Abstract
Inbred strains of mice with differing susceptibilities to atherosclerosis possess widely varying plasma HDL levels. Cholesterol absorption and lipoprotein formation were compared between atherosclerosis-susceptible, low-HDL C57BL6/J mice and atherosclerosis-resistant, high-HDL FVBN/J mice. [3H]cholesterol and triglyceride appeared in the plasma of FVB mice gavaged with cholesterol in olive oil at a much higher rate than in C57 mice. The plasma cholesterol was found almost entirely as HDL-cholesterol in both strains. Inhibition of lipoprotein catabolism with Tyloxapol revealed that the difference in the rate of [3H]cholesterol appearance in the plasma was due entirely to a greater rate of chylomicron secretion from the intestine of the FVB mice. Lipid absorption into the 2nd quarter of the small intestine is greater in the FVB mice and indicates that this region may contain the factors that give rise to the differences in absorption observed between the two mouse strains. Additionally, ad libitum feeding prior to cholesterol gavage accentuates the absorption rate differences compared with fasting. The resultant remodeling of the increased levels of chylomicron in the plasma may contribute to increased plasma HDL. Intestinal gene expression analysis reveals several genes that may play a role in these differences, including microsomal triglyceride transfer protein and ABCG8.
Keywords: enterocyte, triglyceride, lipoprotein, chylomicron
The factors that regulate plasma HDL-cholesterol levels have long been of great interest, inasmuch as elevated plasma HDL-cholesterol is a negative risk factor for cardiovascular disease (1). Plasma steady-state cholesterol levels vary greatly among wild-type inbred mouse strains. Unlike humans, wild-type mice have a plasma lipoprotein profile that is predominantly made up of HDL. Among mice, inbred strains may have quite different HDL levels. The HDL-cholesterol levels among the inbred strains vary from as low as 35 mg/dl to 172 mg/dl (2). We have recently studied the HDL levels, composition, and some properties of the particles derived from C57BL6 (C57) and FVB/N (FVB) mice (3). These strains differ in apoA-I (two amino acid differences) and apoA-II (three amino acid differences). The inbred mouse strains C57BL6 and FVB/N have been widely studied owing to their differing susceptibilities to atherosclerosis, the C57 being one of the most athero-susceptible mouse strains, whereas the FVB is highly resistant (4). These two mouse strains differ greatly in their plasma cholesterol levels, with wild-type FVB mice having HDL levels 2-fold higher than those of wild-type C57 mice. FVB HDL contains about twice as much apoA-II as C57 HDL. Neither hepatic synthesis of apoA-I nor HDL turnover appears to account for the difference in cholesterol levels (3).
Plasma HDL-cholesterol is derived from two major sources: endogenously synthesized cholesterol, mostly in the liver, and HDL-cholesterol that is obtained from the intestine and largely contributed by the diet. In humans, the diet contributes roughly 50% of the plasma cholesterol, whereas in mice, it is closer to 30% (5, 6). In recent years, much has been learned of the factors controlling the absorption of cholesterol in the intestine. In humans, cholesterol absorption efficiency varies greatly within a population (7–9). The same has been found true among inbred strains of rabbits, rats, and mice, indicating genetic regulation of cholesterol absorption (10–18). Crosses of mouse strains that vary in their cholesterol absorption efficiencies have shown that the genetic regulation is found at the enterocyte level (16, 17). Schwarz et al. (16) identified seven quantitative trait loci that affect intestinal cholesterol absorption in mice; however, the identity of the specific genes is not known. Several genes are known to influence the multi-step process of cholesterol absorption, which involves the deesterification of cholesteryl ester and bile salt solubilization in the intestinal lumen. The transporters NPC1L1 and CD36 have been shown to be involved in the uptake of cholesterol from the lumen into the enterocytes (19–21). Inside the enterocytes, the cholesterol is reesterified by ACAT2, packaged with triglyceride and apoB-48 into chylomicrons by microsomal triglyceride transfer protein (MTTP), and then secreted into the circulation (22). Additionally, the ATP binding cassette transporter proteins ABCG5 and ABCG8 have been shown to be involved in resecretion of the enterocyte cholesterol back into the lumen (23, 24). Other factors, including the rate of gastric emptying, have been shown to alter the rate of cholesterol absorption between inbred mouse strains (25).
In further attempts to try to account for the difference in HDL levels in the two strains chosen for study, we here examine the cholesterol absorption differences between C57 and FVB mice and find that the FVB mouse absorbs cholesterol at a higher rate, which is then found at higher levels in circulating HDL. This higher level of absorption of cholesterol is also associated with a higher rate of absorption of triglyceride. Quantitative PCR analysis of intestinal mRNA indicates that several genes, including MTTP and ABCG8, may be involved in differences in rate of lipid absorption between the two strains.
MATERIALS AND METHODS
All chemicals were purchased from Sigma Chemical Co. (St. Louis. MO) unless otherwise noted. Female C57BL/6J, FVBN/J, and LDLR receptor-deficient (LDLR−/−) mice were purchased from Jackson Laboratory, Bar Harbor, ME. The mice were bred in a specific pathogen-free facility. The pups were weaned at 21–28 days and maintained on chow diet #7913 from Harlan Labs (Indianapolis, IN). All other inbred strains were purchased from Jackson Laboratory and maintained on a chow diet, and female mice were used at 8–10 weeks of age for experiments. Body weights were slightly but not significantly higher in FVB mice compared with C57 mice. All procedures performed on the mice were in accordance with National Institutes of Health and institutional guidelines.
Cholesterol absorption
[3H]cholesterol, [3H]triolein, and [14C]cholesterol were purchased from Perkin Elmer (Waltham, MA). Female mice were either fed ad libitum throughout the experiments, or fasted overnight prior to gavage and again through the remainder of the experiment. Either 2 μCi [3H]cholesterol, 2 μCi [3H]triolein, or a combination of 2 μCi [14C]cholesterol and 2 μCi [3H]triolein were gavaged into mice in 200 μl olive oil. The mice were then bled retro-orbitally into tubes containing EDTA at time points ranging from 0 to 24 h postgavage. At the final time point, the mice were exsanguinated and euthanized, and the liver and intestine were collected. Plasma from each time point was mixed with 1 ml of isopropanol in scintillation vials, and this mixture was allowed to evaporate overnight at room temperature. Instafluor scintillation fluid was added, and samples were counted on a Beckman 6895 scintillation counter. In the experiments involving tyloxapol (Triton WR1339) inhibition of lipoprotein catabolism, mice were injected with 100 μl of a 10% solution of tyloxapol in PBS 5 min prior to gavage. Plasma from the final time point was used for NaBr density gradient fractionation to isolate plasma lipoproteins. Plasma (250 μl) mixed with 750 μl 1.386 g/ml NaBr density solution was layered beneath NaBr solutions of decreasing densities from the bottom to the top of the centrifuge tube: 3.2 ml 1.21 g/ml, 3.6 ml 1.063 g/ml, 3.2 ml 1.019 g/ml, and 1 ml 1.006 g/ml. Density gradients were centrifuged for 22 h with no brake at 178,000 g and fractionated manually into 20 fractions. Isopropanol (1 ml) was added to each fraction, evaporated in a 50°C oven for 12 h, and counted on a scintillation counter as described above. To assess lipid absorption into the intestinal cells, gavaged mice were euthanized at 7 or 24 h postgavage. The stomach, intestine, cecum, and colon were harvested from each mouse. The intestine was divided into four equal-length segments, and the lumen contents were collected by wash with 5 ml PBS IFT (intestinal flow-thru). Total feces were also collected at euthanization. Tissues and feces were dissolved in 1 ml 1N NaOH overnight with gentle shaking at 37°C. Tissues, feces, and IFT were resuspended in 5 ml UltimaGold scintillation fluid (Perkin-Elmer) and counted on a scintillation counter.
[3H]cholesterol-labeled chylomicrons
Chylomicrons labeled with [3H]cholesterol were generated by gavaging each of seven LDL receptor-deficient female C57 mice with 2 μCi [3H]cholesterol in 200 μl olive oil. After 7 h, the mice were exsanguinated and the plasma combined. The total volume of plasma (2 ml) was brought up to 6 ml in PBS and centrifuged for 1 h at 103,000 g to bring chylomicrons to the top of the tube. The top chylomicron fraction was removed, brought up to 6 ml again in PBS, and recentrifuged as before. The top chylomicron fraction was removed and concentrated using a centricon-30 concentrator down to 1.6 ml. Ten microliters of the concentrate was counted on the scintillation counter, yielding 75,000 cpm/ml chylomicron concentrate.
Two hundred microliters of [3H]cholesterol-labeled chylomicrons were injected into C57 or FVB mice retro-orbitally. After 3 h, the mice were bled, and plasma was fractionated by density gradient ultracentrifugation as described above. Density fractions were analyzed for radiolabel as described above.
Enterocyte [3H]cholesterol absorption and secretion
Primary enterocytes were prepared from nonfasted C57 and FVB female mice using a protocol modified from Nassir et al. (19). Briefly, the intestine was washed with cold PBS followed by cold HBSS (−Ca2+/−Mg2+). The intestine was then divided into four equal-length segments from stomach to cecum (I1, I2, I3, I4), and segments I3 and I4 were discarded. Segments I1 and I2 were minced into small pieces with a razor blade, and enterocytes were isolated by incubation at 37°C and shaking for 20 min in HBSS (−Ca2+/−Mg2+) + 3 mM EDTA + 0.5 mM dithiothreitol. The cells were filtered through a 70 μm cell filter and pelleted at 1,000 g for 5 min, after which they were resuspended in DMEM and measured for viability by trypan blue exclusion. Cell viability was greater than 90%. Cell protein was measured using BioRad protein reagent (BioRad; Hercules, CA), and 400 μg cell protein was added to each well of 12-well plates in 1 ml DMEM. Absorption studies were initiated by the addition of [3H]cholesterol bile salt micelles (0.2 μCi [3H]cholesterol, 0.14 mM Na cholate, 0.15 mM Na deoxycholate, 0.17 mM 1-palmitoyl-2-oleoylphosphatidylcholine, 0.44 mM oleic acid, and 0.19 mM rac-mono-olein in DMEM). At time points ranging from 5 to 120 min, cells and media were harvested, and cells were washed two times with DMEM. Media cholesterol was extracted with 1 vol isopropanol + 8 vol hexane. Cell cholesterol was extracted two times with 3:2 hexane-isopropanol. Extracts were dried and counted on a scintillation counter as described above.
Secretion studies were done on mice that had first been gavaged with 2 μCi [3H]cholesterol in 200 μl olive oil. Three hours postgavage, the enterocytes were harvested and plated as above. At time points ranging from 5 to 120 min, cells and media were harvested and separated by centrifugation (5 min at 12,000 g). Media cholesterol was extracted with 1 vol isopropanol + 8 vol hexane. Cell cholesterol was extracted two times with 3:2 hexane-isopropanol. Extracts were dried and counted on a scintillation counter as described above.
Intestinal quantitative PCR
C57 and FVB female mice were either fasted for 16 h prior to intestinal harvest, or chow-fed mice were gavaged with 200 μl olive oil followed by 7 h ad libitum access to chow prior to intestinal harvest. The small intestine was harvested from mice from stomach to cecum. Intestine was divided into four equal-length segments designated I1, I2, I3, and I4, with I1 being the segment closest to the stomach. Each section was washed with 5 ml PBS, transferred into cryovials, and frozen in liquid N2 for later RNA preparation. RNA was prepared for each intestinal section using the Trizol extraction method (Invitrogen), and cDNA was prepared from 4 μg RNA using Superscript III (Invitrogen) according to the manufacturer's protocol. Two microliters of 1:100-diluted cDNA was subjected to real-time PCR in a total volume of 14 µl using 7 μl SYBR green mix (SA Biosciences) and 0.5 μl primer mix (SA Biosciences). Gene expression was normalized to hypoxanthine phosphoribosyltransferase (HPRT), with the expression level of each gene expressed relative to intestinal segment I1 of a fed C57 mouse.
Statistical analysis
All statistical analyses were done using Student's t-test in MicroCal Origin Version 4.0 software.
RESULTS
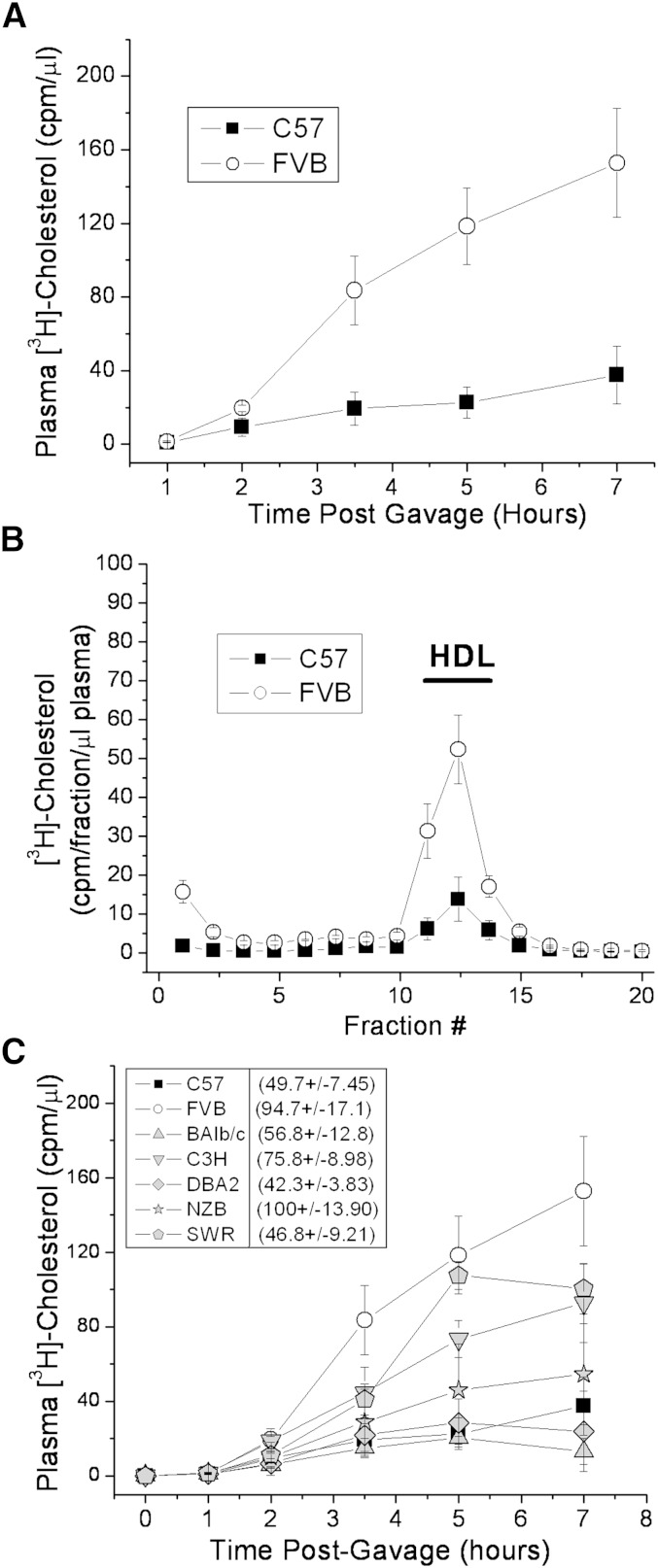
The appearance of labeled cholesterol in the plasma was measured in C57 or FVB female mice over the course of 7 h following gavage of [3H]cholesterol in olive oil (Fig. 1A). The appearance of radiolabeled cholesterol in the plasma was nearly 5-fold greater in the FVB mouse after 7 h. Similar results were found in male C57 and FVB mice (data not shown). This plasma cholesterol was found mainly as HDL (Fig. 1B). Several other strains of mice were tested, and the C57 mouse was found to have among the lowest rate of appearance of radiolabeled cholesterol in the plasma following gavage, whereas the FVB mouse was among the highest (Fig. 1C). Along with C57 and FVB, several strains (C3H, DBA, Balb/c) have HDL levels that reflect their relative cholesterol absorption rates. On the other hand, SWR mice, which have reported plasma HDL levels that are among the lowest of inbred mouse strains (26), were among the highest in cholesterol absorption (Fig. 1C, legend). Similarly, the NZB/BIN strain, which has reported HDL levels similar to those of the FVB, was much lower than FVB in cholesterol absorption. Thus, there may be a rough but imperfect correlation between cholesterol absorption and plasma HDL levels. However, the mechanisms involved in effecting HDL homeostasis probably differ somewhat among all inbred mouse strains.
Fig. 1.
Cholesterol absorption rates in various inbred strains of mice following olive oil gavage. Nonfasted female mice were gavaged with 2 μCi [3H]cholesterol in 200 μl olive oil. A, C: Plasma samples were collected over the course of 7 h, and plasma radiolabel was measured by liquid scintillation counting. B: Plasma samples (7 h) were separated on a 3–20% NaBr gradient and fractionated, and [3H]cholesterol levels in each fraction were determined by liquid scintillation counting. C: Comparison of additional inbred mouse strains. Reported plasma HDL cholesterol levels for females of each strain are shown in parenthesis for comparison (26). Data represent mean ± standard deviation of triplicate mice.
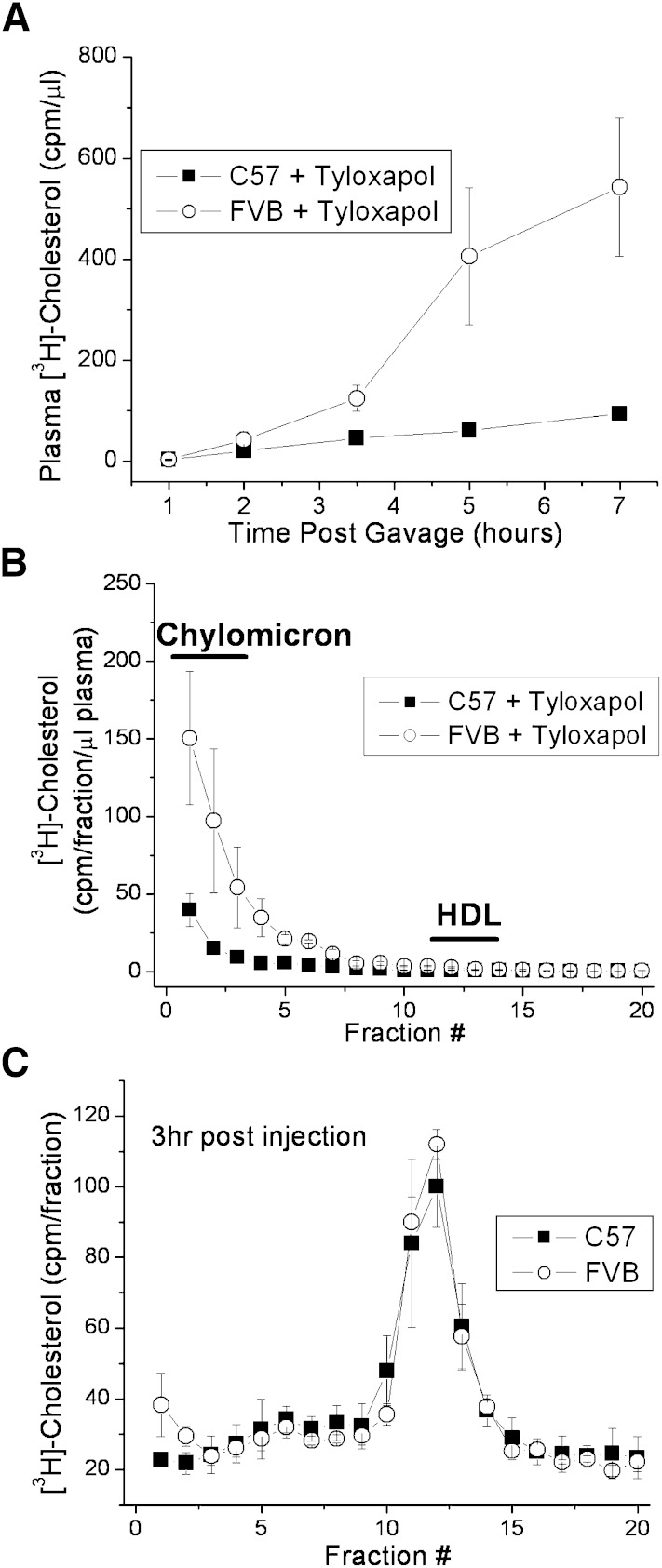
The liver and the intestine are the two major sites of HDL synthesis, with the liver accounting for 70–80% of HDL production (27, 28). It is possible that the differences in HDL production are due to differing HDL production levels at either of these sites. In one case, the HDL would be secreted at differing levels directly from the intestine. In the second case, cholesterol is absorbed and secreted from the intestine at similar rates between the two mouse strains, but the liver production differs. To test this, an experiment similar to that shown in Fig. 1 was conducted, but with the inclusion of an injection of tyloxapol prior to gavage to prevent the catabolism of any newly formed intestinal lipoproteins. Again, nearly 5-fold more of the labeled cholesterol appeared in the plasma of the FVB mice when compared with the C57 mice after 7 h (Fig. 2A). This labeled cholesterol, however, was not found in the HDL lipoprotein fraction, but rather in the chylomicron fraction, with no labeled cholesterol being found in the HDL fraction of the plasma (Fig. 2B), as expected, because chylomicron catabolism is inhibited. This also indicates that the differences in HDL cholesterol shown in Fig. 1 are not arising from direct HDL synthesis by the intestine but rather may be related to differences in the amount of cholesterol secreted in association with chylomicrons. The isolated chylomicrons and the enterocytes from the two mouse strains did not differ in their unlabeled cholesterol/protein content (data not shown), indicating that any cholesterol already present in the intestine at the time of gavage was not differentially diluting the label between the two strains.
Fig. 2.
Absorbed cholesterol is secreted in chylomicrons at different rates in C57 and FVB mice. Nonfasted female C57 and FVB mice were injected retro-orbitally with 100 μl tyloxapol (10% v/v) followed by gavage with 2 μCi [3H]cholesterol in 200 μl olive oil. A: Plasma samples were collected over the course of 7 h, and plasma radiolabel was measured by liquid scintillation counting. B: Plasma samples (7 h) were separated on a 3–20% NaBr gradient and fractionated, and [3H]cholesterol levels in each fraction were determined by liquid scintillation counting. Data represent mean ± standard deviation of triplicate mice. C: Non-fasted mice were injected retro-orbitally with equal amounts of [3H]cholesterol-labeled chylomicrons. Three h plasma samples were separated on a 3-20% NaBr gradient, fractionated, and [3H]cholesterol levels in each fraction determined by liquid scintillation counting. Data represent mean ± SD of triplicate mice.
As further evidence that the difference in HDL is directly a result of differences in chylomicron cholesterol between the two mice, chylomicrons from C57 mice were prepared labeled with [3H]cholesterol. Equal amounts of the labeled chylomicrons were injected into C57 and FVB mice. Within 3 h of injection, more than 95% of the injected label was found in the HDL fraction (Fig. 2C). Importantly, when the mice started with equal amounts of substrate (i.e., chylomicron [3H]cholesterol), there was no difference in the amount of label in the resulting HDL, again indicating that it is the increased production of labeled chylomicron cholesterol that results in increased [3H]cholesterol-labeled HDL in FVB mice.
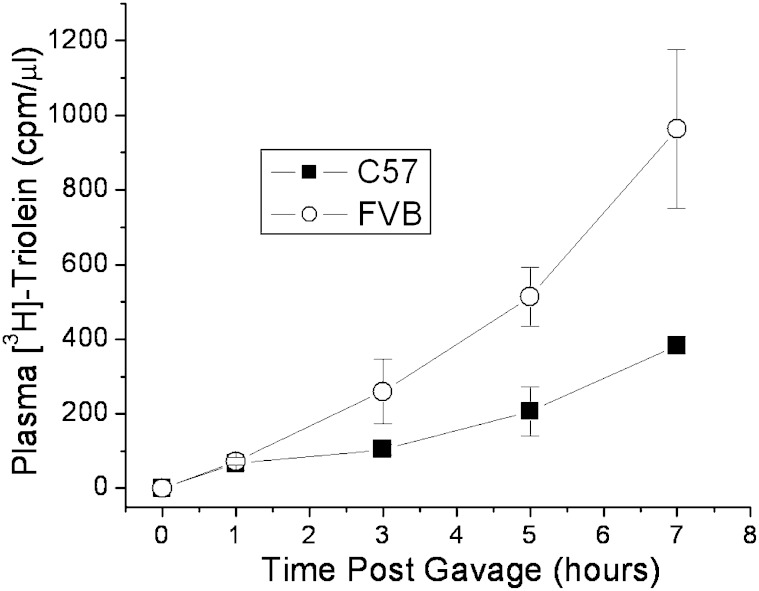
Because triglyceride makes up the bulk of the lipid found in newly secreted chylomicrons, we thought that the absorption of triglycerides might also be greater in the FVB mouse than in C57. Indeed, when mice are gavaged with olive oil containing labeled [3H]triolein after tyloxapol injection, more of the label appears in the plasma of the FVB mice over the 7 h time course (Fig. 3). This suggests a common mechanism influencing the differences in cholesterol and triglyceride absorption observed between the mouse strains.
Fig. 3.
Triglyceride absorption rates in FVB versus C57 mice following olive oil gavage. Nonfasted female C57 and FVB mice were injected retro-orbitally with 100 μl tyloxapol (10% v/v), followed by gavage with 2 μCi [3H]triolein in 200,μl olive oil. Plasma samples were collected over the course of 7 h, and plasma radiolabel was measured by liquid scintillation counting. Data represent mean ± standard deviation of triplicate mice.
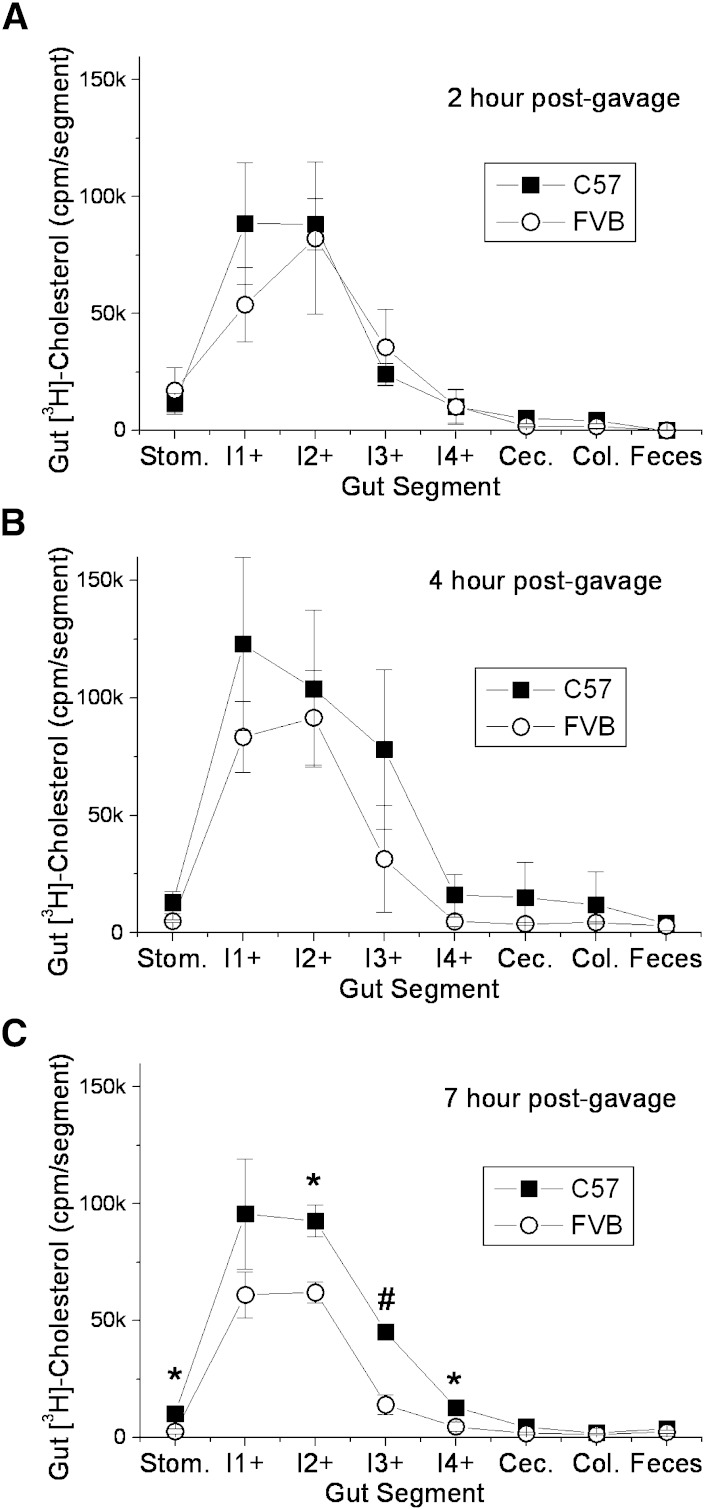
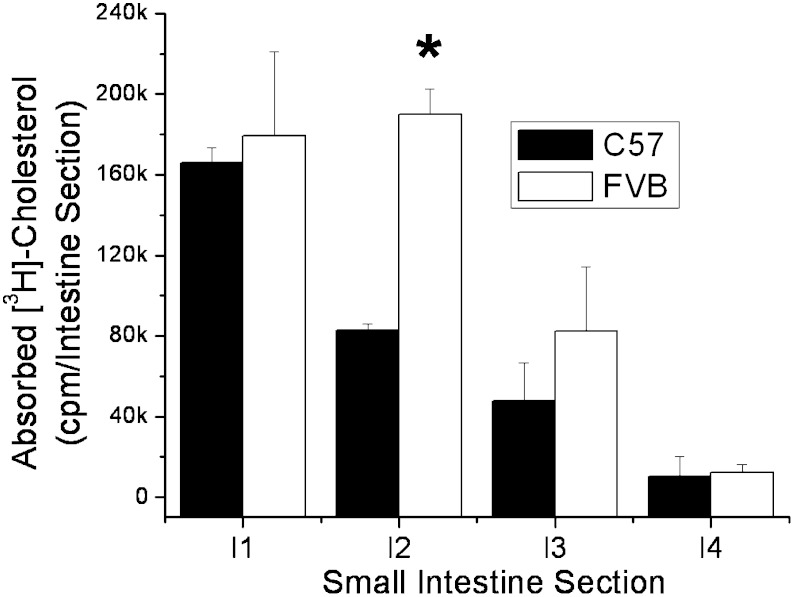
Kirby, Howles, and Hui (25) have previously described large differences in the rate of cholesterol absorption between SJL/J and 129P3/J mice. They also found that the appearance of triglyceride in the plasma was also greater in mice with the higher rate of cholesterol absorption. The higher levels of lipid absorption in SJL/J mice were found to be due to the rate of gastric emptying, which was slower in the 129 mice. However, due to the slower presentation of lipid to the enterocytes of the 129 mice, the 129 mice were then found to absorb greater levels of lipid over a 24 h time period, because the lipid retained in the stomach moved to the intestine and became available for uptake. In our experiments with C57 and FVB mice, the FVB mice had higher plasma [3H]cholesterol even after 24 h, suggesting that the rate of gastric emptying is not the major factor regulating lipid absorption by the enterocytes between these two mouse strains (see supplementary Fig. I). To examine this further, radioactivity remaining in the stomach and intestine and radioactivity in the feces in the cage were examined over the course of 7 h post gavage. By 2 h, more than 90% of the total gut radioactivity had exited the stomach of both mouse strains, demonstrating similar rates of gastric emptying (Fig. 4). No differences were noted in any of the gut segments at 2 or 4 h. However, by 7 h, the levels of [3H]cholesterol in segments 2, 3, and 4 of the FVB mouse (intestinal cells + luminal contents) were significantly lower than in the C57 mouse, indicating that in the FVB mouse, more of the absorbed cholesterol had passed through the enterocytes by this time (Fig. 4C). When the small intestine was washed of its luminal contents (7 h time point), the levels of cell-associated [3H]cholesterol were found to be greater in the FVB mouse intestinal segments, particularly for segment 2, indicating that the FVB enterocytes absorb more lipid from the lumen during that time frame, providing a greater lipid pool for chylomicron assembly and secretion into the circulation (Fig. 5). Unlabeled cholesterol levels were similar in the gut of the two mouse strains, suggesting that this mechanism is not affecting steady-state cholesterol levels within the intestine (data not shown).
Fig. 4.
[3H]cholesterol distribution throughout the gut of C57 and FVB mice following olive oil gavage. Nonfasted female C57 and FVB mice were injected retro-orbitally with 100 μl tyloxapol (7.5% v/v) followed by gavage with 2 μCi [3H]cholesterol in 200 μl olive oil. After 2, 4, or 7 h (panels A–C), the entire gut length (stomach to colon) and excreted feces were harvested and divided into segments, with the small intestine divided into four equal-length segments (I1+ to I4+). Each segment was dissolved in 1 ml 1N NaOH, and [3H]cholesterol levels were measured by liquid scintillation counting. Significant differences between strains are represented by * P < 0.05 or # P < 0.01.
Fig. 5.
Cholesterol absorption into C57 and FVB mouse intestinal cells following olive oil gavage. Nonfasted female C57 and FVB mice were injected retro-orbitally with 100 μl tyloxapol (10% v/v) followed by gavage with 2 μCi [3H]cholesterol in 200 μl olive oil. After 7 h, the small intestine was harvested and divided into four equal-length segments. Each segment was washed with 5 ml PBS to remove luminal contents, dissolved in 1 ml NaOH, and tissue [3H]cholesterol levels measured by liquid scintillation counting. Segment I1 is closest to the stomach. Significant differences between strains are represented by * P < 0.01.
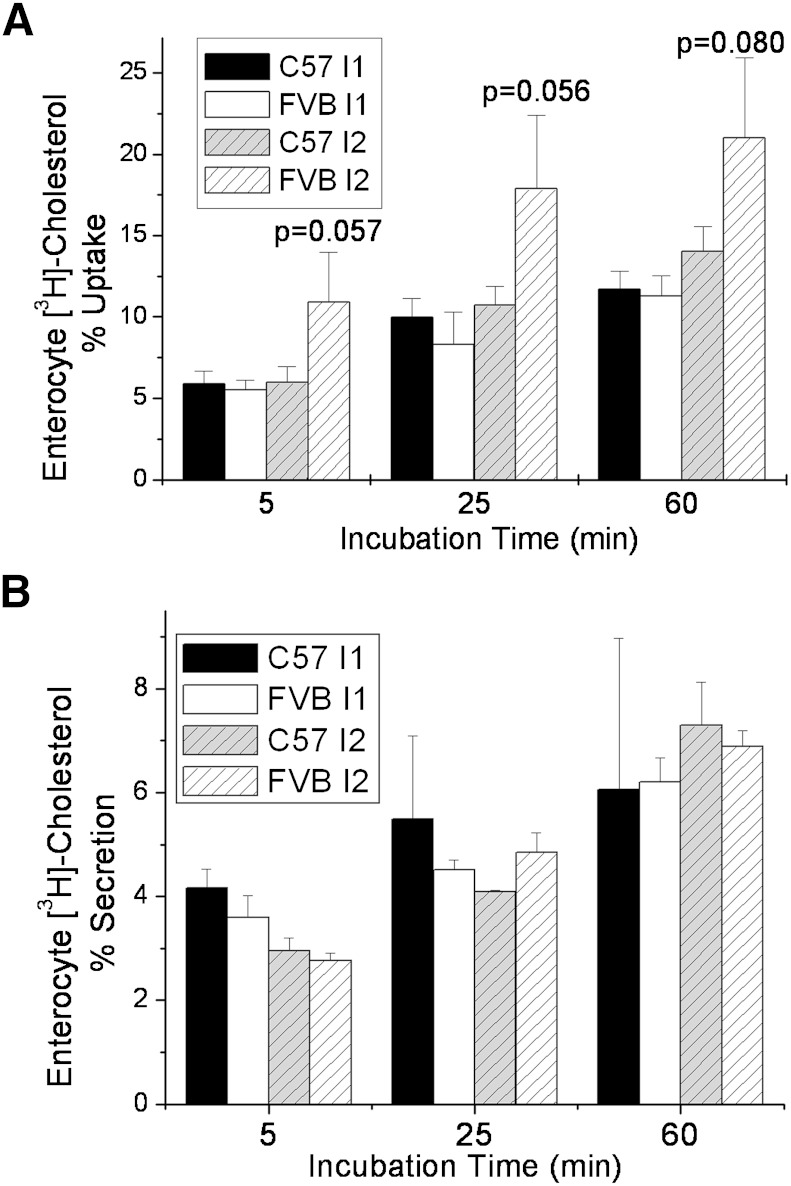
Primary enterocytes from both mouse strains were used to examine cholesterol absorption in cultured cells. No significant differences were noted in the percent [3H]cholesterol taken up by the enterocytes from intestinal segment I1 from the two mice (Fig. 6A). However, as early as 5 min after adding [3H]cholesterol-labeled micelles to primary enterocytes, the percent [3H]cholesterol found in segment I2 of the FVB small intestine was higher than in C57, although this difference did not quite reach significance in any of the repeated experiments. We also isolated primary enterocytes from mice gavaged with [3H]cholesterol and measured the secretion of cholesterol from the cells over time (Fig. 6B). Similar to what we observed with uptake of cholesterol by the cells, there was no significant difference in the percent of cellular radiolabeled cholesterol secreted by small intestinal segments 1 and 2 in the two strains of mice. Taken together, these data suggest that the difference in cholesterol absorption between the two mouse strains lies in the absorption from the lumen into the intestinal cells rather than in the secretion rate from the intestinal cells into the lymph.
Fig. 6.
Cholesterol absorption and secretion from C57 and FVB mouse primary enterocytes. A: Enterocytes from intestinal segments I1 and I2 harvested from nonfasted female C57 and FVB mice were incubated with bile salt micelles containing [3H]cholesterol for various times, and the cell-associated [3H]cholesterol levels were measured at several time points, as described in Materials and Methods. B: Nonfasted C57 and FVB mice were gavaged with 2 μCi [3H]cholesterol in 200 μl olive oil. After 3 h, enterocytes from intestinal segments I1 and I2 were washed, harvested, and plated in DMEM, and secretion of the cell-associated [3H]cholesterol levels were measured at several time points, as described in Materials and Methods. Data represent mean +/− standard deviation of enterocytes from triplicate mice. P values compare C57 I2 to FVB I2.
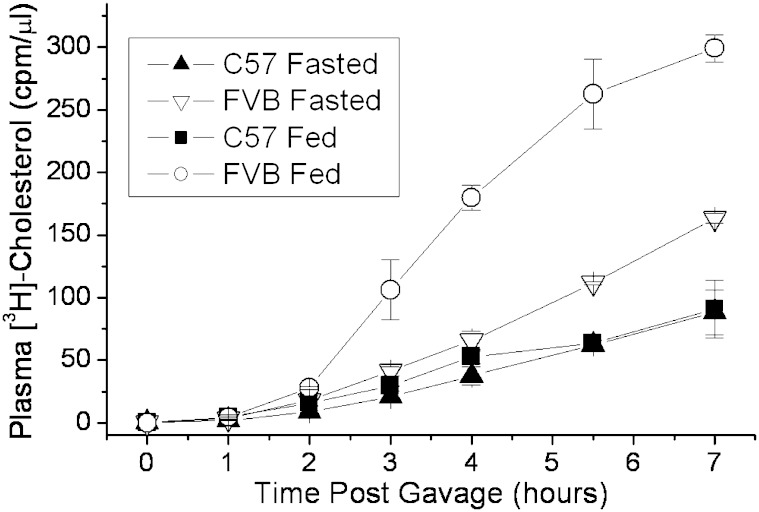
The factors influencing intestinal absorption of lipids may be influenced by the feeding state of the subject (29). We compared the cholesterol absorption of the C57 and FVB mice in both a fed and fasted state prior to [3H]cholesterol/olive oil gavage. The cholesterol absorption of FVB mice fed a chow diet prior to gavage was several-fold greater than those fasted 16 h prior to gavage, whereas the effect of feeding was not particularly notable in the C57 mice (Fig. 7). Although the difference in cholesterol absorption was more dramatic between the two mouse strains under the fed state, even under the fasted state, the difference was significant.
Fig. 7.
The effect of feeding versus fasting on cholesterol absorption in C57 and FVB mice. Female C57 and FVB mice were kept for 16 h with and without standard chow diet. The mice were then injected retro-orbitally with 100 μl tyloxapol (10% v/v), followed by gavage with 2 μCi [3H]cholesterol in 200 μl olive oil. Plasma samples were collected over the course of 7 h, and plasma radiolabel was measured by liquid scintillation counting. Data represent mean ± SD of replicate samples.
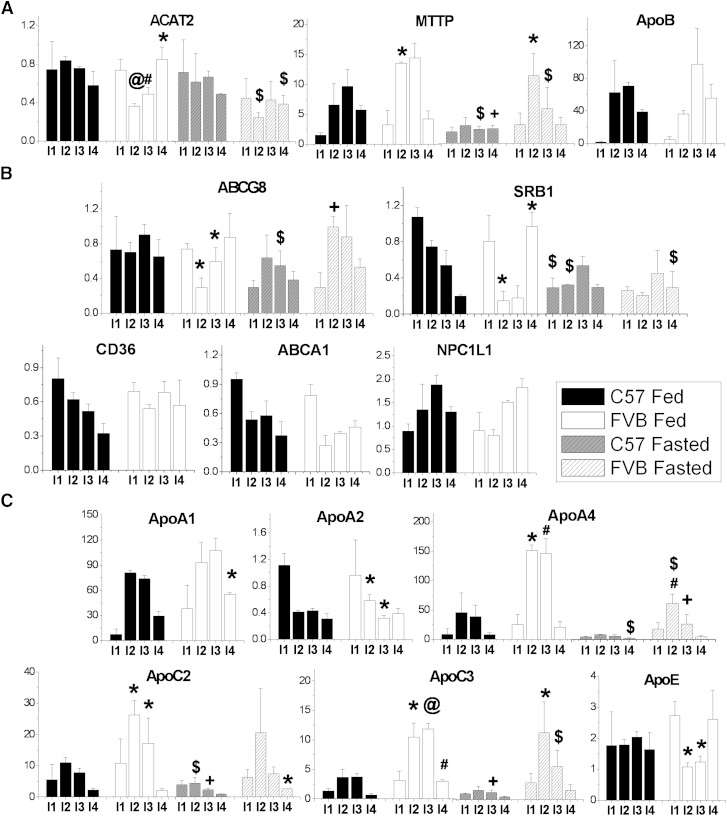
To begin to understand what factors may be regulating the differences in lipid absorption by the enterocytes, we measured the mRNA levels of likely candidates known to be involved in enterocyte lipid absorption throughout the length of the small intestine (Fig. 8). Of the mRNA known to be involved in chylomicron assembly (ACAT2, apoB, MTTP), MTTP was significantly higher in intestinal section 2 of the FVB mouse in both the fed and fasted state. Inversely, ACAT2 expression was higher in sections 2 and 3 in the C57 mouse (Fig. 8A). No differences were seen in apoB expression. The genes known to be involved in cholesterol transport into and out of the enterocytes were also tested. Expression of ABCG8, ABCA1, NPC1L1, and SRB1 were all higher in intestinal sections I2 and I3 of the C57 mouse, but the difference was only significant for ABCG8 and SRB1 (Fig. 8B). Intestinal segment 2 ABCG8 was significantly lower in the FVB mouse under the fed state when compared with the fasted FVB. There were no differences between the mouse strains in expression of the FA transporter CD36 in any of the intestinal sections. The largest differences in expression between the two mouse strains were in apoA-IV, apoC-II, and apoC-III, which were all much higher in intestinal sections I2 and I3 of the FVB mouse (Fig. 8C). Interestingly, apoA-IV and apoC-III lie in the same gene cluster as apoA-I, whose expression in FVB intestine was not significantly different from that in C57 in segments I2 or I3. Also, although apoC-II and apoE share the same gene cluster, there is an inverse pattern of expression when the two mice are compared. The effect of fasting on gene expression was most notable in MTTP, apoA-IV, ABCG8, and SRB1 expression levels.
Fig. 8.
Small intestine mRNA expression levels of various genes associated with lipid absorption/trafficking. The small intestine was harvested from triplicate nonfasted female mice 7 h following a 200 μl olive oil gavage (Fed) and from triplicate female mice fasted 16 h with no gavage (Fasted). Upon harvest, the small intestine was divided into four equal-length segments I1-I4 beginning at the stomach end. Each segment was washed with 5 ml PBS. The values are relative to the I1 segment of one nonfasted C57 mouse. Significant differences between strains are represented by * P < 0.05, # P < 0.005, and @ P < 0.0005. Significant differences between fed and fasted of the same strain are represented by $ P < 0.05 and + P < 0.005.
DISCUSSION
In this manuscript, we set out to explore the potential contribution of the intestine in accounting for the previously reported differences in HDL between C57 and FVB inbred mouse strains. FVB mice have twice the level of HDL-cholesterol, twice the apoA-II component, and an enrichment in apoC-III (3). Given the inverse relationship between plasma cholesterol levels and cardiovascular disease, it is important to understand the genetic factors that regulate these lipoprotein levels. The existence of a variety of inbred mouse strains that possess a wide range of steady-state plasma cholesterol levels provides a useful tool in investigating the genetic causes. The low HDL-cholesterol C57 mice are one of the inbred strains most susceptible to atherosclerotic lesion development whether by means of an atherogenic diet or by knockout of genes that promote lesion development (e.g., LDL receptor or apoE). On the other hand, the high-HDL cholesterol FVB strain is one of the more-atherosclerosis-resistant strains (30). These differences in atherosensitivity are seen even when HDL is not the major plasma lipoprotein, as, for example, in apoE-deficient mice in each strain (31). This may be taken to suggest a difference in total lipid homeostasis between the two mouse strains. Although we report important differences in lipid absorption and handling of absorbed lipids among the two strains, and suggest that these differences may contribute to the observed differences in HDL, we cannot offer a simple mechanistic correlation between these parameters, HDL levels, and atherosensitivity. It may be that primary intestinal lipoprotein particles delivered to the plasma are remodeled there, to give rise to plasma HDL, among other products. Another possibility is that the chylomicron remnants from the two mouse strains (having differing levels of cholesterol label) are taken up by the liver, and the absorbed cholesterol label is then secreted into the plasma within HDL particles.
Numerous studies have examined the variations in lipid absorption found among inbred mouse strains. Wang and Carey (18) have demonstrated that although different methods of measuring cholesterol absorption yield different absolute values, the interexperimental differences were consistent. In our study, we were primarily interested in the appearance of cholesterol in the plasma postgavage. We report a much greater appearance of cholesterol radiolabel in the plasma of FVB mice following oral lipid gavage. This results from the higher levels of cholesterol in the newly secreted chylomicrons, as evidenced by the greater plasma [3H]cholesterol levels in the FVB mouse when chylomicron catabolism is inhibited by tyloxapol. This difference in cholesterol absorption is reflected in the levels of cholesterol found in the plasma HDL. This is especially interesting, inasmuch as the FVB mouse is a high-HDL cholesterol mouse, with HDL-cholesterol levels double those of the C57 mouse (3). When equivalent levels of [3H]cholesterol-labeled chylomicrons are injected into the two mouse strains, within 3 h, the label is found in the plasma almost entirely associated with HDL and at equal levels. This suggests that the genetic factors that regulate the amounts of cholesterol secreted in the chylomicrons may be directly influencing the cholesterol levels of the plasma HDL.
To our knowledge, only one other group has compared the cholesterol absorption of the C57 and FVB mouse (17). In their study, the percent cholesterol absorption in the FVB mouse was not significantly different from that in the C57 mouse. Although the reasons behind the differences between our study and theirs are not completely clear, it may be related to the methodologies used for cholesterol delivery. In our study, the cholesterol is delivered in olive oil, which is mainly long-chain triglyceride, whereas their study used medium-chain triglycerides as a delivery vehicle. This is important, inasmuch as the delivery vehicle significantly affects the amount of cholesterol that is absorbed (18). Indeed, even in our experiments, although the absorption was always greater in the FVB mice, the extent of this difference was modified by the batch of olive oil that was used (data not shown). Additionally, their study looked at a single time point 3 days postgavage, whereas we have examined early time points (0–7 h), as well as throughout a 3 day duration (see supplementary Fig. I). When plasma [3H]cholesterol levels are compared 3 days post gavage using our method, FVB levels are still higher than those of C57, but not as predominantly as at the earlier time points, suggesting that the time frame of the absorption studies is an important consideration.
The mechanisms behind variations in cholesterol absorption between inbred mouse strains have not been elucidated. Although Wang, Paigen, and Carey (17) have shown that cholesterol absorption efficiencies are not determined by intestinal transit times, Kirby, Howles, and Hui (25) have shown that differences in cholesterol absorption in SJL/J and 129 mice are attributable to differences in the rate of gastric emptying. In that study, the 129 mice have a slower rate of gastric emptying and a slower appearance of cholesterol in the plasma, similar to what we see with the C57 mice. However, over longer time periods (24 h), the 129 mice accumulated higher plasma cholesterol from the gut. The authors suggest that the slower gastric emptying in the 129 mice presents less substrate for absorption initially, but the lower concentration presented to the lumen over the course of time allows for more cholesterol to be taken up. This is not occurring in the case of C57 versus FVB, inasmuch as the FVB mouse shows higher plasma [3H]cholesterol, not only at the initial time points, but also as far out as 72 h. The gastric emptying in the C57 and FVB mice was similar (Fig. 4), again indicating that the overall mechanism is different from that found in the SJL/J and 129 mice.
We attempted to ascertain whether some of the in vivo differences noted could be reproduced in cultured enterocytes. Though we cannot say that this was fully achieved, we did observe an increment in cholesterol uptake in the FVB enterocytes of the second intestinal segment, the segment most active in cholesterol absorption, derived from fed animals. This difference approached significance (P < 0.05) and agreed with the in vivo absorption data. We noted no significant differences in the lipid secretion from any of the enterocytes.
Our analysis of transcript levels of proteins involved in lipid absorption highlighted several genes of interest. We have to note the caveat that transcript levels represent just one measure of overall protein product steady state. Our transcript analysis is meant to provide a framework for future studies. That said, several of the genes expected to be involved in chylomicron formation were significantly higher in the FVB mouse, including those for the apolipoproteins A-IV, C-II, and C-III, as well as MTTP. Expression of the main apolipoprotein found on chylomicrons, apoB, was found at similar levels between the two mice, keeping in mind, however, that apoB, at least in the liver, is subjected to significant posttranscriptional regulation (32). ApoC-III and apoA-IV exist on the same gene locus, suggesting that the factors that are causing higher apoA-IV mRNA levels in the FVB mice may also be causing higher apoC-III mRNA levels. However, apoA-I also exists in the same gene cluster, but is expressed at similar levels in the intestine of both strains. Similarly, although apoE and apoC-II lie within the same gene cluster, apoC-II is expressed in the intestine at much higher levels in the FVB mouse, whereas intestinal apoE expression is slightly lower in FVB mice. The variation in level of the transcripts for these apolipoproteins between the two mouse strains is most probably not the root cause of the increased lipid absorption in the FVB mouse, inasmuch as lipid absorption is normal in both apoA-IV and apoC-III knockout mice (33, 34). However, that the differences in expression are not solely in response to lipid feeding is evidenced by the higher expression levels in the FVB mouse even under fasting conditions.
The lower MTTP levels in the C57 mouse under both fed and fasting conditions are notable, given the work by Xie et al. (35) in which MTTP was specifically knocked out in the intestine. These mice displayed decreased intestinal cholesterol absorption and chylomicron secretion with no change in apoB mRNA levels. We expected that the expression levels of MTTP would be increased to an even greater extent in the FVB mouse under fed conditions, given the increased cholesterol absorption (Fig. 7). Although the MTTP expression increased in certain segments of the FVB intestine under fed conditions, the expression levels also increased in the C57 intestine. The cholesterol absorption response to feeding status in the FVB mouse is most probably due to a different mechanism.
The differences in cholesterol absorption are not due to different levels of expression of NPC1L1. On the other hand, the expression pattern of ABCG8 mRNA suggests that it may be involved in regulating cholesterol absorption between the two mouse strains. Under fasted conditions, the levels in FVB intestine are higher than in those in C57. After feeding, the FVB expression levels drop, whereas the C57 levels increase. This change would be expected if the ABCG5/G8 heterodimer is functioning to export cholesterol out of the enterocyte and back into the lumen (22). The decreased expression levels in FVB during feeding would be expected to allow for higher levels of cholesterol to remain in the enterocyte for packaging and secretion in chylomicrons. The inverse would then be true, inasmuch as expression levels rise in C57 during feeding. Other known lipid transporters (ABCA1, SR-B1, and to some extent NPC1L1) followed the pattern of expression of ABCG8, with C57 exhibiting higher expression levels in the middle segments of the small intestine. What effect these differences have on lipid uptake remains to be elucidated. It is also not known whether there is one particular nuclear receptor that is controlling the multiple gene differences between the two mouse strains. The effect of lipid on gene expression in the intestine is documented, and nuclear receptors including SHP and the PPAR, LXR, and FXR families of receptors and SREBP have been implicated in this regulation (36). Future studies examining the effects of nuclear receptor antagonists on lipid uptake are merited.
The impact of the differences in lipid absorption between mouse strains may be the underlying cause of the significantly higher percent body fat of the FVB versus the C57 strains (2, 37). This is, in turn, probably related to the higher blood lipid levels (both cholesterol and triglyceride) in the FVB strain (3). The understanding of the full mechanism behind these differences may involve multiple gene variations between the two mouse strains and may ultimately provide new targets for regulation of lipid absorption. The relationship between these findings and the reported differences in HDL levels and atherosensitivity is complex. Further work will be required to elucidate these relationships.
Supplementary Material
Footnotes
This work was supported by a grant from the LeDucq Foundation.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures.
REFERENCES
- 1.Boden W. E. 2000. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High-Density Lipoprotein Intervention Trial. Am. J. Cardiol. 86: 19L–22L. [DOI] [PubMed] [Google Scholar]
- 2.Svenson K. L., Von Smith R., Magnani P. A., Suetin H. R., Paigen B., Naggert J. K., Li R., Churchill G. A., Peters L. L. 2007. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J. Appl. Physiol. 102: 2369–2378. [DOI] [PubMed] [Google Scholar]
- 3.Sontag T. J., Carnemolla R., Vaisar T., Reardon C. A., Getz G. S. 2012. Naturally occurring variant of mouse apolipoprotein A-I alters the lipid and HDL association properties of the protein. J. Lipid Res. 53: 951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paigen B., Morrow A., Brandon C., Mitchell D., Holmes P. 1985. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 57: 65–73. [DOI] [PubMed] [Google Scholar]
- 5.Dawson P. A., Rudel L. L. 1999. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 10: 315–320. [DOI] [PubMed] [Google Scholar]
- 6.Osono Y., Woollett L. A., Herz J., Dietschy J. M. 1995. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J. Clin. Invest. 95: 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNamara D. J., Kolb R., Parker T. S., Batwin H., Samuel P., Brown C. D., Ahrens E. H. 1987. Heterogeneity of cholesterol homeostasis in man. Response to changes in dietary fat quality and cholesterol quantity. J. Clin. Invest. 79: 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosner M. S., Lange L. G., Stenson W. F., Ostlund R. E. 1999. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 40: 302–308. [PubMed] [Google Scholar]
- 9.Sehayek E., Nath C., Heinemann T., McGee M., Seidman C. E., Samuel P., Breslow J. L. 1998. U-shape relationship between change in dietary cholesterol absorption and plasma lipoprotein responsiveness and evidence for extreme interindividual variation in dietary cholesterol absorption in humans. J. Lipid Res. 39: 2415–2422. [PubMed] [Google Scholar]
- 10.Beynen A. C., Meijer G. W., Lemmens A. G., Glatz J. F., Versluis A., Katan M. B., Van Zutphen L. F. 1989. Sterol balance and cholesterol absorption in inbred strains of rabbits hypo- or hyperresponsive to dietary cholesterol. Atherosclerosis. 77: 151–157. [DOI] [PubMed] [Google Scholar]
- 11.Overturf M. L., Smith S. A., Gotto A. M., Morrisett J. D., Tewson T., Poorman J., Loose-Mitchell D. S. 1990. Dietary cholesterol absorption, and sterol and bile acid excretion in hypercholesterolemia-resistant white rabbits. J. Lipid Res. 31: 2019–2027. [PubMed] [Google Scholar]
- 12.Van Zutphen L. F., Den Bieman M. G. 1981. Cholesterol response in inbred strains of rats, Rattus norvegicus. J. Nutr. 111: 1833–1838. [DOI] [PubMed] [Google Scholar]
- 13.Kirk E. A., Moe G. L., Caldwell M. T., Lernmark J. A., Wilson D. L., LeBoeuf R. C. 1995. Hyper- and hypo-responsiveness to dietary fat and cholesterol among inbred mice: searching for level and variability genes. J. Lipid Res. 36: 1522–1532. [PubMed] [Google Scholar]
- 14.Carter C. P., Howles P. N., Hui D. Y. 1997. Genetic variation in cholesterol absorption efficiency among inbred strains of mice. J. Nutr. 127: 1344–1348. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz M., Davis D. L., Vick B. R., Russell D. W. 2001. Genetic analysis of cholesterol accumulation in inbred mice. J. Lipid Res. 42: 1812–1819. [PubMed] [Google Scholar]
- 16.Schwarz M., Davis D. L., Vick B. R., Russell D. W. 2001. Genetic analysis of intestinal cholesterol absorption in inbred mice. J. Lipid Res. 42: 1801–1811. [PubMed] [Google Scholar]
- 17.Wang D. Q., Paigen B., Carey M. C. 2001. Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J. Lipid Res. 42: 1820–1830. [PubMed] [Google Scholar]
- 18.Wang D. Q., Carey M. C. 2003. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J. Lipid Res. 44: 1042–1059. [DOI] [PubMed] [Google Scholar]
- 19.Nassir F., Wilson B., Han X., Gross R. W., Abumrad N. A. 2007. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 282: 19493–19501. [DOI] [PubMed] [Google Scholar]
- 20.Nauli A. M., Nassir F., Zheng S., Yang Q., Lo C. M., Vonlehmden S. B., Lee D., Jandacek R. J., Abumrad N. A., Tso P. 2006. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 131: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altmann S. W., Davis H. R., Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maguire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 22.Abumrad N. A., Davidson N. O. 2012. Role of the gut in lipid homeostasis. Physiol. Rev. 92: 1061–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen T. M., Sawyer J. K., Kelley K. L., Davis M. A., Kent C. R., Rudel L. L. 2012. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53: 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirby R. J., Howles P. N., Hui D. Y. 2004. Rate of gastric emptying influences dietary cholesterol absorption efficiency in selected inbred strains of mice. J. Lipid Res. 45: 89–98. [DOI] [PubMed] [Google Scholar]
- 26.Paigen B., Svenson K., Peters L. 2012. Diet effects on plasma lipids and susceptibility to atherosclerosis in 43 inbred strains of mice on high-fat atherogenic diet (under pathogen-free conditions). MPD:9904. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, ME. Accessed October 30, 2012, at http://phenome.jax.org.
- 27.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanderson I. R., Naik S. 2000. Dietary regulation of intestinal gene expression. Annu. Rev. Nutr. 20: 311–338. [DOI] [PubMed] [Google Scholar]
- 30.Teupser D., Persky A. D., Breslow J. L. 2003. Induction of atherosclerosis by low-fat, semisynthetic diets in LDL receptor-deficient C57BL/6J and FVB/NJ mice: comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement). Arterioscler. Thromb. Vasc. Biol. 23: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 31.Dansky H. M., Charlton S. A., Sikes J. L., Heath S. C., Simantov R., Levin L. F., Shu P., Moore K. J., Breslow J. L., Smith J. D. 1999. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19: 1960–1968. [DOI] [PubMed] [Google Scholar]
- 32.Adeli K., Mohammadi A., Macri J. 1995. Regulation of apolipoprotein B biogenesis in human hepatocytes: posttranscriptional control mechanisms that determine the hepatic production of apolipoprotein B-containing lipoproteins. Clin. Biochem. 28: 123–130. [DOI] [PubMed] [Google Scholar]
- 33.Weinstock P. H., Bisgaier C. L., Hayek T., Aalto-Setala K., Sehayek E., Wu L., Sheiffele P., Merkel M., Essenburg A. D., Breslow J. L. 1997. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J. Lipid Res. 38: 1782–1794. [PubMed] [Google Scholar]
- 34.Jong M. C., Rensen P. C., Dahlmans V. E., van der Boom H., van Berkel T. J., Havekes L. M. 2001. Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J. Lipid Res. 42: 1578–1585. [PubMed] [Google Scholar]
- 35.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086. [DOI] [PubMed] [Google Scholar]
- 36.Ory D. S. 2004. Nuclear receptor signaling in the control of cholesterol homeostasis: have the orphans found a home? Circ. Res. 95: 660–670. [DOI] [PubMed] [Google Scholar]
- 37.Ackert-Bicknell C., Beamer W., Rosen C., Sundberg J. 2012. Aging study: Bone mineral density and body composition of 32 inbred strains of mice. MPD:25036. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, ME. Accessed October 30, 2012, at http://phenome.jax.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.