsilon. PLoS ONE. 6: e26947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Idris I., Gray S., Donnelly R. 2001. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 44: 659–673. [DOI] [PubMed] [Google Scholar]
- 40.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cintra D. E., Ropelle E. R., Moraes J. C., Pauli J. R., Morari J., Souza C. T., Grimaldi R., Stahl M., Carvalheira J. B., Saad M. J., et al. 2012. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE. 7: e30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piya M. K., McTernan P. G., Kumar S. 2013. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J. Endocrinol. 216: T1–T15. [DOI] [PubMed] [Google Scholar]
- 43.Pillon N. J., Bilan P. J., Fink L. N., Klip A. 2013. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. Metab. 304: E453–E465. [DOI] [PubMed] [Google Scholar]
- 44.Krueger F., Madeja Z., Hemberger M., McMahon M., Cook S. J., Gaunt S. J. 2009. Down-regulation of Cdx2 in colorectal carcinoma cells by the Raf-MEK-ERK 1/2 pathway. Cell. Signal. 21: 1846–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gschwendt M., Dieterich S., Rennecke J., Kittstein W., Mueller H. J., Johannes F. J. 1996. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 392: 77–80. [DOI] [PubMed] [Google Scholar]
- 46.Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marme D., Schachtele C. 1993. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268: 9194–9197. [PubMed] [Google Scholar]
- 47.Boon J., Hoy A. J., Stark R., Brown R. D., Meex R. C., Henstridge D. C., Schenk S., Meikle P. J., Horowitz J. F., Kingwell B. A., et al. 2013. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 62: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvado L., Coll T., Gomez-Foix A. M., Salmeron E., Barroso E., Palomer X., Vazquez-Carrera M. 2013. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 56: 1372–1382. [DOI] [PubMed] [Google Scholar]
- 49.Raciti G. A., Iadicicco C., Ulianich L., Vind B. F., Gaster M., Andreozzi F., Longo M., Teperino R., Ungaro P., Di J. B., et al. 2010. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia. 53: 955–965. [DOI] [PubMed] [Google Scholar]
- 50.Manco M., Mingrone G., Greco A. V., Capristo E., Gniuli D., De G. A., Gasbarrini G. 2000. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism. 49: 220–224. [DOI] [PubMed] [Google Scholar]
- 51.Boden G., Lebed B., Schatz M., Homko C., Lemieux S. 2001. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 50: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 52.Bachmann O. P., Dahl D. B., Brechtel K., Machann J., Haap M., Maier T., Loviscach M., Stumvoll M., Claussen C. D., Schick F., et al. 2001. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 50: 2579–2584. [DOI] [PubMed] [Google Scholar]
- 53.Pan D. A., Lillioja S., Kriketos A. D., Milner M. R., Baur L. A., Bogardus C., Jenkins A. B., Storlien L. H. 1997. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 46: 983–988. [DOI] [PubMed] [Google Scholar]
- 54.Goodpaster B. H., He J., Watkins S., Kelley D. E. 2001. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86: 5755–5761. [DOI] [PubMed] [Google Scholar]
- 55.Schenk S., Cook J. N., Kaufman A. E., Horowitz J. F. 2005. Postexercise insulin sensitivity is not impaired after an overnight lipid infusion. Am. J. Physiol. Endocrinol. Metab. 288: E519–E525. [DOI] [PubMed] [Google Scholar]
- 56.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr, Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corcoran M. P., Lamon-Fava S., Fielding R. A. 2007. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 85: 662–677. [DOI] [PubMed] [Google Scholar]
- 58.Henique C., Mansouri A., Fumey G., Lenoir V., Girard J., Bouillaud F., Prip-Buus C., Cohen I. 2010. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 285: 36818–36827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montell E., Turini M., Marotta M., Roberts M., Noe V., Ciudad C. J., Mace K., Gomez-Foix A. M. 2001. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am. J. Physiol. Endocrinol. Metab. 280: E229–E237. [DOI] [PubMed] [Google Scholar]
- 60.Yuzefovych L. V., Solodushko V. A., Wilson G. L., Rachek L. I. 2012. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology. 153: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jheng H. F., Tsai P. J., Guo S. M., Kuo L. H., Chang C. S., Su I. J., Chang C. R., Tsai Y. S. 2012. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 32: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J. N., Kim H., Yao H., Chen Y., Weng K., Ye J. 2010. Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc. Natl. Acad. Sci. USA. 107: 21424–21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.