Abstract
Adipocyte differentiation is a multistep program under regulation by several factors. Peroxisome proliferator-activated receptor γ (PPARγ) serves as a master regulator of adipogenesis. However, the endogenous ligand for PPARγ remained elusive until 15-keto-PGE2 was identified recently as an endogenous PPARγ ligand. In this study, we demonstrate that zinc-containing alcohol dehydrogenase 2 (ZADH2; here termed prostaglandin reductase-3, PTGR-3) is a new member of prostaglandin reductase family that converts 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2. Adipogenesis is accelerated when endogenous PTGR-3 is silenced in 3T3-L1 preadipocytes, whereas forced expression of PTGR-3 significantly decreases adipogenesis. PTGR-3 expression decreased during adipocyte differentiation, accompanied by an increased level of 15-keto-PGE2. 15-keto-PGE2 exerts a potent proadipogenic effect by enhancing PPARγ activity, whereas overexpression of PTGR-3 in 3T3-L1 preadipocytes markedly suppressed the proadipogenic effect of 15-keto-PGE2 by repressing PPARγ activity. Taken together, these findings demonstrate for the first time that PTGR-3 is a novel 15-oxoprostaglandin-Δ13-reductase and plays a critical role in modulation of normal adipocyte differentiation via regulation of PPARγ activity. Thus, modulation of PTGR-3 might provide a novel avenue for treating obesity and related metabolic disorders.
Keywords: adipocyte differentiation, nuclear receptor, ligand, eicosanoid
The conversion of preadipocytes to adipocytes is regulated by several transcription factors. These factors promote cell morphologic conversion, lipogenic gene expression, and triacylglycerol accumulation. Peroxisome proliferator-activated receptor γ (PPARγ) has been clearly demonstrated to be a master regulator in adipogenesis. To activate PPARγ, a ligand is needed for binding its ligand-binding domain. Thiazolidinedione, an antidiabetic drug, has been known as a potent ligand for PPARγ. However, the natural ligands for PPARγ need to be elucidated.
Prostaglandins are eicosanoid lipid mediators derived from arachidonic acid that are involved in a variety of physiological functions. It has been demonstrated that prostaglandins are potential endogenous ligands for PPARγ that modulate adipocyte differentiation. Several candidates have been identified as potent endogenous ligands for PPARγ, including 15-deoxy-Δ12.14-prostaglandin J2 (15d-PGJ2) (1) and 15-keto-prostaglandin E2 (15-keto-PGE2) (2). We previously reported that the prostaglandin reductase-2 (PTGR-2; previously known as zinc-binding alcohol dehydrogenase domain containing 1, ZADH1) is a 15-oxoprostaglandin-Δ13-reductase that converts 15-keto-PGE2 to inactive 13,14-dihydro-15-keto-PGE2, thereby suppressing PPARγ transcriptional activity and inhibiting adipogenesis (2). PTGR-2 belongs to the zinc-binding alcohol dehydrogenase (ZADH) gene family. In mammal, the ZADH family consists of three members: PTGR-1 (ZADH3), PTGR-2, and PTGR-3 (ZADH2). PTGR-2 is expressed at high levels in white adipose tissue with known 15-oxoprostaglandin-Δ13-reductase activity (2). PTGR-1 is expressed at high levels in kidney and plays a role in preventing cancer growth in vitro and in vivo (3, 4). It has been demonstrated that PTGR-1 is a bifunctional enzyme capable of utilizing leukotriene B4 and 15-keto-prostaglandins as substrates (5). The protein structure of PTGR-3 is computationally predicted to contain an oxidoreductase catalytic site located between amino acids 184 and 320 based on sequence homology and shares 23% amino acid homology to PTGR-2. However, the function of PTGR-3 has not yet been investigated.
In this study, we demonstrate for first time that PTGR-3 is a 15-oxoprostaglandin-Δ13-reductase that converts 15-keto-PGE2 to inactive 13,14-dihydro-15-keto-PGE2, thereby decreasing adipogenesis through regulation of PPARγ transcriptional activity. PTGR-3 was highly expressed in 3T3-L1 preadipocytes, but it decreased during differentiation into adipocytes. Furthermore, PTGR-3 expression was reduced in white adipose tissue of murine models of obesity. These data indicate PTGR-3 is a critical modulator of adiposity.
MATERIALS AND METHODS
Cell culture
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified otherwise. The 3T3-L1 preadipocytes were cultured in Dulbecco modified Eagle's medium (DMEM) with 10% calf serum (CS) at 37°C in an atmosphere of 5% CO2. To induce adipocyte differentiation, confluent cells were cultured in induction medium (DMEM containing 10% FBS, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 1 μg/ml insulin). After 2 days, the cells were maintained in DMEM containing 10% FBS and 1 μg/ml insulin. Two days later, cells were cultured in DMEM containing 10% FBS for 8 days, with a medium change every 2 day. After 8 days of culturing, cells on the plates were stained with Oil Red O to measure the degree of adipocyte differentiation. Total protein lysate was extracted to determine the protein levels of adipogenic genes whose expression increases during adipocyte differentiation: adipocyte fatty acid-binding protein (aP2) and adiponectin.
Plasmids
Expression vector for GAL4-DBD fusion of PPARγ-LBD (CMX-GAL4-PPARγ), CMX-GAL4, and UASG×4-TK-LUC reporter plasmids were generous gifts from R. M. Evans (Howard Hughes Medical Institute, the Salk Institute for Biological Studies, La Jolla, CA). The Aox-TK-luciferase reporter plasmid, which contains three PPRE sites (PPRE-LUC), and the TK-LUC reporter plasmid were kindly provided by C. K. Glass (University of California San Diego, La Jolla, CA). Mouse PTGR-3 cDNA (Flag-PTGR-3) expression vector and control empty vector were purchased from OriGene (Rockville, MD).
Lentiviral infection
Small hairpin RNA (shRNA) plasmids for the mouse PTGR-3 gene and the control vector (shLuc) were obtained from the National RNAi Core Facility of Academia Sinica in Taiwan. For PTGR-3 knockdown, confluent 3T3-L1 preadipocytes were infected with lentivirus carrying control shRNA and shRNA targeting PTGR-3, respectively. After lentiviral infection, cells were selected by antibiotic selection using puromycin for 2 days. Two days later, cells were isolated to culture cells for further propagation. For the PTGR-3 overexpression experiment, confluent 3T3-L1 preadipocytes were infected with lentivirus carrying empty vector (pLVX-IRES-neo, Clontech, Mountain View, CA) and PTGR-3, respectively. After lentiviral infection, cells were maintained in selective medium containing the G418 antibiotics. Cell lines stably expressing empty vector or PTGR-3 were established by antibiotic selection. The selection was continued for at least 1 month. Individual colonies were isolated to culture cells for further propagation. Detailed protocols for lentivirus production and infection of cells were performed following the procedures of the National RNAi Core Facility of Academia Sinica in Taiwan.
In vitro enzymatic reaction
All reagents were purchased from Sigma-Aldrich (St. Louis, MO). Colorimetric method was used to determine PTGR-3 enzyme activity as described previously (2). Briefly, PTGR-3 recombinant protein (OriGene, Rockville, MD) was incubated with 0.5 mM NADPH and 0.6 mM prostaglandins (13,14-dihydro-15-keto-PGE2, 15-keto-PGE2, 15-keto-PGF2α, 15-keto-PGF1α, or 15-keto-PGE1) in 0.1 M Tris-HCl (pH 7.4) at 37°C for 30 min. After enzymatic reaction, color reagent (790 μM indonitrotetrazolium chloride, 60 μM phenazene methosulfate, and 1% Tween 20) was added and incubated at 37°C in the dark for 10 min. To stop the reaction, phthalate buffer (pH 3.0) was added. PTGR-3 activity was determined by measuring the absorbance of formazans, an indicator of remaining NAPDH, at 490 nm with a spectrophotometer. One unit of the enzyme was defined as the amount of enzyme catalyzing the production of 1 μmol NADP+/min. The apparent Km and Vmax values were determined using the Michaelis-Menten equation and calculated by nonlinear regression.
LC-MS/MS analysis of prostaglandins
Details for the prostaglandin extraction and derivation were described by Chou et al. (2). Deuterated 13,14-dihydro-15-keto-PGE2 standard (Cayman Chemical, Ann Arbor, MI) was added to homogenized samples as an internal control. Chromatographic analyses were performed using Accela UHPLC (Thermo Scientific, Hemel Hempstead, UK) and Acquity UPLC systems (Waters, Hertsfordshire, UK). The samples were separated on a C18 reversed-phase LC column (Phenomenex Luna, 150 mm × 2 mm × 3 μm) using a linear mobile phase gradient (A) water and (B) 5% methanol/95% acetonitrile. Mass spectrometry analyses were performed on LTQ Velos (Thermo Scientific) linear ion trap (LIT)-orbitrap and QTRAP 4000 (AB Sciex, Concord, ON, Canada) quadrupole-linear ion trap (QqLIT) mass spectrometers.
Transient transfection and reporter assay
For 3T3-L1 preadipocyte transfection, confluent cells were cotransfected with the PPRE×3-TK-LUC plasmid and phRG-TK plasmid (an internal control) by lipofection (Lipofectamine 2000, Invitrogen, Carlsbad, CA). 3T3-L1 preadipocytes, which were transfected with the TK-LUC reporter plasmid (without the PPRE×3 promoter), were the control group. One day after transfection, media were changed to adipogenic medium without or with chemicals (1 μM troglitazone, 10 μM 15-keto-PGE2, 10 μM 13,14-dihydro-15-keto-PGE2, and 1 μM GW9662). Twenty-four hours after treatment, cells were harvested and luciferase was assayed (Dual-Glo luciferase assay system; Promega, Madison, WI). For 293T cell transfection, confluent cells were incubated with plasmids (GAL4-PPARγ, UASG×4-TK-LUC, and Flag-PTGR-3) and lipofectamine. After 6 h of transient transfection, media were changed to growth media without or with chemicals (1 μM troglitazone, 10 μM 15-keto-PGE2, 10 μM 13,14-dihydro-15-keto-PGE2, and 1 μM GW9662). Twenty-four hours after treatment, cells were harvested for determination of luciferase activity.
Western blot
Total protein from tissue or cells was extracted by radioimmunoprecipitation assay buffer with protease inhibitors. The sample was centrifuged at 12,000 rpm for 10 min, and the supernatant was subjected to Western blot. For Western blot, 20 μg of protein lysate was separated by SDS-PAGE and then transblotted onto a polyvinylidine fluoride membrane (Perkin Elmer, Norwalk, CT). The PTGR-3 and PTGR-2 primary antibodies were purchased from Abcam (Cambridge, MA). Adiponectin antibody, aP2 antibody, and PPARγ were purchased from Cell Signaling (Boston, MA). α-Tubulin and GAPDH antibodies (Cell Signaling, Boston, MA) were used for the loading control in the lysates of total protein. The secondary antibody coupled to horseradish peroxidase was used in the chemiluminescence procedure (Immobilon Western; Millipore, Billerica, MA). The Western blotting procedure was performed according to the manufacturer's instruction.
Approvals for usage of animals
All animal experiments were performed in accordance with protocols approved by the National Taiwan University Institutional Animal Care and Use Committee (IACUC Approval No. 20100329).
Statistical analysis
Results are expressed as means ± SE. Comparison between two groups were performed using unpaired t-test. P < 0.05 was considered statistically significant. A two-way ANOVA (ANOVA) procedure was used to determine the main effects of PPAR ligands, PTGR-3, and their interaction on intracellular triacylglycerol content and luciferase activity. Multiple comparisons between groups were performed using post-hoc Tukey test (SAS Institute Inc., Cary, NC). A significant difference indicated P ≤ 0.05.
RESULTS
PTGR-3 is a negative regulator of adipocyte differentiation
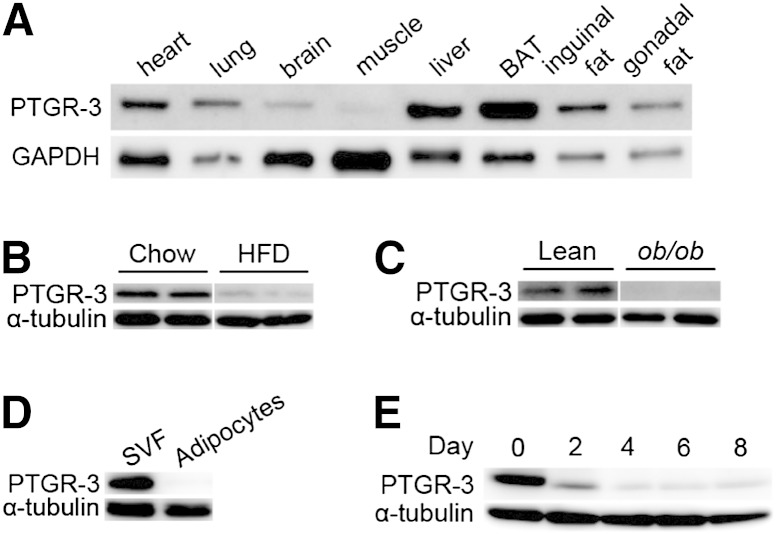
To explore the physiological function of PTGR-3, we first assayed the tissue expression pattern of PTGR-3. PTGR-3 protein and mRNA are ubiquitously expressed in several tissues, including heart, brown adipose tissue, and white adipose tissue (Fig. 1A and supplementary Fig. I-A). We also examined whether PTGR-3 levels were altered in murine models of obesity. We found that PTGR-3 levels were decreased in white adipose tissue of high-fat-diet (HFD) fed mice (Fig. 1B) and ob/ob mice (Fig. 1C and supplementary Fig. I-B). Furthermore, PTGR-3 is specifically expressed in stromal vascular fractions (SVF) rather than mature adipocytes (Fig. 1D), and PTGR-3 levels remarkably decreased during induced adipogenesis of 3T3-L1 cells (Fig. 1E). These results suggest that PTGR-3 may be involved in adipocyte differentiation.
Fig. 1.
PTGR-3 expression pattern in adipose tissue and during adipocyte differentiation. (A) Tissue distribution of PTGR-3 protein from 12-week-old C57BL/6 mice. (B) Expression of PTGR-3 protein from white adipose tissue of 12-week-old chow/HFD-fed mice. (C) Expression of PTGR-3 protein from white adipose tissue of 12-week-old lean and ob/ob mice. (D) Expression of PTGR-3 protein from mouse SVF and adipocytes. (E) Expression of PTGR-3 protein during induced 3T3-L1 adipocyte differentiation.
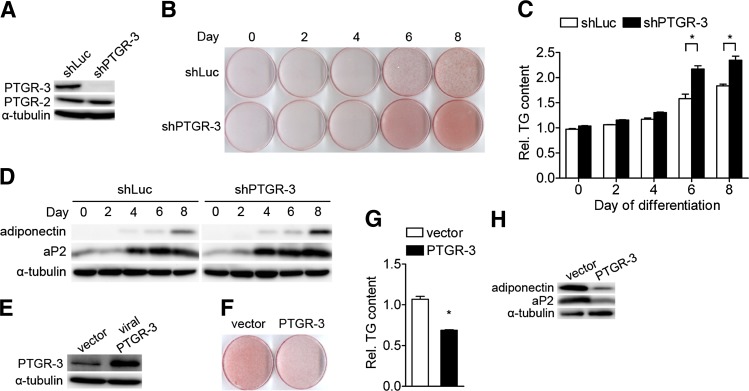
To investigate whether PTGR-3 affects adipocyte differentiation, 3T3-L1 preadipocytes were infected with lentivirus carrying PTGR-3 shRNA and were then induced for adipocyte differentiation. shRNAs targeting PTGR-3 efficiently attenuated PTGR-3 protein levels but not PTGR-2 levels (Fig. 2A). After 8 days of adipogenic induction, adipocyte differentiation was accelerated in PTGR-3-knockdown cells compared with shRNA control cells (shLuc) (Fig. 2B), accompanied by increased intracellular triglyceride levels (Fig. 2C). Consistently, adiponectin and aP2, two markers for adipocyte differentiation, were increased in PTGR-3-knockdown cells compared with shRNA control cells after hormonal induction (Fig. 2D). Quantitative PCR with reverse transcription showed increased expression of PPARγ-targeted genes, including Lpl and Cd36, in PTGR-3-knockdown 3T3-L1 cells after induction (supplementary Fig. II-A). By contrast, adipocyte differentiation was remarkably decreased when we ectopically expressed PTGR-3 protein in 3T3-L1 preadipocytes (Fig. 2E, F), which was accompanied by a decrease in intracellular triglyceride content (Fig. 2G) and in expression of adipocyte differentiation markers (Fig. 2H and supplementary Fig. II-B). Taken together, these results demonstrate PTGR-3 negatively regulates adipocyte differentiation.
Fig. 2.
PTGR-3 is a negative regulator of adipocyte differentiation. (A) Efficiency of shRNA control (shLuc) and PTGR-3 shRNA targeted on mouse PTGR-3 protein levels of 3T3-L1 cells. (B) Oil Red O staining of shRNA control and PTGR-3-knockdown cells during adipocyte differentiation. (C) Quantification of Oil Red O in shRNA control and PTGR-3-knockdown cells during adipocyte differentiation. *P < 0.05 versus shLuc. (D) Expression of adipogenic genes (adiponectin and aP2) in shRNA control and PTGR-3-knockdown cells during adipocyte differentiation. (E) Efficiency of ectopic expression of mouse PTGR-3 protein in 3T3-L1 cells. (F) Oil Red O staining of vector only and PTGR-3-overexpressing cells after 8-day hormonal induction. (G) Quantification of Oil Red O in vector only and PTGR-3-overexpressing cells after 8-day hormonal induction. *P < 0.05 versus vector. (H) Protein level of adipogenic marker genes (adiponectin and aP2) in vector only and PTGR-3-overexpressing cells after 8-day hormonal induction. The bars indicate the means ± SE for cells from three independent replicates (n = 3).
PTGR-3 is a 15-oxoprostaglandin-Δ13-reductase
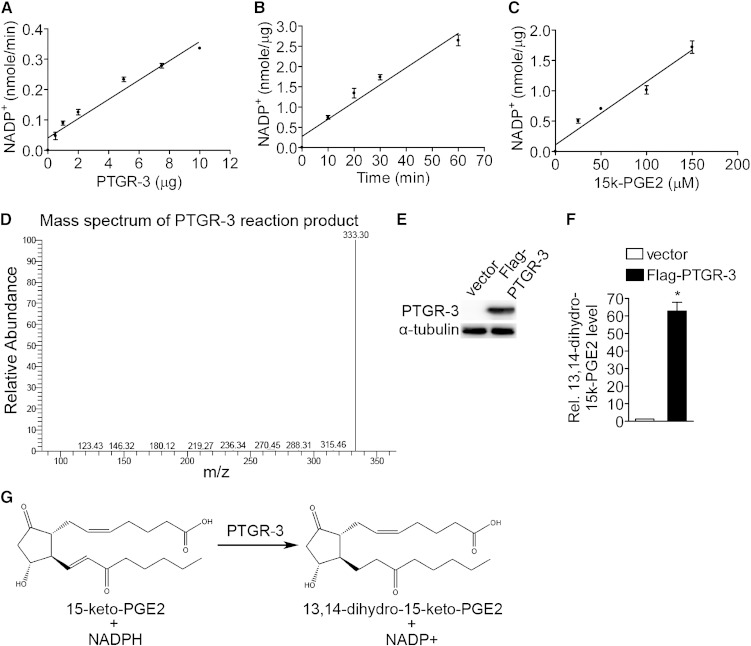
It has been demonstrated that 15-keto-PGE2 can be metabolized to 13,14-dihydro-15-keto-PGE2 by PTGR-2 (2). Therefore, we asked whether PTGR-3 is also an enzyme that catalyzes the same reaction. We first tested various concentrations of PTGR-3 recombinant protein on 15-keto-PGE2 catabolism in vitro. The results showed that PTGR-3 metabolizes 15-keto-PGE2 in a NADPH-dependent reaction and that this reaction was linearly accelerated with increased concentration of PTGR-3 recombinant protein (Fig. 3A), prolonged reaction time, and increased 15-keto-PGE2 concentration (Fig. 3B, C). In addition, we found that PTGR-3 is able to catalyze the metabolism of other prostaglandins, including 15-keto-PGF2α, 15-keto-PGF1α, and 15-keto-PGE1 (Table 1). Enzyme kinetic studies revealed that PTGR-3 had the highest efficiency for 15-keto-PGF2α catabolic reaction (Kcat/Km = 271.76 ± 8.82 mM−1 min−1 compared with 15-keto-PGF1α (214.41 ± 12.92 mM−1 min−1), 15-keto-PGE2 (157.24 ± 14.00 mM−1 min−1) and 15-keto-PGE1 (58.45 ± 4.59 mM−1 min−1) (Table 2). We next asked whether PTGR-3 is a 15-oxoprostaglandin-Δ13-reductase converting 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2. We analyzed the mass spectrum of reaction product obtained from the incubation of 15-keto-PGE2 with PTGR-3 recombinant protein by LC-MS/MS. A specific peak (Fig. 3D) was detected with mass spectrum identical to standard 13,14-dihydro-15-keto-PGE2 (supplementary Fig. III-A) and product obtained by reaction of 15-keto-PGE2 and PTGR-2 recombinant protein (supplementary Fig. III-B), demonstrating 13,14-dihydro-15-keto-PGE2 is a 15-keto-PGE2 metabolite in PTGR-3-mediated reaction. To examine whether PTGR-3 is involved in conversion of 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2 in a cell-based reaction, we ectopically expressed Flag-PTGR-3 protein in 293 cells (Fig. 3E) and treated these cells with 15-keto-PGE2. Intracellular prostaglandins were extracted and analyzed by LC-MS/MS. After 15-keto-PGE2 treatment, a mass spectrum of 13,14-dihydro-15-keto-PGE2 was clearly detected in PTGR-3-overexpressing cells compared with vector-only cells (Fig. 3F, G). Taken together, these results demonstrate that PTGR-3 is a 15-oxoprostaglandin-Δ13-reductase converting 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2 in vitro and in cell-based reactions.
Fig. 3.
PTGR-3 is a 15-oxoprostaglandin-Δ13-reductase. (A) Effect of various dose of PTGR-3 recombinant protein on the PTGR-3 activity in the enzymatic reaction. Each data point represents means ± SE for three independent replicates (n = 3). (B) Time-dependent formation of NADP+ in the PTGR-3 enzymatic reaction. Each data point represents means ± SE for three independent replicates (n = 3). (C) Effect of various dose of 15-keto-PGE2 on PTGR-3 activity in the enzymatic reaction. Each data point represents means ± SE for three independent replicates (n = 3). (D) LC-MS/MS spectrum of PTGR-3 enzymatic reaction product. Mass spectrum of 13,14-dihydro-15-keto-PGE2 from enzymatic reaction product was identified by LC-MS/MS. (E) Efficiency of ectopic expression of Flag-PTGR-3 protein in 293 cells. (F) Verification of intracellular 13,14-dihydro-15-keto-PGE2 level in 15-keto-PGE2-treated Flag-PTGR-3-overexpressing 293 cells. Intracellular 13,14-dihydro-15-keto-PGE2 from 15-keto-PGE2-treated vector only and Flag-PTGR-3-overexpressing 293 cells were extracted and analyzed by LC-MS/MS (n = 3). The bars indicate the means ± SE for three independent replicates. *P < 0.05 versus vector. (G) The scheme of PTGR-3 enzyme reaction mechanism.
TABLE 1.
Specific activities of PTGR-3 on various compounds
| Substrate | Specific Activity(nmol/min·mg protein) |
| 15-Keto-PGE2 | 215.04 ± 1.78 |
| 15-Keto-PGE1 | 109.61 ± 4.40 |
| 15-Keto-PGF2α | 303.34 ± 2.39 |
| 15-Keto-PGF1α | 286.20 ± 3.46 |
| 13,14-Dihydro-15-keto-PGD2 | ND |
| 13,14-Dihydro-15-keto-PGE2 | ND |
| 13,14-Dihydro-15-keto-PGF2α | ND |
| Leukotriene B4 | ND |
ND, nondetectable.
TABLE 2.
Enzymatic substrates and kinetic parameters for PTGR-3
| Km | Vmax | Kcat | Kcat/Km | |
| Substrate | μm | milliunits/mga | min−1 | μM−1 min−1 |
| 15-Keto-PGE2 | 55.03 ± 5.45 | 215.04 ± 1.78 | 8.60 ± 0.07 | 157.24 ± 14.00 |
| 15-Keto-PGE1 | 75.11 ± 7.78 | 109.61 ± 4.40 | 4.37 ± 0.15 | 58.45 ± 4.59 |
| 15-Keto-PGF2α | 42.49 ± 1.00 | 303.34 ± 2.39 | 11.55 ± 0.57 | 271.76 ± 8.82 |
| 15-Keto-PGF1α | 53.54 ± 3.70 | 286.20 ± 3.46 | 11.45 ± 0.14 | 214.41 ± 12.92 |
1 unit = 1 μmol NADP+/min.
15-keto-PGE2, a PTGR-3 substrate, promotes adipogenesis through activation of PPARγ
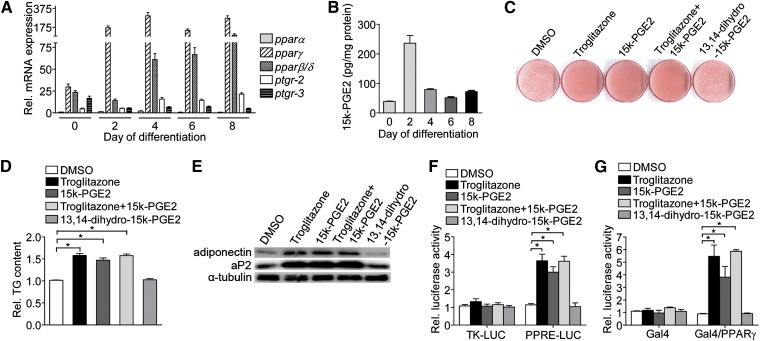
To determine whether 15-keto-PGE2, a substrate of PTGR-3, has a significant effect on adipogenesis, we measured the intracellular 15-keto-PGE2 levels during adipocyte differentiation. Consistent with the reduced expression of PTGR-3 expression in initiation of differentiation (Fig. 1E and Fig. 4A), 15-keto-PGE2 level was remarkably increased to a peak at 48 h after hormonal induction and then declined (Fig. 4B). To examine whether 15-keto-PGE2 has a direct effect on adipocyte differentiation, we treated 3T3-L1 preadipocytes with 15-keto-PGE2 during adipocyte differentiation. After induction of adipocyte differentiation, a remarkable increase in the lipid droplet deposition (Fig. 4C), intracellular triglyceride content (Fig. 4D), and adipogenic marker genes expression (Fig. 4E) was observed in 3T3-L1 preadipocytes treated with 15-keto-PGE2. However, the product of PTGR-3, 13,14-dihydro-15-keto-PGE2, did not cause a significant effect on adipocyte differentiation. The effect of 15-keto-PGE2 on adipocyte differentiation was similar to PPARγ agonist troglitazone. Furthermore, adipocyte differentiation was not further accelerated when 3T3-L1 preadipocytes were simultaneously treated with 15-keto-PGE2 and troglitazone, suggesting that 15-keto-PGE2 and troglitazone positively regulated adipogenesis through similar mechanism.
Fig. 4.
15-keto-PGE2, a PTGR-3 substrate, promotes adipogenesis through activation of PPARγ. (A) Expression of PTGR and PPAR mRNA during adipocyte differentiation. (B) Measurement of 15-keto-PGE2 level during adipocyte differentiation. Intracellular 15-keto-PGE2 level isolated from 3T3-L1 cells were extracted and analyzed by LC-MS/MS (n = 3). (C) 3T3-L1 preadipocytes were maintained in induction medium with or without treatments (1 μM troglitazone, 10 μM 15-keto-PGE2, and 10 μM 13,14-dihydro-15-keto-PGE2) for 2 days. After 8 days of adipogenic stimulation, cells on the plates were stained with Oil Red O and quantified (D), and adipogenic gene (adiponectin and aP2) expression (E) was determined (n = 3). *P < 0.05 versus DMSO. (F) 15-keto-PGE2 triggers PPRE-driven luciferase activity in differentiating 3T3-L1 cells. Differentiating 3T3-L1 cells were transfected with reporter vectors (TK-LUC and PPRE-LUC) for 24 h and then cells were incubated in induction medium with or without treatments (1 μM troglitazone, 10 μM 15-keto-PGE2, and 10 μM 13,14-dihydro-15-keto-PGE2) for 2 days. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. The bars indicate the means ± SE for three independent replicates (n = 3). *P < 0.05 versus DMSO. (G) Transactivation of 15-keto-PGE2 on PPARγ via ligand-binding domain of PPARγ. Differentiating 3T3-L1 cells were transfected with expression vectors (CMX-GAL4 and CMX-GAL4-PPARγ) and UASG×4-TK-LUC reporter plasmid for 24 h, and then cells were incubated in induction medium with or without treatments (1 μM troglitazone, 10 μM 15-keto-PGE2, and 10 μM 13,14-dihydro-15-keto-PGE2) for 2 days. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. The bars indicate the means ± SE for three independent replicates (n = 3). *P < 0.05 versus DMSO.
It has been demonstrated that troglitazone enhances adipogenesis by direct activation of PPARγ via binding to the ligand-binding domain. We tested whether 15-keto-PGE2 promotes adipocyte differentiation by enhancing PPARγ transcriptional activity. We transfected a PPRE-driven luciferase plasmid into 3T3-L1 preadipocytes and then treated these cells with 15-keto-PGE2. The results showed that 15-keto-PGE2 significantly activated the PPRE-driven luciferase activity (Fig. 4F). However, PGE2, the precursor of 15-keto-PGE2, and 13,14-dihydro-15-keto-PGE2, the metabolite of 15-keto-PGE2, do not have any effect on PPRE-driven luciferase activity (supplementary Fig. IV). 15-keto-PGE2 also does not affect the activity of PPARγ’s obligate heterodimer partner, retinoid X receptor (RXR) (supplementary Fig. V). We further asked whether 15-keto-PGE2 directly activates PPARγ via its ligand-binding domain. As expected, 15-keto-PGE2 increased PPARγ activity by direct interaction with activation of PPARγ ligand-binding domain (Fig. 4G). Taken together, these results demonstrate that 15-keto-PGE2, a PTGR-3 substrate, enhances adipogenesis through a PPARγ-dependent pathway.
PTGR-3 decreases proadipogenic effect of 15-keto-PGE2 through regulation of PPARγ activity
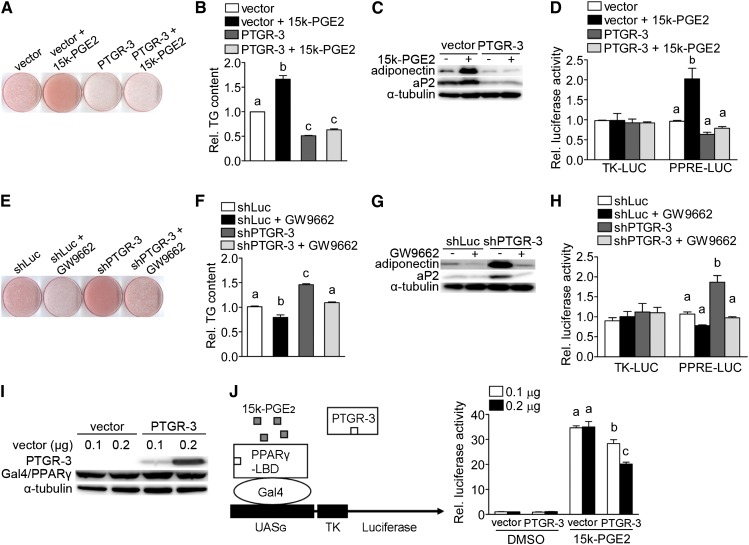
To assess whether PTGR-3 has a direct effect on 15-keto-PGE2-mediated adipogenesis, we treated vector-only and PTGR-3-overexpressing 3T3-L1 cells with or without 15-keto-PGE2. Similar to previous results, 15-keto-PGE2 significantly promoted adipocyte differentiation (Fig. 5A, two-way ANOVA, P < 0.05). Conversely, overexpression of PTGR-3 suppressed adipocyte differentiation in 3T3-L1 cells (Fig. 5A, two-way ANOVA, P < 0.05). However, the proadipogenic effect of 15-keto-PGE2 was remarkably abolished when PTGR-3 protein was overexpressed in 3T3-L1 preadipocytes (Fig. 5A, P < 0.05 for interaction). Similar results were found for intracellular triglyceride content, expression of adipogenic marker genes, and PPRE-driven luciferase activity (Fig. 5B–D).
Fig. 5.
PTGR-3 decreases proadipogenic effect of 15-keto-PGE2 on PPARγ activity. Vector-only and PTGR-3 overexpressing preadipocytes were maintained in induction medium with or without 10 μM 15-keto-PGE2 treatment for 2 days. After 8 days of adipogenic stimulation, cells on the plates were stained with Oil Red O (A) and quantified (B) and adipogenic gene (adiponectin and aP2) expression was determined (C). The bars indicate the means ± SE for three independent replicates (n = 3). Different superscripts indicate significant difference between groups (two-way ANOVA, P ≤ 0.05 with post-hoc Tukey test). The expression of adipogenic markers (adiponectin and aP2) was determined and normalized to α-tubulin. (D) PPRE-driven luciferase activity of vector-only and PTGR-3-overexpressing cells after 15-keto-PGE2 treatment. Vector-only and PTGR-3-overexpressing preadipocytes were transiently transfected with reporter vectors (TK-LUC and PPRE-LUC) for 24 h, and then cells were incubated in induction medium with or without 10 μM 15-keto-PGE2 treatment. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. The bars indicate the means ± SE for three independent replicates (n = 3). Different superscripts indicate significant difference between groups (two-way ANOVA, P ≤ 0.05 with post-hoc Tukey test). shRNA control and PTGR-3-knockdown cells were maintained in induction medium with or without 1 μM GW9662 treatment for 2 days. After 8 days of adipogenic stimulation, cells on the plates were stained with Oil Red O (E) and quantified (F) and adipogenic gene (adiponectin and aP2) expression was determined (G). The bars indicate the means ± SE for three independent replicates (n = 3). Different superscripts indicate significant difference between groups (two-way ANOVA, P ≤ 0.05 with post-hoc Tukey test). Expression of adipogenic genes (adiponectin and aP2) in GW9662-treated control and PTGR-3-knockdown cells. The expression of adipogenic markers (adiponectin and aP2) was determined and normalized to α-tubulin. (H) PPRE-driven luciferase activity of shRNA control and PTGR-3-knockdown cells after GW9662 treatment. shRNA control and PTGR-3-knockdown cells were transiently transfected with reporter vectors (TK-LUC and PPRE-LUC) for 24 h and then cells were incubated in induction medium with or without 1 μM GW9662 treatment. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. The bars indicate the means ± SE for three independent replicates (n = 3). Different superscripts indicate significant difference between groups (two-way ANOVA, P ≤ 0.05 with post-hoc Tukey test). (I) Efficiency of ectopic expression of UASG×4-TK-LUC, CMX-GAL4-PPARγ, and Flag-PTGR-3 protein in 293 cells. The expression of PTGR-3 and Gal/PPARγ was determined and normalized to α-tubulin. (J) Transactivation of 15-keto-PGE2 on PPARγ ligand-binding domain in Flag-PTGR-3-overexpressing 293 cells. 293 cells were transiently transfected with expression vectors (CMX-GAL4, CMX-GAL4-PPARγ, and Flag-PTGR-3) and UASG×4-TK-LUC reporter plasmid for 24 h, and then cells were incubated in growth medium with or without 10 μM 15-keto-PGE2 for 2 days. The activity of firefly luciferase was determined and normalized to the activity of renilla luciferase. The bars indicate the means ± SE for three independent replicates (n = 3). Different superscripts indicate significant difference between groups (one-way ANOVA, P ≤ 0.05 with post-hoc Tukey test).
We further tested whether GW9662, an irreversible competitive PPARγ antagonist that covalently binds to a cysteine residue in the PPARγ ligand-binding domain, can erase the enhanced adipocyte differentiation in PTGR-3-knockdown 3T3-L1 preadipocytes. After GW9662 treatment, adipocyte differentiation, triglyceride content, adipogenic marker genes, and PPRE-driven luciferase activity were significantly decreased in PTGR-3-knockdown cells compared with absence of GW9662 treatment (Fig. 5E–H, two-way ANOVA, P < 0.05 for interaction).
To confirm our hypothesis that PTGR-3 directly suppresses 15-keto-PGE2-mediated transactivation of PPARγ, we cotransfected 293 cells with Flag-PTGR-3, CMX-GAL4-PPARγ, and UASG×4-TK-LUC plasmids (Fig. 5I), and then treated these cells with or without 15-keto-PGE2. Similar to previous results, 15-keto-PGE2 significantly activated luciferase activity, but this effect was remarkably reduced by PTGR-3 overexpression in a dose-dependent manner (Fig. 5J, one-way ANOVA, P < 0.05). Taken together, these results clearly demonstrate that PTGR-3 is involved in 15-keto-PGE2-mediated adipogenesis and plays a negative regulator role in this process.
DISCUSSION
The major finding of this study is PTGR-3, a novel 15-oxoprostaglandin -Δ13-reductase that catalyzes the reaction in converting 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2. We found that PTGR-3 expression in white adipose tissue was reduced in genetic ob/ob and HFD-induced obese mice models. PTGR-3 expression decreased drastically during adipocyte differentiation. Ectopic expression of PTGR-3 in 3T3-L1 preadipocytes decreased PPARγ-dependent transcriptional activity, thereby attenuating adipogenesis. In contrast, suppression of PTGR-3 expression in cells accelerated adipogenesis by increasing PPARγ transcriptional activity. These results reveal that PTGR-3 negatively modulates adipocyte differentiation by regulation of PPARγ transcriptional activity.
It has been demonstrated that several prostaglandins act as bioactive modulators for regulating adipocyte differentiation through either direct binding on the PPARγ ligand-binding domain or indirect mechanisms mediated by signaling transduction. For the past decade, 15-deoxy-Δ12.14-prostaglandin J2 (15d-PGJ2) has been considered a potent natural ligand for PPARγ (1). 15d-PGJ2 is derived from prostaglandin H2 (PGH2), which is converted by PGD synthase to prostaglandin D2 and further chemically dehydrated to form 15d-PGJ2. Several lines of evidence have demonstrated that inhibition of the COX pathway or dysregulation of genes involved in this series of reactions in 3T3-L1 preadipocytes affects adipocyte differentiation (6–9). The highest level of 15d-PGJ2 was detected in 3T3-L1 cells during the maturation phase of adipocytes (10). In contrast, other groups have demonstrated that no differences in formation of 15d-PGJ2 are observed during adipocyte differentiation (11). Thus, it remains uncertain whether 15d-PGJ2 is truly an endogenous ligand for PPARγ that modulates adipocyte differentiation. Prostaglandin F2α (PGF2α) is generated from PGH2 by PGF2α synthase (PGFS), and PGF2α is reported to be an inhibitory modulator of adipocyte differentiation (12). The level of PGF2α rapidly increased to a peak 3 h after initiation of differentiation and then declined, indicating that PGF2α suppresses in the early phase of adipogenesis (13). Knockdown of aldo-keto reductase 1B3 (AKR1B3), a PGFS, decreased de novo PGF2α biosynthesis in 3T3-L1 cells and then further promoted lipid accumulation (13). It has been shown that PGF2α suppressed adipogensis by enhancing phosphorylation of PPARγ via prostaglandin F receptor-activated MEK⁄ERK cascade (14). On the other hand, PGE2, a precursor of 15-keto-PGE2, is the most abundant prostaglandin produced in 3T3-L1 preadipocytes, and the highest level of PGE2 was detected during the early phase of adipogenesis (15, 16). The activity of 15-hydroxy prostaglandin dehydrogenase, an enzyme catalyzing the conversion of PGE2 to 15-keto-PGE2, was also detected in adipose tissue (16), implying that PGE2 metabolites or its catabolizing enzymes may play a potential role in modulating adipocyte differentiation. However, the role of PGE2 metabolites in lipid metabolism has not been widely studied.
In our previous study, we found that 15-keto-PGE2 is a PPARγ ligand and that 15-keto-PGE2 is reduced to 13,14-dihydro-15-keto-PGE2 by PTGR-2 (2). Here, we identified that PTGR-3 is a new member of prostaglandin reductase family. Similar to PTGR-2, PTGR-3 converts 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2 by a similar reductive mechanism, thereby influencing intracellular contents of bioactive 15-keto-PGE2 in cells, which may modulate the process of adipogenesis in 3T3-L1 preadipocytes. Enzyme kinetics studies showed relatively higher efficiency of PTGR-2 in converting 15-keto-PGE2 to 13,14-dihydro-15-keto-PGE2 compared with PTGR-3. Both PTGR-2 and PTGR-3 were expressed abundantly in white adipose tissue (supplementary Fig. I-A) and were downregulated in white adipose tissue of ob/ob mice (supplementary Fig. I-B). Interestingly, expression of PTGR-3 in 3T3-L1 preadipocytes decreased rapidly within 48 h during the course of induced adipocyte differentiation, accompanied by a rise of 15-keto-PGE2 level and PPARγ expression, implying that PTGR-3 may modulate PPARγ activity by regulation of 15-keto-PGE2 production in an early stage of adipocyte differentiation. After 2 days of induction, PTGR-2 expression started to increase and endogenous 15-keto-PGE2 level gradually decreased, indicating PTGR-2 may play a more dominant role in the late stage of adipocyte differentiation than PTGR-3. Cumulatively, these findings demonstrate that these enzymes display different kinetic parameters and different expression patterns during adipogenesis. Whether and how PTGR-2 and PTGR-3 coordinately regulate 15-keto-PGE2 homeostasis and regulate adipogenesis remains to be investigated.
Conversion of preadipocytes to adipocytes is a complicated process, which is regulated by several factors, including hormonal and nutrient stimulation. Preadipocytes have to integrate these signals from the environment for initiation of adipocyte differentiation. Several factors secreted from preadipocytes, such as DLK1/PREF1 and Wnt proteins, play an important role in maintaining the undifferentiated state in preadipocytes by extracellular signaling transduction. It has been demonstrated that dysregulation of these genes promotes adipocyte differentiation by influencing C/EBPα and PPARγ expression in vivo or in vitro (17, 18). These results suggest that maintenance of preadipocytes in an undifferentiated state requires the activity of extracellular signaling molecules. However, little is known about whether intracellular proteins, such as enzymes involved in proadipogenic molecule metabolism, also modulate maintenance of the preadipose state. Here, we demonstrate that expression of PTGR-3, similar to DLK1/PREF1 and Wnt proteins, is predominantly expressed in preadipocytes and decreases remarkably during adipocyte differentiation. In addition, PTGR-3 protein is abundantly expressed in adipose tissue of lean mice, which contains a relatively high percentage of undifferentiated preadipocytes compared with adipose tissue of obese mice, implying that PTGR-3 may play a role in maintaining the undifferentiated state in preadipocytes. Forced PTGR-3 expression attenuates adipocyte differentiation, whereas knocking down PTGR-3 in 3T3-L1 preadipocytes accelerates adipocyte differentiation. Overall, these observations suggest that PTGR-3 protein may facilitate maintaining the undifferentiated state in preadipocytes by inhibiting endogenous 15-keto-PGE2 production and that suppression of PTGR-3 expression is required for normal adipocyte differentiation by regulating PPARγ transcriptional activity.
In conclusion, we provide evidence that PTGR-3 is a novel 15-oxoprostaglandin-Δ13-reductase that affects adipocyte differentiation by regulation of PPARγ activity. PTGR-3 expression is reduced in adipose tissue from murine models of obesity. Modulation of PTGR-3 expression or activity might provide a novel avenue in treating obesity and related metabolic disorders.
Supplementary Material
Acknowledgments
The authors thank the National RNAi Core Facility for providing the RNAi reagents.
Footnotes
Abbreviations:
- aP2
- adipocyte fatty acid-binding protein
- COX
- cyclooxygenase
- DLK1
- delta-like homologue 1
- ERK
- extracellular signal-regulated kinase
- HFD
- high-fat diet
- LC
- liquid chromatography
- LTQ
- linear trap quadrupole
- MEK
- mitogen-activated protein kinase
- PPARγ
- peroxisome proliferator-activated receptor γ
- PPRE
- PPAR response element
- PREF1
- preadipocyte factor 1
- PTGR-3
- prostaglandin reductase-3
- SVF
- stromal vesicular fraction
- UHPLC
- ultra high pressure liquid chromatography
- UPLC
- ultra performance liquid chromatography
This work was supported by National Science Council (NSC 100-2314-B-002-068-MY3) in Taiwan. The mass spectrometry analysis was supported by the Metabolomics Core Facility, Scientific Instrument Center at Academia Sinica.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table, five figures, and methods.
REFERENCES
- 1.Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., Evans R. M. 1995. 15-deoxy-Δ12.14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 83: 803–812. [DOI] [PubMed] [Google Scholar]
- 2.Chou W. L., Chuang L. M., Chou C. C., Wang A. H., Lawson J. A., FitzGerald G. A., Chang Z. F. 2007. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. J. Biol. Chem. 282: 18162–18172. [DOI] [PubMed] [Google Scholar]
- 3.Yokomizo T., Ogawa Y., Uozumi N., Kume K., Izumi T., Shimizu T. 1996. cDNA cloning, expression, and mutagenesis study of leukotriene B4 12-hydroxydehydrogenase. J. Biol. Chem. 271: 2844–2850. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y., Weng C. C., Tong M., Wei J., Tai H. H. 2010. Restoration of leukotriene B4-12-hydroxydehydrogenase/15-oxo-prostaglandin 13-reductase (LTBDH/PGR) expression inhibits lung cancer growth in vitro and in vivo. Lung Cancer. 68: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai H. H., Ensor C. M., Tong M., Zhou H., Yan F. 2002. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 68-69: 483–493. [DOI] [PubMed] [Google Scholar]
- 6.Fajas L., Miard S., Briggs M. R., Auwerx J. 2003. Selective cyclo-oxygenase-2 inhibitors impair adipocyte differentiation through inhibition of the clonal expansion phase. J. Lipid Res. 44: 1652–1659. [DOI] [PubMed] [Google Scholar]
- 7.Ghoshal S., Trivedi D. B., Graf G. A., Loftin C. D. 2011. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J. Biol. Chem. 286: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimori K., Aritake K., Urade Y. 2007. A novel pathway to enhance adipocyte differentiation of 3T3–L1 cells by up-regulation of lipocalin-type prostaglandin D synthase mediated by liver X receptor-activated sterol regulatory element-binding potein-1c. J. Biol. Chem. 282: 18458–18466. [DOI] [PubMed] [Google Scholar]
- 9.Ragolia L., Palaia T., Hall C. E., Maesaka J. K., Eguchi N., Urade Y. 2005. Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J. Biol. Chem. 280: 29946–29955. [DOI] [PubMed] [Google Scholar]
- 10.Mazid M. A., Chowdhury A. A., Nagao K., Nishimura K., Jisaka M., Nagaya T., Yokota K. 2006. Endogenous 15-deoxy-Δ12.14-prostaglandin J2 synthesized by adipocytes during maturation phase contributes to upregulation of fat storage. FEBS Lett. 580: 6885–6890. [DOI] [PubMed] [Google Scholar]
- 11.Bell-Parikh L. C., Ide T., Lawson J. A., McNamara P., Reilly M., FitzGerald G. A. 2003. Biosynthesis of 15-deoxy-Δ12.14-PGJ2 and the ligation of PPARγ. J. Clin. Invest. 112: 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Clipstone N. A. 2007. Prostaglandin F2α inhibits adipocyte differentiation via a Gαq-calcium-calcineurin-dependent signaling pathway. J. Cell. Biochem. 100: 161–173. [DOI] [PubMed] [Google Scholar]
- 13.Fujimori K., Ueno T., Nagata N., Kashiwagi K., Aritake K., Amano F., Urade Y. 2010. Suppression of adipocyte differentiation by aldo-keto reductase 1B3 acting as prostaglandin F2alpha synthase. J. Biol. Chem. 285: 8880–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno T., Fujimori K. 2011. Novel suppression mechanism operating in early phase of adipogenesis by positive feedback loop for enhancement of cyclooxygenase-2 expression through prostaglandin F2α receptor mediated activation of MEK/ERK-CREB cascade. FEBS J. 278: 2901–2912. [DOI] [PubMed] [Google Scholar]
- 15.Hyman B. T., Stoll L. L., Spector A. A. 1982. Prostaglandin production by 3T3–L1 cells in culture. Biochim. Biophys. Acta. 713: 375–385. [DOI] [PubMed] [Google Scholar]
- 16.Anggard E., Larsson C., Samuelsson B. 1971. The distribution of 15-hydroxy prostaglandin dehydrogenase and prostaglandin-delta 13-reductase in tissues of the swine. Acta Physiol. Scand. 81: 396–404. [DOI] [PubMed] [Google Scholar]
- 17.Moon Y. S., Smas C. M., Lee K., Villena J. A., Kim K. H., Yun E. J., Sul H. S. 2002. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 22: 5585–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., MacDougald O. A. 2000. Inhibition of adipogenesis by Wnt signaling. Science. 289: 950–953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.